Hydrological Properties of Soil and Litter Layers of Four Forest Types Restored in the Gully Erosion Area of Latosol in South China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Litter and Soil Samples Collection
2.3. Laboratory Analyses
2.4. Comprehensive Evaluation of Water Conservation Capacity
2.5. Statistical Analysis
3. Results
3.1. Litter Thickness and Mass
3.2. Rm, Weff, and Wmax
3.3. Variations in Water-Holding Capacity of Litter
3.4. Variations in Litter Water Absorption Rate
3.5. Variations in Soil Water-Holding Capacity
3.6. Comprehensive Evaluation of Water Conservation Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borrelli, P.; Robinson, D.A.; Fleischer, L.R.; Lugato, E.; Ballabio, C.; Alewell, C.; Meusburger, K.; Modugno, S.; Schütt, B.; Ferro, V. An assessment of the global impact of 21st century land use change on soil erosion. Nat. Commun. 2017, 8, 2013. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, L.; Ge, P.; Lu, X.; Wei, Y.; Cai, C.; Wang, J. Structure degradation induced by wetting and drying cycles for the hilly granitic soils in collapsing gully erosion areas. Forests 2022, 13, 1426. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, T.; Liu, H.; Ge, P.; Xia, J.; Dai, C.; Zhang, W.; Zhao, X. Effects of AM fungi and grass strips on soil erosion characteristics in red sandstone erosion areas in Southern China. Forests 2022, 13, 1351. [Google Scholar] [CrossRef]
- Ahmad, N.N.S.B.; Mustafa, F.B.; Yusoff, S.Y.M.; Didams, G. A systematic review of soil erosion control practices on the agri-cultural land in Asia. Int. Soil Water Conserv. Res. 2020, 8, 103–115. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Y.; Wang, T.W.; Li, J.W.; Yang, J.W.; Luo, M.Y. Have anthropogenic factors miti-gated or intensified soil erosion over the past three decades in South China? J. Environ. Manag. 2022, 302, 114093. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, D.; Huang, Z.; Fu, Z.; Liu, J.; Hu, Y. Characteristics of soil and foliar N and P concentrations and stoichiometric ratio along restoration ages of Pinus massoniana plantations in red soils erosion regions of southern China. Chin. J. Appl. Environ. Biol. 2019, 25, 768–775. (In Chinese) [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, Q.; Yang, W.; Zhao, J. Characteristics of soil ecological stoichiometry of different vegetation types in ephemeral gully of forestland in Red Soil Region. Res. Soil Water Conserv. 2020, 27, 60–65. (In Chinese) [Google Scholar] [CrossRef]
