Abstract
Plants can produce and release allelochemicals to interfere with the establishment and growth of conspecific and interspecific plants. Such allelopathy is an important mediator among plant species in natural and managed ecosystems. This review focuses on allelopathy and allelochemicals in grasslands and forests. Allelopathy drives plant invasion, exacerbates grassland degradation and contributes to natural forest regeneration. Furthermore, autotoxicity (intraspecific allelopathy) frequently occurs in pastures and tree plantations. Various specialized metabolites, including phenolics, terpenoids and nitrogen-containing compounds from herbaceous and woody species are responsible for allelopathy in grasslands and forests. Terpenoids with a diversity of metabolites are qualitative allelochemicals occurring in annual grasslands, while phenolics with a few specialized metabolites are quantitative allelochemicals occurring in perennial forests. Importantly, allelochemicals mediate below-ground ecological interactions and plant–soil feedback, subsequently affecting the biodiversity, productivity and sustainability of grasslands and forests. Interestingly, allelopathic plants can discriminate the identity of neighbors via signaling chemicals, adjusting the production of allelochemicals. Therefore, allelochemicals and signaling chemicals synergistically interact to regulate interspecific and intraspecific interactions in grasslands and forests. Allelopathy and allelochemicals in grasslands and forests have provided fascinating insights into plant–plant interactions and their consequences for biodiversity, productivity and sustainability, contributing to our understanding of terrestrial ecosystems and global changes.
1. Introduction
Grasslands and forests are integral components of the global ecosystem, totally covering about 70% of the earth’s terrestrial area. Both function as the crucial global pool of biodiversity to supply a wide range of species, and their productivity and sustainability modulate global changes [1,2,3]. Importantly, grasslands and forests play substantial roles in diverse ecological services to generate tremendous benefits for humans, such as water conservation, sand fixation, carbon sequestration, oxygen release and global biogeochemical cycles [4,5]. Understanding the biodiversity, productivity and sustainability of grasslands and forests and their underlying mechanisms has been of great interest to ecologists for decades.
The biodiversity, productivity and sustainability of grasslands and forests are the net outcomes of various biotic versus abiotic feedbacks between plants and their environment. These can arise through a variety of mechanisms such as resource partitioning, niche divergence, plant–soil and other species-specific interactions [6,7,8], but the central driver must be interspecific and intraspecific plant–plant interactions that can be neutral (consummation and recognition), positive (facilitation and kin selection) and negative (competition and allelopathy) to allow local coexistence. The interactions, either beneficial, harmful or commensal, eventually contribute to the biodiversity, productivity and sustainability of grasslands and forests. While most studies have focused on resource competition, environmental factors and global changes, relatively little is known about the importance of allelopathy in grassland and forest ecological processes [9].
A plant may interfere with the growth and establishment of neighboring plants through competition, allelopathy or both. Differing from competition for resources, allelopathy is an interference mechanism in which living or dead plants release allelochemicals exerting an effect (mostly negative) on co-occurring plants [10,11], even within a species (i.e., autotoxicity or intraspecific allelopathy). Four ecological processes, volatilization, leaching, litter decomposition and root exudation, can bring allelochemicals into air or soil. When allelochemicals contact or approach the associated plants, they directly demonstrate allelopathic action by disturbing the systems of photosynthesis, respiration, and metabolism, or indirectly affect target species by altering environmental conditions, particularly for soil physicochemical properties and microbial communities [12,13,14]. In fact, allelopathy originates from interspecific and intraspecific plant–plant interactions in grasslands and forests. The first classical case is black walnut (Juglans nigra), which produced and released a 1,4-naphthoquinone (juglone) to interfere with the growth of understory plants thousands of years ago [15]. The allelopathic interference of shrubs in grass through the release of volatile terpenes into southern California coastal grassland was reported in the 1960s [16]. Subsequently, an increasing number of studies have shown that many ecological events occurring in grasslands and forests are associated with allelopathy and certain allelochemicals [9,10,11,17,18].
Allelopathy in grasslands and forests is key for understanding terrestrial ecosystems and global changes. In recent decades, numerous control experiments and field investigations have been conducted to estimate the functional consequences of allelopathy for plant communities in natural and managed grasslands and forests. However, a comprehensive allelopathy, particularly for allelochemical-mediated below-ground and above-ground interactions in grasslands and forests, is rare. Understanding allelopathy with allelochemicals and their consequences for biodiversity, productivity and sustainability in grasslands and forests can provide new insight into terrestrial ecosystems and global changes. Hence, capturing recent advances and applications in allelopathy and allelochemicals is becoming valuable in advancing interdisciplinary research in grasslands and forests.
2. Allelopathy in Grasslands
2.1. Allelopathy Drives Plant Invasion in Grasslands
The occurrence of invasive plants threatens the structure and function of grassland ecosystems, especially in biodiversity and stability [17]. Several plant species have been confirmed to invade grasslands with an allelopathic mechanism. Spotted knapweed (Centaurea stoebe), native to Europe and introduced into North America, is an example of an invasive plant in western American grasslands. Spotted knapweed can take advantage of root-secreted allelochemicals against local grassland species and alter nutrition availability and underground microbial community composition [18,19]. However, the allelopathy of spotted knapweed is conditional, and there is discrepancy between geographical sites. Spotted knapweed does not exhibit allelopathic invasion in eastern American grasslands [20]. Additionally, sufficient light or infection with fungal endophytes can enhance the allelopathic invasion of spotted knapweed in American grasslands [20,21].
Allelopathic invasion of spotted knapweed in American grasslands results in the novel weapons hypothesis (NWH) that the success of plant invasion can be attributed to the allelochemicals of invaders [22]. Generally, allelochemicals of invasive species have little effect on their original neighbors due to long-term mutual adaptation, but as they are novel to the species of the invaded habitat, they exert a strongly allelopathic interference on the native species [22]. Much evidence has demonstrated that allelochemicals appear to confer a competitive advantage to the invasive plants [23,24,25,26]. However, some studies did not fully support the NWH, and questioned the necessity of secondary metabolites for nonnative species to ensure invasive success [27,28,29]. Another hypothesis, the biochemical recognition hypothesis (BRH), postulates that plant seeds can adaptively detect phytochemicals released from potential competitors and respond by extending their period of dormancy until better establishment conditions occur [30]. Leachates from spotted knapweed reduced the germination rate of grassland species. Importantly, they had no effect on seeding biomass, implying that the allelochemicals in the leachates are non-phytotoxic and do not impede plant growth [30].
