Abstract
Foliar water uptake (FWU) is considered to be a common phenomenon in most terrestrial plants. As a supplementary water source, it plays an important role in the growth and survival of plants in arid areas. However, there is no research to explain the water absorption of plant leaves from the perspective of gender specificity. To this end, we carried out a leaf water absorption capacity experiment and in situ wetting field experiment, respectively, in the early (Initial), middle (Mid) and end (End) of the growth season of male and female Populus euphratica. The results of the leaf water absorption capacity experiment showed that the FWU capacity of male and female P. euphratica showed an increasing trend with the growth period and reached the maximum at the End period. The FWU capacity of female P. euphratica was significantly greater than that of male P. euphratica after the Initial stage. The water absorption speed (k) of male and female leaves also increased with the growth period, but the increase was not significant. The increase in leaf water content per mg of water absorbed per unit of leaf area (LWCA) of male P. euphratica was always greater than that of female P. euphratica. Specific leaf area (SLA), leaf water saturated deficit (WSD) and water absorption parameters (FWU capacity, k) were significantly correlated. The results of the in situ wetting field experiment show that humidification significantly increased the predawn water potential (Mid period) of female and male P. euphratica leaves and the net photosynthetic rate (Mid period) of male P. euphratica leaves, but had no significant effect on chlorophyll fluorescence parameters and anatomical structure. The MFA results show that the water status of male and female P. euphratica leaves was significantly correlated with photosynthetic parameters, fluorescence parameters and anatomical parameters. Our results show that the foliar water uptake capacity of female P. euphratica leaves was stronger than that of male P. euphratica and shows significant dynamic changes during the growing season. This was because female P. euphratica has a developed water storage structure. Foliar water uptake can effectively improve the water status and photosynthetic capacity of male and female P. euphratica, and this improvement was more significant during the most intense period of soil water stress. These findings will deepen our understanding of the ecological adaptation of dioecious plants to foliar water uptake.
1. Introduction
The plant canopy can intercept rain, fog, dew etc., resulting in wet leaves [1,2,3]. The most direct benefit of leaf wetting is water entering the leaf through the absorption of the water accumulated on the leaf surface, which is called foliar water uptake. It can be said that leaf water absorption is a common phenomenon in most terrestrial plants, because this phenomenon is observed in more than 85% of studied species [1,4]. As a complementary water source, although plant leaves absorb less water, this phenomenon still increases leaf water potential [5,6], promotes plant growth [7,8] and improves photosynthetic capacity [9,10].
There are 14,620 species of dioecious plants, which are an important part of terrestrial ecosystems and play an important role in maintaining the stability of regional ecosystems [11]. Studies have shown that the specific adaptation of dioecious plants to soil water demand during the growth period leads to changes in leaf morphology [12,13], structure [14,15] and physiology [16,17,18]. Generally, under drought conditions, female plants show higher sensitivity than male plants, which may be related to the less conservative water-use strategy of the former [19,20], but, when water is sufficient, the physiological differences between the sexes disappear [18,21]. Therefore, we infer that the secondary sexual characteristics of dioecious plants during the growth period have different responses to leaf water absorption, which is more significant in arid areas.
The water absorbed by the leaves can enter the palisade tissue, sponge tissue and epidermal cell wall, and these anatomical characteristics profoundly affect the leaf water absorption capacity of plants [22,23,24]. Boanares et al. [25] found that Leandra australis and Myrcia splendens had a lower leaf water uptake, because the relatively loose sponge tissue in Leandra australis and the higher number of secretory glands in Myrcia splendens reduce the number of parenchyma cells and water storage vacuoles. Dioecious plants exhibit significant differences in their morphological structure [15,26]. For example, the epidermal thickness, palisade tissue thickness and ratio of palisade tissue to spongy tissue of male Podocarpus macrophyllus leaves were greater than those of female plants, showing strong water retention capacity and drought resistance [27]. Combined with the above research, we believe that the morphological structure of dioecious plants is not only an important factor restricting the water absorption capacity of leaves, but also that the differences between male and female plants may lead to different leaf water absorption strategies.
The main pathway of water absorption in plant leaves is the stomata, and the value of water absorption flux is usually consistent with the diffusion of water vapor into the stomata under environmental conditions. Therefore, the water vapor flux into leaves may be partially or completely explained by transpiration, which is often called ‘reverse transpiration’ [28,29]. This theory redefines the leaf water absorption process and links plant leaf water absorption with the gas exchange process. Our previous studies showed that humidification significantly increased the water status of the assimilating branches of Calligonum mongolicum and effectively improved the photosynthetic capacity [10]. However, in other previous studies, it was found that leaf wetting was not beneficial to plant photosynthesis, which inhibited stomatal opening and reduced the carbon assimilation rate [30] or affected the carboxylation of Rubisco [31]. In arid environments, the photosynthetic capacity of female Populus cathayana was more limited than that of male P. cathayana [17,32], but can leaf water input alleviate this photosynthetic limitation of dioecious plants? This question remains to be answered.
Populus euphratica Oliv. is a dioecious tree species with strong drought resistance. It is widely distributed in arid and semi-arid desert areas and plays an important role in water conservation, windbreaking and sand fixation, stabilizing the regional climate and promoting ecosystem functions [33]. A previous study of the research team found that the proportion of dew in the water utilization sources of P. euphratica reached 1% [34]. Later, some scholars found that P. euphratica can transport the absorbed water to the root system to alleviate root stress [35,36]. These results provide a theoretical basis for this study. In general, this paper takes the dioecious plant P. euphratica as the research object, based on the leaf water absorption experiment and wetting experiment at different stages of the growth period, in order to clarify the water absorption characteristics of male and female P. euphratica leaves and the structure and photosynthetic physiological response mechanism of P. euphratica leaves under humidification conditions. This study will help to clarify the strategies of plant water use in arid areas and contribute to our understanding of plant water use patterns. Therefore, we propose the following two scientific hypotheses:
- (1)
- The water absorption capacity of P. euphratica leaves exhibits gender differences, and different water absorption strategies are shown in each growth stage.
- (2)
- The response characteristics of the leaf structure and photosynthetic physiology of male and female P. euphratica to wetting are different and show different rules in the growth stage.
2. Materials and Methods
2.1. Study Area and Plant Materials
The Ebinur Lake Wetland National Nature Reserve (82°36′–83°50′ E, 44°30′–45°09′ N) is located in Jinghe County, Bortala Mongol Autonomous Prefecture, Xinjiang, China. The annual precipitation (100–200 mm) is much lower than the potential evapotranspiration (1500–2000 mm). The average annual precipitation distribution is uneven, with more in summer and less in winter. The annual average temperature is 6–8 °C, the extreme maximum temperature reaches approximately 44 °C, and the extreme minimum temperature is −33 °C (Figure S1). The climate is extremely arid and represents a typical temperate continental arid climate [37]. The flora in the basin belongs to the Junggar Desert sub-region of the northern desert sub-region of the Palaearctic Mengxin District. There are a large number of Tugai forest communities composed of desert plants distributed along the lakes and riverbanks [38].
2.2. Plant Material and Environmental Factors
From 28 March to 28 April 2021, according to the differences between sexes in the phenological growth of P. euphratica, three female and three male P. euphratica with similar individual size were selected from the Tugai forest along the Aqikesu River in the upper reaches of the Ebinur Lake Basin and marked as “Female” and “Male”, respectively (Table S1). The geographical distance between each plant was greater than 100 m, and the distance between the marked plant and the river was approximately 200 m. Then, a VP-4 sensor (Decagon Device Inc., Pullman, WA, USA) was used to measure the environmental factors such as air relative humidity (Rh), air temperature (Tair) and pressure (P), and a leaf humidity sensor (LWS, DECAGON, Pullman, WA, USA) was used to measure the leaf wetness. The data were recorded every 10 min and stored in the EM 50 data collector (EM 50, Decagon, Pullman, WA, USA) (Table 1).

Table 1.
The characteristics of the environmental factors during the experiment period.
The female and male P. euphratica in the growing season were divided into three categories according to the growth period, namely the early growth period (Initial, 16 May 2021–23 May 2021), the middle growth period (Mid, 27 July 2021–3 August 2021) and the end growth period (End, 7 September 2021–14 September 2021). The experiment was carried out in these three time periods, and the contents of the experiments were consistent. Therefore, the first description of the experiment content was taken as an example, and the next were not repeated. The experimental schedule is shown in Table S2.
2.3. Leaf Water Absorption Experiment
According to the experimental method proposed by Liang et al. [39], the leaf water absorption capacity (FWU capacity) and water absorption speed (k) were obtained. The experiment was conducted from 17 May 2021 to 18 May 2021 on female and male P. euphratica.
Sampling steps: Healthy twigs with fresh leaves were removed by pruning and quickly brought back to the laboratory. The average temperature in the laboratory was about 24 °C, the air relative humidity was about 35%, and there was no wind. From each tree of the same sex, 3 twigs were selected as a repeat. Determination steps: The leaves were cut from the twigs and randomly divided into 14 parts, with 4 leaves per part. The fresh weight of each leaf was quickly weighed with a precision balance (AL204, METTLER TOLEDO, Shanghai, China), the result was recorded as the initial mass (IW, g), and each leaf was photographed to calculate the leaf area (A, cm2). The experiment was divided into three stages: for the first two hours, leaves were weighed every 15 min; for the next two hours, leaves were weighed every 30 min; and for the last two hours, leaves were measured every 1 h. The experiment ran for a total of 6 h, collecting data from 14 moments. During the water absorption process, the petiole was sealed with paraffin to prevent water from entering, and then 14 leaves were immersed in 14 cups containing deionized water in turn, and the petiole was fixed on the cup wall to avoid contact between the petiole and water. During each weighing, the leaves removed from the water cup were dried with a dry paper towel, and re-determined the weight after water absorption (FW, g). The fresh weight of the leaves at 6 h of immersion was used as the saturated weight after water absorption and recorded as (SW, g). After the end of the water absorption experiment, the leaves were dried in the oven for 48 h, and then the weight after drying was weighed and recorded as (DW, g). Some indicators refer to Schreel et al. (2019) [40].
