Non-Thermal Plasma Treatment Improves Properties of Dormant Seeds of Black Locust (Robinia pseudoacacia L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Non-Thermal Plasma Apparatus
2.2. Seeds and Non-Thermal Plasma Treatment
2.3. SEM and EDS Analyses
2.4. Contact-Angle Measurement
2.5. Seed Water Uptake
2.6. Seed Germination and Early Growth Seedling
2.7. Data Analysis
3. Results
3.1. SEM and EDS Analyses
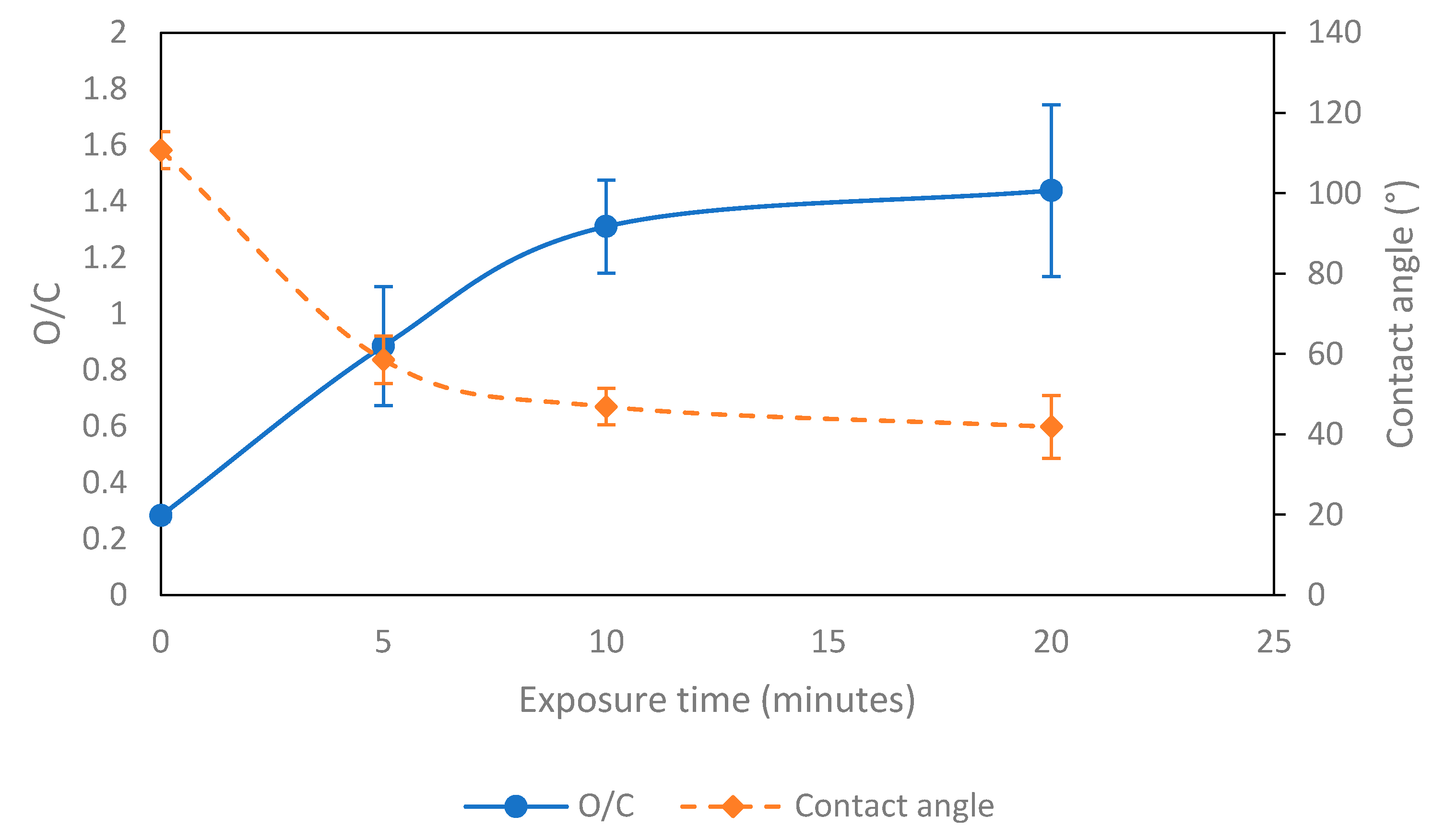
3.2. Contact-Angle Measurement
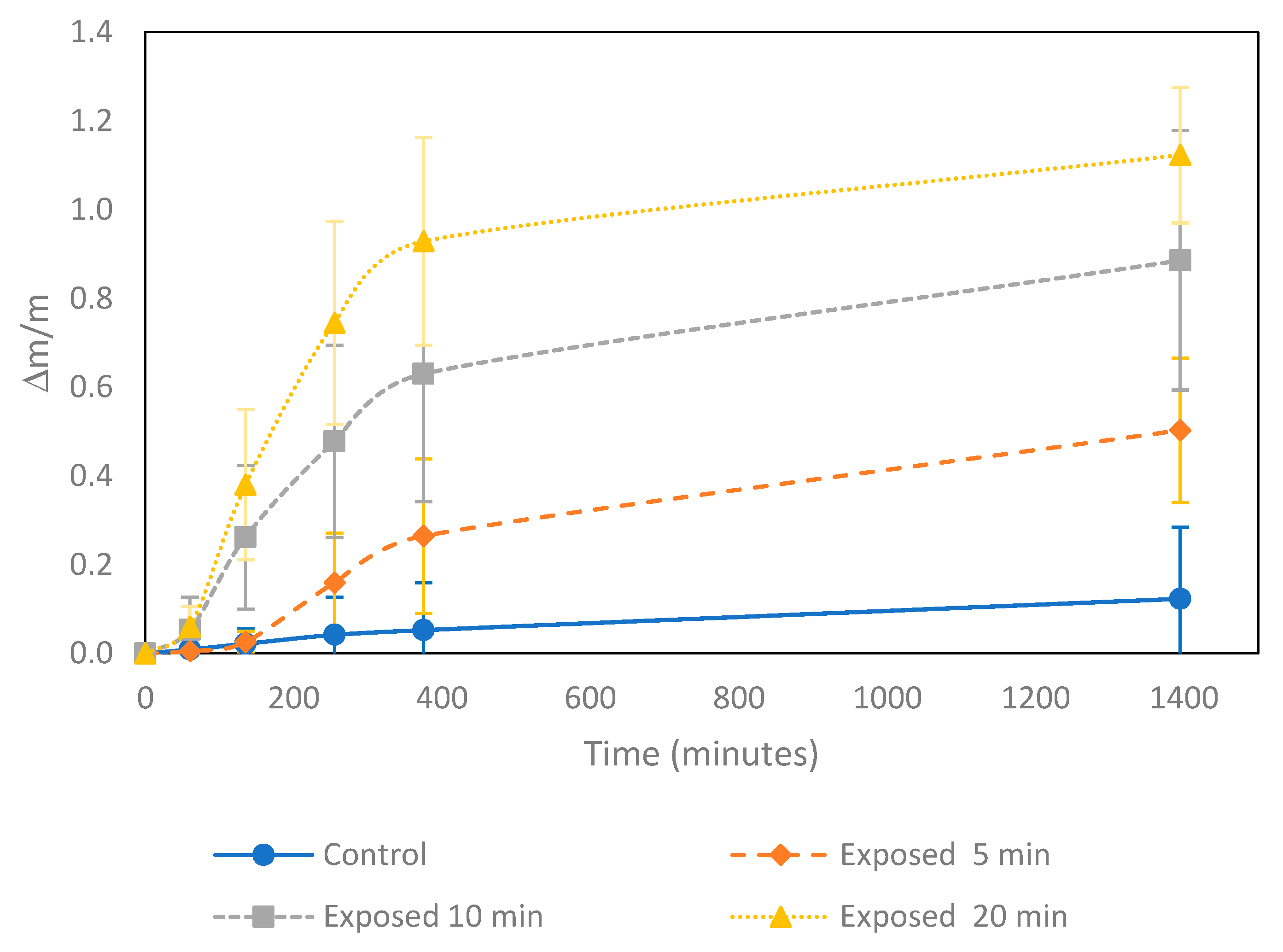
3.3. Seed Water Uptake
3.4. Seed Germination and Early Growth Seedling
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma agriculture from laboratory to farm: A review. Processes 2020, 8, 1002. [Google Scholar] [CrossRef]
- Leti, L.-I.; Gerber, I.C.; Mihaila, I.; Galan, P.-M.; Strajeru, S.; Petrescu, D.-E.; Cimpeanu, M.-M.; Topala, I.; Gorgan, D.-L. The modulatory effects of non-thermal plasma on seed’s morphology, germination and genetics—A review. Plants 2022, 11, 2181. [Google Scholar] [CrossRef]
- Pańka, D.; Jeske, M.; Łukanowski, A.; Baturo-Cieśniewska, A.; Prus, P.; Maitah, M.; Maitah, K.; Malec, K.; Rymarz, D.; Muhire, J.d.D.; et al. Can cold plasma be used for boosting plant growth and plant protection in sustainable plant production? Agronomy 2022, 12, 841. [Google Scholar] [CrossRef]
- Waskow, A.; Howling, A.; Furno, I. Mechanisms of plasma-seed treatments as a potential seed processing technology. Front. Phys. 2021, 9, 174. [Google Scholar] [CrossRef]
- Filatova, I.; Azharonok, V.; Lushkevich, V.; Zhukovsky, A.; Gadzhieva, G.; Spasic, K.; Zivkovic, S.; Puac, N.; Lazovic, S.; Malovic, G. Plasma seeds treatment as a promising technique for seed germination improvement. In Proceedings of the 31st International Conference on Phenomena in Ionized Gases, Granada, Spain, 14–19 July 2013. [Google Scholar]
- Filatova, I.; Lyushkevich, V.; Goncharik, S.; Zhukovsky, A.; Krupenko, N.; Kalatskaja, J. The effect of low-pressure plasma treatment of seeds on the plant resistance to pathogens and crop yields. J. Phys. D Appl. Phys. 2020, 53, 244001. [Google Scholar] [CrossRef]
- Holubová, Ľ.; Kyzek, S.; Ďurovcová, I.; Fabová, J.; Horváthová, E.; Ševčovičová, A.; Gálová, E. Non-thermal plasma—A new green priming agent for plants? Int. J. Mol. Sci. 2020, 21, 9466. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, V.; Šerá, B.; Khun, J.; Šerý, M.; Julak, J. Effects of nonthermal plasma on wheat grains and products. J. Food Qual. 2019, 2019, 7917825. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Cheng, J.-H.; Sun, D.-W. Activities and conformation changes of food enzymes induced by cold plasma: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 794–811. [Google Scholar] [CrossRef]
