Effects of Leaf Hydrophilicity and Stomatal Regulation on Foliar Water Uptake Capacity of Desert Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Sites
2.2. Field Experiments
2.2.1. Sample Plot Design
2.2.2. Plant Selection and Sample Collection with Determination
2.3. Laboratory Experiments
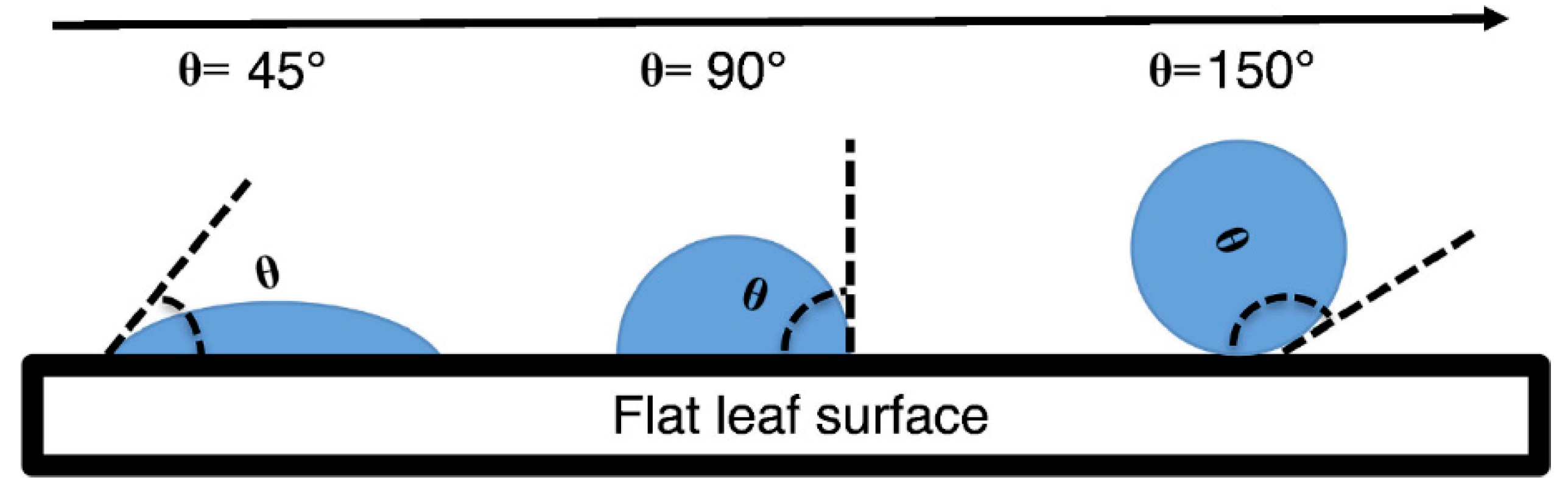
2.3.1. Leaf Wettability
2.3.2. Determination of FWU Parameters
2.3.3. Determination of Pressure–Volume Curves
2.4. Statistical Analyses
3. Results
3.1. Leaf Wettability
3.2. FWU Characteristics of Six Plants
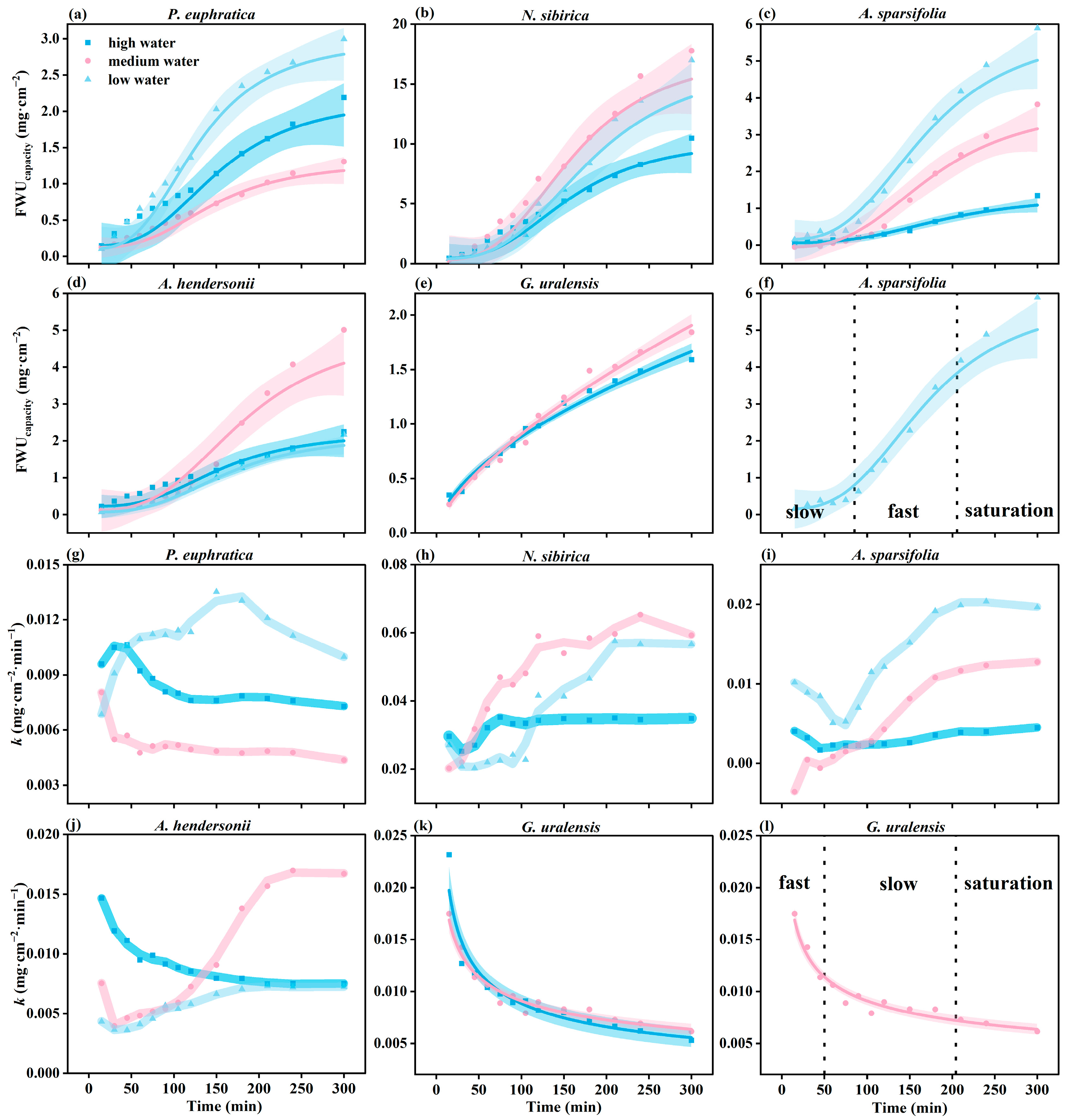
3.2.1. Patterns of FWU Characteristics over Time
3.2.2. Patterns of FWU Characteristics under Water Gradients with Time
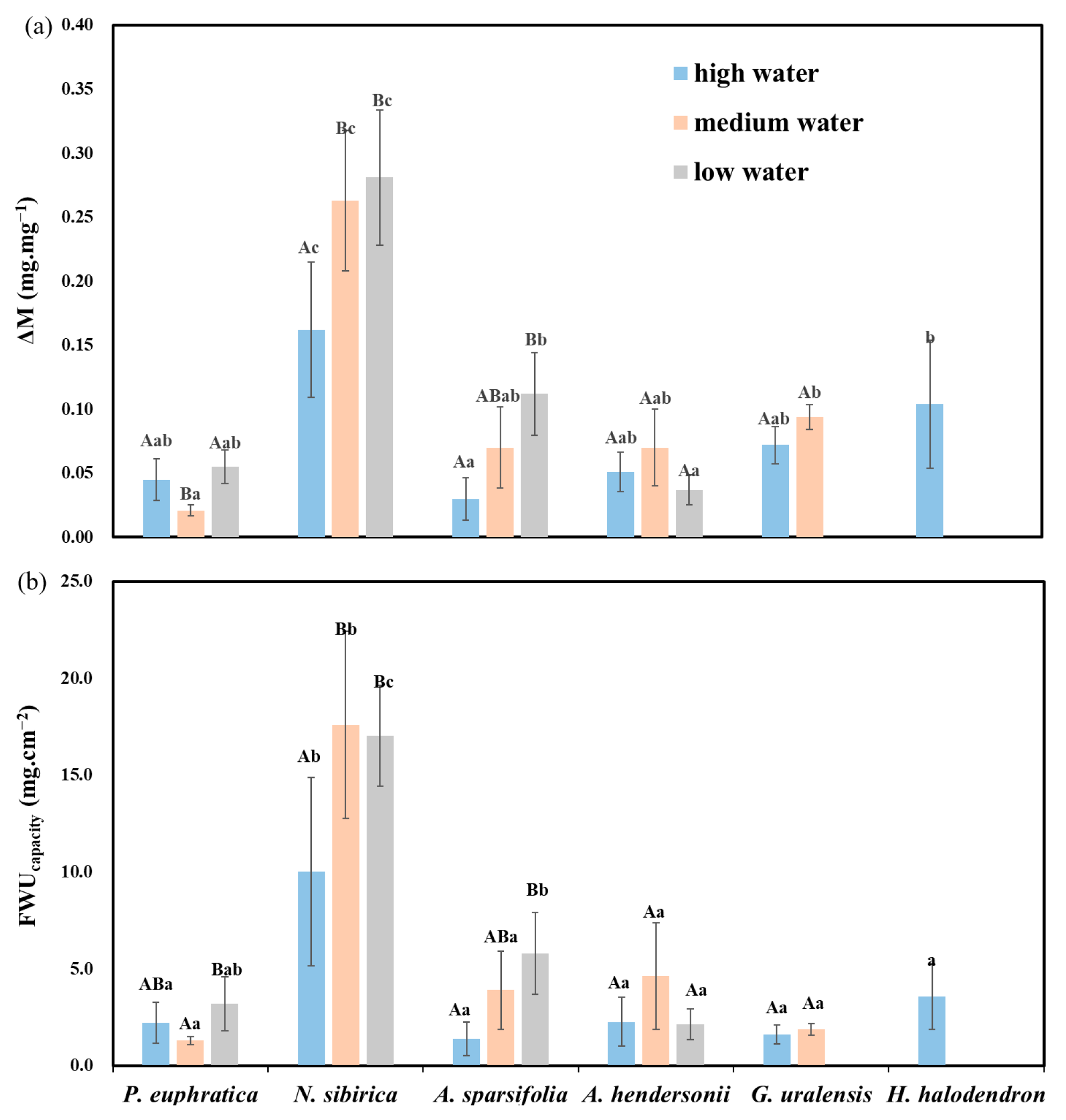
3.2.3. FWU Characteristics under Different Water Gradients and Interspecific Differences
3.3. Leaf Structural Characteristics
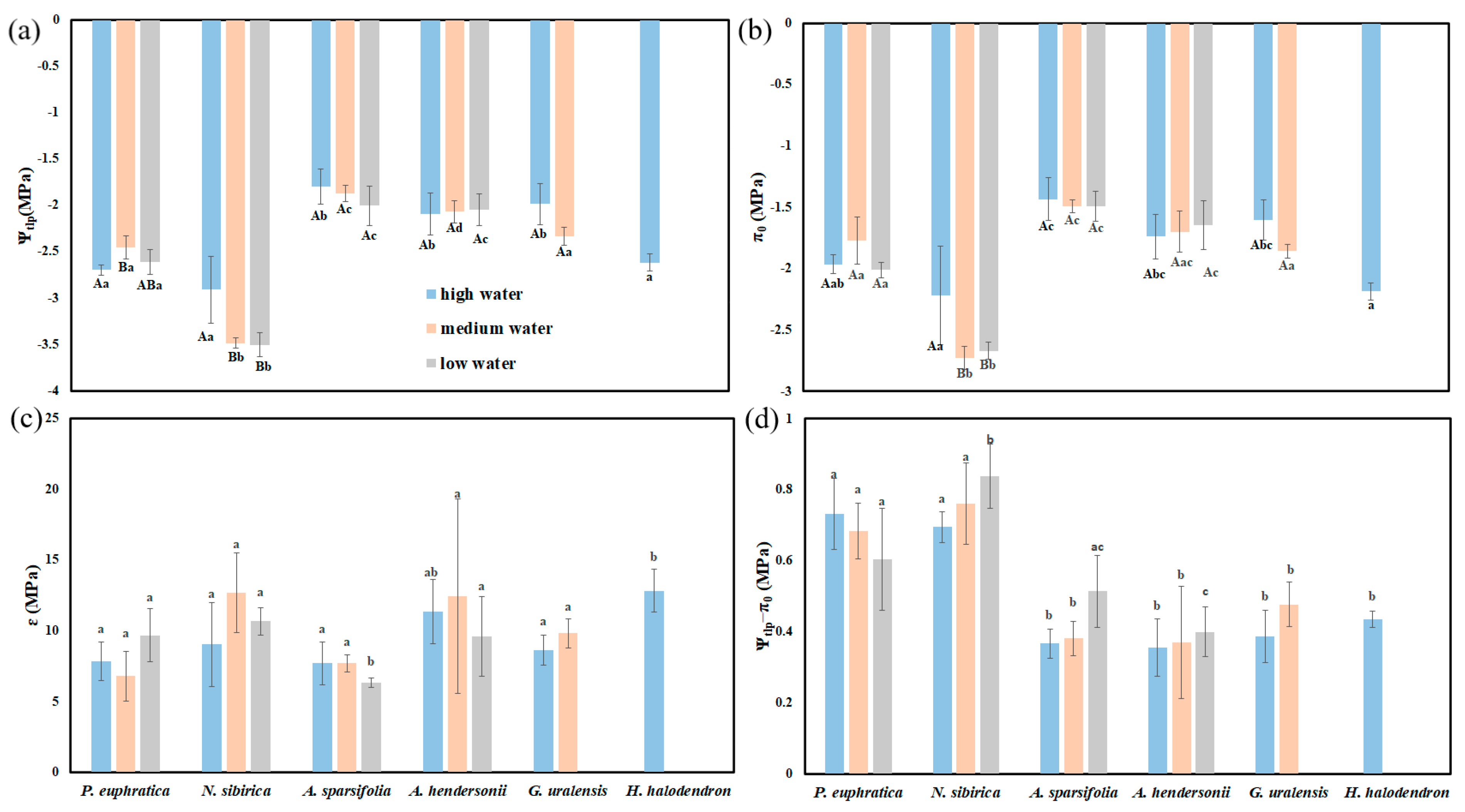
3.4. Hydraulic Parameters of Six Plants
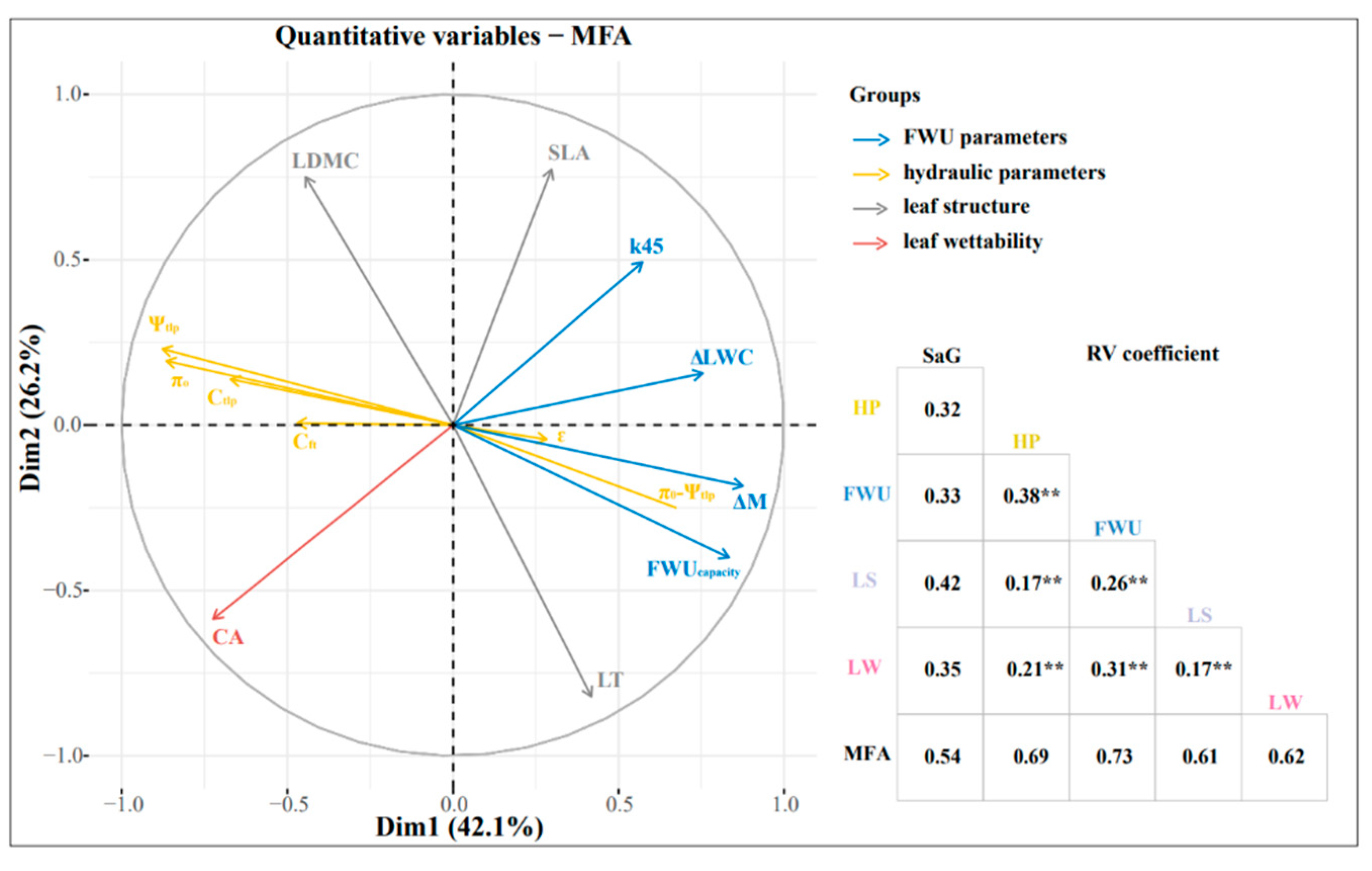
3.5. Relationship between FWU, Leaf Structure, and Hydraulic Parameters
4. Discussion
4.1. FWU Strategies of Six Plants
4.2. Effects of Leaf Wettability and Leaf Structure on FWU Strategies
4.3. Effects of Stomatal Regulation on FWU Strategies
4.4. Relationship between FWU and Leaf Wettability, Leaf Structure, and Stomatal Behavior
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burkhardt, J. Hygroscopic Particles on Leaves: Nutrients or Desiccants? Ecol. Monogr. 2010, 80, 369–399. [Google Scholar] [CrossRef]
- Feng, T.J.; Zhang, Z.Q.; Zhang, L.X.; Xu, W.; He, J.S. Review on the influencing factors and functions of condensated water in arid and semi-arid ecosystems. Acta Ecol. Sin. 2021, 41, 456–468. [Google Scholar]
- Chin, A.R.O.; Guzmán-Delgado, P.; Sillett, S.C.; Kerhoulas, L.P.; Ambrose, A.R.; McElrone, A.R.; Zwieniecki, M.A. Tracheid Buckling Buys Time, Foliar Water Uptake Pays It Back: Coordination of Leaf Structure and Function in Tall Redwood Trees. Plant Cell Env. 2022, 45, 2607–2616. [Google Scholar] [CrossRef]
