A Chemical Explanation for Variations in Antioxidant Capacity across Camellia sinensis L. Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sample Collection
2.3. Antioxidant Activity
2.4. Chemical Analyses
2.5. Data Analysis
3. Results
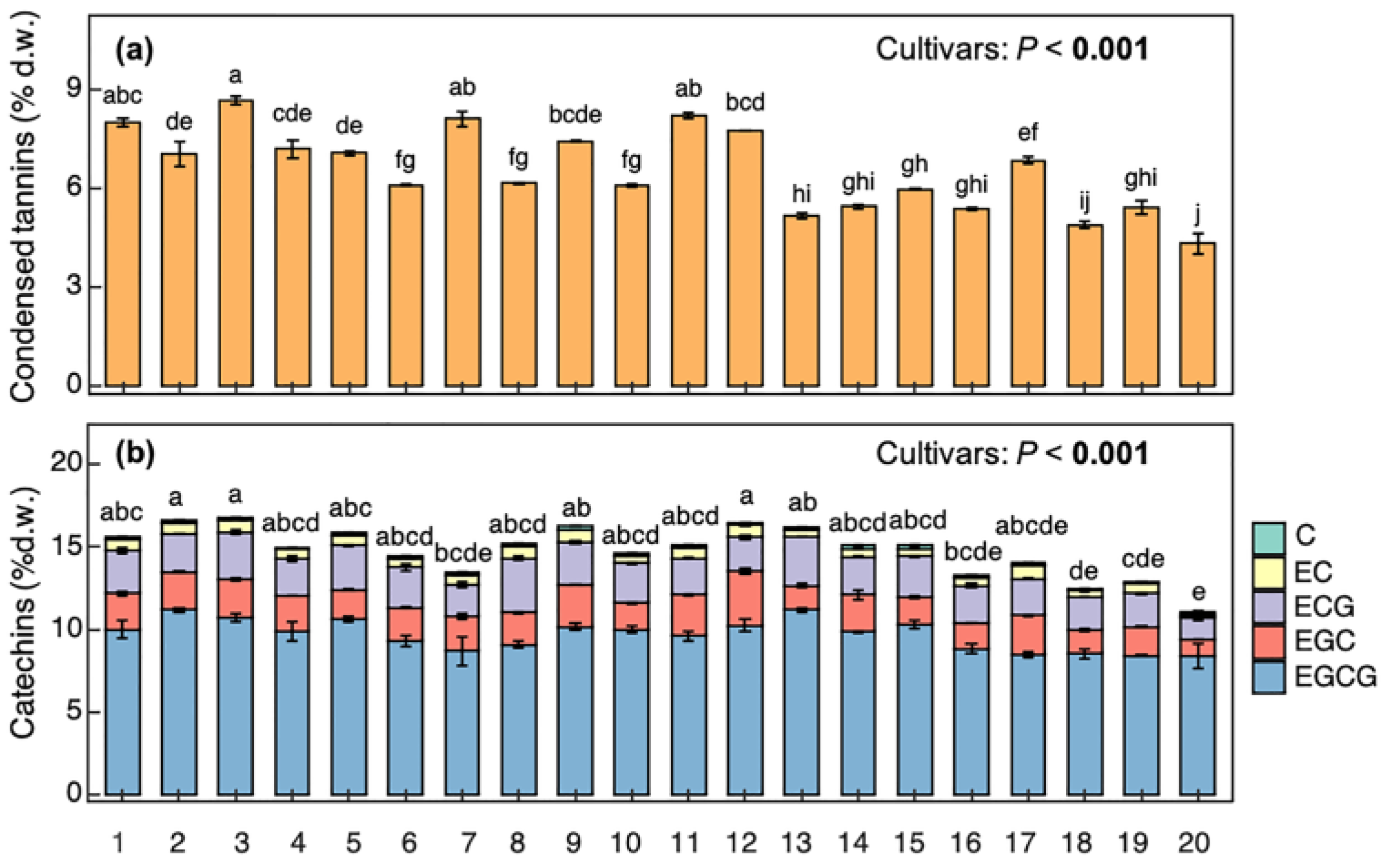
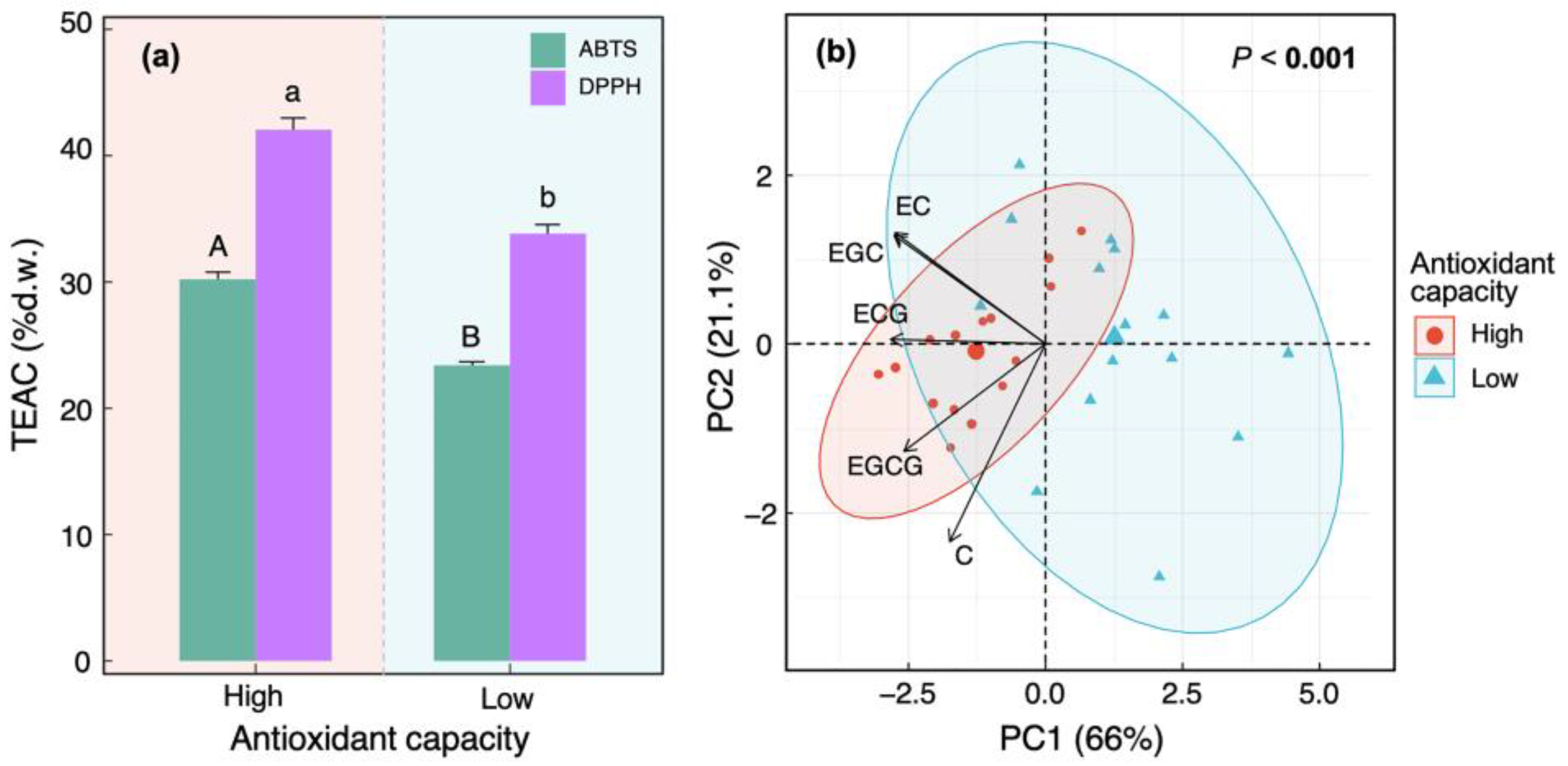
3.1. Variable Aspects of Antioxidant Capacity and Main Flavanol Compounds in Tea Leaves
3.2. Relationships between Antioxidant Capacity and Main Flavanol Compounds
3.3. Contributions of Main Catechin Components to Antioxidant Capacity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, N.; Mukhtar, H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, G.Y.; Meng, X.; Gan, R.Y.; Zhao, C.N.; Liu, Q.; Feng, Y.B.; Li, S.; Wei, X.L.; Atanasov, A.G.; Corke, H.; et al. Health functions and related molecular mechanisms of tea components: An update review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verified Market Research. Global Tea Extract Market Size by Type, by Form, by Application, by Geographic Scope and Forecast. 2021, p. 80758. Available online: https://www.verifiedmarketresearch.com/product/tea-extract-market/ (accessed on 11 October 2022).