- Gao, Y.; Francis, K.; Zhang, X. Review on formation of cold plasma activated water (PAW) and the applications in food and agriculture. Food Res. Int. 2022, 157, 111246. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Jirešová, J.; Scholtz, V.; Julák, J.; Šerá, B. Comparison of the effect of plasma-activated water and artificially prepared plasma-activated water on wheat grain properties. Plants 2022, 11, 1471. [Google Scholar] [CrossRef]
- Šerá, B.; Scholtz, V.; Jirešová, J.; Khun, J.; Julák, J.; Šerý, M. Effects of non-thermal plasma treatment on seed germination and early growth of leguminous plants—A review. Plants 2021, 10, 1616. [Google Scholar] [CrossRef] [PubMed]
- Šerá, B.; Šerý, M.; Zahoranová, A.; Tomeková, J. Germination improvement of three pine species (Pinus) after diffuse coplanar surface barrier discharge plasma treatment. Plasma Chem. Plasma Proc. 2021, 41, 211–226. [Google Scholar] [CrossRef]
- Šerá, B.; Zahoranová, A.; Bujdakova, H.; Šerý, M. Disinfection from pine seeds contaminated with Fusarium circinatum Nirenberg & O’Donnell using non-thermal plasma treatment. Rom. Rep. Phys. 1993, 71, 701. [Google Scholar]
- Šerá, B.; Šerý, M.; Stranak, V.; Špatenka, P.; Tichý, M. Does cold plasma affect breaking dormancy and seed germination? A study on seeds of Lamb's Quarters (Chenopodium album agg.). Plasma Sci. Technol. 2009, 11, 750–754. [Google Scholar] [CrossRef]
- Junior, C.A.; de Oliveira Vitoriano, J.; da Silva, D.L.S.; de Lima Farias, M.; de Lima Dantas, N.B. Water uptake mechanism and germination of Erythrina velutina seeds treated with atmospheric plasma. Sci. Rep. 2016, 6, 33722. [Google Scholar] [CrossRef] [Green Version]
- da Silva, A.; Farias, M.; da Silva, D.; Vitoriano, J.; de Sousa, R.; Alves-Junior, C. Using atmospheric plasma to increase wettability, imbibition and germination of physically dormant seeds of Mimosa caesalpiniafolia. Colloids Surf. B Biointerfaces 2017, 157, 280–285. [Google Scholar] [CrossRef]
- Cui, D.J.; Yin, Y.; Wang, J.Q.; Wang, Z.W.; Ding, H.B.; Ma, R.N.; Jiao, Z. Research on the physio-biochemical mechanism of non-thermal plasma-regulated seed germination and early seedling development in Arabidopsis. Front. Plant Sci. 2019, 10, 1322. [Google Scholar] [CrossRef]