- Darby, A.; Draguljić, D.; Glunk, A.; Gotsch, S.G. Habitat Moisture Is an Important Driver of Patterns of Sap Flow and Water Balance in Tropical Montane Cloud Forest Epiphytes. Oecologia 2016, 182, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Boanares, D.; Kozovits, A.R.; Lemos-Filho, J.P.; Isaias, R.M.S.; Solar, R.R.R.; Duarte, A.A.; Vilas-Boas, T.; França, M.G.C. Foliar Water-Uptake Strategies Are Related to Leaf Water Status and Gas Exchange in Plants from a Ferruginous Rupestrian Field. Am. J. Bot. 2019, 106, 935–942. [Google Scholar] [CrossRef]
- Boanares, D.; Jovelina da-Silva, C.; Mary dos Santos Isaias, R.; Costa França, M.G. Oxidative Metabolism in Plants from Brazilian Rupestrian Fields and Its Relation with Foliar Water Uptake in Dry and Rainy Seasons. Plant Physiol. Biochem. 2020, 146, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Boanares, D.; Isaias, R.R.M.S.; de Sousa, H.C.; Kozovits, A.R. Strategies of Leaf Water Uptake Based on Anatomical Traits. Plant Biol. 2018, 20, 848–856. [Google Scholar] [CrossRef]
- Lima, J.F.; Boanares, D.; Costa, V.E.; Moreira, A.S.F.P. Do Photosynthetic Metabolism and Habitat Influence Foliar Water Uptake in Orchids? Plant Biol. 2023, 25, 257–267. [Google Scholar] [CrossRef]
- Gotsch, S.G.; Nadkarni, N.; Darby, A.; Glunk, A.; Dix, M.; Davidson, K.; Dawson, T.E. Life in the Treetops: Ecophysiological Strategies of Canopy Epiphytes in a Tropical Montane Cloud Forest. Ecol. Monogr. 2015, 85, 393–412. [Google Scholar] [CrossRef]
- Pan, Z.L.; Guo, W.; Wang, T.; Li, Y.P.; Yang, S.J. Research progress on foliar water uptake. Plant Physiol. J. China 2021, 57, 19–32. [Google Scholar] [CrossRef]
- Dawson, T.E.; Goldsmith, G.R. The Value of Wet Leaves. New Phytol. 2018, 219, 1156–1169. [Google Scholar] [CrossRef]
- Li, Z.-K.; Gong, X.-W.; Wang, J.-L.; Chen, Y.-D.; Liu, F.-Y.; Li, H.-P.; Lü, G.-H. Foliar Water Uptake Improves Branch Water Potential and Photosynthetic Capacity in Calligonum mongolicum. Ecol. Indic. 2023, 146, 109825. [Google Scholar] [CrossRef]
- Schreel, J.D.M.; Steppe, K. Foliar Water Uptake Changes the World of Tree Hydraulics. NPJ Clim. Atmos. Sci. 2019, 2, 1–2. [Google Scholar] [CrossRef]
