Belowground Biomass and Root:Shoot Ratios of Three Willow Cultivars at Two Sites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site and Cultivar Selection
2.2. Sampling Procedure
2.3. Sample Processing
2.4. Chemical Analysis
2.5. Data Analysis
3. Results
3.1. Biomass Allocation
3.2. Effect of Measurement Plot Yield
4. Discussion
4.1. Comparing R:S Levels
4.2. Belowground Biomass Quantity and Distribution
4.3. R:S Variance Due to Biomass Production
4.4. Implications for GHG Analysis in Willow Systems
4.5. Breeding Applications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Compliance with Ethical Standards
References
- Volk, T.A.; Berguson, B.; Daly, C.; Halbleib, M.D.; Miller, R.; Rials, T.G.; Abrahamson, L.P.; Buchman, D.; Buford, M.; Cunningham, M.W.; et al. Poplar and Shrub Willow Energy Crops in the United States: Field Trial Results from the Multiyear Regional Feedstock Partnership and Yield Potential Maps Based on the PRISM-ELM Model. GCB Bioenergy 2018, 10, 735–751. [Google Scholar] [CrossRef]
- Eisenbies, M.H.; Volk, T.A.; Posselius, J.; Shi, S.; Patel, A. Quality and Variability of Commercial-Scale Short Rotation Willow Biomass Harvested Using a Single-Pass Cut-and-Chip Forage Harvester. BioEnergy Res. 2014, 8, 546–559. [Google Scholar] [CrossRef]
- Djomo, S.N.; Kasmioui, O.E.; Ceulemans, R. Energy and Greenhouse Gas Balance of Bioenergy Production from Poplar and Willow: A Review: Energy and Greenhouse Gas Balance of Bioenergy Production. GCB Bioenergy 2011, 3, 181–197. [Google Scholar] [CrossRef]
- Rowe, R.; Street, N.; Taylor, G. Identifying Potential Environmental Impacts of Large-Scale Deployment of Dedicated Bioenergy Crops in the UK. Renew. Sustain. Energy Rev. 2009, 13, 271–290. [Google Scholar] [CrossRef]
- Volk, T.A.; Heavey, J.P.; Eisenbies, M.H. Advances in Shrub-Willow Crops for Bioenergy, Renewable Products, and Environmental Benefits. Food Energy Secur. 2016, 5, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Heaton, R.J.; Randerson, P.F.; Slater, F.M. The Economics of Growing Short Rotation Coppice in the Uplands of Mid-Wales and an Economic Comparison with Sheep Production. Biomass Bioenergy 1999, 17, 59–71. [Google Scholar] [CrossRef]