- Degutytė-Fomins, L.; Paužaitė, G.; Žūkienė, R.; Mildažienė, V.; Koga, K.; Shiratani, M. Relationship between cold plasma treatment-induced changes in radish seed germination and phytohormone balance. Jpn. J. Appl. Phys. 2020, 59, SH1001. [Google Scholar] [CrossRef]
- Alves-Junior, C.; da Silva, D.L.S.; Vitoriano, J.O.; Barbalho, A.P.C.B.; de Sousa, R.C. The water path in plasma-treated Leucaena seeds. Seed Sci. Res. 2020, 30, 13–20. [Google Scholar] [CrossRef]
- Jiresova, J.; Sera, B.; Scholtz, V.; Khun, J.; Sery, M. The dormancy overcoming and affection of early growth of alfalfa (Medicago sativa L.) seeds by non-thermal plasma and plasma activated water. Rom. Rep. Phys. 2021, 73, 711. [Google Scholar]
- Grainge, G.; Nakabayashi, K.; Steinbrecher, T.; Kennedy, S.; Ren, J.; Iza, F.; Leubner-Metzger, G. Molecular mechanisms of seed dormancy release by gas plasma-activated water technology. J. Exp. Bot. 2022, 73, 4065–4078. [Google Scholar] [CrossRef]
- Nicolau, J.P.B.; Pereira, M.D.; Silva, F.E.d.; Souza, D.L.d.S.; Medeiros, A.D.d.; Alves, C.Z. Atmospheric plasma overcomes dormancy of Pityrocarpa moniliformis (Benth.) Luckow & R. W. Jobson seeds. J. Seed Sci. 2022, 44, 1. [Google Scholar] [CrossRef]
- Mildaziene, V.; Ivankov, A.; Sera, B.; Baniulis, D. Biochemical and physiological plant processes affected by seed treatment with non-thermal plasma. Plants 2022, 11, 856. [Google Scholar] [CrossRef]
- Huntley, B. European vegetation history: Palaeovegetation maps from pollen data-13000 yr BP to present. J. Quat. Sci. 1990, 5, 103–122. [Google Scholar] [CrossRef]
- Vítková, M.; Müllerová, J.; Sádlo, J.; Pergl, J.; Pyšek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. For. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef]
- Sukopp, H.; Wurzel, A. The effects of climate change on the vegetation of central European cities. Urban Habitats 2003, 1, 66–86. [Google Scholar]
- Mantovani, D.; Veste, M.; Freese, D. Black locust (Robinia pseudoacacia L.) ecophysiological and morphological adaptations to drought and their consequence on biomass production and water-use efficiency. N. Z. J. For. Sci. 2014, 44, 29. [Google Scholar] [CrossRef] [Green Version]
- Sitzia, T.; Cierjacks, A.; De Rigo, D.; Caudullo, G. Robinia pseudoacacia in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; pp. 166–167. [Google Scholar]
- Cierjacks, A.; Kowarik, I.; Joshi, J.; Hempel, S.; Ristow, M.; von der Lippe, M.; Weber, E. Biological flora of the British Isles: Robinia pseudoacacia. J. Ecol. 2013, 101, 1623–1640. [Google Scholar] [CrossRef]