Although both the NWH and BRH focus on plant-derived chemicals and predict similar results that phytochemicals released from invasive plants inhibit the emergence of native plants, their fundamental mechanisms are distinct. This can be explained either a negative exposure to toxic chemicals by NWH or a positive recognition of facilitative chemicals by BRH [22,30]. Nevertheless, whether the success of invasive plants is attributed to allelochemicals has been debated. Actually, allelopathy is pervasive in invasive plants [31]. Interestingly, allelopathy of native grassland communities seems to increase their resistance to invasion by introduced plants [32], but there was no evidence that native plant communities’ tolerance to allelopathy contributes to the degree of invasiveness of introduced plants. A more vital linkage between allelopathic traits and invasive performance needs to be explored in further studies.
2.2. Allelopathy Exacerbates Grassland Degradation
Grassland degradation is a phenomenon in which grass struggles to grow or hardly survives, which usually leads to an irreversible reduction in grassland productivity and biodiversity [33]. Many factors have been regarded as the drivers of grassland degradation, of which the main factors are natural climate change and human disturbance [34,35]. One early sign of degraded grassland is that the originally dominant species are gradually replaced by other adaptable plants, such as toxic weeds with allelopathic traits [36,37,38]. Toxic weeds in degraded grassland are adapted to extremely harsh environmental conditions and exhibit high aggression toward surrounding plants, even poisoning livestock or humans [39,40].
In the process of grassland degradation, toxic weeds not only vigorously compete with forage plants for water and nutrition resources, but also produce a wide range of secondary metabolites to exert allelopathic effects on the establishment of the co-occurring plants, subsequently reducing species richness and exacerbating grassland degradation [41,42,43]. Several studies have shown that extracts of toxic weeds, regardless of plant tissues or growing soil, can reduce the seed germination rate and seedling biomass of the receiving plants [38,44,45]. However, the allelopathic effects have distinct differences among the extract concentration, extract source and tested species [44]. Many phytotoxic compounds, such as coumarins, flavonoids and terpenoids, have been isolated and identified from toxic weeds. These potential allelochemicals could jeopardize the photosynthesis, respiration, and metabolic system of plants [46,47,48].
Stellera chamaejasme and Artemisia frigida are representatives of toxic weeds and generally serve as bioindicators to characterize the degree of grassland degradation. S. chamaejasme is a common toxic weed in the degraded grasslands of northern China, which can restrict the growth of co-occurring plants via root exudates [38,49]. A. frigida, a perennial dicotyledonous semi-shrub species, has a wide distribution range in the global temperate grasslands, covering Eurasian steppes and northern mixed-grass prairies. Differing from the mainly allelopathic pathway of S. chamaejasme, A. frigida can significantly decrease seed germination and seedling growth by emitting volatile organic compounds (VOCs) as allelochemicals [50,51]. This environmental disturbance may severely influence the composition and abundance of VOCs emitted from A. frigida. Artificial damage can induce A. frigida to release more categories and greater concentrations of VOCs [51]. In particular, grazing activity can enhance the allelopathic effect on the growth of other grassland species, suggesting that allelopathy may interact with over-grazing grassland to accelerate the grassland deterioration by frequently simulating A. frigida [52].
Overall, allelopathy is one of the critical factors driving grassland degradation. Comprehensively understanding of how allelochemicals from toxic weeds mediate intraspecific and interspecific plant–plant interactions would be useful for rehabilitating degraded grassland.
2.3. Allelopathy in Pasture Management
A pasture is a piece of grassland that mainly grows forage grass for livestock. Its quantity and quality are closely related to grassland ecosystem health and animal husbandry development. Hence, the management of pasture, whether natural or managed, is essential to ensure adequate forage grass and to support livestock production.
Allelopathy-based interspecific and intraspecific interactions have ecological consequences for the productivity and biodiversity of a pasture. Particularly in a managed pasture, pasture weeds can immensely decrease forage yield and quality, negatively affecting livestock production. Fortunately, some forage species can take full advantage of allelopathy and allelochemicals to retard the emergence and growth of co-occurring weeds, from which they will obtain growing benefits [53,54]. For example, rye (Secale cereale) is a cool-season forage species with high frost and drought resistance; it is generally planted in infertile or acid soils due to its strong adaptability. Rye can produce and release benzoxazinoids to selectively inhibit broadleaf weeds, modifying the spectrum of weed species in the pasture [55,56]. Therefore, some fine forage cultivars with allelopathic traits can be used for weed control. In particular, natural allelochemicals released from allelopathic forage cultivars may act as biological herbicides to a large extent, lowering the consumption of chemical herbicides and the cost of pasture management [57,58]. Many studies have shown that the application of allelopathic forage cultivars can effectively control pasture weeds and increase pasture productivity [55,57,59]. Notably, allelopathic forage species such as rye not only suppressed the pasture weeds but also succeeding forage species. To avoid failure in rotation systems, it is warranted to select resistant succeeding forage species [60].
Autotoxicity (intraspecific allelopathy) is ubiquitous in pastures. Autotoxicity in pasture has been well verified in alfalfa (Medicago sativa) [61,62,63]. Alfalfa is a major forage legume used as a high-quality livestock feed and cultivated in pastures throughout the world. Several phytotoxic phenolics, saponins and medicarpin in alfalfa can remarkably suppress their own seed germination. To attenuate the autotoxicity, the most obvious solution is to develop a new autotoxicity-tolerant alfalfa cultivar. A recent study has picked out the most autotoxicity-tolerant alfalfa from 22 cultivars based on a technique for order of preference by similarity to ideal solution analysis [64], which provides a theoretical basis for the breeding of autotoxicity-tolerant alfalfa cultivars. However, a long-term and large-scale field verification is needed to assess the tolerance of different alfalfa cultivars to autotoxicity.
A mixture of diverse forage species is considered as another option to experimentally prove effectiveness in improving forage productivity [65,66]. Directly, some highly allelopathy-tolerant forage seeds can be used as a subsequent alternative for restoring sparse natural grassland caused by allelopathy [67]. Additionally, the pattern of mixing species also has another benefit for pastures. The mixture of rye with berseem clover (Trifolium alexandrinum) may promote rye pathogen-resistant capabilities [68]. In the coexistence system of Artemisia adamsii with Stipa krylovii, volatiles emitted by A. adamsii can strengthen photosynthesis of S. krylovii by enhancing stomatal conductance even with water deficiency [69]. When grown with the P-mobilizing species Filifolium sibiricum, Leymus chinensis exhibited greater shoot and root P content [70]. These positive interactions are prevalent in pastures and mostly attributed to plant–plant chemical communication.