- Liang, Y.; Li, D.; Lu, X.; Xuan, Y.; Pan, X.; Mu, H.; Shi, D.; Zhang, B. Soil erosion changes over the past five decades in the red soil region of southern China. J. Mt. Sci.-Engl. 2010, 7, 92–99. [Google Scholar] [CrossRef]
- Mao, Y.T.; Hu, W.; Chau, H.W.; Lei, B.K.; Di, H.J.; Chen, A.Q.; Hou, M.T.; Whitley, S. Combined cultivation pattern reduces soil erosion and nutrient loss from sloping farmland on red soil in Southwestern China. Agronomy 2020, 10, 1071. [Google Scholar] [CrossRef]
- Pereira, L.C.; Balbinot, L.; Lima, M.T.; Bramorski, J.; Tonello, K.C. Aspects of forest restoration and hydrology: The hydrological function of litter. J. For. Res. 2022, 33, 543–552. [Google Scholar] [CrossRef]
- Crouzeilles, R.; Curran, M.; Ferreira, M.S.; Lindenmayer, D.B.; Grelle, C.E.V.; Rey Benayas, J.M. A global meta-analysis on the ecological drivers of forest restoration success. Nat. Commun. 2016, 7, 11666. [Google Scholar] [CrossRef] [PubMed]
- Bonner, M.T.L.; Herbohn, J.; Gregorio, N.; Pasa, A.; Avela, M.S.; Solano, C.; Moreno, M.O.M.; Almendras-Ferraren, A.; Wills, J.; Shoo, L.P.; et al. Soil organic carbon recovery in tropical tree plantations may depend on restoration of soil microbial composition and function. Geoderma 2019, 353, 70–80. [Google Scholar] [CrossRef]
- Bastin, J.-F.; Finegold, Y.; Garcia, C.; Mollicone, D.; Rezende, M.; Routh, D.; Zohner, C.M.; Crowther, T.W. The global tree restoration potential. Science 2019, 365, 76–79. [Google Scholar] [CrossRef]
- Yan, M.; Fan, L.; Wang, L. Restoration of soil carbon with different tree species in a post-mining land in eastern Loess Plateau, China. Ecol. Eng. 2020, 158, 106025. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, D.; Deng, Y.; Ding, S.; Cai, C.; Huang, Z. Soil physicochemical properties and fertility evolution of permanent gully during ecological restoration in granite hilly region of South China. Forests 2021, 12, 510. [Google Scholar] [CrossRef]
- Ran, S.; Jin, J. Evolvement and control of vulnerable ecological region―A case study in ongniud banner and aohan banner, inner mongolia. Chinese Geogr. Sci. 2004, 14, 135–141. [Google Scholar] [CrossRef]