The total amount of leaf water absorption (TFWU, g·g−1) is
Leaf water absorption per unit area (FWU capcacity, mg·cm−2) is
Leaf water content (LWC, %) is calculated as follows:
In the above formula, LWCI is the initial leaf water content, LWCS is the saturated leaf water content, LWC is the leaf water content at a certain moment, k is the water absorption speed and t is the water absorption time.
The difference in leaf water content (ΔLWC, %) is
The increase in leaf water content per mg of water absorbed per unit of leaf area (LWCA; %·cm2·mg−1) is
The increase in FWU capacity per MPa decrease in Ψleaf (FWUi; mg·cm−2·MPa−1) is
The potential relative importance of FWU to alleviate drought stress at leaf level (PRI; %) is
The actual relative importance of FWU to alleviate drought stress (ARI; %·MPa−1) is
2.4. In Situ Wetting Experiment
- (1)
- Experimental design
According to the selected female and male P. euphratica, two parts of healthy branches with leaves were selected for each tree and marked as treatment (TR) and (CK), respectively. The in situ wetting experiment began on 16 May 2021. The leaves on TR branches were sprayed with distilled water at predawn to ensure that the surface of the leaves was moist, and the wetting effect lasted for 1 h. Branch leaves marked as CK were not treated for natural control. The above humidification process was repeated every day for the next 6 days, and the number of humidification days throughout the experimental period was 7. The test method is based on Cavallaro et al. [41].
- (2)
- Determination of leaf water potential at predawn and midday
On 23 May 2021 (the last day of the experimental period), leaf water potential samples of female and male P. euphratica under TR and CK treatments were collected at predawn (4:00, local time) and midday (10:00) on that day. The specific collection method was to use branch scissors to remove the twigs with leaves of female and male P. euphratica under the wetting and control treatments and immediately put them in a self-sealing bag before placing them in an incubator (4 °C) for preservation, and then quickly bring them back to measure the leaf water potential with a dew point water potential meter (WP4C, Decagon Device Inc., Pullman, WA, USA). From each tree, three twigs were cut as repeats.
- (3)
- Determination of photosynthetic and chlorophyll fluorescence parameters
In order to explore the response of the photosynthetic physiology and fluorescence physiology of female and male P. euphratica leaves to humidification, at midday (10:00, local time) on 23 May 2021, the net photosynthetic rate (Pn, μmol CO2·m−2·s−1), stomatal conductance (gs, mol H2O·m−2·s−1), intercellular carbon dioxide concentration (Ci, μmol CO2·m−2·s−1) and transpiration rate (Tr, mmol H2O·m−2·s−1) of the leaves of female and male P. euphratica were measured using a photosynthetic measurement system (LI-6400XT, Li-COR, Inc., Lincoln, NE, USA). Water use efficiency (WUE, μmol·mmol−1) is the ratio of net photosynthetic rate to transpiration rate. Three healthy leaves were selected for each P. euphratica, and each leaf was measured 10 times. After the determination of photosynthetic parameters, the labeled leaves were wrapped with tinfoil paper for 30 min of dark adaptation. After the dark adaptation, the chlorophyll fluorescence parameters of each P. euphratica were measured. These parameters include minimum initial fluorescence (Fo), maximum fluorescence under dark adaptation (Fm), maximal photochemical efficiency (Fv/Fm), minimum fluorescence under light adaptation (Fo’), maximum fluorescence under light adaptation (Fm’), light energy capture efficiency in light system II (Fv’/Fm’), photochemical quenching coefficient (qP) and electron transfer efficiency (ETR).
- (4)
- Leaf anatomical sample collection and determination
After the determination of photosynthetic and chlorophyll fluorescence parameters, we collected anatomical samples, and the samples of P. euphratica leaves were collected using a mixed sampling method. A total of 15 female P. euphratica leaves were collected (5 leaves were collected for each female P. euphratica individual), and 15 leaves were fully mixed and divided into 3 samples as repetition; then, each sample collected was immediately placed in a sample bottle containing FAA fixed solution (70% ethanol 90 mL + formaldehyde 5 mL + acetic acid 5 mL). Then, the samples for dissection were taken to the laboratory for the determination of anatomical parameters such as leaf thickness (LT), upper and lower epidermal cell thickness (TUE; TLE), upper and lower palisade tissue thickness (TUP; TLP) and sponge tissue thickness (TS).
2.5. Statistical Analysis
Before data analysis, a normality test was carried out. If it did not conform to the normal distribution, logarithmic transformation was carried out to meet the requirements of data analysis. An independent-sample t-test was used to test the difference in the water relationship index between male and female P. euphratica, and a paired-sample t-test was used to test the differences in the same sex in different growth periods.
General linear mixed models (GLMMs) were built to evaluate the influence of FWU capacity on oxidative response related to leaf traits. The leaf traits and sex were used as fixed explanatory variables, and the response variables were water uptake per unit area (FWU capacity) and water uptake speed (k). The identity of each measured plant was used as a random effect variable to control for potential pseudo-replication biases in our models.
Three-way repeated-measures analysis of variance was used to evaluate gender (S), time (T), treatment (W) and their interaction effects. The Kolmogorov–Smirnov test was used to determine whether the data obeyed the normal distribution. The spherical test was used to analyze whether the interaction term exhibited any interaction. If the outcome of the spherical test was p > 0.05, the spherical hypothesis was satisfied. If p < 0.05, the spherical hypothesis was not satisfied. At this time, ε needed to be corrected. When ε < 0.75, the Greenhouse–Geisser method was used for correction; when ε > 0.75, the Huynh–Feldt method was used for correction [42], and the Bonferroni test was used for multiple comparisons. The differences between the 12 groups were analyzed by one-way ANOVA.
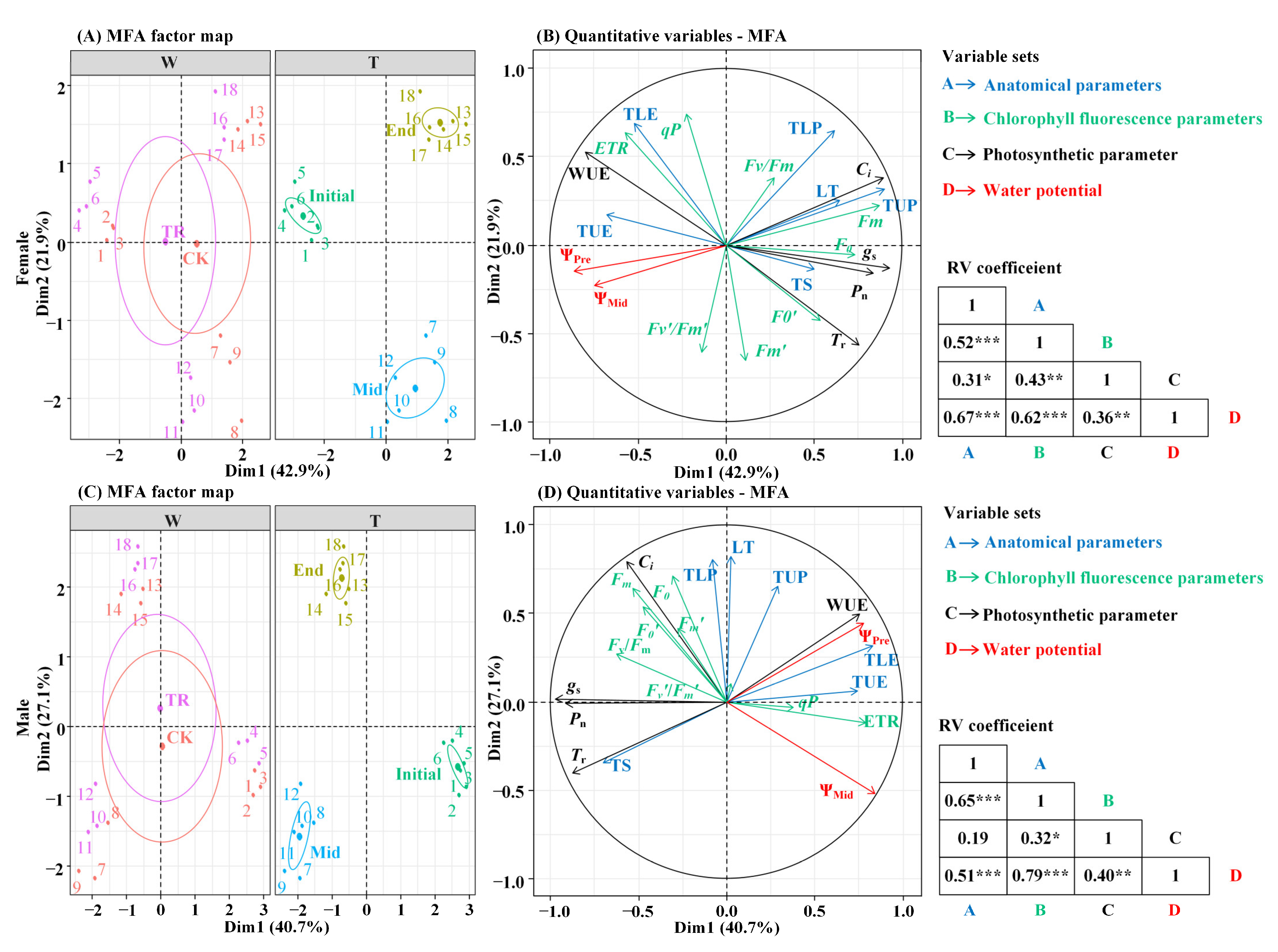
Multivariate factor analysis was performed using the FactoMineR package in R (R v.4.2.1, http://cran.rproject.org). Taking the sex of P. euphratica as the classification variable and using water potential data, photosynthesis parameters, the chlorophyll fluorescence parameter and anatomic parameter data as the variable set, the relationship between the variable sets of male and female P. euphratica are discussed.