3. Allelopathy in Forests
3.1. Allelopathy in Natural Forests
Natural forests usually possess plant diversity and stable productivity. The role of allelopathy and the mechanisms underpinning it remain poorly resolved in species-rich forests, but allelopathy does contribute to natural forest regeneration. Forest regeneration is commonly considered as a critical ecological process that sustains resource reproduction through the establishment of saplings and the replacement of dead trees; it has profound implications for the perpetuation of tree species in the temporal and spatial dimensions. However, long-term exposure to allelochemicals from woody species may create a barrier effect on the understory-regenerated saplings, resulting in forest regeneration failure. In particular, endangered and rare plant species are inherently difficult to generate due to their scarce propagules and low adaptability. Allelopathy additively reduces the likelihood of the sapling establishment and probably leads to locally rare species’ extinction. Cinnamomum migao and Metasequoia glyptostroboides are two endangered woody species. Their regeneration is extremely restrained, and the natural population would be gradually diminished over time without active management. Generally, most natural populations only occasionally have 1~2 saplings in their understories [71,72]. Recent studies found that leaf extracts or litters of C. migao and M. glyptostroboides dramatically impeded their seedling growth by impairing the lipid structure of the cell membrane, suggesting that autotoxicity might aggravate the obstruction of the natural forest regeneration among some endangered tree species [72,73].
Apart from autotoxicity or self-inhibition, allelochemical-mediated interspecific interactions also hinder natural forest regeneration and impact the plant community’s composition. In the context of forests dominated by two tree species, dominant tree species may chemically inhibit the sapling regeneration of the others. For example, Kandelia obovate and Aegiceras corniculatum are two dominant species in mangrove forests. Leaf litter leachates of K. obovate are detrimental to the propagule germination and sapling growth of A. corniculatum, ultimately modulating the natural regeneration of the whole mangrove forest [74]. In the later successional forests of maple-beech codominance (Acer saccharum and Fagus grandifolia), the abundance of beech progressively increases as maple decreases with the years. This result, in part, can be explained by the allelopathic advantage of beech leading to the regeneration failure of maple [75,76].
Monopolistic herbaceous plants grown in the floor layer may inhibit natural forest regeneration. For example, the natural regeneration of sessile oak (Quercus petraea) is often hampered by the dense moor grass (Molinia caerulea) understory [77]. When watered with root exudates of moor grass, a significant decrease in oak biomass occurred, suggesting the allelopathic interference of moor grass in oak growth. Even though this negative impact was lower than that of resource depletion, it demonstrated the crucial contribution of herbaceous allelopathy to natural forest regeneration [78].
Based on the understanding of the allelopathic mechanisms underlying natural forest regeneration, some appropriate methods of forest management are proposed to alleviate the adverse effects of allelopathy and promote long-term natural regeneration. One of the most direct and efficient ways is to reduce the frequency of allelopathic interactions by removing litter, or eradicating the allelopathic species. Prevention of saplings from potential allelochemicals facilitates the sustainability of forest health [78,79]. In addition, attempts to enhance the diversity of the shrub layer and floor layer may be an alternative way to promote natural forest regeneration [80].
3.2. Allelopathy in Tree Plantations
A tree plantation is an artificial forest for the large-scale production of wood; usually, easily established and fast-growing tree species are selected as a monoculture forest. The productivity and sustainability of tree plantations intimately links the economic and ecological benefits of forestry. However, successive rotations of some forestry species may cause a replanting problem or soil disease, resulting in a decline in productivity and the loss of biodiversity in plantations [81,82]. Although the underlying mechanism for this issue is still being disentangled, a growing amount of evidence has shown that allelochemicals enriched in soil are mainly responsible for this problem [83,84].
Eucalyptus is one of the most widely planted forestry genera on the planet, but it has suffered from autotoxicity for a long time. Most studies have demonstrated that allelochemicals of Eucalyptus penetrate into the soil through the decomposition of litter and leachates, exerting an allelopathic effect on understory plants, thus limiting the regeneration of native vegetation [85,86]. However, Zhang et al. (2016) argued that the poor establishment of indigenous vegetation on plantations mainly arose from Eucalyptus roots rather than Eucalyptus litter. Retention of understory litter is more likely to facilitate the performance of native species [87]. Whatever the case is, a consensus is that allelopathy is more crucial than resource competition in the replanting problem of Eucalyptus plantations [88]. Chinese fir (Cunninghamia lanceolata) is another tree plantation severely disrupted by autotoxicity. Regeneration failure and poor establishment have remained critical problems in monocultural plantations of this species [89]. However, root exudates contribute more to soil allelochemicals than the litter in Chinese fir plantations. Root-secreted allelochemicals, therefore, are considered a primary source leading to the decline in the plantation of Chinese fir [90].
The mixture of multiple tree species is an effective way to improve the self-inhibition and soil deterioration caused by allelopathy and allelochemicals in plantations [91,92,93]. In Eucalyptus plantations, Albizia lebbeck, an introduced N-fixing species, has been regarded as a ’good partner’ to Eucalyptus. Mixed-species plantations of Eucalyptus with A. lebbeck increase productivity and maintain soil fertility compared with pure Eucalyptus stands [91]. Similarly, the establishment and productivity of autotoxic Manchurian walnut (Juglans mandshurica) can be improved in the presence of larch (Larix gmelini). Larch root exudates and soil in mixed-species plantations greatly stimulated the growth of Manchurian walnut seedlings and rapidly degraded the allelochemical juglone [92]. The growth and regeneration of Chinese fir is improved in Michelia macclurei and Chinese fir mixed-species plantations. One of the explanations for this beneficial promotion is that there may be interspecific facilitation mediated by the root exudates from M. macclurei, which not only attenuate the release of allelochemicals from Chinese fir roots but also induce a microbial shift to accelerate the decomposition rates of allelochemicals [93]. These studies illustrate the importance of mixed-species stands in plantations. However, most successful mixtures were empirically established from traditional practices, or were assessed from haphazard experimental combinations. We lack effective strategies for a priori selection of mixtures to achieve relevant benefits. Therefore, understanding intraspecific or interspecific allelopathy will be a key step in screening appropriate combinations of tree species to design plantations.
3.3. Tree-Understory Vegetation Allelopathic Interactions
The canopy position and soil occupancy of dominant forest trees remarkably reduce light and soil nutrient availability for understory vegetation. Even so, some shrub and herbaceous species in understory vegetation can adapt to these diverse conditions and coexist with trees. Apart from competition for resources, the allelopathy of the trees is an interference mechanism for the growth of understory vegetation [94,95]. The allelopathic trait of some trees is highly associated with forest abundance and biodiversity, particularly for woody invasive species. The presence of allelopathic tree species in forests can reduce the abundance of understory vegetation, ultimately becoming dense monospecific stands and extending to the whole forests [96,97,98]. In this process, allelochemicals may act as a meditator [99].