- Liu, G.; Shangguan, Z.; Yao, W.; Yang, Q.; Zhao, M.; Dang, X.; Guo, M.; Wang, G.; Wang, B. Ecological Effects of Soil Conservation in Loess Plateau. Bull. Chin. Acad. Sci. 2017, 32, 11–19. (In Chinese) [Google Scholar] [CrossRef]
- Ma, X.; Zhao, C.; Zhu, J. Aggravated risk of soil erosion with global warming—A global meta-analysis. Catena 2021, 200, 105129. [Google Scholar] [CrossRef]
- Kumar, R.; Bhardwaj, A.K.; Rao, B.K.; Vishwakarma, A.K.; Kakade, V.; Dinesh, D.; Singh, G.; Kumar, G.; Pande, V.C.; Bhatnagar, P.R.; et al. Soil loss hinders the restoration potential of tree plantations on highly eroded ravine slopes. J. Soil. Sediment. 2021, 21, 1232–1242. [Google Scholar] [CrossRef]
- Poesen, J.; Nachtergaele, J.; Verstraeten, G.; Valentin, C. Gully erosion and environmental change: Importance and research needs. Catena 2003, 50, 91–133. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, T.; Liu, B.; Liu, G.; Wilson, G.V. Effects of gully erosion and gully filling on soil depth and crop production in the black soil region, northeast China. Environ. Earth Sci. 2013, 68, 1723–1732. [Google Scholar] [CrossRef]
- Mbaya, L.A.; Ayuba, H.K.; Abdullahi, J. An assessment of gully erosion in gombe town, gombe state, Nigeria. J. Geogr. Geol. 2012, 4, 110–121. [Google Scholar] [CrossRef]
- Tang, F.; Yao, Y.; Song, J.; Wang, C.; Liu, Y. Interactive influence of soil erosion and cropland revegetation on soil enzyme activities and microbial nutrient limitations in the Loess Hilly-Gully Region of China. Agronomy 2022, 12, 2796. [Google Scholar] [CrossRef]
- Austin, A.T.; Vivanco, L. Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 2006, 442, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Mackay, D.S.; Band, L.E. Forest ecosystem processes at the watershed scale: Dynamic coupling of distributed hydrology and canopy growth. Hydrol. Process. 1997, 11, 1197–1217. [Google Scholar] [CrossRef]
- Ilek, A.; Kucza, J.; Szostek, M. The effect of stand species composition on water storage capacity of the organic layers of forest soils. Eur. J. Forest Res. 2015, 134, 187–197. [Google Scholar] [CrossRef]
- Gomyo, M.; Kuraji, K. Effect of the litter layer on runoff and evapotranspiration using the paired watershed method. J. Forest Res. 2016, 21, 306–313. [Google Scholar] [CrossRef]
- Chen, S.; Cao, T.; Tanaka, N.; Gao, T.; Zhu, L.; Zou, C. Hydrological properties of litter layers in mixed forests in Mt. Qinling, China. iForest 2018, 11, 243–250. [Google Scholar] [CrossRef]