- Nicolescu, V.-N.; Hernea, C.; Bakti, B.; Keserű, Z.; Antal, B.; Rédei, K. Black locust (Robinia pseudoacacia L.) as a multi-purpose tree species in Hungary and Romania: A review. J. For. Res. 2018, 29, 1449–1463. [Google Scholar] [CrossRef]
- Rédei, K.; Csiha, I.; Keserű, Z.; Végh, Á.K.; Győri, J. The silviculture of black locust (Robinia pseudoacacia L.) in Hungary: A review. South-East Eur. For. 2011, 2, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Kolbek, J.; Vítková, M.; Větvička, V. From history of Central European Robinia growths and its communities. Zprávy České Bot. Spol. 2004, 39, 287–298. [Google Scholar]
- Keresztesi, B. Robinia pseudoacacia: The basis of commercial honey production in Hungary. Bee World 1977, 58, 144–150. [Google Scholar] [CrossRef]
- Thompson, K. Seed Persistence in Soil. In Methods in Comparative Plant Ecology; Hendry, G.A., Grime, J.P., Eds.; Chapman and Hall: London, UK, 1993; pp. 199–202. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Baskin, J.M.; Baskin, C.C.; Li, X. Taxonomy, ecology, and evolution of physical dormancy in seeds. Plant Species Biol. 2000, 15, 139–152. [Google Scholar] [CrossRef]
- Abdullah, K.H.; Duhok University; Ahmed, G.B.; Selah-Alden, M.T.; Hassan, H.N.; Mahmood, M.J.; Hameed, N.A.; Amin, S.M. Overcoming seed dormancy of Robinia pseudoacacia L. and Ceratonia siliqua L. species using different pretreatments in Malta Forest Nursery–DUHOK. J. Duhok Univ. 2018, 21, 1–7. [Google Scholar] [CrossRef]
- Masaka, K.; Yamada, K. Variation in germination character of Robinia pseudoacacia L. (Leguminosae) seeds at individual tree level. J. For. Res. 2009, 14, 167–177. [Google Scholar] [CrossRef]
- Mirzaei, M.; Moghadam, A.R.L.; Ardebili, Z.O. The induction of seed germination using sulfuric acid, gibberellic acid and hot water in Robinia pseudoacacia L. Int. Res. J. Appl. Basic Sci. 2013, 4, 96–98. [Google Scholar]
- Roman, A.M.; Truta, A.M.; Viman, O.; Morar, I.M.; Spalevic, V.; Dan, C.; Sestras, R.E.; Holonec, L.; Sestras, A.F. Seed germination and seedling growth of Robinia pseudoacacia depending on the origin of different geographic provenances. Diversity 2022, 14, 34. [Google Scholar] [CrossRef]
- Khun, J.; Machková, A.; Kašparová, P.; Klenivskyi, M.; Vaňková, E.; Galář, P.; Julák, J.; Scholtz, V. Non-thermal plasma sources based on cometary and point-to-ring discharges. Molecules 2021, 27, 238. [Google Scholar] [CrossRef]
- Kohlík, V. Plán péče o přírodní památku Královská obora na období 2010–2019. City Hall of Prague. 2009. Available online: http://www.praha-priroda.cz/priloha/51cda1b540d7c/planpece-pp-kralovska-obora-2010-2019-51cda1f37de07.pdf (accessed on 2 February 2023).
