Abstract
Since its infestation was first discovered in Wuhan, Hubei Province, China, in October 2006, Corythurcha ciliata has become an important piercing-sucking pest of Platanus occidentalis. In this paper, the content of secondary plant products, nutrients, and resistance enzymes in damaged leaves of P. occidentalis was determined, and the leaves infested by different numbers of adults (0 head, 1–3 heads, or >7 heads) were tested via spectrum analysis. The results showed that the tannin content of the damaged leaves was 1.47 mg/100 g, which was higher than that of the undamaged leaves (1.20 mg/g). The tannin content in the control poplar (Populus × euramericana cv.‘74/76’) leaves was 0.2 mg/100 g higher than that in damaged leaves. The soluble sugar content in intact leaves of P. occidentalis (1.35 mg/100 g) was significantly higher than that of damaged leaves and poplar leaves. C. ciliata feeding can induce an increase in defense enzymes, such as peroxidase, polyphenol oxidase, and phenylalanine ammonia lyase, in host leaves. In the west and south part of the crown, 580–680 nm is a sensitive band for monitoring C. ciliata. The results of this study can be used to reveal the host-selection mechanism of C. ciliata and to explore new infestation-monitoring technologies.
1. Introduction
After being damaged by pests, host plants produce defensive secondary metabolites, including alkaloids, phenols, flavonoids, and tannins, which may have an adverse effect on the feeding behavior of pests and even may have a toxic killing effect [1,2,3]. For example, phenolic glycosides in Populus have long been recognized as a defensive secondary metabolite to lepidopteran pests [4,5], glucosinolates are typical defensive secondary metabolites in cruciferous plants [6], and the phenolics in leaves of Poplus nigra inhibit the growth of Lymantria dispar [5]. In addition to the above changes, the chlorophyll content, water content, physical structure, and cell activity of plant leaves change when some pests (especially piercing-sucking pests) invade host plants, and these changes can manifest as changes in the reflection spectrum [7]. If a standard spectral library based on a spectral index database of different degrees of pest damage in host plants is established, then these target pests could be monitored by hyperspectral remote sensing. There have been experimental studies by many scholars in the monitoring of diseases and insect pests in agriculture and forestry crops, such as Cnaphalocrocis medialis, Puccinia striiformis, Leptinotarsa decemlineata, Bursaphelenchus xylophus, Dendrolimus tabulaeformis, and Tetranychus cinnbarinus [8,9,10,11,12,13].
Corythurcha ciliata (SAY) (Hemiptera:Tingidae), native to temperate areas of the middle eastern United States, mainly infests the Platanus plant. Since its infestation was first discovered in Wuhan, Hubei Province, China, in October 2006 [14,15], C. ciliata has become an important piercing-sucking pest of Platanus. The damage to Platanus leaves is particularly serious. At present, the situation in the Yangtze River basin in north and central China has had a negative impact on seedling breeding, urban landscapes, and urban sanitation in the country [16]. In order to reveal the host-selection mechanism by C. ciliata and explore new monitoring technology, in this paper, the secondary metabolites, nutrients, and spectral characteristics of leaves infested by P. occidentalis were analyzed.
2. Materials and Methods
2.1. Determination of Secondary Metabolites and Nutrients in C. ciliata Host Plant Leaves
Sample collection: The sample Platanus occidentalis forest is located in Xinglong, Linyi, Shandong Province, China (37.116° N; 116.745° E). It is eight years old, the average DBH is 15 cm, and the average tree height is 7.5 m. This sample forest was planted in November 2014. In August 2015, an infestation of C. ciliata occurred, and no control measures were taken. Five sample trees were randomly selected, and a branch with a length of 80 cm damaged by C. ciliata was randomly cut at the center of the canopy of each plant with a pole tree pruner. One damaged and one undamaged mature leaf on the branch were selected, and they were then placed into an insulation bucket with ice. After returning to the laboratory, it was placed in a refrigerator at −20 °C for storage. Using the same method, Populus×euramericana cv.‘74/76’ (nonhost plant) leaves were randomly collected around the forest as a control group.