For the allelopathic effect of trees on understory plants, leaf litter and leachates have long been considered the main source [100,101]. Leaf litter and leachates from trees falling into the ground may prevent the colonization and development of understory vegetation [102,103]. This suppression is mainly attributed to their physical and chemical effects [104,105]. However, allelochemicals from leaf litter and leachates also have a measurable effect on understory vegetation [106,107,108]. Through the decomposition of leaf litter, allelochemicals can be gradually liberated into the soil and come into effect by altering soil pH, nutrient availability, the nitrogen cycle and microbial community structures [109,110]. Especially intriguing is leaf litter and leachates that may modify plant coexistence in the grass layer. For example, spotted knapweed and Bromus tectorum exhibit strong competition with each other, while leaf litter and leachates of Pinus ponderosa can mitigate the competitive effect of spotted knapweed on B. tectorum. In other words, the presence of P. ponderosa shifted competitive outcomes through physical and allelopathic effects, thereby indirectly facilitating B. tectorum by more strongly inhibiting spotted knapweed [111].
In some cases, leaf litter and leachates cannot solely show allelopathic potential. It must unite other biotic or abiotic factors to jointly impact the ecological process [102,112]. Prosopis juliflora is one of the world’s most aggressive invasive species, the leaf litter of which causes the increase of total phenolics in soil and toxifies understory vegetation [113]. When incubated with similar levels of leaf leachate from P. juliflora, the content of allelochemicals varies in different soil textures. Sandy soil accumulates higher levels of phenolics than sandy loam soil due to the greater absorption of inactive phenolics fettered in sandy loam soil [112]. In addition, the allelopathic effect of P. juliflora is also limited by soil moisture because their water-soluble allelochemicals in the soil are more likely to be washed away by rain. Therefore, P. juliflora could not manifest their allelopathic potential in humid soil. Only in dry environments, P. juliflora can create a depressive impact on understory plants [114].
Dense understory species with highly allelopathic potential, in turn, may directly slow the growth of trees and indirectly cause trouble by dissolving the fungal hyphae of trees. Garlic mustard (Alliaria petiolata) is a typical understory invasive species that may suppress fungal mutualists via allelochemicals, leading to significant declines in a series of physiological and metabolic functions [115,116,117]. Nevertheless, arbuscular mycorrhizal fungi (AMF) strains can be quickly selected by the allelopathic stress from garlic mustard. After the initial decline in AMF abundance, resistant AMF strains gradually displace sensitive AMF strains and the abundance rises again after the long-term invasion of garlic mustard [118,119]. Moreover, as an invader, the novelty of allelochemicals to resident species, regardless of the plant or microorganism, diminishes over time. Ultimately, garlic mustard may enter a new coevolutionary relationship with native competitors and slowly be integrated into the native community [120,121].
4. Allelochemicals in Grasslands and Forests
4.1. Category of Allelochemicals
All the occurrences of allelopathic phenomena can be attributed to a certain or a set of allelochemicals. Allelochemicals and their properties largely determine the allelopathic effectiveness. In the past decades, numerous old and new allelochemicals have been detected and identified from herbaceous and woody species in grasslands and forests. These allelochemicals involve a diversity of plant secondary metabolites, but are mainly divided into three categories of phenolics, terpenoids and nitrogen-containing compounds.
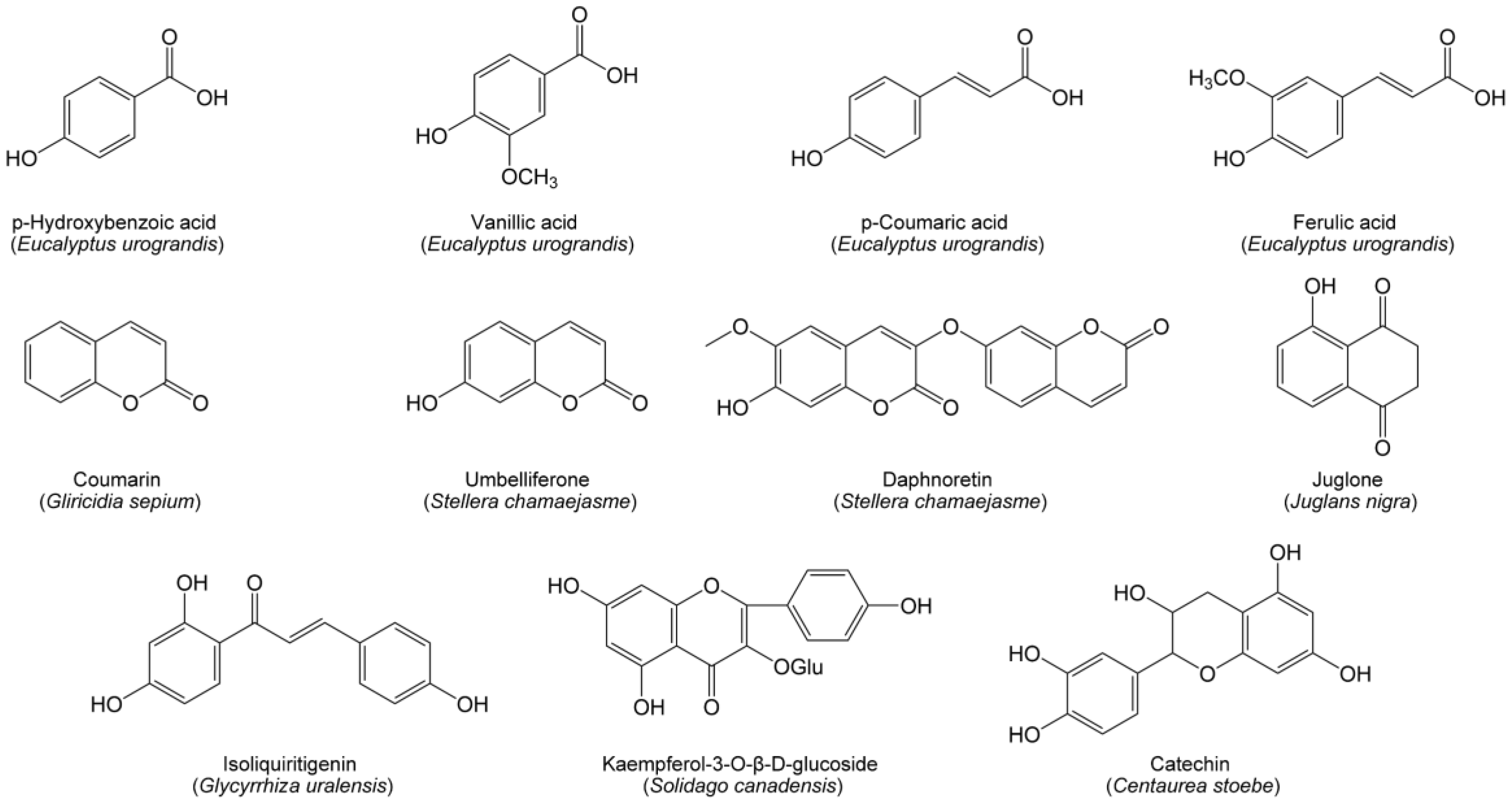
Phenolics have a wide distribution in plants and represent a diverse group of compounds with an aromatic ring possessing at least one hydroxyl group and possibly other substituents, including simple phenolic acids, coumarins, flavonoids and quinones. In forests, a tremendous amount of lignin from litter is decomposed into a variety of phenolic acids. These lignin-derived phenolic acids are main allelochemicals in forest soil, leading to a decline in forest species’ abundance and biodiversity. For example, the soil of the Eucalyptus urograndis plantation contains high levels of hydroxybenzoic, vanillic, coumaric and ferulic acids (Figure 1), resulting in autotoxicity of E. urograndis [85]. However, the allelopathic effect of phenolic acids is concentration-dependent. In particular, individual phenolic acids are insufficient to effectively suppress the growth of co-occurring plants, but their mixtures exhibit phytotoxic effects [122].