The above analysis was performed in SPSS 19.0 (IBM, Chicago, IL, USA) and R (R v.4.2.1, http://cran.rproject.org). Charts were produced in Origin 2021 (OriginLab, Northampton, MA, USA), Excel 2013 (Microsoft, Redmond, WA, USA) and Visio 2010 (Microsoft, Redmond, WA, USA). The data are expressed as mean + standard deviation.
3. Results
3.1. Dynamics of Foliar Water Uptake
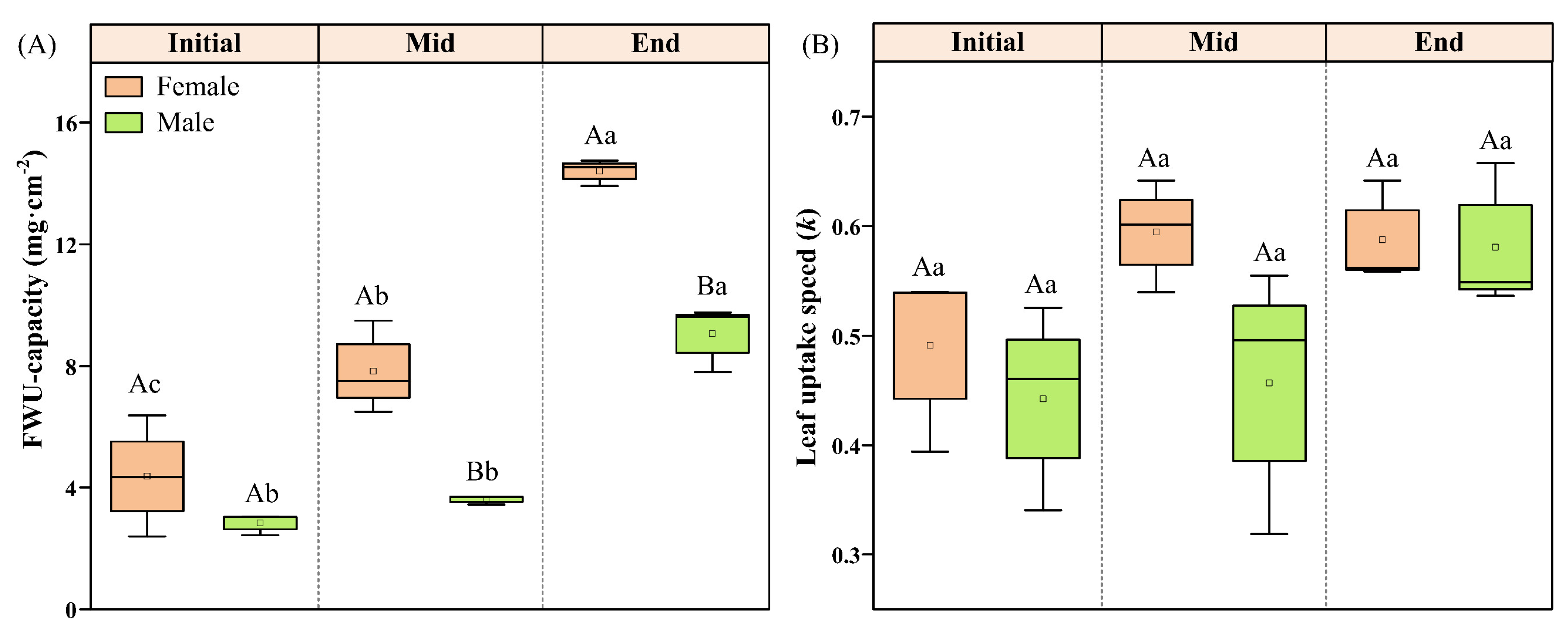
With the growth period, the FWU capacity of male and female P. euphratica showed an increasing trend, reaching the maximum at the End period. The FWU capacity of female P. euphratica showed significant differences in the three periods (p < 0.05). The FWU capacity of male P. euphratica in the End period was significantly greater than in the Initial and Mid periods (p < 0.05). The difference in FWU capacity between male and female P. euphratica occurred only during the Mid and End periods and reached the maximum at the End period (Figure 1A). The k of male and female leaves also increased with the growth period, but the increase was not significant (p > 0.05). In each period, there was little difference in water absorption rate between male and female leaves (Figure 1B).
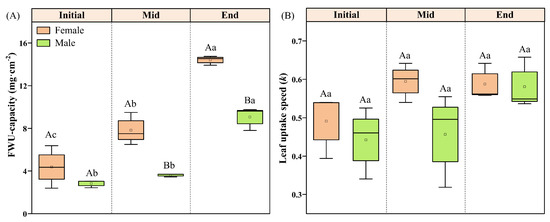
Figure 1.
FWU capacity (A) and leaf uptake speed (B) of female and male P. euphratica in different growth stages. Note: Capital letters indicate significant gender differences between males and females at the same growth stage (independent-sample t-test, p < 0.05), and the lowercase letters indicate significance differences in the same sex in different growth stages (paired-sample t-test, p < 0.05).
The TFWU and FWUi of female P. euphratica were significantly higher than those of male P. euphratica in each period (p < 0.05) (Table 2). Those of both male and female P. euphratica in the End period were significantly higher than those in the Initial and Mid periods (p < 0.05), and there was no significant difference between the Initial and Mid periods (p > 0.05). There was no significant gender difference in ΔLWC between female P. euphratica and male P. euphratica in each period (p > 0.05). After the initial stage of growth, the LWCA of male P. euphratica was always significantly higher than that of female P. euphratica (p < 0.05). The PRI and ARI of male and female P. euphratica showed significant differences only in the Mid period (p < 0.05). The PRI of female P. euphratica in the Initial period was significantly lower than that in the Mid and End periods, while the PRI of male P. euphratica increased with the growth period, and there were significant differences between each growth period (p < 0.05). The ARI of male and female P. euphratica in the End period was significantly higher than that in the Initial and Mid periods (p < 0.05) (Table 2).

Table 2.
The parameters of leaf water uptake in different growth stages of female and male P. euphratica.
The SLW was significantly correlated with FWU capacity and k (F = 12.515, p = 0.003; F = 5.281, p = 0.035); in addition to SLW, WSD was also significantly correlated with FWU capacity and k (F = 38.372, p < 0.001; F = 7.472, p = 0.015). SLA and LWC only had significant effects on FWU capacity (F = 10.652, p = 0.005; F = 7.591, p = 0.014) (Table 3).

Table 3.
Correlation analysis between leaf traits and water absorption parameters.
3.2. The Effects of Wetting on Water Potential
Single factors (T, S and W), two-factor interactions (T × S, T × W and S × W) and the three-factor interaction (T × S × W) had significant effects on the ΨPre of P. euphratica leaves. Further analysis of the effects of various factors on the interaction term found that the interaction effect of S × W in the End period was significant (F1,2 = 46.443, p = 0.021), and the ΨPre of the control and treatment was significantly different for female P. euphratica. Under the wetting treatment conditions, there was a significant difference in Ψpre between the Initial period and the End period (F1,2 = 155.769, p = 0.006). There were significant differences in ΨPre between female and male P. euphratica during the Mid period. T and S had significantly separate effects on ΨMid (F2,4 = 145.004, p < 0.001; F1,2 = 53.353, p = 0.018). The interaction between T and S during the Initial period had a significant main effect on the ΨMid of P. euphratica (F2,4 = 49.787, p = 0.001), but there was no significant difference between the female and male P. euphratica treatments and the control (Table 4).

Table 4.
The leaf water potential of female and male P. euphratica in the wetting experiment.