- Fan, X.; Hao, X.; Zhang, S.; Zhao, Z.; Zhang, J.; Li, Y. Populus Euphratica Counteracts Drought Stress through the Dew Coupling and Root Hydraulic Redistribution Processes. Ann. Bot. 2023, mcac159. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.A.; Zhang, Y.; Wang, X.; Shrestha, N.; Malik, K.; Khan, I.; Ma, W.; Sun, Y.; Li, F.; Ran, J.; et al. Phylogenetic Independence in the Variations in Leaf Functional Traits among Different Plant Life Forms in an Arid Environment. J. Plant Physiol. 2022, 272, 153671. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Garcia, J.; Boanares, D.; França, M.G.C.; Sershen; López-Portillo, J. Foliar Water Uptake in Eight Mangrove Species: Implications of Morpho-Anatomical Traits. Flora 2022, 293, 152100. [Google Scholar] [CrossRef]
- Bryant, C.; Fuenzalida, T.I.; Zavafer, A.; Nguyen, H.T.; Brothers, N.; Harris, R.J.; Beckett, H.A.A.; Holmlund, H.I.; Binks, O.; Ball, M.C. Foliar Water Uptake via Cork Warts in Mangroves of the Sonneratia Genus. Plant Cell Environ. 2021, 44, 2925–2937. [Google Scholar] [CrossRef]
- Martin, C.E.; von Willert, D.J. Leaf Epidermal Hydathodes and the Ecophysiological Consequences of Foliar Water Uptake in Species of Crassula from the Namib Desert in Southern Africa. Plant Biol. 2000, 2, 229–242. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Seasonal Changes of Leaf Surface Contamination in Beech, Oak, and Ginkgo in Relation to Leaf Micromorphology and Wettability. New Phytol. 1998, 138, 91–98. [Google Scholar] [CrossRef]
- Wagner, P.; Furstner, R.; Barthlott, W.; Neinhuis, C. Quantitative Assessment to the Structural Basis of Water Repellency in Natural and Technical Surfaces. J. Exp. Bot. 2003, 54, 1295–1303. [Google Scholar] [CrossRef]
- Fernandez, V.; Brown, P.H. From Plant Surface to Plant Metabolism: The Uncertain Fate of Foliar-Applied Nutrients. Front. Plant Sci. 2013, 4, 289. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.; Sancho-Knapik, D.; Guzmán, P.; Peguero-Pina, J.J.; Gil, L.; Karabourniotis, G.; Khayet, M.; Fasseas, C.; Heredia-Guerrero, J.A.; Heredia, A.; et al. Wettability, Polarity, and Water Absorption of Holm Oak Leaves: Effect of Leaf Side and Age. Plant Physiol. 