- Forester, S.C.; Lamber, J.D. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol. Nutr. Food Res. 2011, 55, 844–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kottawa-Arachchi, J.D.; Gunasekare, M.T.K.; Ranatunga, M.A.B. Biochemical diversity of global tea Camellia sinensis (L.) O. Kuntze germplasm and its exploitation: A review. Genet. Resour. Crop Evol. 2019, 66, 259–273. [Google Scholar] [CrossRef]
- Ma, J.; Yao, M.; Liang, C. Research progress on germplasms of tea plant (Camellia sinensis). J. Tea Sci. 2015, 35, 11–16. [Google Scholar]
- Jin, J.Q.; Ma, J.Q.; Ma, C.L.; Yao, M.Z.; Chen, L. Determination of catechin content in representative Chinese tea germplasms. J. Agric. Food Chem. 2014, 62, 9436–9441. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Z.X. Variations of main quality components of tea genetic resources Camellia sinensis (L.) O. Kuntze preserved in the China national germplasm tea repository. Plant Food Hum. Nutr. 2005, 60, 31–35. [Google Scholar] [CrossRef]
- Almajano, M.P.; Carbo, R.; Jimenez, J.A.L.; Gordon, M.H. Antioxidant and antimicrobial activities of tea infusions. Food Chem. 2008, 108, 55–63. [Google Scholar] [CrossRef]
- Nibir, Y.M.; Sumit, A.F.; Akhand, A.A.; Ahsan, N.; Hossain, M.S. Comparative assessment of total polyphenols, antioxidant and antimicrobial activity of different tea varieties of Bangladesh. Asian Pac. J. Trop. Biomed. 2017, 7, 352–357. [Google Scholar] [CrossRef]
- Guo, Y.J.; Sun, L.Q.; Yu, B.Y.; Qi, J. An integrated antioxidant activity fingerprint for commercial teas based on their capacities to scavenge reactive oxygen species. Food Chem. 2017, 237, 645–653. [Google Scholar] [CrossRef]
- Tang, G.Y.; Zhao, C.N.; Xu, X.Y.; Gan, R.Y.; Cao, S.Y.; Liu, Q.; Shang, A.; Mao, Q.Q.; Li, H.B. Phytochemical composition and antioxidant capacity of 30 Chinese teas. Antioxidants 2019, 8, 180. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Edit. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.H.; Li, N.; Zhu, H.T.; Wang, D.; Yang, C.R.; Zhang, Y.J. Plant resources, chemical constituents, and bioactivities of tea plants from the genus Camellia section Thea. J. Agric. Food Chem. 2019, 67, 5318–5349. [Google Scholar] [CrossRef] [PubMed]
- Teixeria, A.M.; Sousa, C. A review on the biological activity of Camellia species. Molecules 2021, 26, 2178. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.F.; Zhang, Y.J.; Yang, C.R.; Chen, K.K.; Jiang, H.J. Phenolic antioxidants from green tea produced from Camellia taliensis. J. Agric. Food Chem. 2008, 56, 7517–7521. [Google Scholar] [CrossRef]
- Zhu, L.F.; Xu, M.; Zhu, H.T.; Wang, D.; Yang, S.X.; Yang, C.R.; Zhang, Y.J. New flavan-3-ol dimer from green tea produced from Camellia taliensis in the Ai-Lao mountains of Southwest China. J. Agric. Food Chem. 2012, 60, 12170–12176. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Shang, B.; Agathokleous, E.; Rubert-Nason, K.F.; Xu, Y.; Feng, Z. Nonlinear responses of foliar phenylpropanoids to increasing O3 exposure: Ecological implications in a Populus model system. Sci. Total Environ. 2021, 767, 144358. [Google Scholar] [CrossRef]