3.3. The Effects of Wetting on Plant Photosynthesis and Fluorescence
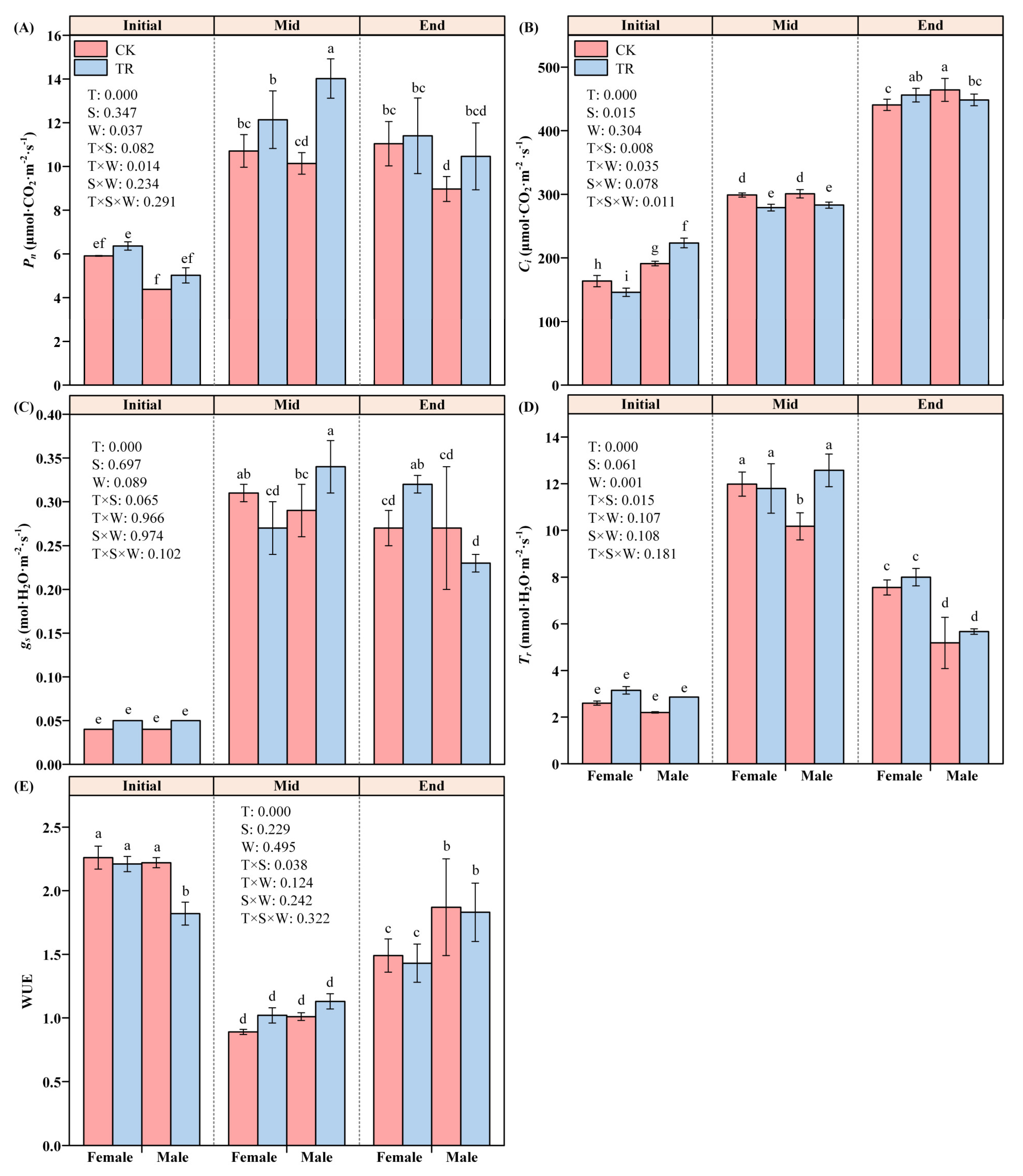
Among the variation characteristics of Pn in male and female P. euphratica, T and W had significant effects on Pn (F2,4 = 326.156, p < 0.001; F1,2 = 25.374, p = 0.037), and there was also an interaction effect (F2,4 = 14.882, p = 0.014), while the gender S factor did not show a significant effect (F1,2 = 1.483, p = 0.347). Further analysis of its individual effects showed that, under CK conditions, the net photosynthetic rates of female and male P. euphratica in the Initial period were significantly different from those in the Mid and End periods (F2,4 = 34.927, p = 0.042; F2,4 = 86.637, p = 0.013). There was no significant difference in Pn between the Mid and End period. Under the TR condition, only the Initial and Mid periods showed significant differences. Only male P. euphratica showed differences in wetting treatment during the Mid period (F1,2 = 23.430, p = 0.040) (Figure 2A).
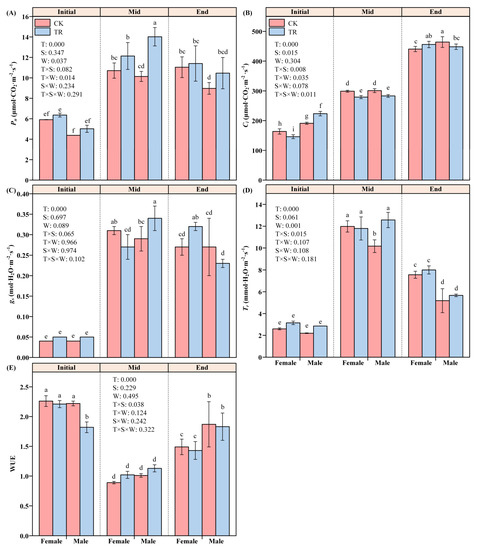
Figure 2.
The photosynthetic parameters of female and male P. euphratica in the wetting experiment. Pn: net photosynthetic rate (A); Ci: intercellular carbon dioxide concentration (B); gs: stomatal conductance (C); Tr: transpiration rate (D); WUE: water use efficiency (E). Note: Different lowercase letters indicate statistically significant differences between treatments (one-way ANOVA, p < 0.05) (Table S4).
Among the variation characteristics of Ci in female and male P. euphratica, time T and S had significant effects on Ci (F2,4 = 1781.667, p < 0.001; F1,4 = 65.707, p = 0.015). T × S × W had a significant three-factor interaction effect on Ci (F2,4 = 16.967, p = 0.011). T × S and T × W had significant two-factor interaction effects on Ci (F2,4 = 19.999, p = 0.008; F2,4 = 8.691, p = 0.035). There were significant differences between TR and CK in each period (F2,4 = 938.950, p < 0.001; F2,4 = 671.835, p < 0.001). The Ci of P. euphratica under the TR condition had significant gender differences in the Initial and Mid periods (Figure 2B).
Among the variation characteristics of gs in male and female P. euphratica, only time T had a significant effect on gs (F2,4 = 274.322, p < 0.001). Other factors or the interaction between factors had no significant effect on gs (Figure 2C). Among the variation characteristics of Tr in male and female P. euphratica, T and W had significant effects on Tr (F2,4 = 326.156, p < 0.001; F1,2 = 25.374, p = 0.037). When P. euphratica was under the TR condition, T × S had a significant two-factor interaction effect on Tr (F2,4 = 326.156, p < 0.001). Further analysis of its individual effects found that the Tr of male and female P. euphratica in the Initial period was significantly different from that in the Mid and End periods. Under the TR condition, the Tr of male and female P. euphratica showed a significant difference only at the End stage (F1,2 = 82.507, p = 0.012) (Figure 2D). In the variation characteristics of male and female P. euphratica, T had a significant single effect on WUE (F2,4 = 259.077, p < 0.001). There was a significant two-factor interaction effect of T × S on Tr (F2,4 = 8.300, p = 0.038). Further analysis of its individual effect found that under both CK and TR conditions, the WUE of male and female P. euphratica showed differences between the Initial period and End period and Mid period (Figure 2E).
In terms of the results for the chlorophyll fluorescence parameters, the time factor T only had a significant single effect on Fo, Fm, Fo’, qP and ETR (p < 0.05). The sex factor S had a significant single effect on Fv’/Fm’ (p < 0.05), and the treatment factor W had no significant single effect on any fluorescence parameter (Table 5).

Table 5.
The fluorescence parameters of female and male P. euphratica in the wetting experiment.
There was an interaction effect of T × S on the Fo’ of P. euphratica under the condition of TR. It was found that the Fo’ of P. euphratica in the Initial period was significantly different from that in the Mid period (F2,4 = 7.628, p = 0.021) (Table 5).
Fm’, Fv’/Fm’ and qP had significant three-factor interaction effects. Among them, P. euphratica Fm’ had an interaction with T × S under TR conditions (F2,4 = 7.887, p = 0.041). Further analysis of its individual effects found that the Fm’ of females at the Initial period was significantly different from that at the Mid period, and the Fm’ of females at the Mid and End periods was significantly different, while the Initial and End period had no significant difference (Table 5).
For female P. euphratica, there was a significant difference between the treatment and the control only at the Initial stage (F1,2 = 43.925, p = 0.022), while for males, there was a significant difference between the treatment and the control at the End stage (F1,2 = 206.660, p = 0.005). After wetting treatment, the Fv’/Fm’ of female P. euphratica was significantly different between the Initial and Mid periods (p < 0.05) (Table 5).
There was interaction between T × S in qP under TR conditions (F2,4 = 35.698, p = 0.003). Further analysis of its individual effect showed that there was no significant gender difference in the qP of P. euphratica under TR conditions. Comparing the differences between the growth periods, it was found that the qP of female and male P. euphratica in the Initial period showed significant differences from that in the Mid period (F2,4 = 9.854, p = 0.025; F2,4 = 16.309, p = 0.044) (Table 5).