- Sery, M.; Zahoranova, A.; Kerdik, A.; Sera, B. Seed germination of black pine (Pinus nigra Arnold) after diffuse coplanar surface barrier discharge plasma treatment. IEEE Trans. Plasma Sci. 2020, 48, 939–945. [Google Scholar] [CrossRef]
- Gómez-Ramírez, A.; López-Santos, C.; Cantos, M.; García, J.L.; Molina, R.; Cotrino, J.; Espinós, J.P.; González-Elipe, A.R. Surface chemistry and germination improvement of Quinoa seeds subjected to plasma activation. Sci. Rep. 2017, 7, 5924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holc, M.; Mozetič, M.; Recek, N.; Primc, G.; Vesel, A.; Zaplotnik, R.; Gselman, P. Wettability increase in plasma-treated agricultural seeds and its relation to germination improvement. Agronomy 2021, 11, 1467. [Google Scholar] [CrossRef]
- Juliette, F.V.D.S.; Luís, C.N.D.S.; Isabel, R.D.S.A.; Alex; Correia, M.T.D.S.; Re, J.M.; Janete, M.D.A.J.; Maria, T.D.S.C.; Rcia, V.D.S.M. Antimicrobial activity of Pityrocarpa monilifomis leaves and its capacity to enhance the activity of four antibiotics against Staphylococcus aureus strains. J. Med. Plants Res. 2013, 7, 2067–2072. [Google Scholar] [CrossRef]
- Felix, F.C.; de Medeiros, J.A.D.; Ferrari, C.d.S.; das Chagas, K.P.T.; Castro, M.L.; de Souza, W.M.A.T.; Vieira, F.D.A.; Pacheco, M.V. Selection of Pityrocarpa moniliformis (Benth.) Luckow & R. W. Jobson mother trees for seeds production. Rev. Bras. Cienc. Agrar. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Świecimska, M.; Tulik, M.; Šerá, B.; Golińska, P.; Tomeková, J.; Medvecká, V.; Bujdáková, H.; Oszako, T.; Zahoranová, A.; Šerý, M. Non-thermal plasma can be used in disinfection of Scots pine (Pinus sylvestris L.) seeds infected with Fusarium oxysporum. Forests 2020, 11, 837. [Google Scholar] [CrossRef]


| Treatment | SG (%) | SVI_I (mm) | SVI_II (g) | SVI_III (g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | HSD | Mean | SE | HSD | Mean | SE | HSD | Mean | SE | HSD | |
| 0 min | 5.56 | 2.22 | a | 404.44 | 287.84 | a | 0.43 | 0.26 | a | 0.06 | 0.03 | a |
| 5 min | 22 | 3.43 | a | 1499.33 | 263.46 | ac | 1.86 | 0.26 | ac | 0.31 | 0.06 | ab |
| 10 min | 43.33 | 4.59 | b | 3058 | 564.37 | bc | 3.92 | 0.65 | bc | 0.65 | 0.12 | b |
| 20 min | 44.67 | 6.72 | b | 3698.67 | 467.22 | b | 4.41 | 0.67 | b | 0.6 | 0.1 | b |
| Treatment | L (mm) | LS (mm) | LR (mm) | R/S_L (mm) | ||||||||
| Mean | SE | HSD | Mean | SE | HSD | Mean | SE | HSD | Mean | SE | HSD | |
| 0 min | 56 | 21.07 | a | 35.89 | 11.27 | a | 20.11 | 10.18 | a | 0.52 | 0.13 | a |