Determination methods: The tannin content in the leaves was measured by the spectrophotometric method with sodium tungstate-phosphomolybdic acid [17], the total phenol content was measured by the Folin–Ciocalteu method [18], the total flavonoids were measured by the ultraviolet spectrometric method [19], the anthrone colorimetry method was adopted to determine the carbohydrate content [20], and the soluble protein content was determined by the Bradford method [21].
2.2. Changes in Defense Enzymes in Host Leaves after Being Eaten by C. ciliata
Sample collection: Five annual potted seedlings with the same growth conditions were selected and placed in the open forest space described in Section 2.1. All potted seedlings were covered with 40 pieces of mesh plastic gauze. On 10 July 2020, 500 fifth instar nymphs were collected in the forest and inoculated on five potted seedlings covered with plastic gauze, with 100 nymphs per plant. From 20 July to 18 August 2017, one leaf was cut off from each plant every 10–15 days and immediately placed into an insulation bucket with ice. After returning to the laboratory, it was placed in a refrigerator at −20 °C for storage.
Determination methods: The activities of polyphenol oxidase (PPO), peroxidase (POD), and phenylalanine ammonia lyase (PAL) in P. occidentalis leaves at different stages were determined by UV absorption [22,23,24].
2.3. Determination of Spectral Characteristics of Host Leaves
The leaves infested with 0, 1–3, 4–6, or ≥7 adult insects were observed with a telescope under the damaged plants in the forest described in Section 2.1, and the leaves were then severed with a pole tree pruner. Five leaves were severed in four physical directions with different infestation quantities. The samples were immediately sealed with zip-lock bags and placed into the incubator in standby mode. According to the measurement method of Dou Zhiguo et al., the spectrum of the leaves was measured with an ASD Field Spec 4 portable hyper-spectrometer [25].
The spectral band was 350–2500 nm, there were 2151 spectrum channels, the operating center wavelength was 350–1000 nm, the spectral bandwidth was 1.4 nm, the operating center wavelength was 1001–2500 nm, the spectrum width was about 1.1 nm, the minimum integration time was 1 ms, and the full field-of-view angle was 25°. The embedded light source was a hand-held blade clamp with a built-in tungsten halogen lamp for direct measurement. Before each measurement, the instrument was calibrated with a diffuse reference plate. During the measurement, each leaf sample was measured 10 times with an interval of 0.1 s, and the average value was taken as the spectral reflection value of that leaf. A white board correction was performed every 15 min.
2.4. Data Analysis Methods
All data were processed using IBM SPSS Statistics 20 for statistical analysis. The differences in leaf (the damaged and undamaged leaves) secondary metabolite and nutrient content were analyzed with a univariate one-way ANOVA.
3. Results
3.1. Secondary Biomass and Nutrient Content in Host Plant Leaves affected by C. ciliata
From the secondary biomass content in leaves (Figure 1), there were significant differences in tannin content among the three treatments (damaged leaves, undamaged leaves, and control (107 poplar leaves) (F2,6 = 6.435, P = 0.032). The tannin content of P. occidentalis leaves infested by C. ciliata was 1.47mg/100 g, which was higher than the undamaged leaf tannin content (1.20 mg/100 g), while the control (107 poplars) leaf tannin content was higher than the damaged leaf tannin content (0.2 mg/g). There was no significant difference in total flavonoids and total phenolics among treatments (total flavonoids: F2,6 = 2.715, P = 0.145; total phenolics: F2,6 = 2.352, P = 0.176).
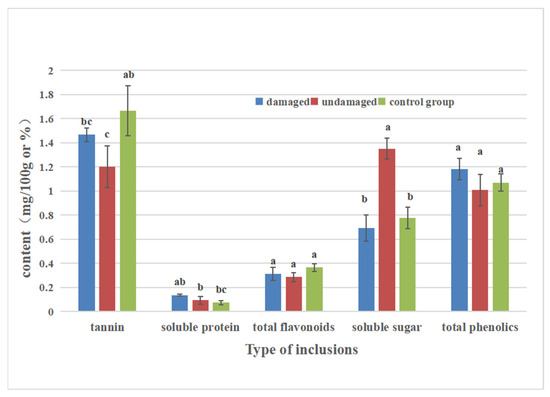
Figure 1.
Contents of secondary biomass and nutrients in host plant leaves affected by C. ciliata.