3.4. The Effects of Wetting on Leaf Anatomical Structure
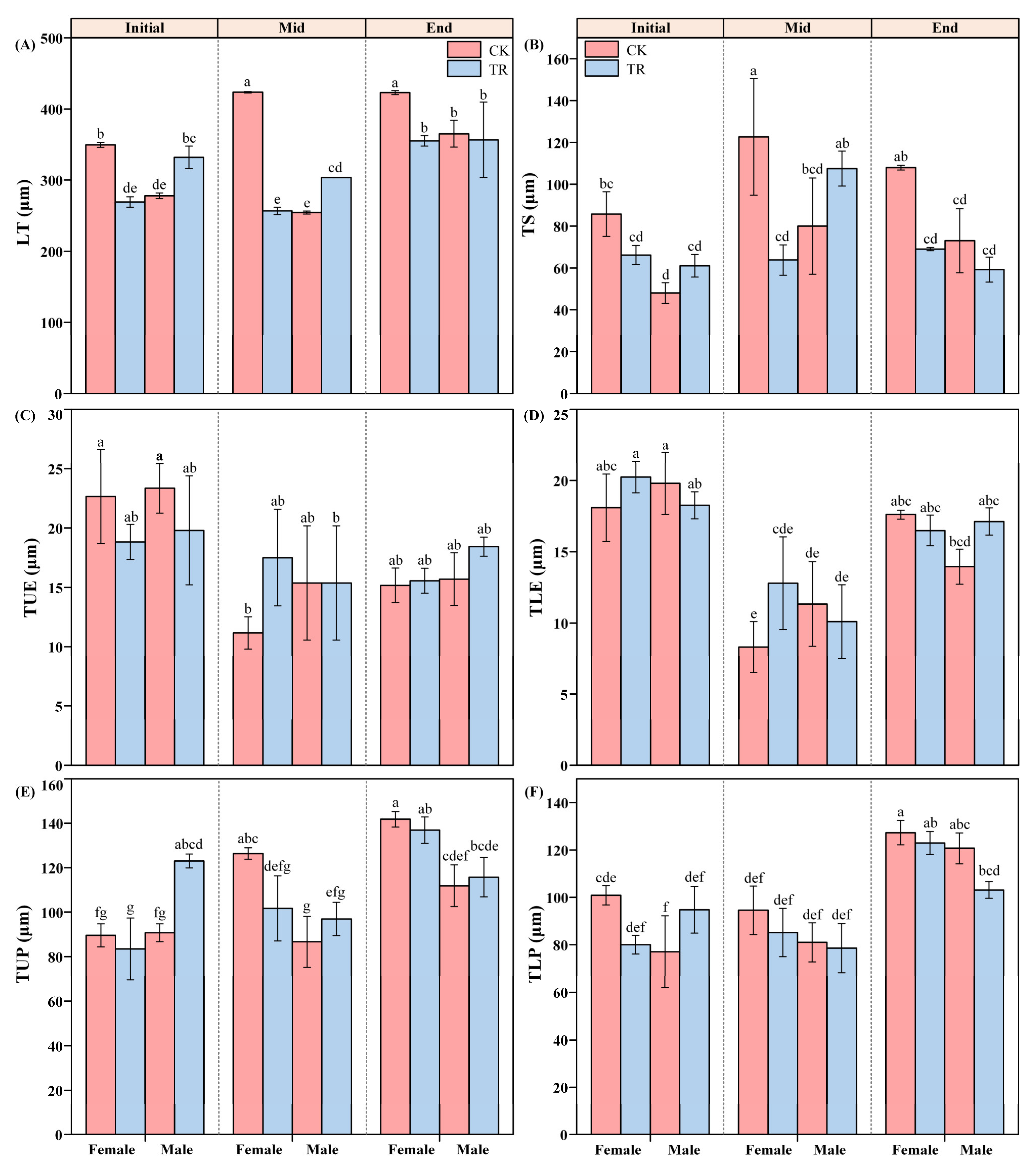
Time T had significant individual effects on LT, TUE, TLE, TUP, TLP and TS (Figure 3 and Figure 4). Among the variation in the characteristics of LT for male and female P. euphratica, T, S and W had significant individual effects on the LT of P. euphratica (F2,4 = 30.114, p = 0.004; F1,2 = 63.500, p = 0.015; F1,2 = 58.224, p = 0.017). Secondly, T × S × W had a significant interaction with LT (F2,4 = 28.222, p = 0.004). Further analysis found that S × W had a significant interaction effect only in the Initial and Mid periods. In both periods, the following rules were demonstrated: the LT of female and male P. euphratica was significantly different under the same treatment, and the LT of P. euphratica was also significantly different under different treatments for the same sex (Figure 4A). The two-factor interaction effects of T × S and S × W showed significant effects on the TUP and TS of P. euphratica leaves (Figure 4B, E). The difference in TUP between male and female P. euphratica under CK conditions was manifested in the Mid and End periods. The difference in TUP between male and female P. euphratica under the TR condition only appeared in the End period. The interaction effect of S × W on TS only worked in the Initial period, and the difference between male and female TS was only reflected under CK conditions (Figure 4B).
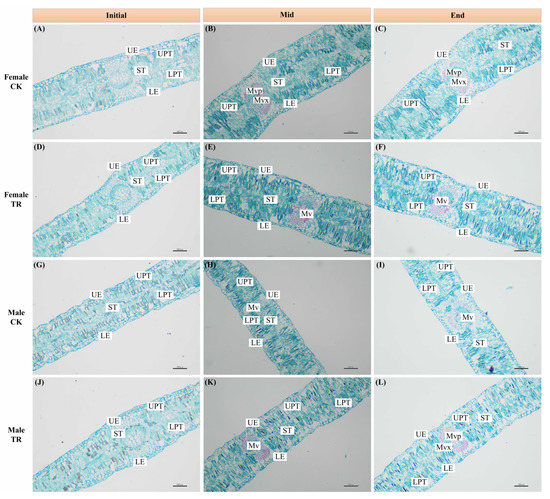
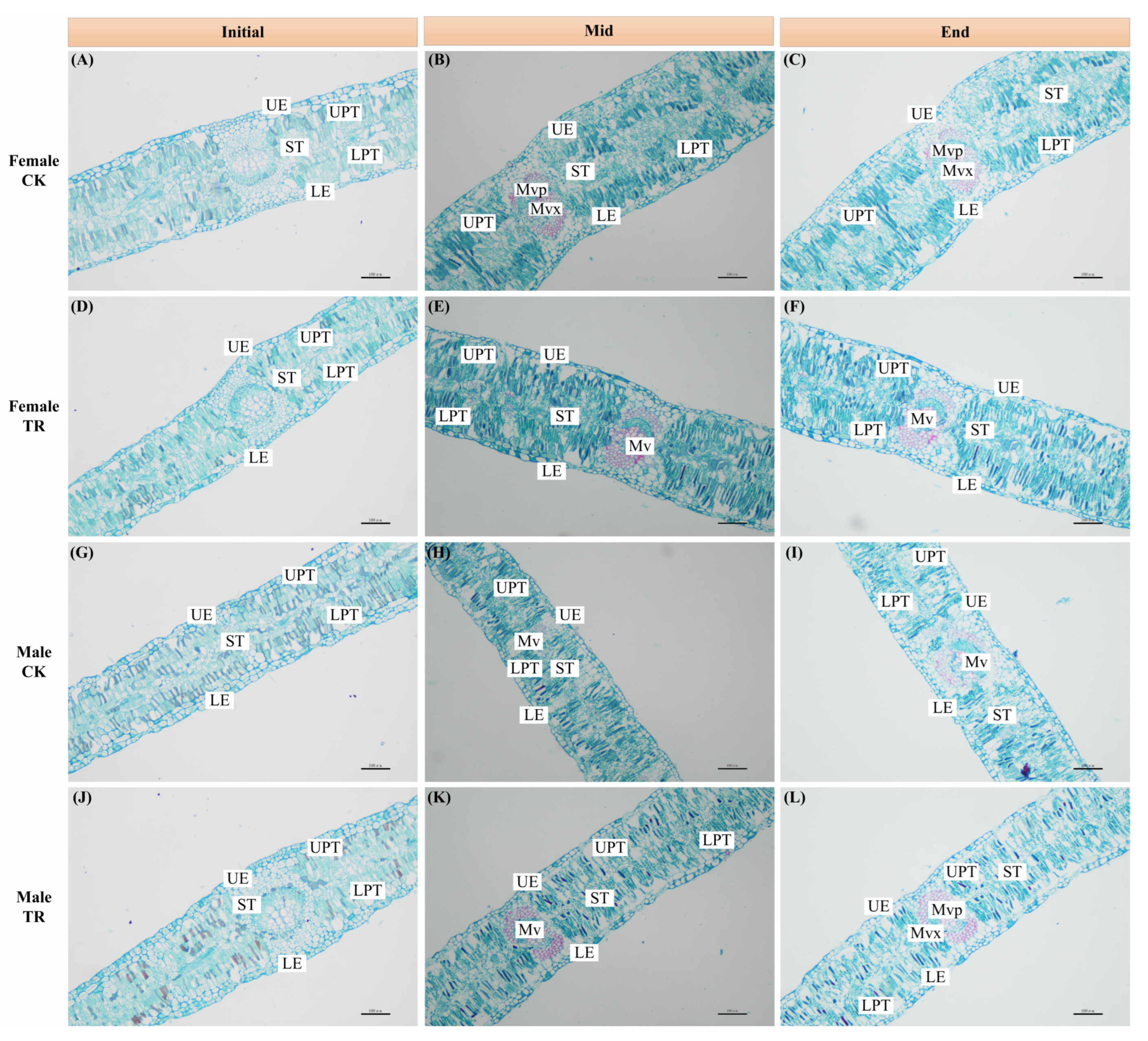
Figure 3.
Leaf anatomical structure of female (A–C) and male (G–I) P. euphratica under CK conditions; leaf anatomical structure of female (D–F) and male (J–L) P. euphratica under TR conditions. Ue: Upper epidermal; LE: Lower epidermal; ST: Spongy tissue; UPT: Upper palisade tissue; LPT: Lower palisade tissue; Mv: Main vein; Mvp: Main vein phloem; Mvx: Main vein xylem.
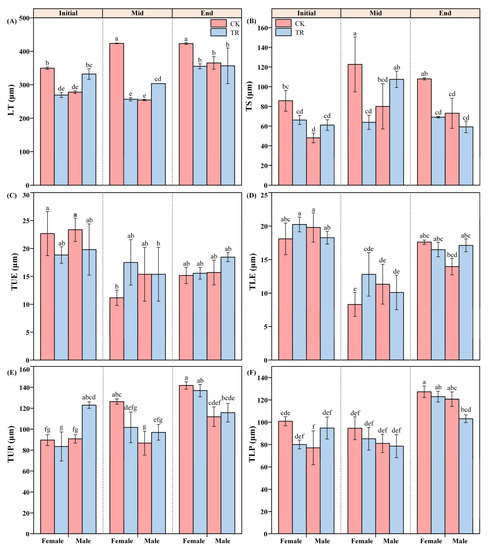
Figure 4.
The anatomic parameters of female and male P. euphratica in the wetting experiment. LT: leaf thickness (A), TS: sponge tissue thickness (B), TUE: upper epidermal cell thickness (C), TLE: lower epidermal cell thickness (D), TUP: upper palisade tissue thickness (E), TLP: lower palisade tissue thickness (F). Note: Different lowercase letters indicate statistically significant differences between treatments (one-way ANOVA, p < 0.05) (Table S6).