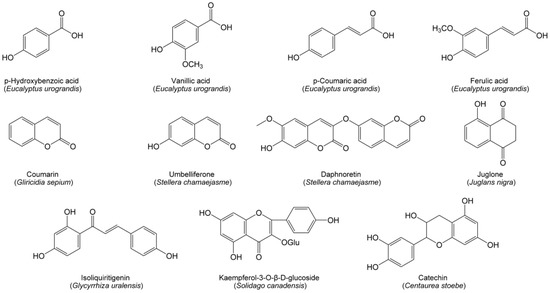
Figure 1.
Phenolic allelochemicals from herbaceous and woody species in grasslands and forests.
Many coumarins possess phytotoxicity and act as potential allelochemicals in grasslands and forests. Coumarin exacted from the leaf of Gliricidia sepium was identified as an allelochemical to inhibit the growth of plants [123]. Umbelliferone and daphnoretin (Figure 1) are two coumarin allelochemicals in Stellera chamaejasme [38]. Umbelliferone can inhibit plant growth by inducing membrane lipid peroxidation and retarding cell division, while daphnoretin inhibits plant growth by arresting the mitosis process [124].
Flavonoids generally perform a broad range of ecological functions. Several flavonoids have proved to be allelochemicals in grasslands and forests. Isoliquiritigenin (Figure 1) is a flavonoid allelochemical in Glycyrrhiza uralensis. It is able to trigger a chain of reactions in plant cells, including the overproduction of reactive oxygen species, lipid peroxidation, and a decline in cell vitality and chlorophyll content, ultimately resulting in seedling growth inhibition [125]. Another flavonoid, kaempferol-3-O-β-D-glucoside (Figure 1), is an allelochemical of Solidago canadensis [126]. Catechin (Figure 1) is a controversial flavonoid allelochemical secreted by spotted knapweed. Many studies have found high catechin concentrations in spotted knapweed soils [127,128] and proposed that catechin acts as a novel allelochemical of spotted knapweed, which contributes to growth limitation of the native plants [18]. However, several studies pointed out that catechin was hardly present in the bulk soils of spotted knapweed, and possessed low phytotoxicity to a variety of plant species [27,28].
Quinones are the classical allelochemicals in forests. Juglone (Figure 1) is an exclusive allelochemical of Juglandaceae family and represents one of the best-known members of quinones [129]. Initially, juglone is stored in leaves, barks and roots in the form of non-toxic naphthol O-glycoside. When released from plant living tissues to the environment, it is hydrolyzed into a less phytotoxic naphthol, and subsequently oxidized into phytotoxic juglone. The allelopathic mechanisms of juglone are associate with the disruption of leaf photosynthesis, transpiration, respiration and stomatal conductance. Additionally, juglone has high stability in soil. The toxicity of juglone can maintain for up to a year in spite of the removal of the walnut trees [130].
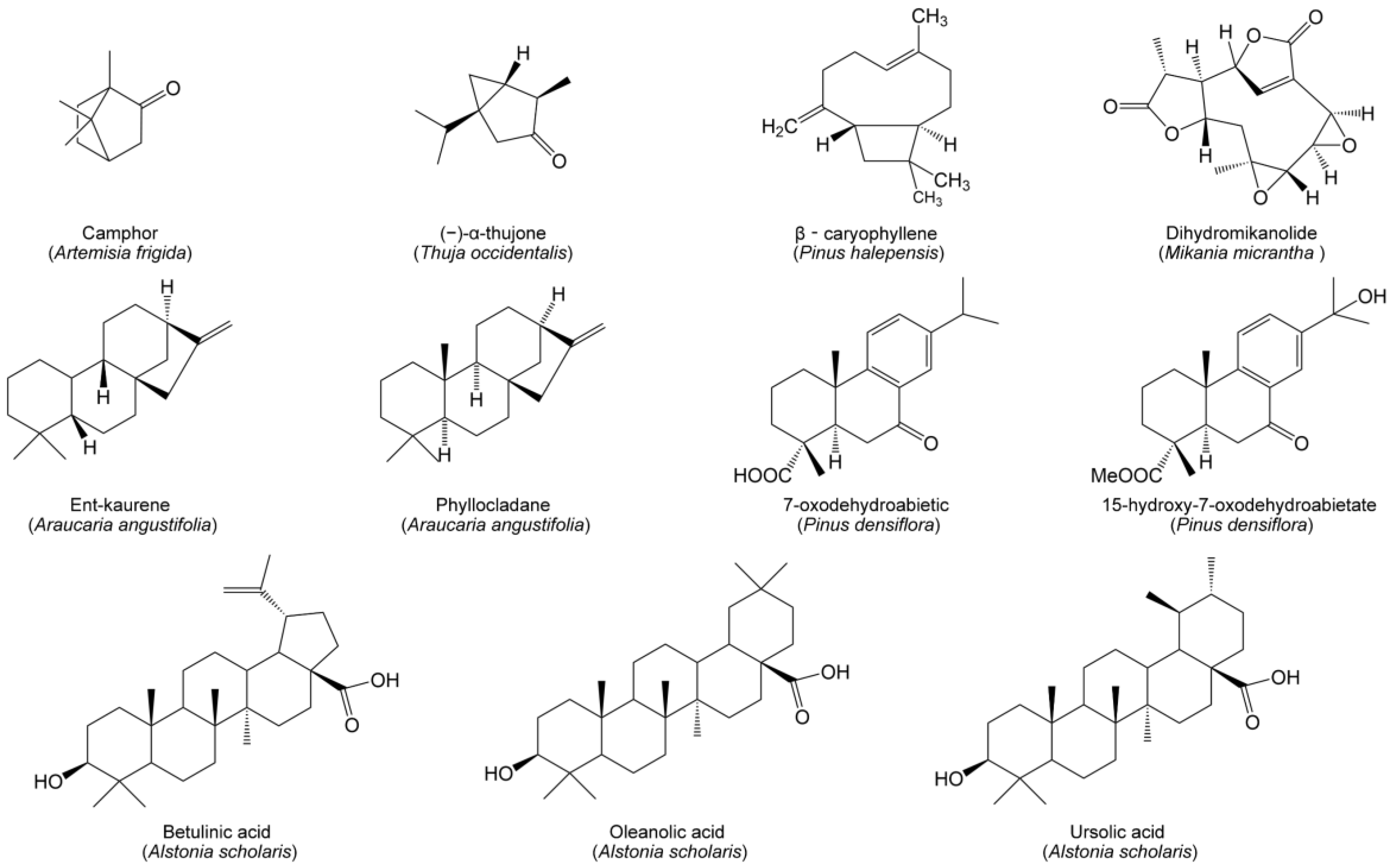
Terpenoids, including monoterpenes, sesquiterpenes, diterpenes, triterpenes and steroids, are a class of compounds derived from the 5-carbon isoprene. Monoterpenes and their derivatives possess strong volatility and may interact with neighboring plants in their gaseous phase. Volatile allelochemicals emitted by donor plants generally impact surrounding plants through two main pathways, either forming ’terpene clouds’ of directly impacted target plants [16], or leaching into the soil of indirectly affected target plants. The volatiles of A. frigida contain a copious quantity of terpenoids, among which monoterpene camphor is a key allelochemical affecting the neighboring species [36]. Another monoterpene, (−)-α-thujone, emitted from Thuja occidentalis (Figure 2), can display phytotoxic activities against seed germination and seedling growth of Taraxacum mongolicum