In terms of leaf nutrient content (Figure 1, Table 1), the soluble protein content of damaged leaves (0.14 mg/100 g) was significantly higher than that of the control (0.07 mg/100 g) (F2,6 = 7.256, P = 0.025), but there was no significant difference between undamaged leaves (0.09 mg/100 g) and damaged leaves. The soluble sugar content (1.35 mg/100 g) of the undamaged leaves was significantly higher than that of the other two treatments (F2,6 = 41.279, P = 0.000). The content of soluble sugar in the damaged leaves and control leaves was 0.69 mg/100 g and 0.78 mg/100 g, respectively.

Table 1.
Measuring data of secondary biomass and nutrient content in host plant leaves affected by C. ciliata.
3.2. Changes in Host Defense Enzymes Induced by the Feeding of C. ciliata
The results regarding host defense enzymes induced by the feeding of C. ciliata at different periods showed that after the host plant was damaged, the peroxidase in the leaves decreased first. The peak period of occurrence of C. ciliata (mid-August) reached a maximum value of 4.64 mu/g in the forest (Figure 2, Table 2). Polyphenol oxidase and phenylalanine ammonia lyase increased to a certain extent after injury, and the content then tended to be stable, with the highest increase values of 43.42% and 31.86%, respectively (Figure 3 and Figure 4, Table 3 and Table 4).
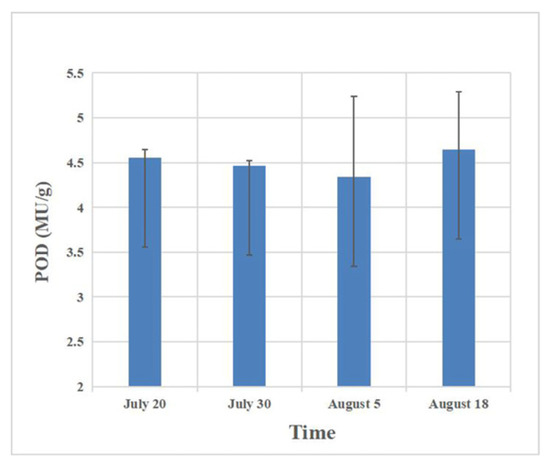
Figure 2.
Changes in peroxidase in host leaves at different times of injury.

Table 2.
Measuring peroxidase in host leaves at different times of injury.
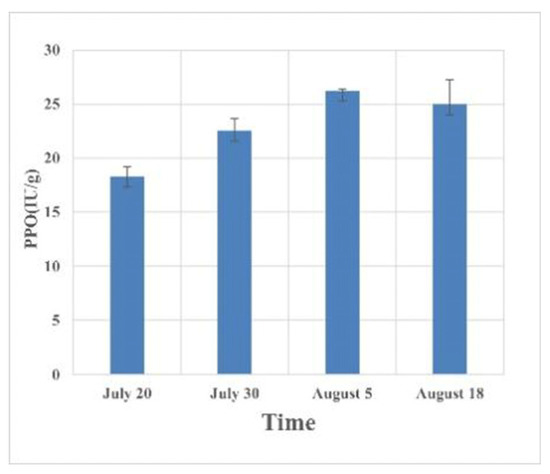
Figure 3.
Changes in polyphenol oxidase in host leaves at different times of injury.
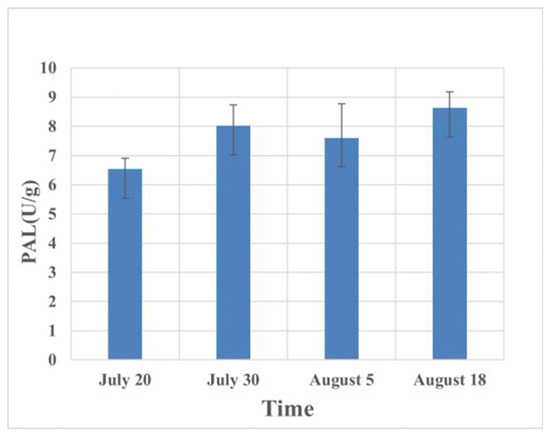
Figure 4.
Changes in phenylalanine ammonia lyase in host leaves at different times of injury.

Table 3.