- Buchholz, T.; Volk, T.A. Improving the Profitability of Willow Crops—Identifying Opportunities with a Crop Budget Model. BioEnergy Res. 2011, 4, 85–95. [Google Scholar] [CrossRef]
- Frank, J.R.; Brown, T.R.; Volk, T.A.; Heavey, J.P.; Malmsheimer, R.W. A Stochastic Techno-Economic Analysis of Shrub Willow Production Using EcoWillow 3.0S: A Stochastic Techno-Economic Analysis of Shrub Willow Production Using EcoWillow 3.0S. Biofuels Bioprod. Bioref. 2018, 12, 846–856. [Google Scholar] [CrossRef]
- Addo-Danso, S.D.; Prescott, C.E.; Smith, A.R. Methods for Estimating Root Biomass and Production in Forest and Woodland Ecosystem Carbon Studies: A Review. For. Ecol. Manag. 2016, 359, 332–351. [Google Scholar] [CrossRef]
- Cunniff, J.; Purdy, S.J.; Barraclough, T.J.P.; Castle, M.; Maddison, A.L.; Jones, L.E.; Shield, I.F.; Gregory, A.S.; Karp, A. High Yielding Biomass Genotypes of Willow (Salix spp.) Show Differences in below Ground Biomass Allocation. Biomass Bioenergy 2015, 80, 114–127. [Google Scholar] [CrossRef] [Green Version]
- Fontana, M.; Collin, A.; Courchesne, F.; Labrecque, M.; Bélanger, N. Root System Architecture of Salix Miyabeana “SX67” and Relationships with Aboveground Biomass Yields. Bioenerg. Res. 2020, 13, 183–196. [Google Scholar] [CrossRef]
- Gamble, J.D.; Johnson, G.A.; Current, D.A.; Wyse, D.L.; Zamora, D.S.; Sheaffer, C.C. Alley Cropping Affects Perennial Bioenergy Crop Root Distribution, Carbon, and Nutrient Stocks. Agron. J. 2020, 112, 3718–3732. [Google Scholar] [CrossRef]
- Ghestem, M.; Sidle, R.C.; Stokes, A. The Influence of Plant Root Systems on Subsurface Flow: Implications for Slope Stability. BioScience 2011, 61, 869–879. [Google Scholar] [CrossRef]