- Karori, S.M.; Wachira, F.N.; Ngure, R.M.; Mireji, P.O. Polyphenolic composition and antioxidant activity of Kenyan tea cultivars. J. Pharmacogn. Phytochem. 2014, 3, 105–116. [Google Scholar]
- Joshi, R.; Rana, A.; Gulati, A. Studies on quality of orthodox teas made from anthocyanin-rich tea clones growing in Kangra valley, India. Food Chem. 2015, 176, 357–366. [Google Scholar] [CrossRef]
- Chen, L.; Yao, M.Z.; Wang, X.C.; Yang, Y.J. Tea genetic resources in China. Int. J. Tea Sci. 2012, 8, 55–64. [Google Scholar]
- Han, W.Y.; Huang, J.G.; Li, X.; Li, Z.X.; Ahammed, G.J.; Yan, P.; Stepp, J.R. Altitudinal effects on the quality of green tea in east China: A climate change perspective. Eur. Food Res. Technol. 2017, 243, 323–330. [Google Scholar] [CrossRef]
- Deka, H.; Barman, T.; Dutta, J.; Devi, A.; Tamuly, P.; Pau, R.K.; Karak, T. Catechin and caffeine content of tea (Camellia sinensis L.) leaf significantly differ with seasonal variation: A study on popular cultivars in North East India. J. Food Compost. Anal. 2021, 96, 103684. [Google Scholar] [CrossRef]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1986, 25, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Erol, N.T.; Sari, F.; Polat, G.; Velioglu, Y.S. Antioxidant and antibacterial activities of various extracts and fractions of fresh tea leaves and green tea. Tarim. Bilim. Derg. 2009, 15, 371–378. [Google Scholar]
- Zhao, C.; Tang, G.; Cao, S.; Xu, X.; Gan, R.; Liu, Q.; Mao, Q.; Shang, A.; Li, H. Phenolic profiles and antioxidant activities of 30 tea infusions from green, black, oolong, white, yellow and dark teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- Henning, S.M.; Fajardo-Lira, C.; Lee, H.W.; Youssefian, A.A.; Go, V.L.W.; Heber, D. Catechin content of 18 teas and a green tea extract supplement correlates with the antioxidant capacity. Nutr. Cancer 2003, 45, 226–235. [Google Scholar] [CrossRef]
- Roy, M.K.; Koide, M.; Rao, T.P.; Okubo, T.; Ogasawara, Y.; Juneja, L.R. ORAC and DPPH assay comparison to assess antioxidant capacity of tea infusions: Relationship between total polyphenol and individual catechin content. Int. J. Food Sci. Nutr. 2010, 61, 109–124. [Google Scholar] [CrossRef]
- Ding, J.; Mei, S.; Gao, L.; Wang, Q.; Ma, H.; Chen, X. Tea processing steps affect chemical compositions, enzyme activities, and antioxidant and anti-inflammatory activities of coffee leaves. Food Front. 2022, 3, 505–516. [Google Scholar] [CrossRef]
- Enko, J.; Gliszczyńska-Świgło, A. Influence of the interactions between tea (Camellia sinensis) extracts and ascorbic acid on their antioxidant activity: Analysis with interaction indexes and isobolograms. Food Addi. Contam. 2015, 32, 1234–1242. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernandez, T.Y.; Regolo, L.; Alvarez-Suarez, J.M.; Navarro-Hortal, M.D.; Xiao, J.; Quiles, J.L.; Battino, M.; Giampieri, E. The reciprocal interaction between polyphenols and other dietary compounds: Impact on bioavailability, antioxidant capacity and other physico-chemical and nutritional parameters. Food Chem. 2022, 375, 131904. [Google Scholar] [CrossRef]