Measuring polyphenol oxidase in host leaves at different times of injury.

Table 4.
Measuring phenylalanine ammonia lyase in host leaves at different times of injury.
3.3. Reflection Spectral Characteristics of Host Leaves with Different Numbers of Adult C. ciliata
The reflection spectral characteristics of host leaves with different numbers of adult C. ciliata are shown in Figure 5. In the visible light band (400–700 nm), at about 580 nm, there is a reflection peak (green peak) in the east, south, and west directions and a red light absorption valley at about 680 nm. The reflectance of leaves without adult infestation in the three directions is higher than that of leaves with such damage and is between the reflection peak and the absorption valley. In the south and west directions, the reflectance increased with the increase of adult colonization. In the wavelength range of 1660–1860 nm, the reflectivity of the leaves in the west direction also increased with the increase in adult infestation. In the north direction, as the wavelength increased, the reflection peak of the damaged leaf was always earlier than that of the undamaged leaf. It can be seen that, in the canopy of the west and south, 580–680 nm was a sensitive band for monitoring C. ciliata populations.
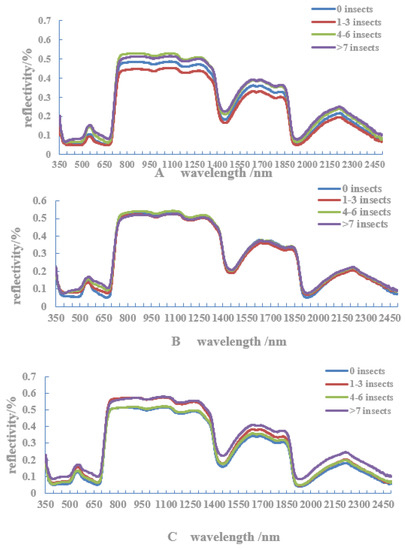
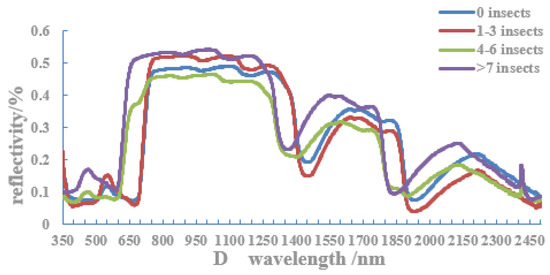
Figure 5.
Spectral curves of host leaves with different numbers of adult C. ciliata in different directions ((A) east; (B) south; (C) west; and (D) north).
4. Discussion and Conclusions
Changes of some nutrients and secondary metabolites in plants have important effects on the growth, development, and physiological metabolism of pests [26]. The content of flavonoids, tannins, and total phenols in the phloem of Pinus massoniana increased significantly, while the content of soluble sugar and protein decreased after being damaged by Hypobitelus xiaoi [27]. Li Yanyan (2013) also showed that, after Aphis gossypii was infested, the content of soluble sugar and soluble protein in the leaves of five host plants such as cucumber, Hami melon, pumpkin, gourd, and stir melon significantly decreased, while the content of tannins and flavonoids was significantly increased [28]. Similarly, the content of tannins and flavonoids in the leaves of Phyllostachys heterocycla (Carr.) Mitford cv. Pubescens was also significantly increased after being damaged by Besaia goddrica (Schaus, 1928). The content of total sugar, soluble sugar, protein, nitrogen, and phosphorus was significantly decreased, and the decline increased with the aggravation of the infestation [29]. The nitrogen content in the leaves of Tsuga canadensis was negatively correlated with the density of Fiorinia externa in the previous year [30]. The results of our study showed that the tannin content in the leaves of P. occidentalis was also significantly increased. The difference was that there was no significant difference in the content of the flavonoids and total phenols between the damaged and undamaged leaves. It is worth noting that the tannin content in the leaves of non-host plants is higher than that in the leaves of P. occidentalis. This may be one of the reasons why the C. ciliata does not feed on poplar leaves. The soluble sugar content in the undamaged leaves of P. occidentalis was significantly higher than that in the non-host leaves, which may be one of the reasons why P. occidentalis is chosen instead of poplars as the host.