2014, 166, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.L.; Guo, W.; Zhang, Y.J.; Schreel, J.D.M.; Gao, J.Y.; Li, Y.P.; Yang, S.J. Leaf Trichomes of Dendrobium Species (Epiphytic Orchids) in Relation to Foliar Water Uptake, Leaf Surface Wettability, and Water Balance. Environ. Exp. Bot. 2021, 190, 104568. [Google Scholar] [CrossRef]
- Li, J.J.; Bai, G.S.; Zhang, R. Water absorption of common trees leaves in loess hilly and gully region of Northern Shaanxi. Chin. Soil Water Conserv. Sci. 2013, 11, 99–102. [Google Scholar] [CrossRef]
- Guzmán-Delgado, P.; Laca, E.; Zwieniecki, M.A. Unravelling Foliar Water Uptake Pathways: The Contribution of Stomata and the Cuticle. Plant Cell Environ. 2021, 44, 1728–1740. [Google Scholar] [CrossRef]
- Koch, K.; Hartmann, K.D.; Schreiber, L.; Barthlott, W.; Neinhuis, C. Influences of Air Humidity during the Cultivation of Plants on Wax Chemical Composition, Morphology and Leaf Surface Wettability. Environ. Exp. Bot. 2006, 56, 1–9. [Google Scholar] [CrossRef]
- Chin, A.R.O.; Guzmán-Delgado, P.; Kerhoulas, L.P.; Zwieniecki, M.A. Acclimation of Interacting Leaf Surface Traits Affects Foliar Water Uptake. Tree Physiol. 2022, tpac120. [Google Scholar] [CrossRef]
- Roth-Nebelsick, A.; Hacke, U.G.; Voigt, D.; Schreiber, S.G.; Krause, M. Foliar Water Uptake in Pinus Species Depends on Needle Age and Stomatal Wax Structures. Ann. Bot. 2022, mcac141. [Google Scholar] [CrossRef]
- Goldsmith, G.R.; Bentley, L.P.; Shenkin, A.; Salinas, N.; Blonder, B.; Martin, R.E.; Castro-Ccossco, R.; Chambi-Porroa, P.; Diaz, S.; Enquist, B.J.; et al. Variation in Leaf Wettability Traits along a Tropical Montane Elevation Gradient. New Phytol. 2017, 214, 989–1001. [Google Scholar] [CrossRef]
- Burkhardt, J.; Basi, S.; Pariyar, S.; Hunsche, M. Stomatal Penetration by Aqueous Solutions–an Update Involving Leaf Surface Particles. New Phytol. 2012, 196, 774–787. [Google Scholar] [CrossRef]
- Luo, D.D.; Wang, C.K.; Jin, Y. Plant water-regulation strategies: Isohydric versus anisohydric behavior. Chin. J. Plant Ecol. 2017, 41, 1020–1032. [Google Scholar]