3.5. Driving Factor Analysis of Wetting Treatment for P. euphratica
In Figure 5A,B, the eigenvalues of axis 1 and axis 2 are 4.03 and 2.06, respectively, and the contribution rates of each variable set in the first and second axes are 42.85% and 21.88%, respectively(Tables S7 and S8). The cumulative contribution rate of the two axes reaches 64.73% (Figure 5A). In Figure 5C,D, the eigenvalues of axis 1 and axis 2 are 3.86 and 2.57, respectively, and the contribution rates of each variable set in the first and second axes are 40.65% and 27.06%, respectively. The cumulative contribution rate of the two axes reached 67.71% (Figure 5B), so the first two axes were selected to explain the relationship between the variable sets. There were significant correlations between leaf water potential and anatomical parameters, fluorescence parameters and photosynthetic parameters (p < 0.01). Since the water potential was negative, the angle of correlation between the indicators demonstrates a negative correlation if it is an acute angle and a positive correlation if it is an obtuse angle. The analysis found that the angle between the predawn water potential of female and male P. euphratica and TE in the leaf anatomical parameters was the smallest, and the angle between the predawn water potential and the WUE in the photosynthetic parameters was the smallest. It can be seen that the predawn water potential has a strong correlation with these parameters. Comparing the distribution of the fluorescence parameters of male and female P. euphratica, it can be seen that the angle between predawn water potential and Fv/Fm is an obtuse angle, indicating that the two are significantly positively correlated (Figure 5B,D).
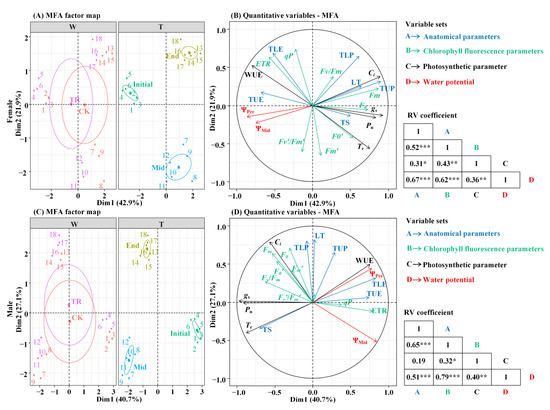
Figure 5.
Multivariate factor analysis of variable sets in female and male P. euphratica. Note: The individual map of MFA (A,C) and the quantitative variables map of MFA (B,D). ***, p < 0.001; **, p < 0.01; and *, p < 0.05.
4. Discussion
4.1. Foliar Water Uptake Capacity
As long as the external water potential of the leaf is greater than the internal water potential, water will enter the leaf from the outside [1,2]. The water absorption test conducted for P. euphratica leaves sets an ideal condition, which reflects the maximum potential of the water absorption and water absorption rate of P. euphratica, so this index is often used to evaluate the capacity of leaf water absorption. The results of the water absorption experiment show that the water absorption capacity of P. euphratica leaves increased with the growth period, and the FWU capacity of female P. euphratica exhibited a significant time difference. After the Initial period, the FWU capacity of female P. euphratica increased significantly, becoming much larger than that of male P. euphratica at approximately twice the value; k also showed a gradual increase during the growth period, although the difference was not significant. This shows that the water absorption potential of P. euphratica leaves increases during the growth period, and the water absorption potential of female P. euphratica is stronger than that of male P. euphratica in the middle and later growth stages. There are two kinds of water absorption strategies for plants, namely, the storage efficiency of high water absorption and a low water absorption rate, and the speed efficiency of low water absorption and a high water absorption rate [6,25,43]. However, the results from P. euphratica presented in this paper show that storage efficiency and speed efficiency can coexist, which is consistent with the results of Boanares et al. [44] on Senna reniformis.
The difference in foliar water uptake also leads to significant changes in the degree of drought stress (LWCA), which reduces FWU at the leaf level [40] (Table 2). Although the water absorption capacity of female P. euphratica leaves was stronger than that of male P. euphratica, from the change in LWCA, the water absorption of male P. euphratica leaves alleviates the water deficit at the leaf level more strongly than that of female P. euphratica. During the Mid period, the atmospheric temperature was the highest and the precipitation was relatively less (Table 1; Figure S1), so the plants suffered the greatest risk of drought stress during this period. The LWCA of male and female P. euphratica in this period was larger than that in the other two periods, indicating that the water absorption strategy of male and female P. euphratica leaves showed a high degree of environmental adaptability. Coincidentally, the advantage of male P. euphratica in alleviating leaf water deficit was the largest in the Mid period, indicating that male P. euphratica had a stronger adaptability to arid environments [45]. After further evaluating the potential (PRI) and actual relative importance (ARI) of FWU in alleviating plant water deficit, it was found that these two parameters of female P. euphratica were significantly greater than those of male P. euphratica, but this difference was only shown in the Mid period. The results show that FWU was more important than male P. euphratica in alleviating the leaf water deficit of female P. euphratica, that is, the potential value and actual value of FWU for female P. euphratica were higher.
Leaf traits profoundly affect leaf water absorption capacity [7,46,47]. SLW is one of the important indicators used to reflect the morphological characteristics of plant leaves. Plants with a higher SLW have a higher mesophyll density than those with a lower SLW [47], while WSD reflects the state of the leaf water saturation deficit. These two indicators are significantly correlated with FWU capacity and k, respectively. Boanares et al. [43] believed that a higher RWC was the result of a higher water absorption rate. The results reflected by WSD were actually consistent with their conclusions, that is, the higher the degree of leaf water deficit, the greater the water absorption and water absorption rate of leaves. This may be a strategy to avoid the effects of drought.
4.2. Response of Leaf Ecophysiology to Wetting
Studies have shown that FWU improves plant water status [5,48,49,50,51]. The effect of wetting on plant ΨPre is more significant than for ΨMid [10,52], and our results confirm this. Humidification significantly increased the predawn water potential of P. euphratica leaves during the Mid period, minimizing the risk of water stress caused by scarce precipitation during this period [10]. Boanares et al. [43] found that there is a relationship between leaf water uptake and plant response to wetting. Specifically, the water uptake rate is negatively correlated with ΨPre, and the water uptake is positively correlated with ΨPre, because plants with higher leaf water uptake slowly absorb more water, lose less water than plants that absorb it faster and keep more water in their tissues for longer. In this paper, the ΨPre of P. euphratica did not change due to the gender differences in FWU capacity after humidification, which indicated that the process may be dominated by water absorption rate. This is because rapid water absorption can improve water status more quickly, which is very important for plants in arid areas.
In addition to Ci, there was no significant gender difference in the photosynthetic parameters of P. euphratica in each growth stage, but humidification treatment improved the photosynthetic capacity of P. euphratica in each growth stage, and this effect reached the maximum in the Mid period. Male P. euphratica had a higher carbon benefit in the Mid period. Although the Ci of male P. euphratica was smaller in this period, male P. euphratica maintained higher Pn through larger stomatal conductance [40,53]. The transpiration of P. euphratica was very strong in the Mid period, and WUE was significantly lower than that in the other two periods. It can be seen that the WUE of male and female P. euphratica leaves was improved after humidification induction, although the change was not significant. Humidification has a compensatory effect on the carbon assimilation process of P. euphratica. Improved leaf water status can effectively alleviate the water loss caused by transpiration [43], which is beneficial to P. euphratica.
Plant chlorophyll fluorescence parameters are helpful to understand the photosynthetic mechanism of plants against environmental stress, and are good indicators reflecting the relationship between plant photosynthesis and environmental stress [54,55]. Fv/Fm is the conversion efficiency of intrinsic light energy, which can reflect the ability of light system II to use light energy. The higher the value, the less likely it is that photoinhibition occurs. Under non-stress physiological conditions, the Fv/Fm of most plants is between 0.8 and 0.85, and the lower the value, the higher the degree of stress [52,56]. In addition to the End period, the Fv/Fm value of P. euphratica under the control condition reached 0.82, and the Fv/Fm values of P. euphratica in other growth stages were less than this interval, indicating that the photosynthetic organs of both were subject to different degrees of stress. After comparing the results for the fluorescence parameters, it was found that humidification did not significantly improve the light energy utilization process of plants.
Although humidification could not significantly induce a change in leaf anatomical structure, the effect of time on each anatomical parameter was very significant. During the growth period, the development of leaf thickness, spongy tissue and upper palisade tissue of female P. euphratica was stronger than that of male P. euphratica. Generally, plants with thicker leaves often have more developed water storage tissues, such as neat palisade tissue, parenchyma cells and water-containing parenchyma at the vascular end [57]; this may be one of the reasons why the FWU capacity of female P. euphratica leaves is greater than that of male P. euphratica, that is, the water storage structure contributes to leaf water absorption [58].
The change in water absorption was inseparable from cell structure support and physiological regulation (Figure 5). During the whole growth period, the response of the leaf structure and physiology of male and female P. euphratica to humidification exhibited both similarities and differences. In addition to WUE, there was a positive correlation between predawn water potential and photosynthetic capacity in male and female P. euphratica, indicating that an improvement in leaf water status due to humidification was beneficial to the process of carbon assimilation [59]. After comparing the fluorescence parameters, we found that qP and ETR were negatively correlated with the leaf predawn water potential. The larger the qP value, the greater the electron transport activity of photosystem II, which can not only reflect the redox state of the original electron acceptor QA, but also reflect the ability of the photon energy captured by photosystem II to be used for photochemical reaction. ETR reflects the apparent electron transfer efficiency under actual light intensity conditions [60,61]. Through the relationship between qP, ETR and predawn water potential, we believe that humidification inhibits the electron transfer process of the photosynthetic apparatus of P. euphratica, which may explain why humidification does not significantly improve chlorophyll fluorescence parameters. The predawn water potential of female P. euphratica was significantly positively correlated with TUP, while the predawn water potential of male P. euphratica was significantly positively correlated with TS, which may be due to the demand for water storage in the palisade tissue of female P. euphratica, while the sponge tissue of male P. euphratica provides a larger intercellular space and promotes gas exchange [62]. The studies have shown that male P. euphratica shows stronger drought resistance than female P. euphratica [45], and the stronger leaf water absorption capacity of female P. euphratica may benefit it at the individual or population level [63]. At present, we do not have sufficient data to prove that climate change has a greater impact on female or male P. euphratica. Especially in the past 50 years, the climate in Xinjiang has shown a trend of warming and wetting [64], which may make the mechanism of climate influence on plants more complicated. Therefore, in the future, we will focus on the role of long-term changes in climate on the structural, physiological and ecological adaptability of dioecious plants.