- Martani, E.; Ferrarini, A.; Serra, P.; Pilla, M.; Marcone, A.; Amaducci, S. Belowground Biomass C Outweighs Soil Organic C of Perennial Energy Crops: Insights from a Long-term Multispecies Trial. GCB Bioenergy 2021, 13, 459–472. [Google Scholar] [CrossRef]
- Pacaldo, R.S.; Volk, T.A.; Briggs, R.D. No Significant Differences in Soil Organic Carbon Contents along a Chronosequence of Shrub Willow Biomass Crop Fields. Biomass Bioenergy 2013, 58, 136–142. [Google Scholar] [CrossRef]
- Wilkinson, A.G. Poplars and Willows for Soil Erosion Control in New Zealand. Biomass Bioenergy 1999, 16, 263–274. [Google Scholar] [CrossRef]
- Rytter, R.-M.; Rytter, L. Growth, Decay and Turnover of Fine Roots of Basket Willows. Can. J. For. Res. 1998, 28, 863–902. [Google Scholar] [CrossRef]
- Rytter, R.-M. Biomass Production and Allocation, Including Fine-Root Turnover, and Annual N Uptake in Lysimeter-Grown Basket Willows. For. Ecol. Manag. 2001, 140, 177–192. [Google Scholar] [CrossRef]
- Zan, C.S.; Fyles, J.W.; Girouard, P.; Samson, R.A. Carbon Sequestration in Perennial Bioenergy, Annual Corn and Uncultivated Systems in Southern Quebec. Agric. Ecosyst. Environ. 2001, 86, 135–144. [Google Scholar] [CrossRef]
- Matthews, R. Modelling of Energy and Carbon Budgets of Wood Fuel Coppice Systems. Biomass Bioenergy 2001, 21, 1–19. [Google Scholar] [CrossRef]
- Volk, T.A. Alternative Site Preparation and Coppice Management during the Establishment of Short Rotation Woody Crops. PhD Dissertation, State University of New York College of Environmental Science and Forestry, Syracuse, NY, USA, 2002. [Google Scholar]
- Hangs, R.D.; Schoenau, J.J.; Van Rees, K.C.J.; Bélanger, N.; Volk, T.; Jensen, T. First Rotation Biomass Production and Nutrient Cycling within Short-Rotation Coppice Willow Plantations in Saskatchewan, Canada. BioEnergy Res. 2014, 7, 1091–1111. [Google Scholar] [CrossRef] [Green Version]
- Johnson, I.R.; Thornley, J.H.M. A Model of Shoot: Root Partitioning with Optimal Growth. Ann. Bot. 1987, 60, 133–142. [Google Scholar] [CrossRef]