- Tardieu, F.; Simonneau, T. Variability among Species of Stomatal Control under Fluctuating Soil Water Status and Evaporative Demand: Modelling Isohydric and Anisohydric Behaviours. J. Exp. Bot. 1998, 49, 419–432. [Google Scholar] [CrossRef]
- Klein, T. The Variability of Stomatal Sensitivity to Leaf Water Potential across Tree Species Indicates a Continuum between Isohydric and Anisohydric Behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Eller, C.B.; Lima, A.L.; Oliveira, R.S. Cloud Forest Trees with Higher Foliar Water Uptake Capacity and Anisohydric Behavior Are More Vulnerable to Drought and Climate Change. New Phytol. 2016, 211, 489–501. [Google Scholar] [CrossRef]
- Schreel, J.D.M.; von der Crone, J.S.; Kangur, O.; Steppe, K. Influence of Drought on Foliar Water Uptake Capacity of Temperate Tree Species. Forests 2019, 10, 562. [Google Scholar] [CrossRef]
- Meng, X.Y.; Meng, B.C.; Wang, Y.J.; Liu, Z.H.; Ji, X.N.; Yu, D.L. Influence of Climate Change and Human Activities on Water Resources in Ebinur Lake in Recent 60 Years. Hydrol. China 2015, 35, 90–96. [Google Scholar]
- Qin, W.H.; Meng, W.Q. Desert Salt Lake–Ebinur Lake National Nature Reserve. Lifeworld China 2020, 7, 16–27. [Google Scholar]
- Wang, X.F. Development Countermeasures of Psammophytes in Northwest China. Rural Technol. 2022, 13, 115–117. [Google Scholar] [CrossRef]
- Zhang, X.N.; Li, Y.; He, X.M.; Lv, G.H. Effects of soil water and salinity on relationships between desert plant functional diversity and species diversity. Chin. J. Ecol. 2019, 38, 2354–2360. [Google Scholar] [CrossRef]
- Li, Y.; Sima, Y.Z.B.B.; Dong, Y.; Sheng, Y.C.; Tan, J. Analysis of Variation Rules and Abrupt Changes of Precipitation in Aibi Lake Oasis. Water-Sav. Irrig. China 2017, 10, 41–45. [Google Scholar]
- Wang, S.Y.; Lv, G.H.; Jiang, L.M.; Wang, H.F.; Li, Y.; Wang, J.L. Multi-scale Analysis on Functional Diversity and Phylogenetic Diversity of Typical Plant Community in Ebinur Lake. Chin. J. Ecol. Environ. 2020, 29, 889–900. [Google Scholar] [CrossRef]