The results showed that the feeding of C. ciliata can induce the increase of defense enzymes such as peroxidase, polyphenol oxidase, and phenylalanine ammonia lyase in host leaves. It is well known that insects respond to the adverse effects of plant secondary biomass through detoxification and metabolism. For example, Spodoptera littoralis could be adapted to the defense of plant protease inhibitors by the overexpression of the tissue protein cysteine digestive enzyme [31]. Callosobruchus maculatus could also enhance their proteolytic ability by overexpressing the sensitive L-type histidine cysteine digestive enzymes (CmCPs) when they are affected by soybean cystatin [32]. The detoxification mechanism of C. ciliata to defenses of Platanus occidentalis will require further research.
Existing results show that the monitoring-sensitive spectral bands of different pest species are also different; 670–690 nm and 760–900 nm were sensitive bands for wheat aphid monitoring [33], 750–900 nm was a sensitive band for the monitoring of Ostrinia furnacalis [34], 694–716 nm and 732–829 nm were sensitive bands for the monitoring of Epilachna vigintioctopunctata (Fabricius) [35], and 450–890nm and 1400–1800nm were sensitive bands for forecasting potential bark beetle outbreaks [36]. Hyperspectral bands such as 685 nm, 970 nm, and 1200 nm were used to discover the early hazards of the Mountain Pine Beetle [37]. White (2007) also showed that Eo-1 Hyperion moisture indices can better monitor the detection of Mountain Pine Beetle Red attack damage in large areas [38]. Our results show that, in the crown of the west and south of Platanus occidentalis, 580–680 nm was a sensitive band for the monitoring of C. ciliata.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14030449/s1.
Author Contributions
Conceptualization, X.Z. and H.W.; methodology, X.Z., K.W. and H.W.; software, X.Z. and M.W.; validation, X.Z. and M.W.; formal analysis, X.Z.; investigation, Y.Z. and Y.L.; resources, K.W. and Y.L.; data curation, K.W.; writing—original draft preparation, X.Z.; writing—review and editing, X.Z. and M.W.; visualization, M.W. and H.W.; supervision, K.W.; project administration, H.W.; funding acquisition, H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Natural Science Foundation of Shandong Province, China (grant number: ZR2019MC021).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
The study did not involve humans.
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Ehrlich, P.R.; Raven, P.H. Butterflies and plants: A study in coevolution. Evolution 1964, 18, 586–608. [Google Scholar] [CrossRef]
- Li, D.M.; Zhou, L.Y. Coevolution—The relationship between insects and plants. Entomol. Knowl. 1997, 34, 45–49. [Google Scholar]
- Wang, C.Z. Effects of Gossypol and tannic acid on the growth and digestion physiology of cotton bollworm larvae. J. Plant Prot. 1997, 24, 13–18. [Google Scholar]
- Hemming, J.; Lindroth, R.L. Effects of phenolic glycosides and protein on Gypsy moth (Lepidoptera: Lymantriidae) and forest tent Caterpillar (Lepidoptera: Lasiocampidae) performance and detoxication activities. Environ. Entomol. 2000, 29, 1108–1115. [Google Scholar] [CrossRef]
- Hale, B.K.; Herms, D.A.; Hansen, R.C.; Clausen, T.P.; Arnold, D. Effects of drought stress and nutrient availability on dry matter allocation, phenolic glycosides, and rapid induced resistance of poplar to two lymantriid defoliators. J. Chem. Ecol. 2005, 31, 2601–2620. [Google Scholar] [CrossRef] [PubMed]