- Thornley, J.H.M. A Balanced Quantitative Model for Root: Shoot Ratios in Vegetative Plants. Ann. Bot. 1972, 36, 431–441. [Google Scholar] [CrossRef]
- Rytter, R.-M. The Potential of Willow and Poplar Plantations as Carbon Sinks in Sweden. Biomass Bioenergy 2012, 36, 86–95. [Google Scholar] [CrossRef]
- Stadnyk, C. Root Dynamics and Carbon Accumulation of Six Willow Clones in Saskatchewan. Master’s Thesis, University of Saskatchewan, Saskatoon, Saskatchewan, Canada, 2010. [Google Scholar]
- Yang, S.; Volk, T.; Fortier, M.-O. Willow Biomass Crops Are a Carbon Negative or Low-Carbon Feedstock Depending on Prior Land Use and Transportation Distances to End Users. Energies 2020, 13, 4251. [Google Scholar] [CrossRef]
- Heller, M.C.; Keoleian, G.A.; Volk, T.A. Life Cycle Assessment of a Willow Bioenergy Cropping System. Biomass Bioenergy 2003, 25, 147–165. [Google Scholar] [CrossRef]
- Caputo, J.; Balogh, S.B.; Volk, T.A.; Johnson, L.; Puettmann, M.; Lippke, B.; Oneil, E. Incorporating Uncertainty into a Life Cycle Assessment (LCA) Model of Short-Rotation Willow Biomass (Salix spp.) Crops. BioEnergy Res. 2013, 7, 48–59. [Google Scholar] [CrossRef]
- Fabio, E.S.; Volk, T.A.; Miller, R.O.; Serapiglia, M.J.; Kemanian, A.R.; Montes, F.; Kuzovkina, Y.A.; Kling, G.J.; Smart, L.B. Contributions of Environment and Genotype to Variation in Shrub Willow Biomass Composition. Ind. Crop. Prod. 2017, 108, 149–161. [Google Scholar] [CrossRef]
- Volk, T.A.; Abrahamson, L.P.; Cameron, K.D.; Castellano, P.; Corbin, T.; Fabio, E.; Johnson, G.; Kuzovikina-Eischen, Y.; Labrecque, M.; Miller, R.; et al. Yields of Biomass Crops across a Range of Sites in North America. Asspects Appl. Biol. 2011, 112, 67–74. [Google Scholar]
- Sleight, N.J.; Volk, T.A. Recently Bred Willow (Salix spp.) Biomass Crops Show Stable Yield Trends Over Three Rotations at Two Sites. Bioenerg. Res. 2016, 9, 782–797. [Google Scholar] [CrossRef]
- USDA Natural Resources Conservation Service Web Soil Survey. Available online: https://websoilsurvey.sc.egov.usda.gov/ (accessed on 11 June 2017).