- Rosado, B.H.P.; Holder, C.D. The Significance of Leaf Water Repellency in Ecohydrological Research: A Review. Ecohydrology 2013, 6, 150–161. [Google Scholar] [CrossRef]
- Holder, C.D. Leaf Water Repellency of Species in Guatemala and Colorado (USA) and Its Significance to Forest Hydrology Studies. J. Hydrol. 2007, 336, 147–154. [Google Scholar] [CrossRef]
- Shi, H.; Wang, H.X.; Li, Y.Y. Wettability on plant leaf surface and its ecological significance. Acta Ecol. Sin. 2011, 31, 4287–4298. [Google Scholar]
- Liang, X.; Su, D.; Yin, S.; Wang, Z. Leaf Water Absorption and Desorption Functions for Three Turfgrasses. J. Hydrol. 2009, 376, 243–248. [Google Scholar] [CrossRef]
- Tyree, M.T.; Hammel, H.T. The Measurement of the Turgor Pressure and the Water Relations of Plants by the Pressure-Bomb Technique. J. Exp. Bot. 1972, 23, 267–282. [Google Scholar] [CrossRef]
- Leaf Pressure-Volume Curve Parameters. PROMETHEUS. Available online: https://prometheusprotocols.net/function/water-relations/pressure-volume-curves/leaf-pressure-volume-curve-parameters/ (accessed on 24 October 2022).
- Reich, P.B.; Wright, I.J.; Cavender-Bares, J.; Craine, J.M.; Oleksyn, J.; Westoby, M.; Walters, M.B. The Evolution of Plant Functional Variation: Traits, Spectra, and Strategies. Int. J. Plant Sci. 2003, 164, 143–164. [Google Scholar] [CrossRef]
- Guzmán-Delgado, P.; Mason Earles, J.; Zwieniecki, M.A. Insight into the Physiological Role of Water Absorption via the Leaf Surface from a Rehydration Kinetics Perspective. Plant Cell Environ. 2018, 41, 1886–1894. [Google Scholar] [CrossRef]
- Ma, C.Y.; Wang, W.Q.; Zhao, Y.X.; Xiao, K. Study on leaf anatomical structure of Glycyrrhiza uralensis. Chin. J. Tradit. Chin. Med. 2009, 34, 1034–1037. [Google Scholar]
- Yin, Q.L. Leaf Anatomical Structure of Main Plants and Its Environmental Adaptations in the Hilly-Gullied Platrau Region. Master’s Thesis, Northwest University, Kirkland, WA, USA, 2015. Available online: http://kns.cnki.net/kcms/detail/frame/list.aspx?dbcode=CMFD&filename=1015333288.nh&dbname=CMFD201601&RefType=1&vl=p3BxlfZtEZV6ZlMXQ2T_6zj4w92WuEZpqvC4gPiHsafUhB_cklND0PZDZUpK1FZy (accessed on 26 November 2022).