- Ilek, A.; Szostek, M.; Mikołajczyk, A.; Rajtar, M. Does mixing tree species affect water storage capacity of the forest floor? Laboratory test of pine-oak and fir-beech litter layers. Forests 2021, 12, 1674. [Google Scholar] [CrossRef]
- Bai, Y.X.; Zhou, Y.C.; Zhang, X.Y.; Du, J.J. Water conservation capacity of litter and soil in mixed plantation of Pinus massoniana and Broadleaved Trees. Sci. Silvae Sin. 2021, 57, 24–36. (In Chinese) [Google Scholar] [CrossRef]
- Guevara-Escobar, A.; Gonzalez-Sosa, E.; Ramos-Salinas, M.; Hernandez-Delgado, G.D. Experimental analysis of drainage and water storage of litter layers. Hydrol. Earth Syst. Sci. 2007, 11, 1703–1716. [Google Scholar] [CrossRef]
- Zagyvai-Kiss, K.A.; Kalicz, P.; Szilágyi, J.; Gribovszki, Z. On the specific water holding capacity of litter for three forest ecosystems in the eastern foothills of the Alps. Agr. Forest Meteorol. 2019, 278, 107656. [Google Scholar] [CrossRef]
- Cheng, C.; He, K.N.; Yu, G.F.; Chai, S.X. Comparative study on water conservation capacity of different forest types of artificial forest in arid and semi-arid area. Acta Ecol. Sin. 2021, 41, 1979–1990. (In Chinese) [Google Scholar] [CrossRef]
- Wang, C.; Zhao, C.; Xu, Z.; Wang, Y.; Peng, H. Effect of vegetation on soil water retention and storage in a semi-arid alpine forest catchment. J. Arid Land 2013, 5, 207–219. [Google Scholar] [CrossRef]
- Dunkerley, D. Percolation through leaf litter: What happens during rainfall events of varying intensity? J. Hydrol. 2015, 525, 737–746. [Google Scholar] [CrossRef]
- Pang, X.; Bao, W. Effect of substituting plantation species for native shrubs on the water-holding characteristics of the forest floor on the eastern Tibetan Plateau. J Resour. Ecol. 2011, 2, 217–224. [Google Scholar]
- Li, Y.; Li, B.; Zhang, X.; Chen, J.J.; Zhan, F.D.; Guo, X.H.; Zu, Y.Q. Differential water and soil conservation capacity and associated processes in four forest ecosystems in Dianchi Watershed, Yunnan Province, China. J. Soil Water Conserv. 2015, 70, 198–206. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhang, H.; Xia, Y.; Xiao, J.; Wu, Y. Research on litter hydrology characteristic of typical vegetation in Jinyun mountain in Chongqing City. J. Soil Water Conserv. 2004, 4, 41–44. (In Chinese) [Google Scholar] [CrossRef]