and Arabidopsis thaliana [131]. β-Caryophyllene (Figure 2), a sesquiterpene within the needle litter of Pinus halepensis, exerts a deleterious effect on the germination and growth of herbaceous target species [132]. Dihydromikanolide (Figure 2) is another sesquiterpene allelochemical from Mikania micrantha, which contributes to promoting soil bacterial diversity but reduces fungal diversity [133]. Two diterpenes, ent-kaurene and phyllocladane (Figure 2), isolated from senescent needles of Araucaria angustifolia can act as allelochemicals to inhibit the germination and seedling growth of neighboring plants [134]. Similarly, diterpene allelochemicals, 7-oxodehydroabietic acid and 15-hydroxy-7-oxodehydroabietate (Figure 2), were found in the understory soil of Pinus densiflora. Both allelochemicals may be the underlying cause of sparse understory vegetation within the P. densiflora canopy [135]. Besides, some pentacyclic triterpenoids may function as allelochemicals, such as betulinic, oleanolic and ursolic acids (Figure 2) within the litter of Alstonia scholaris, limiting the growth of co-occurring species by inhibiting seed germination, radicle growth and the functioning of photosystem II [136].
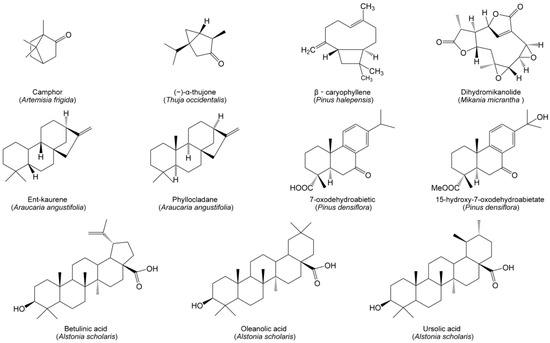
Figure 2.
Allelopathic terpenoids from herbaceous and woody species in grasslands and forests.
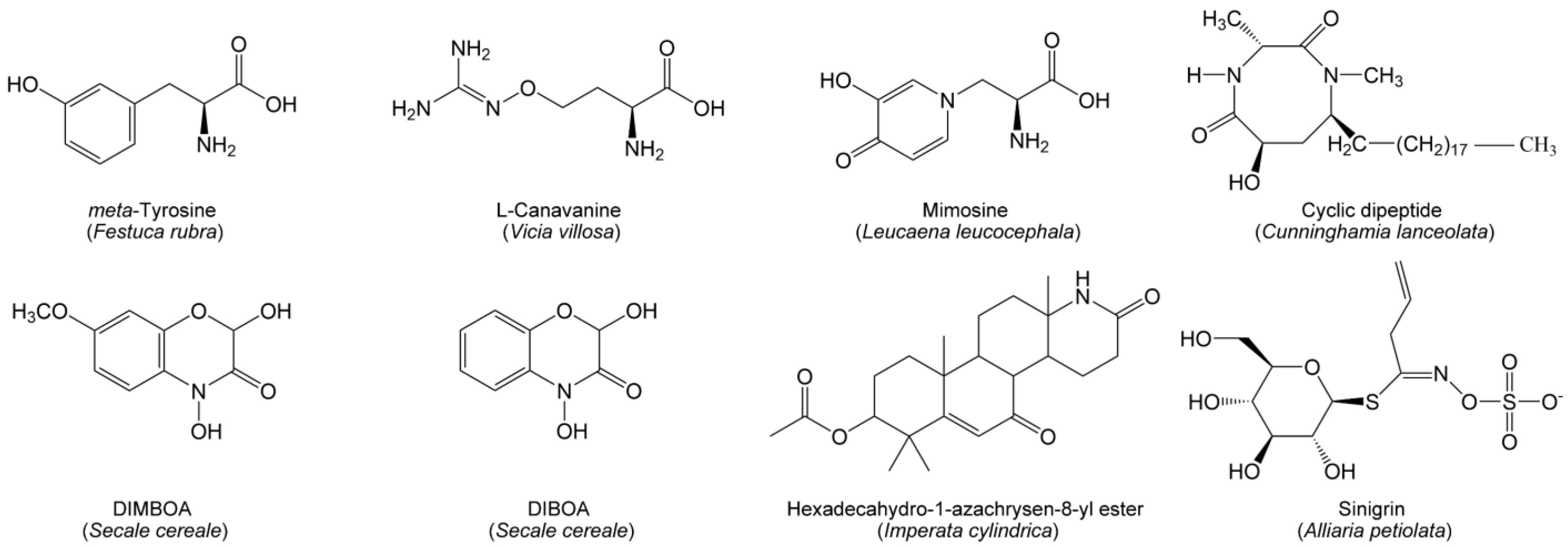
Nitrogen-containing compounds mainly include alkaloids, non-protein amino acids, benzoxazinoids and cyanogenic glycosides. Compared with phenolics and terpenoids, nitrogen-containing allelochemicals are relatively unknown. However, several specialized nitrogen-containing metabolites have been identified as allelochemicals that have significant ecological implications for grasslands and forests. Hexadecahydro-1-azachrysen-8-yl ester (Figure 3), identified as a potential alkaloid allelochemical in Imperata cylindrica, can reduce root growth and mycorrhizal colonization [137]. There are many non-protein amino acids involving allelopathic interferences with co-occurring species in grasslands (Figure 3). meta-Tyrosine of fine fescue grasses (Festuca rubra) can interfere with the root development of competing plants [138]. Mimosine of Leucaena leucocephala can retard plant growth by blocking the cell division of protoplasts and disturbing the associated enzyme activity [139]. L-Canavanine of Vicia villosa not only exerts the phytotoxic effect by disrupting the arginine metabolism in the plants but also significantly alters the microbial community composition and diversity in soil [140,141]. A novel cyclic dipeptide (6-Hydroxy-1,3-dimethyl-8-nonadecyl-[1,4]-diazocane-2,5-diketone) (Figure 3) has been found in Chinese fir; it is a highly active allelochemical to be responsible for serious replanting problems in plantations [142]. Benzoxazinoids are a class of well-known nitrogen-containing allelochemicals, among which 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one (DIMBOA) and 2,4-dihydroxy-(2H)-1,4-benzoxazin-3(4H)-one (DIBOA) (Figure 3) can be released by rye and exert strong suppression of plant growth [143,144]. Cyanogenic glycosides are specialized metabolites derived from amino acids. Sinigrin (Figure 3), as an allelochemical of cyanogenic glycosides from garlic mustard and broccoli (Brassica oleracea); it can lead to the poor establishment of North American forests by disrupting the AMF symbionts [145].