- Cipollini, D. Variation in the expression of chemical defenses in Alliaria petiolata (Brassicaceae) in the field and common garden. Am. J. Bot. 2002, 89, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.D. Remote-sensing of biotic and abiotic plant stress. Annu. Rev. Phytopathol. 1986, 24, 265–287. [Google Scholar] [CrossRef]
- Qi, J. Study on Remote Sensing Monitoring Technology of Colorado Potato Beetle Monitoring; Chinese Academy of Agricultural Sciences: Beijing, China, 2010. [Google Scholar]
- Fu, Y.M.; Xue, H.J.; Xu, J.M. Study on remote sensing monitoring technique for Dendrolimus tabulaeformis Tsai et Liu by TM imagery in western liaoning. Mod. Agric. Sci. Technol. 2011, 544, 235–236. [Google Scholar]
- Sun, H.M.; Liu, S.J. Design and application of remote sensing monitoring database of pine wood nematode in Qianxinan Prefecture. J. Xingyi Norm. Univ. Natl. 2019, 26, 111–115. [Google Scholar]
- Duan, W.N.; Jing, X.; Liu, L.Y.; Zhang, T.; Zhang, L.H. Remote sensing monitoring of wheat stripe rust by fusion of SIF and reflectance spectra. Spectrosc. Spectr. Anal. 2022, 42, 859–865. [Google Scholar]
- Bao, Y.X.; Li, Y.T.; Wang, L.; Gao, W.T.; Zhu, F. Monitoring on the occurrence of Cnaphalocrocis medinalis based on multi-temporal HJ Satellite’s remotesensing images. Chin. J. Agrometeorol. 2016, 37, 464–470. [Google Scholar]
- Chan Aland, H.Y.; Barnes, C.; Swinfield, T.; Coomes, D.A. Monitoring ash dieback (Hymenoscyphus fraxineus) in British forests using hyperspectral remote sensing. Remote Sens. Ecol. Conserv. 2020, 7, 306–320. [Google Scholar] [CrossRef]
- Ju, R.; Li, Y.; Feng, W.; Du, Y. Spread of and damage by an exotic lacebug, Corythuca ciliata (say, 1832) (Hemiptera: Tingidae), in China. Entomol. News 2009, 120, 409–414. [Google Scholar] [CrossRef]
- Li, C.R.; Xia, W.S.; Wang, F.L. First records of Corythucha ciliata (Say) in China (Hemiptera, tingidae). Acta Zootaxonomica Sin. 2007, 32, 944–946. [Google Scholar]
- Wu, H.W.; Li, D.J.; Wang, X.N.; Wang, L.D.; Li, X.C.; Liu, H.X. Development situation and control measures for Corythucha ciliata in Shandong. J. Shandong For. Sci. Technol. 2013, 43, 108–110. [Google Scholar]
- Deng, Z.D. Inclusions Changes in Different Families Pinus massoniana Lamb Damaged by Hemiberlesia pitysophila Takagi; Fujian Agriculture and Forestry University: Fuzhou, China, 2009. [Google Scholar]
- Cao, Y.P.; Dai, H.Z.; Cao, W.; Han, Z.P. Determination of total phenols in Zizyphus jujuba Mill. by Folin-Ciocaileu colorimetry. J. Anhui Agric. Sci. 2008, 36, 299–1299,1302. [Google Scholar]
- Zheng, J.X.; Hu, Y.J.; Liang, W.Q.; Pu, J.B.; Wei, K.M. Determination of total flavonoids in Trifolium pratense by UV-Vis spectrophotometry. Chin. J. Tradit. Med. Sci. Technol. 2009, 23, 386–387. [Google Scholar]
- Li, J.B.; Fang, L.P.; Lv, Z.Z.; Zhang, Z. Relationships between the cotton resistance to the cotton aphid (Aphis gossypii) and the content of soluble sugars. Plant Prot. 2008, 34, 26–30. [Google Scholar]
- Shan, W.R.; Zhao, J.J.; Zhao, M. Effect of chemical treatment on soluble protein content and peroxidase activity of cotton. Guangdong Agric. Sci. 2010, 29, 77–78. [Google Scholar]
- Lv, M.; Sun, H.H.; Gao, X.W. The Study on the activities of Polyphenol Oxidase and Peroxidase in cotton and corn induced by insects. Chin. Agric. Sci. Bull. 2012, 28, 21–216. [Google Scholar]
- Mao, H.; Chen, H.; Liu, X.X.; Zhang, Q.W. Effects of Apolygus lucorum feeding and mechanical damage on defense enzyme activities in cotton leaves. Chin. J. Appl. Entomol. 2011, 48, 1431–1436. [Google Scholar]