- Serapiglia, M.J.; Cameron, K.D.; Stipanovic, A.J.; Abrahamson, L.P.; Volk, T.A.; Smart, L.B. Yield and Woody Biomass Traits of Novel Shrub Willow Hybrids at Two Contrasting Sites. BioEnergy Res. 2012, 6, 533–546. [Google Scholar] [CrossRef] [Green Version]
- SAS. Institute SAS/STAT 9.2 User’s Guide, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2009. [Google Scholar]
- Amichev, B.Y.; Hangs, R.D.; Bélanger, N.; Volk, T.A.; Vujanovic, V.; Schoenau, J.J.; Van Rees, K.C.J. First-Rotation Yields of 30 Short-Rotation Willow Cultivars in Central Saskatchewan, Canada. BioEnergy Res. 2014, 8, 292–306. [Google Scholar] [CrossRef]
- Guidi, W.; Labrecque, M. Effects of High Water Supply on Growth, Water Use, and Nutrient Allocation in Willow and Poplar Grown in a 1-Year Pot Trial. Water Air Soil Pollut. 2010, 207, 85–101. [Google Scholar] [CrossRef]
- Phillips, C.J.; Marden, M.; Suzanne, L.M. Observations of Root Growth of Young Poplar and Willow Planting Types. N.Z. J. For. Sci. 2014, 44, 15. [Google Scholar] [CrossRef] [Green Version]
- Tylek, P.; Pietrzykowski, M.; Walczyk, J.; Juliszewski, T.; Kwasniewski, D. Root Biomass and Morphological Characterization of Energy Willow Stumps. Croat. J. For. Eng. 2017, 38, 47–54. [Google Scholar]
- Chimento, C.; Amaducci, S. Characterization of Fine Root System and Potential Contribution to Soil Organic Carbon of Six Perennial Bioenergy Crops. Biomass Bioenergy 2015, 83, 116–122. [Google Scholar] [CrossRef]
- Keyes, M.R.; Grier, C.C. Above- and below-Ground Net Production in 40-Year-Old Douglas-Fir Stands on Low and High Productivity Sites. Can. J. For. Res. 1981, 11, 599–605. [Google Scholar] [CrossRef]
- Kibet, L.C.; Blanco-Canqui, H.; Jasa, P. Long-Term Tillage Impacts on Soil Organic Matter Components and Related Properties on a Typic Argiudoll. Soil Tillage Res. 2016, 155, 78–84. [Google Scholar] [CrossRef]