| 5 min | 68.31 | 7.4 | a | 47.78 | 4.12 | a | 20.54 | 3.88 | a | 0.42 | 0.06 | a |
| 10 min | 68.31 | 7.97 | a | 47.54 | 4.67 | a | 20.76 | 3.7 | a | 0.43 | 0.05 | a |
| 20 min | 84.95 | 7.79 | a | 57.71 | 6.52 | a | 27.24 | 1.39 | a | 0.48 | 0.03 | a |
| Treatment | FW (g) | FWS (g) | FWR (g) | R/S_FW (g) | ||||||||
| Mean | SE | HSD | Mean | SE | HSD | Mean | SE | HSD | Mean | SE | HSD | |
| 0 min | 0.07 | 0.02 | a | 0.06 | 0.01 | a | 0.01 | 0 | a | 0.14 | 0.05 | a |
| 5 min | 0.09 | 0 | ab | 0.08 | 0 | ab | 0.01 | 0 | a | 0.11 | 0.02 | a |
| 10 min | 0.09 | 0.01 | ab | 0.08 | 0.01 | ab | 0.01 | 0 | a | 0.13 | 0.01 | a |
| 20 min | 0.1 | 0.01 | b | 0.09 | 0.01 | b | 0.01 | 0 | a | 0.13 | 0.01 | a |
| Treatment | DW (g) | DWS (g) | DWR (g) | R/S_DW (g) | ||||||||
| Mean | SE | HSD | Mean | SE | HSD | Mean | SE | HSD | Mean | SE | HSD | |
| 0 min | 0.011 | 0.002 | a | 0.01 | 0.002 | a | 0.001 | 0 | a | 0.061 | 0.035 | a |
| 5 min | 0.014 | 0.001 | a | 0.013 | 0.001 | a | 0.001 | 0 | a | 0.086 | 0.015 | a |
| 10 min | 0.014 | 0.001 | a | 0.013 | 0.001 | a | 0.001 | 0 | a | 0.098 | 0.005 | a |
| 20 min | 0.013 | 0.001 | a | 0.012 | 0.001 | a | 0.001 | 0 | a | 0.104 | 0.007 | a |
| Plant | NTP Treatment | Changes in Seed Testa | Seed Germination | Comments | References |
|---|---|---|---|---|---|
| Robinia pseudoacacia | Point-to-plane corona discharge in regime of transient spark; discharge voltage 9.65 kV, discharge current 150 µA, source voltage 10.1 kV; 3 mm distance; 5, 10, and 20 min | Eroded surface (SEM), increased content of oxygen (EDS), increase of hydrophilicity (contact angle), higher water absorption (seed water uptake) | Significant difference (p < 0.05) in seed germination (8×) in seeds treated for 20 min | Fresh weights of seedling root, fresh weights of seedling shoot, and all three indexes of seedling vitality were significantly better than in control samples. | Present manuscript |
| Pityrocarpa moniliformis | DBD; power of 10 kV and frequency of 400 kHz; duration 1.5, 2.0, 3.0, 4.0, and 5.0 min | Lowest contact angles of 64 and 61° after 5.0 and 4.0 min; electrical conductivity of soaking liquid and faster reaching phase III were higher in treated seeds | Highest cumulative germination after 5 min | NTP can be used in P. moniliformis seed-stimulation. | [24] |