5. Conclusions
During the growing season, the foliar water uptake capacity of male and female P. euphratica leaves increased with the growth period. Among them, the water absorption capacity of female P. euphratica leaves was stronger than that of male P. euphratica, and showed a strategy of coexistence between storage and rate efficiency, which was inseparable from the developed water storage tissue of female P. euphratica. Leaf water absorption can effectively improve the leaf water status of male and female P. euphratica, and FWU effectively enhances the ecological adaptability of male and female P. euphratica. The leaves of female P. euphratica are more dependent on FWU, and the leaf water potential and net photosynthetic rate are effectively improved, but the ability of male P. euphratica to alleviate leaf water deficit and carbon benefit was stronger than that of male P. euphratica. This advantage was the largest in the middle of growth, indicating that male P. euphratica was more adaptable to the drought environment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14071444/s1, Figure S1: Monthly precipitation and mean monthly temperature at study site in 2021; Table S1: The characteristics of female and male P. euphratica; Table S2: Experimental Schedule; Table S3: The leaf water potential of female and male P. euphratica in the wetting experiment; Table S4: The photosynthetic parameters of female and male P. euphratica in the wetting experiment; Table S5: The fluorescence parameters of female and male P. euphratica in the wetting experiment; Table S6: The anatomical parameters of female and male P. euphratica in the wetting experiment; Table S7: The extracted axis features of MFA for female P. euphratica; Table S8: The extracted axis features of MFA for male P. euphratica.
Author Contributions
Investigation, Z.L. and Y.C.; conceptualization, Z.L.; methodology, Z.L. and H.W.; software, Z.L. and Y.C.; writing—original draft preparation, Z.L.; writing—review and editing, Z.L.; supervision, X.Z.; funding acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (42171026; 31700354), and the Xinjiang Uygur Autonomous Region Graduate Research and Innovation Project (XJ2021G04).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Berry, Z.C.; Emery, N.C.; Gotsch, S.G.; Goldsmith, G.R. Foliar Water Uptake: Processes, Pathways, and Integration into Plant Water Budgets. Plant Cell Environ. 2019, 42, 410–423. [Google Scholar] [CrossRef]
- Dawson, T.E.; Goldsmith, G.R. The Value of Wet Leaves. New Phytol. 2018, 219, 1156–1169. [Google Scholar] [CrossRef]
- Schreel, J.D.M.; Steppe, K. Foliar Water Uptake in Trees: Negligible or Necessary? Trends Plant Sci. 2020, 25, 590–603. [Google Scholar] [CrossRef]
- Goldsmith, G.R.; Matzke, N.J.; Dawson, T.E. The Incidence and Implications of Clouds for Cloud Forest Plant Water Relations. Ecol. Lett. 2013, 16, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.-W.; Lü, G.-H.; He, X.-M.; Sarkar, B.; Yang, X.-D. High Air Humidity Causes Atmospheric Water Absorption via Assimilating Branches in the Deep-Rooted Tree Haloxylon Ammodendron in an Arid Desert Region of Northwest China. Front. Plant Sci. 2019, 10, 573. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Z.; Yang, J. Effects of Leaf Hydrophilicity and Stomatal Regulation on Foliar Water Uptake Capacity of Desert Plants. Forests 2023, 14, 551. [Google Scholar] [CrossRef]
- Yang, X.-D.; Lv, G.-H.; Ali, A.; Ran, Q.-Y.; Gong, X.-W.; Wang, F.; Liu, Z.-D.; Qin, L.; Liu, W.-G. Experimental Variations in Functional and Demographic Traits of Lappula Semiglabra among Dew Amount Treatments in an Arid Region. Ecohydrology 2017, 10, e1858. [Google Scholar] [CrossRef]
- Steppe, K.; Vandegehuchte, M.W.; Van De Wal, B.A.E.; Hoste, P.; Guyot, A.; Lovelock, C.E.; Lockington, D.A. Direct Uptake of Canopy Rainwater Causes Turgor-Driven Growth Spurts in the Mangrove Avicennia Marina. Tree Physiol. 2018, 38, 979–991. [Google Scholar] [CrossRef]
- Carmichael, M.J.; White, J.C.; Cory, S.T.; Berry, Z.C.; Smith, W.K. Foliar Water Uptake of Fog Confers Ecophysiological Benefits to Four Common Tree Species of Southeastern Freshwater Forested Wetlands. Ecohydrology 2020, 13, e2240. [Google Scholar] [CrossRef]
- Li, Z.-K.; Gong, X.-W.; Wang, J.-L.; Chen, Y.-D.; Liu, F.-Y.; Li, H.-P.; Lü, G.-H. Foliar Water Uptake Improves Branch Water Potential and Photosynthetic Capacity in Calligonum Mongolicum. Ecol. Indic. 2023, 146, 109825. [Google Scholar] [CrossRef]
- Renner, S.S. The Relative and Absolute Frequencies of Angiosperm Sexual Systems: Dioecy, Monoecy, Gynodioecy, and an Updated Online Database. Am. J. Bot. 2014, 101, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, G.; Zang, R.; Korpelainen, H.; Berninger, F. Sex-Related Differences in Leaf Morphological and Physiological Responses in Hippophae rhamnoides along an Altitudinal Gradient. Tree Physiol. 2007, 27, 399–406. [Google Scholar] [CrossRef]
- Yu, L.; Dong, H.; Li, Z.; Han, Z.; Korpelainen, H.; Li, C. Species-Specific Responses to Drought, Salinity and Their Interactions in Populus Euphratica and P. Pruinosa Seedlings. J. Plant Ecol. 2020, 13, 563–573. [Google Scholar] [CrossRef]
- Olano, J.M.; González-Muñoz, N.; Arzac, A.; Rozas, V.; von Arx, G.; Delzon, S.; García-Cervigón, A.I. Sex Determines Xylem Anatomy in a Dioecious Conifer: Hydraulic Consequences in a Drier World. Tree Physiol. 2017, 37, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Korpelainen, H.; Li, C. Sexual Differences and Sex Ratios of Dioecious Plants under Stressful Environments. J. Plant Ecol. 2021, 14, 920–933. [Google Scholar] [CrossRef]
- Xu, X.; Peng, G.; Wu, C.; Korpelainen, H.; Li, C. Drought Inhibits Photosynthetic Capacity More in Females than in Males of Populus Cathayana. Tree Physiol. 2008, 28, 1751–1759. [Google Scholar] [CrossRef]
- Xu, X.; Yang, F.; Xiao, X.; Zhang, S.; Korpelainen, H.; Li, C. Sex-Specific Responses of Populus Cathayana to Drought and Elevated Temperatures. Plant Cell Environ. 2008, 31, 850–860. [Google Scholar] [CrossRef]
- Juvany, M.; Munné-Bosch, S. Sex-Related Differences in Stress Tolerance in Dioecious Plants: A Critical Appraisal in a Physiological Context. J. Exp. Bot. 2015, 66, 6083–6092. [Google Scholar] [CrossRef]
- Leigh, A.; Nicotra, A. Sexual Dimorphism in Reproductive Allocation and Water Use Efficiency in Maireana Pyramidata (Chenopodiaceae), a Dioecious, Semi-Arid Shrub. Aust. J. Bot. 2003, 51, 509–514. [Google Scholar] [CrossRef]
- Rozas, V.; DeSoto, L.; Olano, J. Sex-Specific, Age-Dependent Sensitivity of Tree-Ring Growth to Climate in the Dioecious Tree Juniperus Thurifera. New Phytol. 2009, 182, 687–697. [Google Scholar] [CrossRef]
- Li, C.; Ren, J.; Luo, J.; Lu, R. Sex-Specific Physiological and Growth Responses to Water Stress in Hippophae rhamnoides L. Populations. Acta Physiol. Plant. 2004, 26, 123–129. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Nogués, S.; Alegre, L. Diurnal Variations of Photosynthesis and Dew Absorption by Leaves in Two Evergreen Shrubs Growing in Mediterranean Field Conditions. New Phytol. 2002, 144, 109–119. [Google Scholar] [CrossRef]
- Gouvra, E.; Grammatikopoulos, G. Beneficial Effects of Direct Foliar Water Uptake on Shoot Water Potential of Five Chasmophytes. Can. J. Bot. 2011, 81, 1278–1284. [Google Scholar] [CrossRef]
- dos Santos Garcia, J.; Boanares, D.; França, M.G.C.; Sershen; López-Portillo, J. Foliar Water Uptake in Eight Mangrove Species: Implications of Morpho-Anatomical Traits. Flora 2022, 293, 152100. [Google Scholar] [CrossRef]
- Boanares, D.; Isaias, R.R.M.S.; De Sousa, H.C.; Kozovits, A.R. Strategies of Leaf Water Uptake Based on Anatomical Traits. Plant Biol. 2018, 20, 848–856. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Chen, G.; Li, M.; Liu, M.; Liu, D. Epidermal Micromorphology and Mesophyll Structure of Populus Euphratica Heteromorphic Leaves at Different Development Stages. PLoS ONE 2015, 10, e0137701. [Google Scholar] [CrossRef]
- Yang, F.; Deng, D.; Zhao, L.; Zhu, L. Comparative study on leaf morphological and structural characteristics of male and female Podocarpus macrophyllus. Acta Agric. Jiangxi 2021, 33, 42–47. [Google Scholar] [CrossRef]