- Wang, S.J.; Ren, L.Q.; Han, Z.W.; Qiu, Z.M.; Zhou, C.H. non–smooth morphology of typical plant leaf surface and its anti–adhesion and hydrophobicity. Chin. J. Agric. Eng. 2005, 9, 16–19. [Google Scholar]
- Liu, Y.X.; Ma, Y.L.; Lan, H.Y. Advances in morphology and function of plant non-glandular trichomes. Plant Physiol. China 2018, 54, 1527–1534. [Google Scholar] [CrossRef]
- Schwerbrock, R.; Leuschner, C. Air Humidity as Key Determinant of Morphogenesis and Productivity of the Rare Temperate Woodland Fern Polystichum Braunii. Plant Biol. 2016, 18, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Meng, N.; Zuo, F.Y.; Liu, Y.J.; Li, J.K. Leaf water potential, anatomical structure of epidermis and leaf salt-tolerant characteristics of wild Nitraria tangutorum B.and Suaeda glauca B. J. Tianjin Agric. Univ. China 2013, 20, 5–8. [Google Scholar]
- Chen, L.; Yang, X.G.; Song, N.P.; Yang, M.X.; Xiao, X.P.; Wang, X. Leaf water uptake strategy of plant in the arid and semi–arid region of Ningxia. J. Zhejiang Univ. China (Agric. Life Sci.) 2013, 39, 565–574. [Google Scholar]
- Boanares, D.; Ferreira, B.G.; Kozovits, A.R.; Sousa, H.C.; Isaias, R.M.S.; França, M.G.C. Pectin and Cellulose Cell Wall Composition Enables Different Strategies to Leaf Water Uptake in Plants from Tropical Fog Mountain. Plant Physiol. Biochem. 2018, 122, 57–64. [Google Scholar] [CrossRef]
- Berry, Z.C.; Emery, N.C.; Gotsch, S.G.; Goldsmith, G.R. Foliar Water Uptake: Processes, Pathways, and Integration into Plant Water Budgets. Plant Cell Environ. 2019, 42, 410–423. [Google Scholar] [CrossRef]
- Rascio, A.; Nicastro, G.; Carlino, E.; Di Fonzo, N. Differences for Bound Water Content as Estimated by Pressure-Volume and Adsorption Isotherm Curves. Plant Sci. 2005, 169, 395–401. [Google Scholar] [CrossRef]
- Powell, T.L.; Wheeler, J.K.; de Oliveira, A.A.R.; Lola da Costa, A.C.; Saleska, S.R.; Meir, P.; Moorcroft, P.R. Differences in Xylem and Leaf Hydraulic Traits Explain Differences in Drought Tolerance among Mature Amazon Rainforest Trees. Glob. Change Biol. 2017, 23, 4280–4293. [Google Scholar] [CrossRef]
- Zhu, S.-D.; Chen, Y.-J.; Ye, Q.; He, P.-C.; Liu, H.; Li, R.-H.; Fu, P.-L.; Jiang, G.-F.; Cao, K.-F. Leaf Turgor Loss Point Is Correlated with Drought Tolerance and Leaf Carbon Economics Traits. Tree Physiol. 2018, 38, 658–663. [Google Scholar] [CrossRef]
- Huo, J.; Shi, Y.; Zhang, H.; Hu, R.; Huang, L.; Zhao, Y.; Zhang, Z. More Sensitive to Drought of Young Tissues with Weak Water Potential Adjustment Capacity in Two Desert Shrubs. Sci. Total Environ. 2021, 790, 148103. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Scoffoni, C.; Sack, L. The Determinants of Leaf Turgor Loss Point and Prediction of Drought Tolerance of Species and Biomes: A Global Meta-Analysis. Ecol. Lett. 2012, 15, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Meinzer, F.C.; Woodruff, D.R.; Marias, D.E.; Smith, D.D.; McCulloh, K.A.; Howard, A.R.; Magedman, A.L. Mapping ‘Hydroscapes’ along the Iso-to Anisohydric Continuum of Stomatal Regulation of Plant Water Status. Ecol. Lett. 2016, 19, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Proxies for Stringency of Regulation of Plant Water Status (Iso/Anisohydry): A Global Data Set Reveals Coordination and Trade-Offs among Water Transport Traits. Tree Physiol. 2019, 39, 122–134. [CrossRef] [PubMed]
- Li, X.; Blackman, C.J.; Peters, J.M.R.; Choat, B.; Rymer, P.D.; Medlyn, B.E.; Tissue, D.T. More than Iso/Anisohydry: Hydroscapes Integrate Plant Water Use and Drought Tolerance Traits in 10 Eucalypt Species from Contrasting Climates. Funct. Ecol. 2019, 33, 1035–1049. [Google Scholar] [CrossRef]
- Eller, C.B.; Burgess, S.S.O.; Oliveira, R.S. Environmental Controls in the Water Use Patterns of a Tropical Cloud Forest Tree Species, Drimys Brasiliensis (Winteraceae). Tree Physiol. 2015, 35, 387–399. [Google Scholar] [CrossRef]