- Chen, Q.S.; Guo, Z.M.; Zhao, J.W. Identification of green tea’s (Camellia sinensis (L.)) quality level according to measurement of main catechins and caffeine contents by HPLC and support vector classification pattern recognition. J. Pharmaceut. Biomed. 2008, 48, 1321–1325. [Google Scholar] [CrossRef]
- van Acker, S.A.; van den Berg, D.J.; Tromp, M.N.; Griffioen, D.H.; van Bennekom, W.P.; van der Vijgh, W.J.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Xu, J.Z.; Yeung, S.Y.; Chang, Q.; Huang, Y.; Chen, Z.Y. Comparison of antioxidant activity and bioavailability of tea epicatechins with their epimers. Br. J. Nutr. 2004, 91, 873–881. [Google Scholar]
- Cai, Y.Z.; Mei, S.; Jie, X.; Luo, Q.; Corke, H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef]


| Cultivars | Total Phenolic Concentrations (% d.w.) | Origin |
|---|---|---|
| Biyun | 25.33 ± 0.76 | Zhejiang |
| Cui Yun | 25.20 ± 0.52 | Anhui |
| Da Hong Pao | 23.96 ± 0.56 | Fujian |
| Fuan Da Bai | 22.47 ± 0.96 | Fujian |
| Fuding Da Bai | 20.17 ± 1.00 | Fujian |
| Hanlv | 30.59 ± 1.70 | Zhejiang |
| Jin Guanyin | 23.00 ± 0.66 | Fujian |
| Jin Mudan | 31.10 ± 0.36 | Fujian |
| Jin Xuan | 21.06 ± 0.89 | Taiwan |
| Ju Hua Xiang | 25.64 ± 1.05 | Zhejiang |
| Longjing 43 | 26.38 ± 1.78 | Zhejiang |
| Longjing Changye | 23.64 ± 0.53 | Zhejiang |
| Tai Xiang Zi | 26.53 ± 0.62 | Zhejiang |
| Tie Guanyin | 29.86 ± 2.05 | Fujian |
| Zhongcha 102 | 24.38 ± 0.68 | Zhejiang |
| Zhongcha 108 | 22.87 ± 0.83 | Zhejiang |
| Zhongcha 111 | 25.81 ± 0.10 | Zhejiang |
| Zhongcha 302 | 25.44 ± 1.00 | Zhejiang |
| Zhonghuang No. 1 | 26.29 ± 2.15 | Zhejiang |
| Zhonghuang No. 2 | 25.93 ± 0.77 | Zhejiang |
| Dependent Variable | Compounds | Estimate | SE | df | p-Value | % Variance Explained |
|---|---|---|---|---|---|---|
| DPPH TEAC | C | −0.10 | 0.34 | 59 | 0.778 | 56.03 |
| EC | 0.26 | 0.72 | 59 | 0.714 | ||
| ECG | 1.15 | 0.51 | 59 | 0.028 | ||
| EGC | 0.77 | 0.62 | 59 | 0.216 | ||
| EGCG | 1.89 | 0.46 | 59 | <0.001 | ||
| ABTS TEAC | C | −0.18 | 0.20 | 59 | 0.370 | 54.94 |
| EC | 0.20 | 0.46 | 59 | 0.663 | ||
| ECG | 0.56 | 0.32 | 59 | 0.084 | ||
| EGC | 0.65 | 0.38 | 59 | 0.087 | ||
| EGCG | 1.23 | 0.30 | 59 | <0.001 |
| Catechin Components | High-Antioxidative Cultivar (% d.w.) | Low-Antioxidative Cultivar (% d.w.) | Contribution (%) |
|---|---|---|---|
| EGCG | 10.48 | 8.53 | 57.0 |
| ECG | 2.14 | 1.62 | 18.0 |
| EGC | 2.54 | 1.97 | 17.5 |
| EC | 0.65 | 0.49 | 6.5 |
| C | 0.16 | 0.14 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wu, Y.; Zhang, L.; Hasan, M.K.; Zhang, L.; Yan, P.; Fu, J.; Han, W.; Li, X. A Chemical Explanation for Variations in Antioxidant Capacity across Camellia sinensis L. Cultivars. Forests 2023, 14, 249. https://doi.org/10.3390/f14020249
Li Z, Wu Y, Zhang L, Hasan MK, Zhang L, Yan P, Fu J, Han W, Li X. A Chemical Explanation for Variations in Antioxidant Capacity across Camellia sinensis L. Cultivars. Forests. 2023; 14(2):249. https://doi.org/10.3390/f14020249
Chicago/Turabian StyleLi, Zhengzhen, Yaxian Wu, Lan Zhang, Md. Kamrul Hasan, Liping Zhang, Peng Yan, Jianyu Fu, Wenyan Han, and Xin Li. 2023. "A Chemical Explanation for Variations in Antioxidant Capacity across Camellia sinensis L. Cultivars" Forests 14, no. 2: 249. https://doi.org/10.3390/f14020249