- Frank, J.; Therasme, O.; Volk, T.A.; Brown, T.; Malmsheimer, R.W.; Fortier, M.-O.; Eisenbies, M.H.; Ha, H.; Heavey, J. Integrated Stochastic Life Cycle Assessment and Techno-Economic Analysis for Shrub Willow Production in the Northeastern United States. Sustainability 2022, 14, 9007. [Google Scholar] [CrossRef]
- Senerby-Forsse, L.; Ferm, A.; Kauppi, A. Coppicing Ability and Sustainability. In Ecophysiology of Short Rotation Forest Crops; Elsevier: Essex, UK, 1992; pp. 146–184. [Google Scholar]
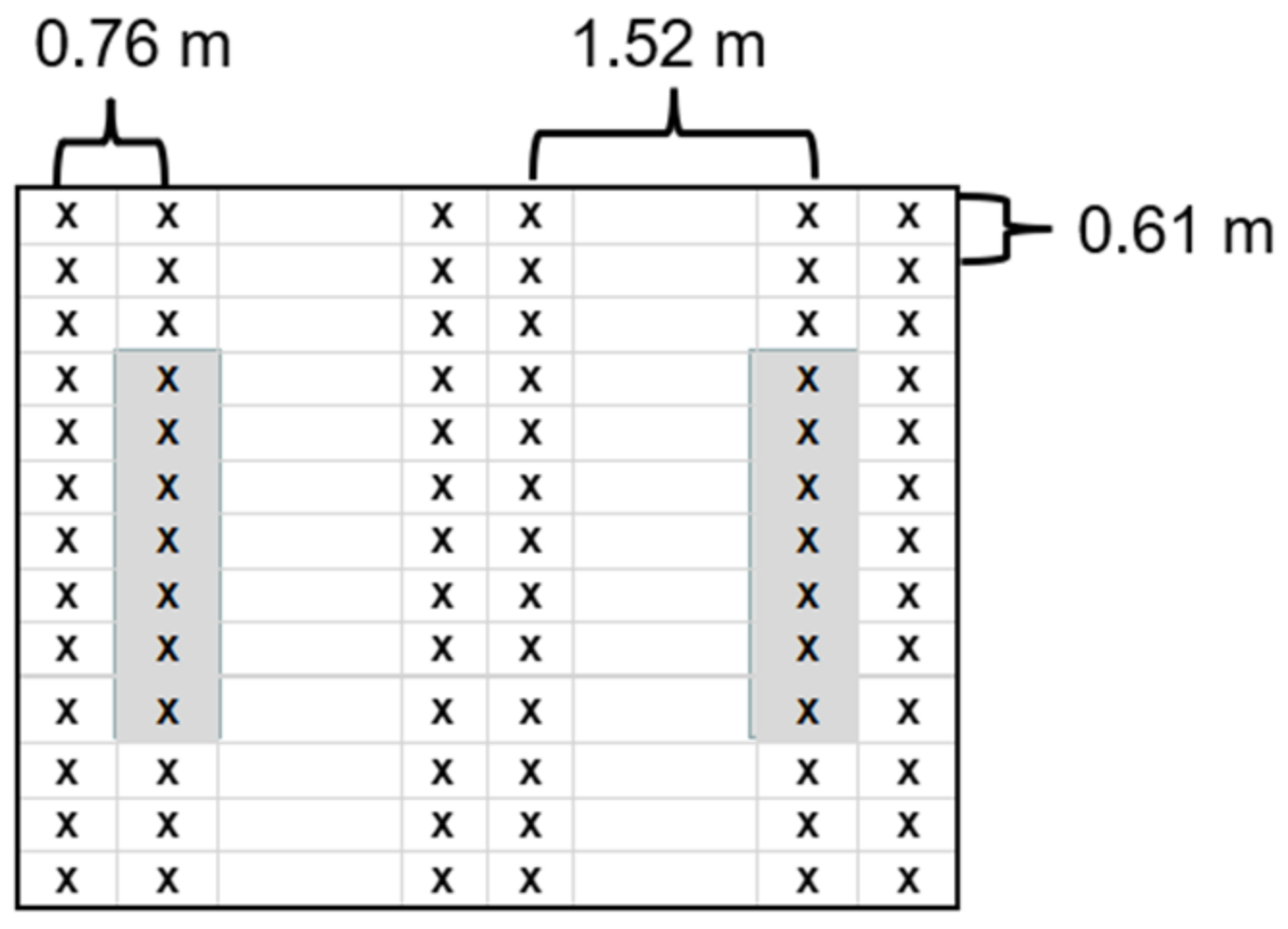
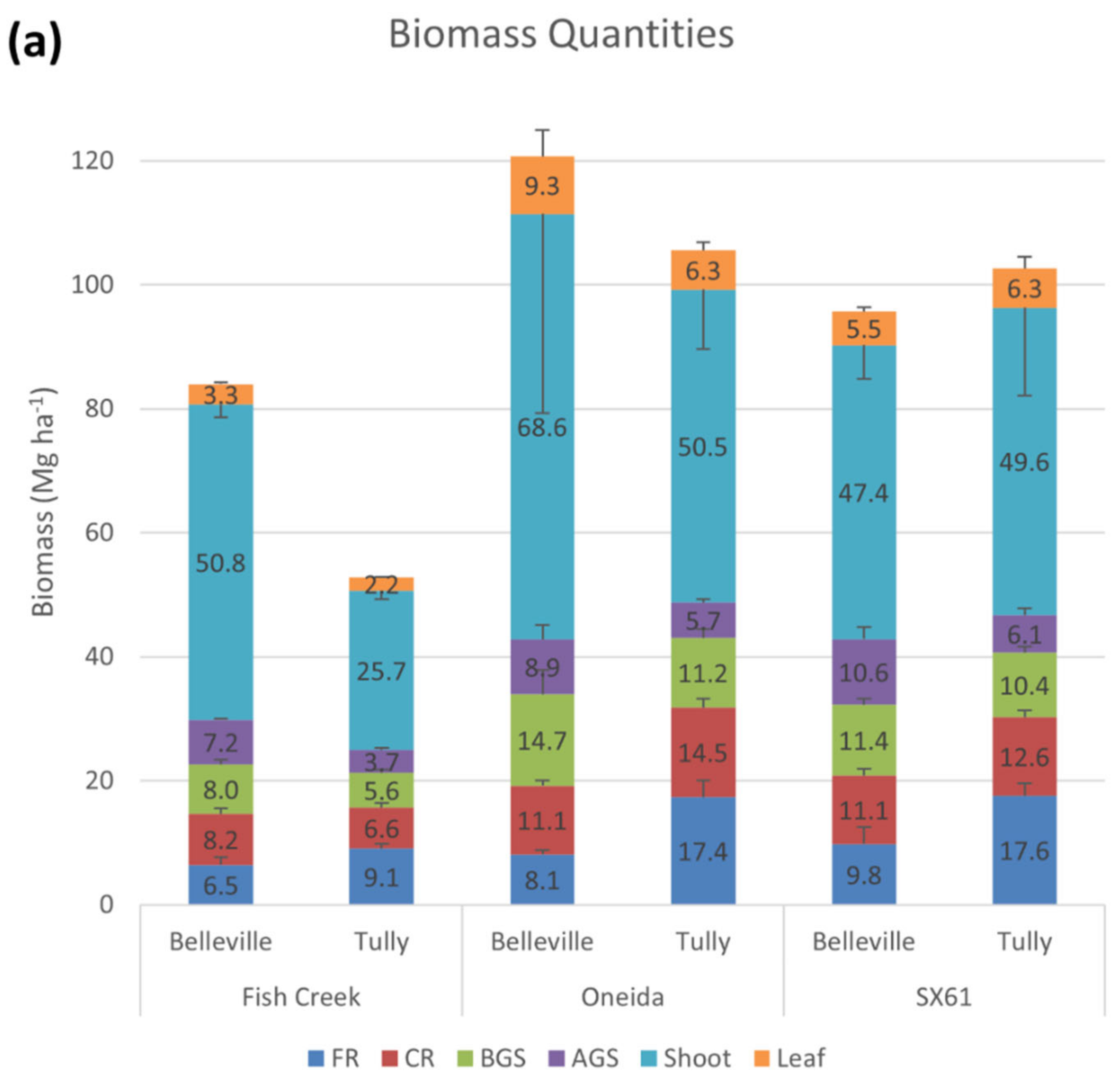
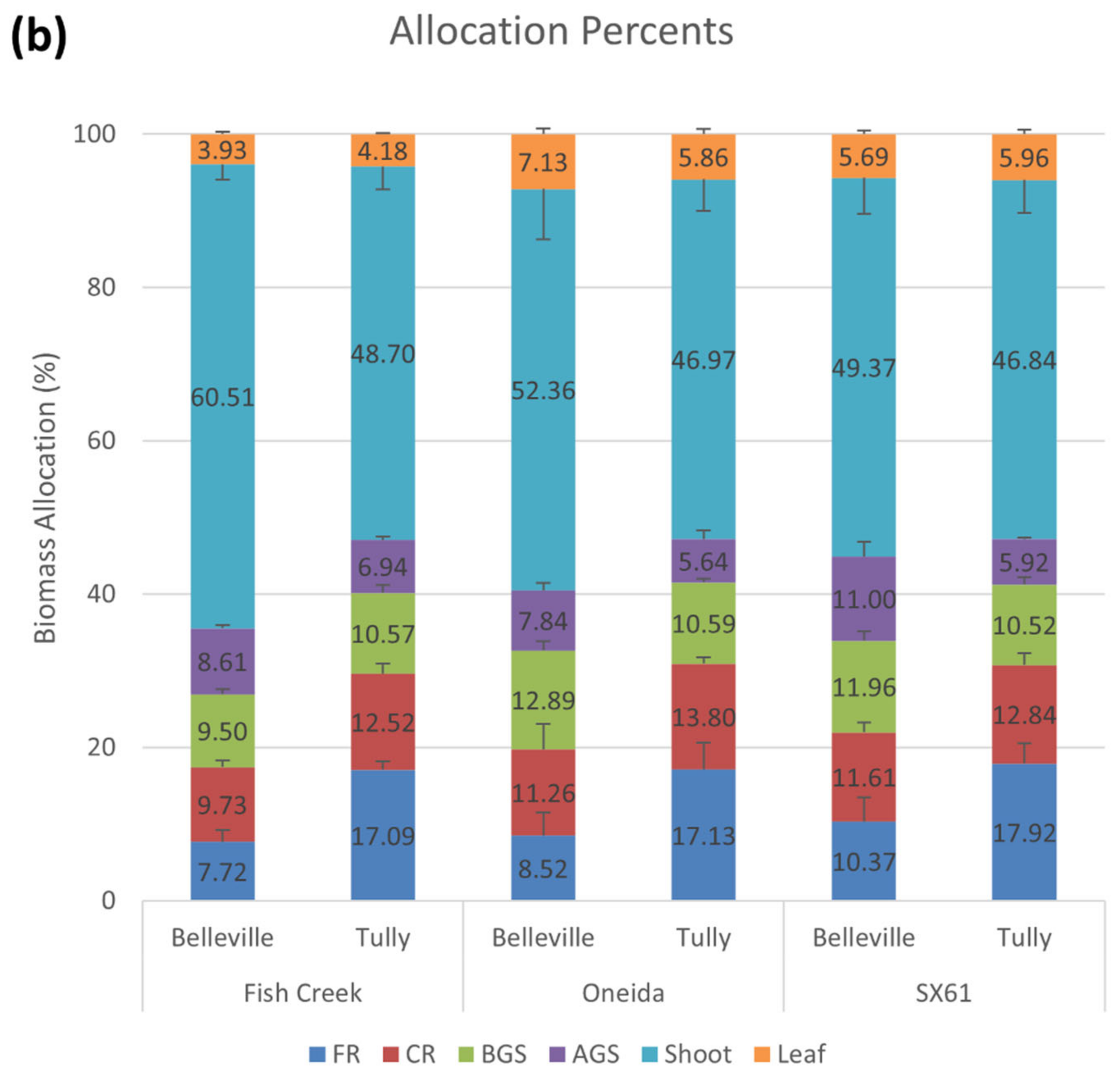

| Parameter | Mean (SE) | p-Values from ANOVA for Effects of Site, Cultivar, and Site × Cultivar Interactions a | Least Squares Means Separations for Significant ANOVA Parameters in Column to the Left (a = 0.1) b | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Site | Cultivar | Site × Cultivar | Measurement Plot Yield | Belleville | Tully | Fish Creek | Oneida | SX61 | ||
| % Aboveground Biomass | 56.3 (2.0) | 0.2608 | 0.4059 | 0.9423 | 0.0435 | |||||
| % Belowground Biomass | 43.7 (2.0) | 0.2608 | 0.4059 | 0.9423 | 0.0435 | |||||
| % Leaf | 5.5 (0.3) | 0.9275 | 0.0031 | 0.1647 | 0.0897 | 4.1 b | 6.6 a | 5.7 a | ||
| % Shoot | 50.8 (1.9) | 0.2276 | 0.1835 | 0.9331 | 0.0443 | |||||
| % AGS | 7.7 (0.6) | 0.0258 | 0.3071 | 0.0805 | 0.0538 | SX: 10.9 a; FC: 8.0 b; ON: 7.9 b | FC: 7.6 b; ON: 5.9 b; SX: 5.7 b | |||
| % BGS | 11.0 (0.4) | 0.4622 | 0.0808 | 0.0808 | x | T: 10.6 ab; B: 9.5 b | B: 12.9 a; T: 10.6 ab | B: 12.0 a; T: 10.5 ab | ||
| % CRs | 12.0 (0.7) | 0.3575 | 0.4553 | 0.7318 | 0.0155 | |||||
| % FRs | 13.1 (1.4) | 0.0130 | 0.4384 | 0.7561 | 0.0137 | 9.6b | 16.7a | |||
| % AGS of Belowground Biomass | 18.2 (1.6) | 0.0024 | 0.0323 | 0.0177 | 0.0005 | SX: 24.8 a; FC: 22.2 ab; ON: 20.3 bc | FC: 17.4 c; ON: 12.7 d; SX: 12.0 d | |||
| % BGS of Belowground Biomass | 25.8 (1.2) | 0.0300 | 0.2558 | 0.3930 | x | 28.9 a | 22.6 b | |||
| % CR of Belowground Biomass | 27.2 (0.7) | 0.8998 | 0.7719 | 0.2881 | 0.0723 | |||||