- Keith, D.M.; Johnson, E.A.; Valeo, C. A hillslope forest floor (duff) water budget and the transition to local control. Hydrol. Process. 2010, 24, 2738–2751. [Google Scholar] [CrossRef]
- Neris, J.; Tejedor, M.; Rodríguez, M.; Fuentes, J.; Jiménez, C. Effect of forest floor characteristics on water repellency, infiltration, runoff and soil loss in Andisols of Tenerife (Canary Islands, Spain). Catena 2013, 108, 50–57. [Google Scholar] [CrossRef]
- Tu, Z.; Chen, S.; Ruan, D.; Chen, Z.; Huang, Y.; Chen, J. Differential hydrological properties of forest litter layers in artificial afforestation of eroded areas of latosol in China. Sustainability 2022, 14, 14869. [Google Scholar] [CrossRef]
- Sato, Y.; Kumagai, T.O.; Kume, A.; Otsuki, K.; Ogawa, S. Experimental analysis of moisture dynamics of litter layers—The effects of rainfall conditions and leaf shapes. Hydrol. Process. 2004, 18, 3007–3018. [Google Scholar] [CrossRef]
- Sun, J.; Yu, X.; Wang, H.; Jia, G.; Zhao, Y.; Tu, Z.; Deng, W.; Jia, J.; Chen, J. Effects of forest structure on hydrological processes in China. J. Hydrol. 2018, 561, 187–199. [Google Scholar] [CrossRef]
- Farahnak, M.; Mitsuyasu, K.; Otsuki, K.; Shimizu, K.; Kume, A. Factors determining soil water repellency in two coniferous plantations on a hillslope. Forests 2019, 10, 730. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Y.; Liu, P.; Zhang, Q.; Njoroge, B.; Zhou, Q.; Gan, X.; Zhang, W.; Li, Y. Soil moisture dominated the temporal dynamics of litter moisture content in subtropical forests: A 7-year observation in south China. J. Hydrol.-Reg. Stud. 2022, 41, 101102. [Google Scholar] [CrossRef]
- Hua, W.; Jianli, Z.; Lifei, Y.; Lingbin, Y.; Congjun, Y.; Tengyong, L. Study on water conservation capacity of litter from different types of forest in Caohai Basin. Meteorol. Environ. Res. 2013, 4, 17–22, 26. [Google Scholar]
- Zhou, Q.; Keith, D.M.; Zhou, X.; Cai, M.; Cui, X.; Wei, X.; Luo, Y. Comparing the water-holding characteristics of broadleaved, coniferous, and mixed forest litter layers in a Karst Region. Mt. Res. Dev. 2018, 38, 220–229. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, D.; Lei, Y.; Su, K.; Wang, G.; Ma, H. Hydrological characteristics of litter in different forest succession stages at Liuxihe Watershed, southern China. Front. For. China 2009, 4, 317–322. [Google Scholar] [CrossRef]