- Vesala, T.; Sevanto, S.; Grönholm, T.; Salmon, Y.; Nikinmaa, E.; Hari, P.; Hölttä, T. Effect of Leaf Water Potential on Internal Humidity and CO2 Dissolution: Reverse Transpiration and Improved Water Use Efficiency under Negative Pressure. Front. Plant Sci. 2017, 8, 54. [Google Scholar] [CrossRef]
- Binks, O.; Coughlin, I.; Mencuccini, M.; Meir, P. Equivalence of Foliar Water Uptake and Stomatal Conductance? Plant Cell Environ. 2020, 43, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Terashima, I. Effects of Continuous Leaf Wetness on Photosynthesis: Adverse Aspects of Rainfall. Plant Cell Environ. 1995, 18, 431–438. [Google Scholar] [CrossRef]
- Hanba, Y.; Moriya, A.; Kimura, K. Effect of Leaf Surface Wetness and Wetability on Photosynthesis in Bean and Pea. Plant Cell Environ. 2004, 27, 413–421. [Google Scholar] [CrossRef]
- Li, Z.; Wu, N.; Liu, T.; Chen, H.; Tang, M. Effect of arbuscular mycorrhizal inoculation on water status and photosynthesis of Populus cathayana males and females under water stress. Physiol. Plant 2015, 155, 192–204. [Google Scholar] [CrossRef]
- Keyimu, M.; Halik, Ü.; Betz, F.; Dulamsuren, C. Vitality Variation and Population Structure of a Riparian Forest in the Lower Reaches of the Tarim River, NW China. J. For. Res. 2017, 29, 749–760. [Google Scholar] [CrossRef]
- Li, W.; Lü, G.; Zhang, L.; Wang, H.; Li, Z.; Wang, J.; Ma, H.; Liu, Z. Analysis of Potential Water Source Differences and Utilization Strategies of Desert Plants in Arid Regions. Ecol. Environ. Sci. 2019, 28, 1557–1566. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, X.; Sun, H.; Hua, D.; Qin, J. How Populus Euphratica Utilizes Dew in an Extremely Arid Region. Plant Soil 2019, 443, 493–508. [Google Scholar] [CrossRef]
- Fan, X.; Hao, X.; Zhang, S.; Zhao, Z.; Zhang, J.; Li, Y. Populus euphratica Counteracts Drought Stress through the Dew Coupling and Root Hydraulic Redistribution Processes. Ann. Bot. 2023, 131, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lü, G.; Wang, Y.; Zhang, X. Water use efficiency of halophytes in Ebinur Lake Wetland Nature Reserve of Xinjiang. Chin. J. Econ. 2010, 29, 2341–2346. [Google Scholar] [CrossRef]
- Gong, X.; Lü, G. Species Diversity and Dominant Species’ Niches of eremophyte communities of the Tugai forest in the Ebinur basin of Xinjiang, China. Biodivers. Sci. 2017, 25, 34–45. [Google Scholar] [CrossRef]
- Liang, X.; Su, D.; Yin, S.; Wang, Z. Leaf Water Absorption and Desorption Functions for Three Turfgrasses. J. Hydrol. 2009, 376, 243–248. [Google Scholar] [CrossRef]
- Schreel, J.D.M.; von der Crone, J.S.; Kangur, O.; Steppe, K. Influence of Drought on Foliar Water Uptake Capacity of Temperate Tree Species. Forests 2019, 10, 562. [Google Scholar] [CrossRef]
- Cavallaro, A.; Carbonell Silleta, L.; Pereyra, D.A.; Goldstein, G.; Scholz, F.G.; Bucci, S.J. Foliar Water Uptake in Arid Ecosystems: Seasonal Variability and Ecophysiological Consequences. Oecologia 2020, 193, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.; Delaney, H. Designing Experiments and Analyzing Data: A Model Comparison Perspective; Routledge Academic Inc. Publisher: London, UK, 2003; ISBN 978-1-4106-0924-3. [Google Scholar]
- Boanares, D.; Kozovits, A.R.; Lemos-Filho, J.P.; Isaias, R.M.S.; Solar, R.R.R.; Duarte, A.A.; Vilas-Boas, T.; França, M.G.C. Foliar Water-uptake Strategies Are Related to Leaf Water Status and Gas Exchange in Plants from a Ferruginous Rupestrian Field. Am. J. Bot. 2019, 106, 935–942. [Google Scholar] [CrossRef]
- Boanares, D.; Ferreira, B.G.; Kozovits, A.R.; Sousa, H.C.; Isaias, R.M.S.; França, M.G.C. Pectin and Cellulose Cell Wall Composition Enables Different Strategies to Leaf Water Uptake in Plants from Tropical Fog Mountain. Plant Physiol. Biochem. 2018, 122, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Huang, Z.; Tang, S.; Korpelainen, H.; Li, C. Populus Euphratica Males Exhibit Stronger Drought and Salt Stress Resistance than Females. Environ. Exp. Bot. 2023, 205, 105114. [Google Scholar] [CrossRef]
- Chin, A.R.O.; Guzmán-Delgado, P.; Görlich, A.; HilleRisLambers, J. Towards multivariate functional trait syndromes: Predicting foliar water uptake in trees. Ecology, 2023; online version of record. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.R.O.; Guzmán-Delgado, P.; Kerhoulas, L.P.; Zwieniecki, M.A. Acclimation of interacting leaf surface traits affects foliar water uptake. Tree Physiol. 2023, 43, 418–429. [Google Scholar] [CrossRef]
- Losso, A.; Dämon, B.; Hacke, U.; Mayr, S. High potential for foliar water uptake in early stages of leaf development of three woody angiosperms. Physiol. Plant 2023, 175, e13961. [Google Scholar] [CrossRef]
- Ru, C.; Hu, X.; Chen, D.; Wang, W.; Song, T. Heat and Drought Priming Induce Tolerance to Subsequent Heat and Drought Stress by Regulating Leaf Photosynthesis, Root Morphology, and Antioxidant Defense in Maize Seedlings. Environ. Exp. Bot. 2022, 202, 105010. [Google Scholar] [CrossRef]
- Eller, C.B.; Lima, A.L.; Oliveira, R.S. Foliar Uptake of Fog Water and Transport Belowground Alleviates Drought Effects in the Cloud Forest Tree Species, Drimys brasiliensis (Winteraceae). New Phytol. 2013, 199, 151–162. [Google Scholar] [CrossRef]
- Holanda, A.E.R.; Souza, B.C.; Carvalho, E.C.D.; Oliveira, R.S.; Martins, F.R.; Muniz, C.R.; Costa, R.C.; Soares, A.A. How Do Leaf Wetting Events Affect Gas Exchange and Leaf Lifespan of Plants from Seasonally Dry Tropical Vegetation? Plant Biol. 2019, 21, 1097–1109. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Qin, L.; Gong, X.; Lü, G. Influence of Dew on Fluorescence Parameter and Water Use Efficiency of Halostachys caspica in Different Salinity Habitats. Arid Zone Res. 2017, 34, 1124–1132. [Google Scholar] [CrossRef]
- Yu, L.; Tang, S.; Guo, C.; Korpelainen, H.; Li, C. Differences in Ecophysiological Responses of Populus euphratica Females and Males Exposed to Salinity and Alkali Stress. Plant Physiol. Biochem. 2023, 198, 107707. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.H.; Weis, E. Chlorophyll Fluorescence and Photosynthesis: The Basics. Annu. Rev. Plant Biol. 2003, 42, 313–349. [Google Scholar] [CrossRef]
- Wu, M.; Deng, P.; Zhao, Y.; Zhao, S.-H.; Chen, J.-N.; Shu, Y.; Huang, T.-F. Effects of drought on leaf growth and chlorophyll fluorescence kinetics parameters in Cyclobalanopsis glauca seedlings of Karst areas. J. Appl. Ecol. 2019, 30, 4071–4081. [Google Scholar] [CrossRef]
- Howarth, J.F.; Durako, M.J. Diurnal Variation in Chlorophyll Fluorescence of Thalassia testudinum Seedlings in Response to Controlled Salinity and Light Conditions. Mar. Biol. 2013, 160, 591–605. [Google Scholar] [CrossRef]
- Bryant, C.; Fuenzalida, T.I.; Zavafer, A.; Nguyen, H.T.; Brothers, N.; Harris, R.J.; Beckett, H.A.A.; Holmlund, H.I.; Binks, O.; Ball, M.C. Foliar Water Uptake via Cork Warts in Mangroves of the Sonneratia Genus. Plant Cell Environ. 2021, 44, 2925–2937. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.A.; Chapman, S.; Jesse, A.; O’Brien, E.; Langley, J.A.; Bardou, R.; Devaney, J.; Parker, J.D.; Cavanaugh, K.C. Foliar Water Uptake by Coastal Wetland Plants: A Novel Water Acquisition Mechanism in Arid and Humid Subtropical Mangroves. J. Ecol. 2020, 108, 2625–2637. [Google Scholar] [CrossRef]
- Ferreira, J.L.D.; Daniela, B.; Eliodoro, V.C.; Pinheiro, A.S.F. Do photosynthetic metabolism and habitat influence foliar water uptake in orchids? Plant Biol. 2022, 25, 257–267. [Google Scholar]
- Richardson, A.D.; Berlyn, G.P. Spectral Reflectance and Photosynthetic Properties of Betula Papyrifera (Betulaceae) Leaves along an Elevational Gradient on Mt. Mansfield, Vermont, USA. Am. J. Bot. 2002, 89, 88–94. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2010; ISBN 978-0-87893-866-7. [Google Scholar]
- Chartzoulakis, K.; Patakas, A.; Kofidis, G.; Bosabalidis, A.; Nastou, A. Water Stress Affects Leaf Anatomy, Gas Exchange, Water Relations and Growth of Two Avocado Cultivars. Sci. Hortic. 2002, 95, 39–50. [Google Scholar] [CrossRef]
- Li, Z.-K.; Chen, Y.-D.; Wang, J.-L.; Jiang, L.-M.; Fan, Y.-X.; Lü, G.-H. Foliar water uptake and its influencing factors differ between female and male Populus euphratica. Environ. Exp. Bot. 2023, 213, 105419. [Google Scholar] [CrossRef]
- Yao, J.-Q.; Chen, Y.-N.; Guan, X.-F.; Zhao, Y.; Chen, J.; Mao, W.-Y. Recent climate and hydrological changes in a mountain–basin system in Xinjiang, China. Earth-Sci. Rev. 2022, 226, 103957. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).