| % FR of Belowground Biomass | 28.8 (2.2) | 0.0050 | 0.4676 | 0.5414 | 0.0275 | 22.0 b | 35.6 a | |||
| root:shoot ratio | 0.63 (0.04) | 0.8970 | 0.2834 | 0.8481 | 0.0978 | |||||
| leaf:stem ratio | 0.109 (0.006) | 0.0806 | <0.0001 | 0.0041 | x | T: 0.086 d; 0.065 e | B: 0.137 a; T: 0.125 bc | T: 0.127 b; B: 0.116 c | ||
| FR:CR ratio | 1.07 (0.08) | 0.0142 | 0.5007 | 0.9566 | x | 0.80 b | 1.33 a | |||
| Site | Cultivar | R:S Ratio | SE | Adjusted a R:S Ratios |
|---|---|---|---|---|
| Belleville | Fish Creek | 0.46 | 0.03 | 0.52 |
| Belleville | Oneida | 0.65 | 0.16 | 0.65 |
| Belleville | SX61 | 0.72 | 0.12 | 0.73 |
| Tully | Fish Creek | 0.63 | 0.08 | 0.57 |
| Tully | Oneida | 0.65 | 0.09 | 0.63 |
| Tully | SX61 | 0.64 | 0.10 | 0.66 |
| Source | Site(s) | Cultivar | Root Age (years) | Shoot Age (years) | Rotation | R:S Ratio | R:S Ratio (Including FRs) |
|---|---|---|---|---|---|---|---|
| Rytter 2001 [18] | Clay site; Sandy site | S. viminalis a | 1 2 or 3 | 1 2 or 3 | 1 1 | 0.22–0.29 0.16–0.21 | 0.55–0.68 0.33–0.36 |
| Zan et al., 2001 [19] | high and low-quality sites | S. alba × glatfelteri | 4 | 1 | 2 | 0.2–0.4 | 0.5–1.0 |
| Matthews 2001 [20] | Ingerthorpe | Bowles hybrid | 4; 6; 11 | 1; 2; 3 | 3; 4; 5 | 0.23–0.48 | 0.27–0.56 |
| Matthews 2001 [20] | Long Ashton | Gigantea | 22 | 1 | 22 | 2.7 | 3.0 |
| Volk 2002 [21] | Tully, NY, USA | SV1 SX61 | 2 | 1 | 1 | 5.7–6.2 2.4–3.0 | |
| Volk 2002 [21] | Wolcott, NY, USA | SV1 | 2 | 1 | 1 | 0.9–1.1 | |
| Guidi and Labrecque 2010 [37] | 0.54 | ||||||
| Stadnyk 2010 [26] | Saskatoon, SK, USA | CAN SHE FC ALL SX61 SX64 | 2 | 2 | 1 | 0.38 0.34 0.22 0.30 0.32 0.28 | |
| Stadnyk 2010 [26] | Prince Albert, SK, USA | CAN SHE FC ALL SX61 SX64 | 2 | 2 | 1 | 0.22 0.33 0.18 0.81 0.13 0.28 | |
| Pacaldo et al., 2013 [15] | Tully, NY, USA | SV1 | 5; 14; 19 | 2 | 2; 5; 6 | 1.0–1.3 | 1.9–2.5 |
| Pacaldo et al., 2013 [15] | Lafayette, NY, USA | SV1 | 12 | 2 | 4 | 1.9 | 4.1 |