- Patima, W.H.; Chen, L.H.; Cui, Y.H.; Li, Y.; Feng, H.Z. Effects of Tetraychus urticae damage on stress defense-enzymes activity of cotton. J. Environ. Entomol. 2016, 38, 1185–1191. [Google Scholar]
- Dou, Z.G.; Cui, L.J.; Wu, G.J.; Li, J.; Pan, X.; Cai, Z.J.; Lei, Y.R.; Li, W. Estimation of the hyperspectral inversion of reed chlorophyll under Hyalopterus pruni attack. Chin. J. Ecol. 2018, 37, 3163–3170. [Google Scholar]
- Mattson, W.J.; Scriber, J.M. Nutritional ecology of insect folivores of woody plants: Nitrogen, water, fiber, and mineral considerations. Nutr. Ecol. Insects Mites Spiders Relat. Invertebr. 1987, 105–146. [Google Scholar]
- Chen, Z.L. The Effect of the Harm of the Pine Stem of Xiao on the Sub-Biomass and Nutrient Content of Pinus massoniana; Fujian Agriculture and Forestry University: Fuzhou, China, 2010. [Google Scholar]
- Li, Y.Y.; Zhou, X.R.; Pang, B.P.; Chang, J. Influences of Aphis gossypii Glover feeding on the contents of main nutrients and secondary compounds in host plants. J. Environ. Entomol. 2013, 35, 49–54. [Google Scholar]
- Zhang, S.L. The Effect of the damage caused by Besaia goddrica (Schaus)on the content of main secondary substance and nutrition substance of Phyllostachys heterocycla cv. Pubescens. Bamboo Res. Trans. 2008, 27, 4. [Google Scholar]
- McClure, M.S. Foliar nitrogen: A basis for host suitability for elongate hemlock scale, Fionnia extema (Homoptera: Diaspididae). Ecology 1980, 61, 72–79. [Google Scholar] [CrossRef]
- Leo, F.D.; MA, B.B.; Ceci, L.R.; Gallerani, R.; Jouanin, L. Opposite effects on Spodoptera littoralis larvae of high expression level of a trypsin proteinase inhibitor in transgenic plants. Plant Physiol. 1998, 118, 997–1004. [Google Scholar] [CrossRef]
- Zhu-Salzman, K.; Zeng, R.S. Molecular mechanisms of insect adaptation to plant defense: Lessons learned from a bruchid beetle. Insect Sci. 2008, 15, 4777–4811. [Google Scholar] [CrossRef]
- He, G.J.; Hu, D.Y.; Jin, X.H.; Yang, J.G.; Li, G.Y. The spectra measurement and analysis of wheat aphid disaster in Beijing. Remote Sens. Technol. Appl. 2002, 17, 119–124. [Google Scholar]
- Zhang, Y.Q.; Wen, L.P.; Shi, J.; Wang, Z.Y.; He, K.L.; Cheng, D.F. Change in canopy reflectance and yield loss of corns damaged by Asian corn borer (Ostrinia furnacalis). Plant Prot. 2007, 33, 54–57. [Google Scholar]
- Apan, A.; Datt, B.; Kelly, R. Detection of pests and diseases in vegetable crops using hyperspectral sensing: A comparison of reflectance data for different sets of symptoms. In Proceedings of the SSC 2005 Spatial Intelligence, Innovation and Praxis: The National Biennial Conference of the Spatial Sciences Institute, Melbourne, Australia, 28 September 2005; Spatial Sciences Institute: Melbourne, Australia, 2005. ISBN 0-9581366-2-9. [Google Scholar]
- Lausch, A.; Heurich, M.; Gordalla, D.; Dobner, H.J.; Gwillym-Margianto, S.; Salbach, C. Forecasting potential bark beetle outbreaks based on spruce forest vitality using hyperspectral remote-sensing techniques at different scales. For. Ecol. Manag. 2013, 308, 76–89. [Google Scholar] [CrossRef]
- Niemann, K.O.; Quinn, G.; Stephen, R.; Visintini, F.; Parton, D. Hyperspectral remote sensing of Mountain Pine Beetle with an emphasis on previsual assessment. Can. J. Remote Sens. 2015, 41, 191–202. [Google Scholar] [CrossRef]
- White, J.C.; Coops, N.C.; Hilker, T.; Wulder, M.A.; Carroll, A.L. Detecting Mountain Pine Beetle red attack damage with Eo-1 hyperion moisture indices. Int. J. Remote Sens. 2007, 28, 2111–2121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).