- Wu, Y.; Song, L.; Liu, W.; Liu, W.; Li, S.; Fu, P.; Shen, Y.; Wu, J.; Wang, P.; Chen, Q.; et al. Fog Water Is Important in Maintaining the Water Budgets of Vascular Epiphytes in an Asian Tropical Karst Forests during the Dry Season. Forests 2018, 9, 260. [Google Scholar] [CrossRef]
- Schaepdryver, K.H.D.; Goossens, W.; Naseef, A.; Kalpuzha Ashtamoorthy, S.; Steppe, K. Foliar Water Uptake Capacity in Six Mangrove Species. Forests 2022, 13, 951. [Google Scholar] [CrossRef]
- Carmichael, M.J.; White, J.C.; Cory, S.T.; Berry, Z.C.; Smith, W.K. Foliar Water Uptake of Fog Confers Ecophysiological Benefits to Four Common Tree Species of Southeastern Freshwater Forested Wetlands. Ecohydrology 2020, 13, 2240. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, H.; Yu, X.; Jia, G.; Jiang, J. Evidence of Foliar Water Uptake in a Conifer Species. Agric. Water Manag. 2021, 255, 106993. [Google Scholar] [CrossRef]
- Maréchaux, I.; Bartlett, M.K.; Iribar, A.; Sack, L.; Chave, J. Stronger Seasonal Adjustment in Leaf Turgor Loss Point in Lianas than Trees in an Amazonian Forest. Biol Lett. 2017, 13, 20160819. [Google Scholar] [CrossRef]
- Long, Y.X. Water Regulation Strategies of Five Dominant Woody Plants in Desert Forest of Ebinur Lake Basin. Master’s Thesis, Xinjiang University, Urumqi, China, 2021. [Google Scholar] [CrossRef]


| Water Gradient | Soil Sample Depth | Soil Water Content |
|---|---|---|
| High water | 0–20 cm | 0.131 ± 0.026 a |
| 20–40 cm | 0.144 ± 0.013 a | |
| 40–60 cm | 0.137 ± 0.009 a | |
| Medium water | 0–20 cm | 0.093 ± 0.013 b |
| 20–40 cm | 0.119 ± 0.022 b | |
| 40–60 cm | 0.128 ± 0.018 a | |
| Low water | 0–20 cm | 0.028 ± 0.007 c |
| 20–40 cm | 0.026 ± 0.005 c | |
| 40–60 cm | 0.035 ± 0.003 b |
| Species | 5 μL Contact Angle (°) | ||
|---|---|---|---|
| High Water | Medium Water | Low Water | |
| P. euphratica | 101.69 ± 2.22 Aa | 101.62 ± 2.28 Aa | 99.33 ± 3.7 Aa |
| N. sibirica | 83.56 ± 4.62 Ab | 81.82 ± 5.57 Ab | 80.58 ± 3.67 Ab |
| A. sparsifolia | 122.08 ± 6.96 Ac | 127.27 ± 6.12 Ac | 125.82 ± 4.48 Ac |
| A. hendersonii | 129.22 ± 3.63 Ad | 131.97 ± 4.33 Ac | 131.23 ± 6.16 Ad |
| G. uralensis | 59.42 ± 4.28 Ac | 61.37 ± 3.44 Ad | - |
| H. halodendron | 123.58 ± 4.17 e | - | - |
| Gradient | Species | LT (mm) | LDMC (%) |
|---|---|---|---|
| High water | P. euphratica | 0.463 ± 0.038 ABd | 0.357 ± 0.037 Ac |
| N. sibirica | 0.652 ± 0.009 Ae | 0.198 ± 0.026 Aa | |
| A. sparsifolia | 0.382 ± 0.014 Ac | 0.350 ± 0.017 Ac | |
| A. hendersonii | 0.435 ± 0.078 Acd | 0.276 ± 0.032 Ab | |
| G. uralensis | 0.208 ± 0.008 Aa | 0.400 ± 0.012 Ac | |
| H. halodendron | 0.296 ± 0.037 b | 0.389 ± 0.031 c | |
| Medium water | P. euphratica | 0.513 ± 0.080 Abc | 0.253 ± 0.008 Bb |
| N. sibirica | 0.682 ± 0.115 Ad | 0.215 ± 0.019 Aa | |
| A. sparsifolia | 0.469 ± 0.016 Bb | 0.312 ± 0.018 Bc | |
| A. hendersonii | 0.635 ± 0.072 Bcd | 0.234 ± 0.017 Aab | |
| G. uralensis | 0.203 ± 0.004 Aa | 0.393 ± 0.019 Ad | |
| Low water | P. euphratica | 0.410 ± 0.019 Ba | 0.334 ± 0.026 Aa |
| N. sibirica | 0.728 ± 0.217 Ab | 0.214 ± 0.038 Ab | |
| A. sparsifolia | 0.489 ± 0.028 Ba | 0.312 ± 0.019 Ba | |
| A. hendersonii | 0.483 ± 0.046 Aa | 0.282 ± 0.026 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, Z.; Yang, J. Effects of Leaf Hydrophilicity and Stomatal Regulation on Foliar Water Uptake Capacity of Desert Plants. Forests 2023, 14, 551. https://doi.org/10.3390/f14030551
Wang H, Li Z, Yang J. Effects of Leaf Hydrophilicity and Stomatal Regulation on Foliar Water Uptake Capacity of Desert Plants. Forests. 2023; 14(3):551. https://doi.org/10.3390/f14030551
Chicago/Turabian StyleWang, Huimin, Zhoukang Li, and Jianjun Yang. 2023. "Effects of Leaf Hydrophilicity and Stomatal Regulation on Foliar Water Uptake Capacity of Desert Plants" Forests 14, no. 3: 551. https://doi.org/10.3390/f14030551
APA StyleWang, H., Li, Z., & Yang, J. (2023). Effects of Leaf Hydrophilicity and Stomatal Regulation on Foliar Water Uptake Capacity of Desert Plants. Forests, 14(3), 551. https://doi.org/10.3390/f14030551