| Phillips et al., 2014 [38] | Gisborne, New Zealand | Hiwinui and Tangoio | 1 | 1 | 1 | 0.47–0.93 | |
| Hangs et al., 2014 b [22] | 4 sites in Saskatchewan | 6 cultivars | 4 | 3 | 1 | 0.11 | 0.38–0.98 |
| Cunniff et al., 2015 [10] | Harpenden | Endurance, Resolution, Terra Nova, Tora | 3; 5 | 2 | 1 or 2 | 0.1–0.2 c | |
| Cunniff et al., 2015 [10] | Aberystwyth | Endurance, Resolution, Terra Nova, Tora | 3; 5 | 2 | 1 or 2 | 0.2–0.3 c | |
| Tylek et al., 2017 [39] | Kaniów | Salix spp. | 12 | 3 | 4 | 1.19 | 1.23 |
| Current Study | Tully and Belleville, NY | Fish Creek, Oneida, SX61 | 10 | 3 | 2 | ||
| Position | Belleville | Tully | ||||
|---|---|---|---|---|---|---|
| 9882-34 | 9980-005 | SX61 | 9882-34 | 9980-005 | SX61 | |
| Percent Carbon (Mean and Standard Error) | ||||||
| Foliage | 47.1 (0.06) | 45.1 (0.28) | 45.5 (0.15) | 47.5 (0.12) | 46.3 (0.24) | 45.3 (0.51) |
| Stem | 46.5 (0.11) | 46.3 (0.12) | 45.8 (0.22) | 47.0 (0.38) | 46.6 (0.03) | 46.3 (0.01) |
| Aboveground Stool | 47.3 (0.68) | 46.0 (0.19) | 46.2 (0.08) | 47.7 (0.63) | 46.5 (0.09) | 46.5 (0.03) |
| Belowground Stool | 46.7 (0.29) | 46.0 (0.12) | 45.4 (0.23) | 46.2 (0.42) | 46.0 (0.20) | 45.4 (0.23) |
| Coarse Roots | 44.3 (0.19) | 44.6 (0.16) | 44.5 (0.44) | 45.1 (0.11) | 45.3 (0.14) | 44.8 (0.10) |
| Fine Roots | 45.1 (0.38) | 45.5 (0.37) | 44.6 (0.42) | 45.2 (0.41) | 45.6 (0.22) | 46.0 (0.41) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sleight, N.J.; Volk, T.A.; Eisenbies, M. Belowground Biomass and Root:Shoot Ratios of Three Willow Cultivars at Two Sites. Forests 2023, 14, 525. https://doi.org/10.3390/f14030525
Sleight NJ, Volk TA, Eisenbies M. Belowground Biomass and Root:Shoot Ratios of Three Willow Cultivars at Two Sites. Forests. 2023; 14(3):525. https://doi.org/10.3390/f14030525
Chicago/Turabian StyleSleight, Nathan J., Timothy A. Volk, and Mark Eisenbies. 2023. "Belowground Biomass and Root:Shoot Ratios of Three Willow Cultivars at Two Sites" Forests 14, no. 3: 525. https://doi.org/10.3390/f14030525