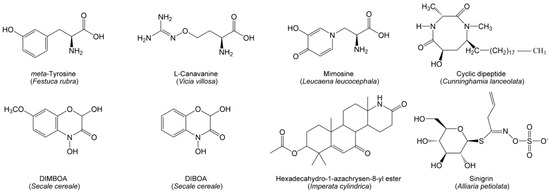
Figure 3.
Nitrogen-containing allelochemicals from herbaceous and woody species in grasslands and forests.
4.2. Identification and Detection of Allelochemicals
Allelochemicals can be either unknown or known in plants and their environments. Unknown allelochemicals have to be identified by non-targeted analysis, while known allelochemicals can be detected by targeted analysis. The identification of unknown allelochemicals first isolates pure individuals from sample components, and then the individuals can be determined and analyzed by mass spectrum, infrared spectrum and nuclear magnetic resonance [11]. Such non-targeted analysis may investigate which allelochemicals are responsible for the allelopathic interactions in a given system. Therefore, applying non-targeted analysis for identification of unknown allelochemicals has been key to understanding the ecological role of allelopathy in grasslands and forests.
Compared with the identification of unknown allelochemicals, detection of known allelochemicals is straight forward. Targeted analysis of known allelochemicals is usually conducted by means of gas or liquid chromatography coupled with tandem mass spectrometry (GC-MS/MS, LC-MS/MS). GC-MS/MS is the most preferred technique for qualitative and quantitative assessment of volatile allelochemicals. In contrast, non-volatile allelochemicals with relatively high molecular weight, mainly produced and released from root exudation and plant decomposition, can be analyzed with LC-MS/MS.
Understanding the functional significance of allelopathic plant–plant interactions and processes occurring in grasslands and forests requires accurate information about the quantity, quality and spatiotemporal dynamics of allelochemicals. The best way to trap and detect allelochemicals in vivo, in situ and real time from living plants and their environments remains a problem. Accordingly, it is warranted to develop analytical methods that are more realistic or closer to the actual field situation [146]. Phillips et al. (2008) designed an experimental system employed to trap root exudates from intact tree roots in situ. This method can account for the spatial heterogeneity and temporal dynamics of forest soils and root systems [147]. A recent study has developed quick and in situ detection of allelochemicals in Taxus soil by microdialysis combined with UPLC-MS/MS [148], providing a more finely tuned picture of allelochemical dynamics in grasslands and forests.
4.3. Activity-Concentration Relationship of Allelochemicals
The action of allelochemicals is concentration-dependent. Thus, the activity–concentration relationship is crucial for allelochemical interference, particularly for their presence at the phytotoxic level in the soil. Although soil abiotic factors such as pH, enzyme activities, organic matter and nutrient availability contribute to the change of allelopathic activity, microbial effects are undoubtedly the most crucial factor that affect allelochemicals in the soil. Soil microbes determine the below-ground transportation and intensity of allelochemicals. Accordingly, the fate and dynamics of allelochemicals are mainly attributed to soil biological processes, and potential abiotic controls. For instance, flavonoid allelochemicals have high persistence in soil because they are decomposed very slowly and last a long time in soil, which favors suppression of the emergence and growth of plants and modification of the soil’s properties, even at the low levels [149]. However, a recent study has found that soil organic carbon decreases the lifetime of flavonoids underlying plant–microbe interactions. In particular, the dissolved organic carbon in soils can repress flavonoid bioavailability and attenuates the efficacy of flavonoid-based plant–microbe communication [150].
Allelochemicals in grasslands and forests have differential concentrations, activities and categories. Most phenolics at a high concentration show allelopathic activities. Terpenoids and nitrogen-containing allelochemicals may impact plant species at a low concentration (Table 1). Accordingly, the action of phenolics involved in allelopathy requires a considerable amount of them, representing quantitative allelochemicals. In contrast, the act of terpenoids and nitrogen-containing allelochemicals greatly depends on their category rather than their amounts, representing qualitative allelochemicals. In addition, qualitative terpenoids with a diversity of allelochemicals frequently occur in annual grasslands, while quantitative phenolics with a few specialized allelochemicals occur in perennial forests. This is due to the production and release of allelochemicals in perennial forests by large-scale litter decomposition.

Table 1.
The phytotoxic level of important allelochemicals in grasslands and forests.
In many studies, the applied concentrations of allelochemicals were greater than those detected in the environment. This issue was because the concentrations of allelochemicals detected would be locally much higher in intact soils. Extractions would have diluted the allelochemicals over large soil volumes. Additionally, frequent allelochemicals provided over a long term at low concentrations can have powerful effects. Thus, even if the actual concentration of allelochemicals in the environment was still substantially lower than the necessary concentration to impact neighboring plant species, an effect would still be expected, because in the environment, there would be a constant release of allelochemicals.