- Lin, Y. Present situation and countermeasures of soil and water conservation in Hainan Province. Soil Water Conserv. China 2015, 3, 7–9. (In Chinese) [Google Scholar] [CrossRef]
- Carnol, M.; Bazgir, M. Nutrient return to the forest floor through litter and throughfall under 7 forest species after conversion from Norway spruce. For. Ecol. Manag. 2013, 309, 66–75. [Google Scholar] [CrossRef]
- Lentz, R.D.; Ippolito, J.A. Biochar and manure affect calcareous soil and corn silage nutrient concentrations and uptake. J. Environ. Qual. 2014, 43, 775. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, F.; Chen, Y.; Hu, Z. Optimal kinematics design of macpherson suspension: Integrated use of grey relational analysis and improved entropy weight method. J. Harbin Inst. Technol. (New Ser.) 2022, 29, 41–51. [Google Scholar]
- Acharya, B.S.; Stebler, E.; Zou, C.B. Monitoring litter interception of rainfall using leaf wetness sensor under controlled and field conditions. Hydrol. Process. 2017, 31, 240–249. [Google Scholar] [CrossRef]
- Martin, W.K.E.; Timmer, V.R. Capturing spatial variability of soil and litter properties in a forest stand by landform segmentation procedures. Geoderma 2006, 132, 169–181. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Y.; Du, J.; Zhang, X.; Di, N. Effects of a broadleaf-oriented transformation of coniferous plantations on the hydrological characteristics of litter layers in subtropical China. Glob. Ecol. Conserv. 2021, 25, e01400. [Google Scholar] [CrossRef]
- Dong, H.; Yang, C.; Su, C.; Cao, H. Litter and Soil Hydrological Effects of Five No-commercial Forests in Dongguan. J. Soil Water Conserv. 2021, 35, 144–149, 160. (In Chinese) [Google Scholar] [CrossRef]
- Levia, D.F.; Bollinger, W.C.; Hrabik, R.A. Evaporation of intercepted precipitation from fruit litter of Liquidambar styraciflua L. (sweetgum) in a clearing as a function of meteorological conditions. Int. J. Biometeorol. 2005, 49, 325–331. [Google Scholar] [CrossRef] [PubMed]

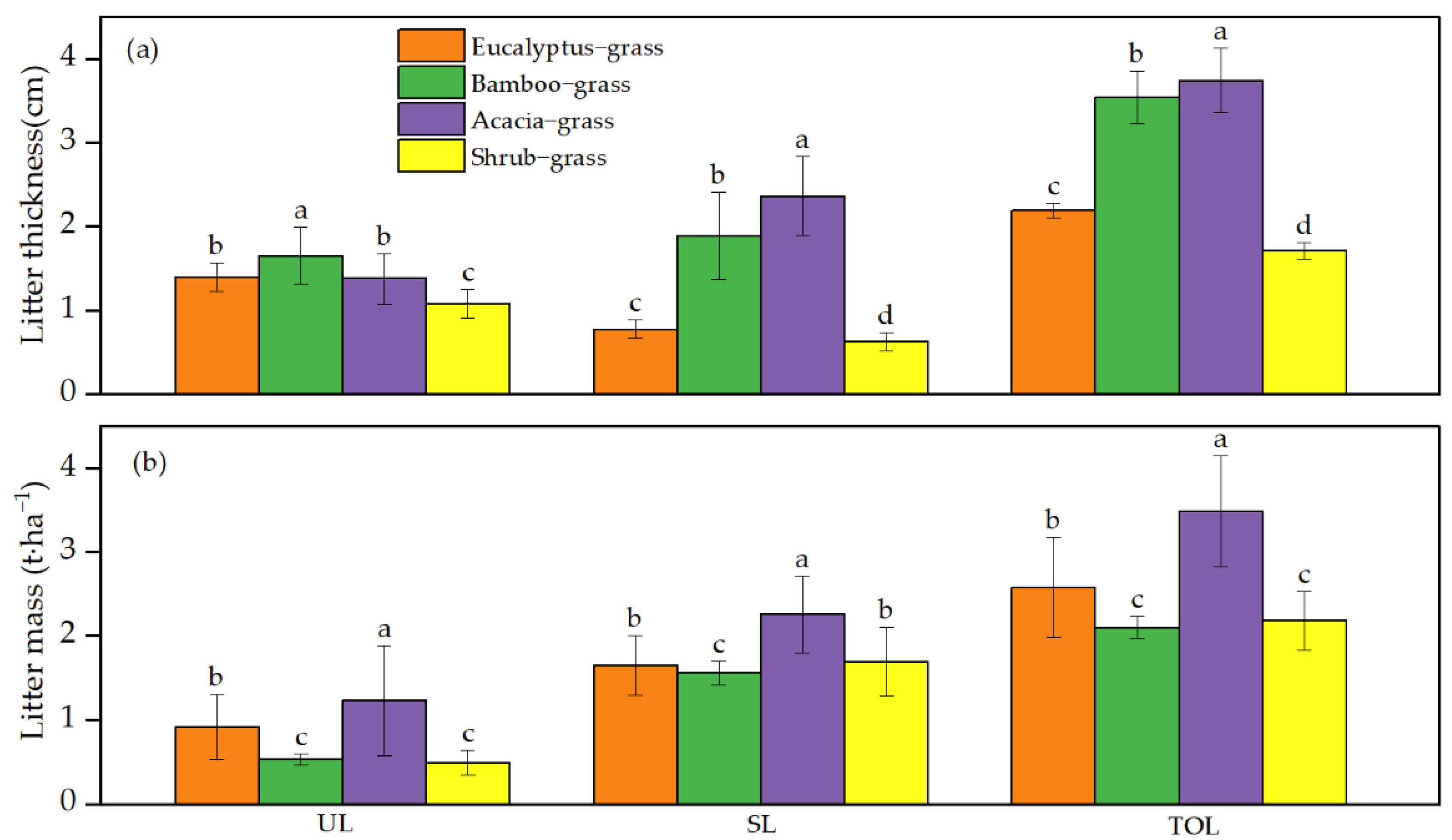
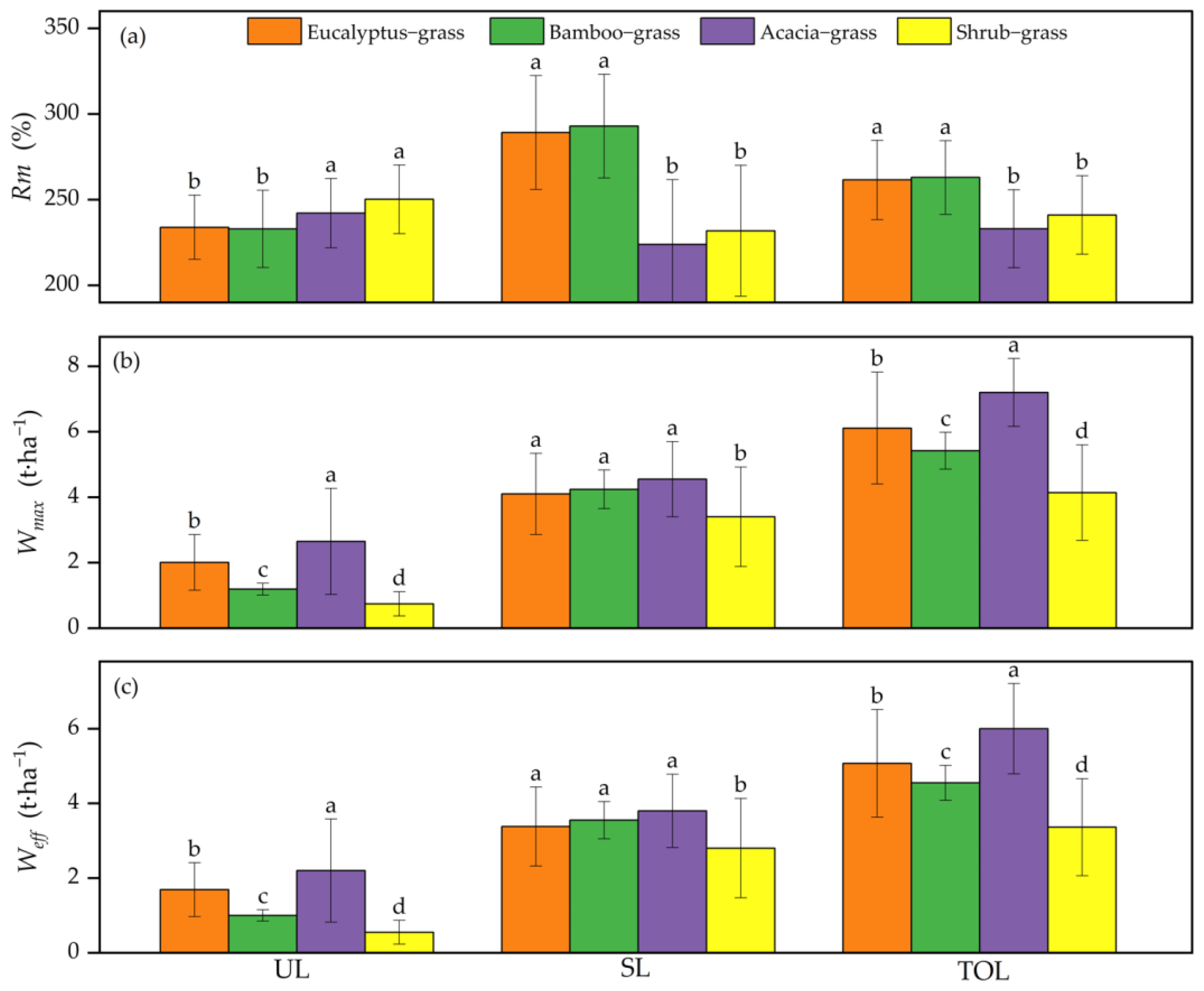
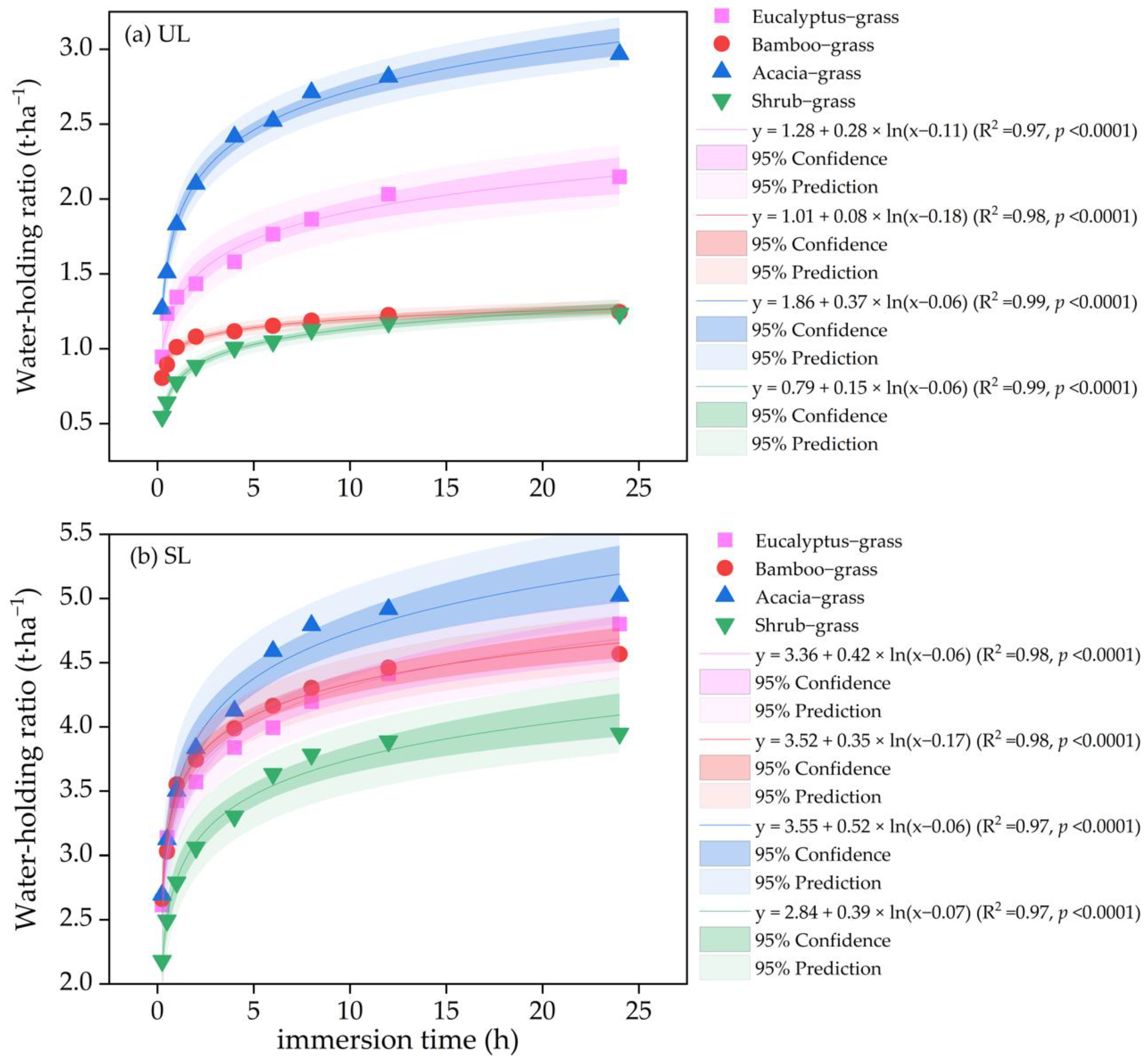
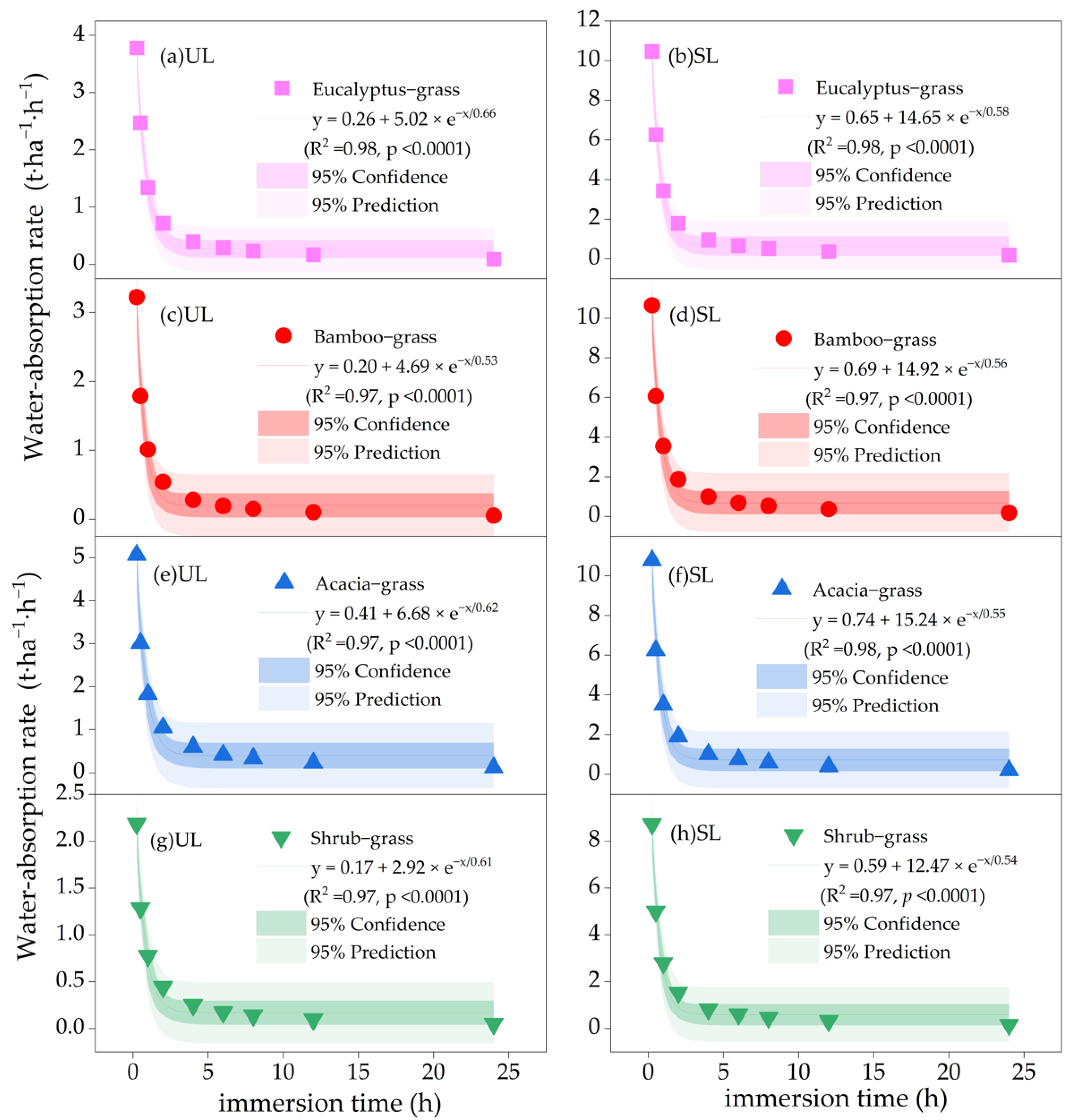
| Forest Type | Dominant Tree Species | Stand Age (Years) | Average Tree Height (m) | Average Diameter at Breast Height (cm) | Tree Density (Trees·ha−1) | Canopy Density | Slope Gradient (°) |
|---|---|---|---|---|---|---|---|
| Eucalyptus–grass | E. robusta | 10 | 10.95 ± 2.15 | 10.10 ± 1.31 | 2500 | 0.53 | 2~5 |