| Medicago sativa (cultivars + wild plants) | Point-to-plane corona discharge in regime of transient spark; voltage of 4.6 kV; 3 mm distance; TS treatment: 5, 10, and 20 min; PAW treatment: pH≈ 3–4; c(H2O2) ≈ 100 mg/L; c(NO3−) ≈500 mg/L; c(NO2−)≈1 mg/L | Not tested | Seed germination increased (not significantly) from 21% (control) to 34% (TS + PAW treatment, 10 min) in wild plant | Significant increase in some initial growth parameters of seedlings in cultivars. | [22] |
| Leucaena leucocephala | DBD; pulses at 17.5 kV, frequency of 990 Hz; duration of 3, 9, and 15 min | Microcracks and contour in the hilar region and micropyle; electrical conductivity and pH of leached water correlated to imbibition time | Germination percentage changed from 4% (control) to 7% (treated) | Used NTP not sufficient to overcome dormancy. There are two resistance barriers to water penetration: integument surface and region of the macrosclereid cell wall in the seed. | [21] |
| Mimosa caesalpiniafolia | DBD; pulses at 17.5 kV, frequency of 990 Hz; duration of 3, 9, and 15 min | Saturation of the seed imbibition after 9 min | Germination rate was 8 times higher than control after 3 min; the best proportion of Richard curve after 30 min | Wettability and imbibition were directly related to the treatment duration. | [18] |
| Erythrina velutina | DBD; voltage of 10 kV, frequency of 750 Hz, power of 150 W; gas He, flux of 0.03 L/s; distance of 13 mm for 60 s | Contact angle decreased from 112° by 48% (p < 0.05); higher imbibition of 75% in micropyle and hilum | Germination rate significantly (p < 0.05) increase to 75 ± 3.8%; differences (p < 0.05) between Richards and sigmoidal curve parameters Vi, Qu, and Sk | Micropyle and hilum cooperate in the water absorption. | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šerá, B.; Jirešová, J.; Scholtz, V.; Julák, J.; Khun, J. Non-Thermal Plasma Treatment Improves Properties of Dormant Seeds of Black Locust (Robinia pseudoacacia L.). Forests 2023, 14, 471. https://doi.org/10.3390/f14030471
Šerá B, Jirešová J, Scholtz V, Julák J, Khun J. Non-Thermal Plasma Treatment Improves Properties of Dormant Seeds of Black Locust (Robinia pseudoacacia L.). Forests. 2023; 14(3):471. https://doi.org/10.3390/f14030471
Chicago/Turabian StyleŠerá, Božena, Jana Jirešová, Vladimír Scholtz, Jaroslav Julák, and Josef Khun. 2023. "Non-Thermal Plasma Treatment Improves Properties of Dormant Seeds of Black Locust (Robinia pseudoacacia L.)" Forests 14, no. 3: 471. https://doi.org/10.3390/f14030471
APA StyleŠerá, B., Jirešová, J., Scholtz, V., Julák, J., & Khun, J. (2023). Non-Thermal Plasma Treatment Improves Properties of Dormant Seeds of Black Locust (Robinia pseudoacacia L.). Forests, 14(3), 471. https://doi.org/10.3390/f14030471