| Bamboo–grass | Bambusa oldhamii | 10 | 3.52 ± 0.71 | 6.90 ± 0.32 | 2500 | 0.42 | 2~5 |
| Acacia–grass | A. mangium | 10 | 8.51 ± 1.13 | 8.70 ± 0.52 | 2500 | 0.75 | 2~5 |
| Shrub–grass | Melastoma candidum | 10 | 1.52 ± 0.25 | 1.70 ± 0.21 ★ | 2500 | 0.35 | 2~5 |
| Variable | Factor | df | SS | MS | F | p |
|---|---|---|---|---|---|---|
| Litter thickness | tree | 3 112 | 17.48 | 5.83 | 68.36 | <0.001 *** |
| layer | 1 112 | 1.80 | 1.80 | 21.15 | <0.001 *** | |
| tree × layer | 3 112 | 15.94 | 5.31 | 62.33 | <0.001 *** | |
| Litter mass | tree | 3 112 | 9.20 | 3.07 | 21.35 | <0.001 *** |
| layer | 1 112 | 29.88 | 29.88 | 208.11 | <0.001 *** | |
| tree × layer | 3 112 | 0.87 | 0.29 | 2.01 | 0.117 | |
| Total thickness | tree | 3 56 | 44.88 | 14.96 | 233.34 | <0.001 *** |
| Total mass | tree | 3 56 | 18.42 | 6.14 | 26.27 | <0.001 *** |
| Forest Type | Soil Depth (cm) | Bulk Density (g·cm−3) | Non-Capillary Porosity (%) | Capillary Porosity (%) | Total Porosity (%) | Soil Water-Holding Capacity (t·ha−1) |
|---|---|---|---|---|---|---|
| 0 to 10 | 1.43 ± 0.08 a | 4.40 ± 2.10 a | 32.26 ± 2.65 a | 36.66 ± 1.91 a | 44.00 ± 5.82 b | |
| 10 to 20 | 1.53 ± 0.03 a | 4.17 ± 2.36 a | 31.32 ± 1.72 a | 35.48 ± 0.98 a | 41.70 ± 13.78 b | |
| Eucalyptus–grass | 20 to 40 | 1.59 ± 0.06 a | 4.74 ± 1.38 a | 30.46 ± 3.12 a | 35.19 ± 2.83 a | 94.80 ± 11.92 a |
| 40 to 60 | 1.60 ± 0.08 a | 4.15 ± 0.58 a | 22.14 ± 10.18 a | 26.29 ± 9.74 b | 83.03 ± 11.28 a | |
| 0 to 10 | 1.45 ± 0.12 a | 5.16 ± 0.52 a | 34.33 ± 2.54 a | 39.48 ± 2.74 a | 51.60 ± 30.05 b | |
| 10 to 20 | 1.50 ± 0.02 a | 4.37 ± 2.52 a | 34.15 ± 1.94 a | 38.52 ± 2.00 a | 43.77 ± 7.13 b | |
| Bamboo–grass | 20 to 40 | 1.51 ± 0.09 a | 3.11 ± 0.71 a | 31.75 ± 0.74 a | 34.85 ± 0.29 a | 62.21 ± 10.37 a |
| 40 to 60 | 1.45 ± 0.09 a | 3.16 ± 3.01 a | 23.26 ± 3.64 b | 26.42 ± 4.30 b | 63.20 ± 10.4 a | |
| 0 to 10 | 1.41 ± 0.10 a | 5.96 ± 3.55 a | 33.12 ± 0.53 a | 39.07 ± 3.75 a | 59.61 ± 10.64 c | |
| 10 to 20 | 1.44 ± 0.06 a | 4.95 ± 1.51 a | 32.46 ± 4.24 a | 37.40 ± 3.05 a | 49.51 ± 9.17 c | |
| Acacia–grass | 20 to 40 | 1.54 ± 0.04 a | 4.54 ± 0.92 a | 32.00 ± 1.84 a | 36.54 ± 2.04 a | 90.81 ± 10.16 b |
| 40 to 60 | 1.46 ± 0.13 a | 5.09 ± 1.06 a | 30.41 ± 3.12 a | 35.50 ± 3.02 a | 101.83 ± 11.07 a | |
| 0 to 10 | 1.44 ± 0.12 a | 4.81 ± 3.55 a | 28.74 ± 1.78 a | 33.54 ± 5.11 a | 48.11 ± 11.01 b | |
| 10 to 20 | 1.47 ± 0.05 a | 3.80 ± 1.51 a | 29.06 ± 5.02 a | 32.85 ± 4.01 a | 38.03 ± 9.17 b | |
| Shrub–grass | 20 to 40 | 1.56 ± 0.04 a | 3.98 ± 1.10 a | 26.93 ± 1.78 a | 30.91 ± 1.56 a | 79.63 ± 10.16 a |
| 40 to 60 | 1.50 ± 0.14 a | 3.39 ± 0.92 a | 27.53 ± 1.53 a | 30.93 ± 0.67 a | 67.81 ± 11.07 a |
| Grade I Index | Weight | Serial No. | Grade II Index | Weight |
|---|---|---|---|---|
| X1 | Litter thickness | 0.0936 | ||
| X2 | Litter mass | 0.1467 | ||
| Litter layer | 0.4826 | X3 | Rm | 0.0903 |
| X4 | Weff | 0.0770 | ||
| X5 | Wmax | 0.0751 | ||
| X6 | Bulk density | 0.0678 | ||
| X7 | Non-capillary porosity | 0.1579 | ||
| Soil layer | 0.5174 | X8 | Capillary porosity | 0.0913 |
| X9 | Total porosity | 0.0918 | ||
| X10 | Soil water-holding capacity | 0.1085 |
| Forest Type | Water Conservation Capacity | Comprehensive Evaluation Value | Rank | |
|---|---|---|---|---|
| Litter Layer | Soil Layer | |||
| Eucalyptus–grass | 0.1218 | 0.1092 | 0.2310 | 2 |
| Bamboo–grass | 0.0938 | 0.1003 | 0.1941 | 3 |
| Acacia–grass | 0.2061 | 0.3197 | 0.5257 | 1 |
| Shrub–grass | 0.0352 | 0.0140 | 0.0492 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, Z.; Chen, S.; Chen, Z.; Ruan, D.; Zhang, W.; Han, Y.; Han, L.; Wang, K.; Huang, Y.; Chen, J. Hydrological Properties of Soil and Litter Layers of Four Forest Types Restored in the Gully Erosion Area of Latosol in South China. Forests 2023, 14, 360. https://doi.org/10.3390/f14020360
Tu Z, Chen S, Chen Z, Ruan D, Zhang W, Han Y, Han L, Wang K, Huang Y, Chen J. Hydrological Properties of Soil and Litter Layers of Four Forest Types Restored in the Gully Erosion Area of Latosol in South China. Forests. 2023; 14(2):360. https://doi.org/10.3390/f14020360
Chicago/Turabian StyleTu, Zhihua, Suyi Chen, Zexian Chen, Dongshuo Ruan, Wei Zhang, Yujie Han, Lin Han, Kang Wang, Yanping Huang, and Jinhui Chen. 2023. "Hydrological Properties of Soil and Litter Layers of Four Forest Types Restored in the Gully Erosion Area of Latosol in South China" Forests 14, no. 2: 360. https://doi.org/10.3390/f14020360
APA StyleTu, Z., Chen, S., Chen, Z., Ruan, D., Zhang, W., Han, Y., Han, L., Wang, K., Huang, Y., & Chen, J. (2023). Hydrological Properties of Soil and Litter Layers of Four Forest Types Restored in the Gully Erosion Area of Latosol in South China. Forests, 14(2), 360. https://doi.org/10.3390/f14020360