5. Allelochemicals Mediate Below-Ground Interactions and Plant–Soil Feedback
5.1. Below-Ground Chemical Interactions
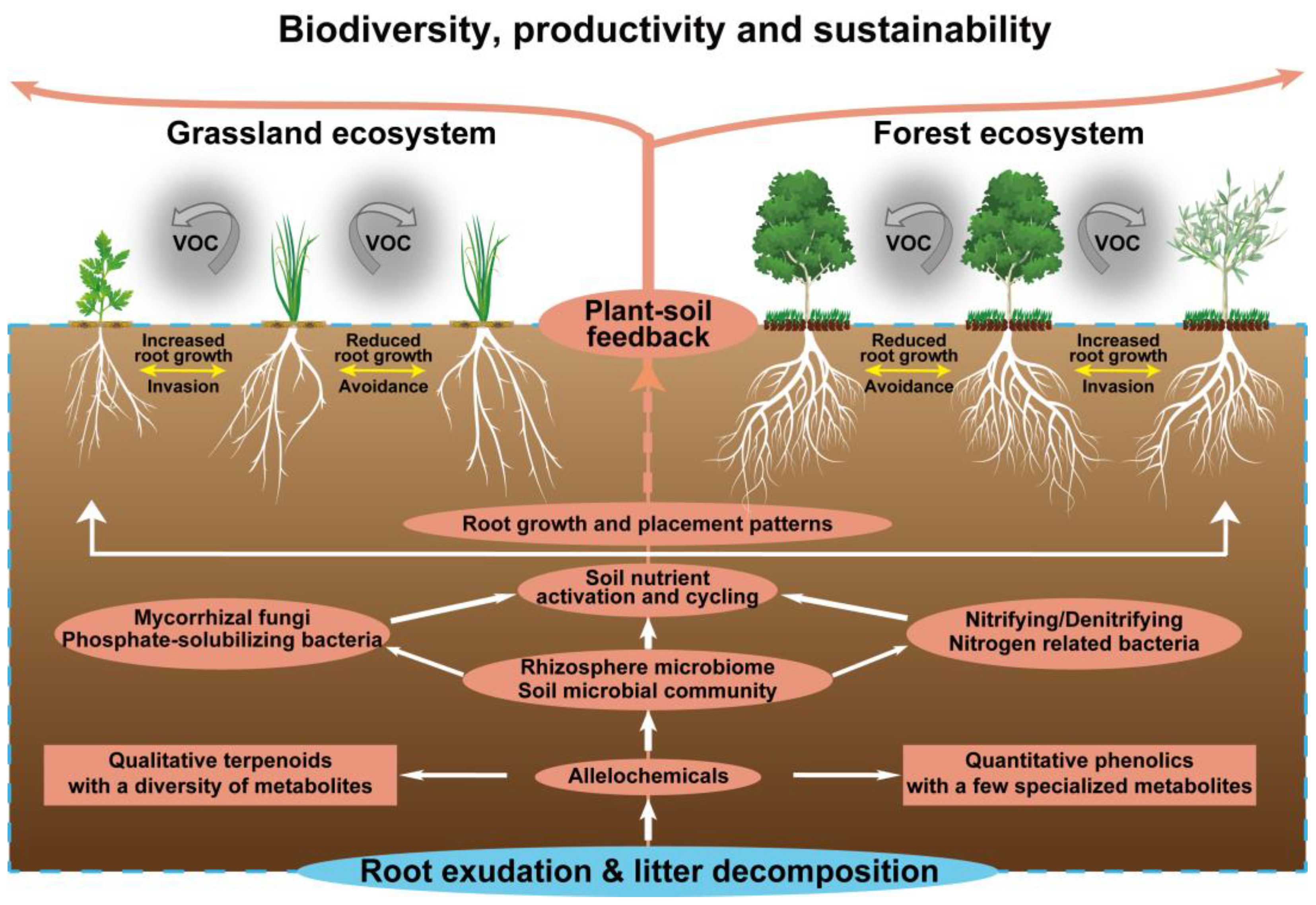
The action of allelochemicals requires their presence in the environment. Environmental factors such as temperature, light, soil nutrients and microorganisms may strengthen or alleviate the allelochemical activity. This adjustment of the action of allelochemicals in response to the environment reflects the adaptability of the allelopathic plants [9]. Most allelochemicals shift from plants into the soil following root exudation, decomposition, volatilization and leaching. These allelochemicals dispersing in the soil inevitably interact with a variety of below-ground components particularly for root placement pattern [158], soil nutrient availability, microbial community structure, mycorrhizal fungi colonization, and subsequent plant–soil feedback [8]. Therefore, the biodiversity, productivity and sustainability of grasslands and forests may be driven by allelochemical-mediated below-ground interactions and plant–soil feedback. Such a conceptual framework is outlined in Figure 4.
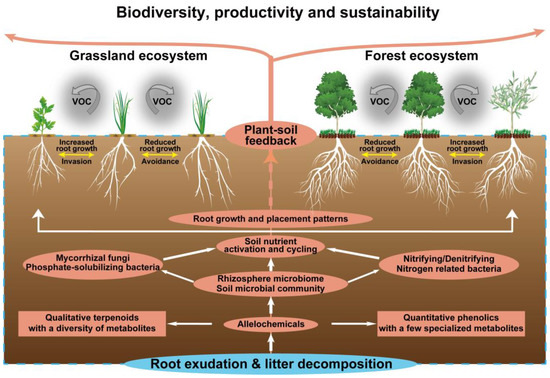
Figure 4.
Allelochemical-mediated below-ground interactions and plant–soil feedback.
The root is a vital organ interacted with soil. In response to the soil environment, a plant may place its roots in intrusive (approaching), avoidant (repelling) or unresponsive patterns [159]. Such root placement patterns, particularly for intrusive and avoidant patterns, may be driven by allelochemicals [160,161], altering below-ground ecological interactions and ultimately affecting plant performance and productivity (Figure 4). A recent study has revealed that pairwise allelopathic plant–plant interactions generate all possible combinations of intrusive, avoidant and unresponsive root placement [158]. Allelopathic species showed a general tendency toward root intrusion, while most target species adjusted root placement to avoid root-secreted allelochemicals from allelopathic species [158]. Similar allelochemical-mediated root responses have been observed in forage grass and tree species, such as avoidant response of annual ryegrass (Lolium rigidum) roots to neighboring allelopathic canola (Brassica napus) [162], and root avoidance in mixed-species plantations of Chinese fir and Michelia macclurei [93].
Allelochemical-mediated root placement patterns may contribute to plant–microbe interactions that control vital below-ground processes [158,163]. Allelochemicals are important carbon sources of soil microorganisms that determine the changes in microbial composition and community, and then affect the activation and circulation of soil nutrients (Figure 4). Cinnamic acid can significantly alter soil microbial community functional diversity and genetic diversity [164]. The hyphal branching of AMF is induced and stimulated by flavonoids, and flavonoid-associated microorganisms can colonize the roots of a very wide range of plants in order to increase nutrient uptake, especially that of P, and enhance the plant health [165]. Allelochemicals also directly participate in the activation and cycling of soil nutrients. p-Hydroxybenzoic acid can alter the form of soil N, causing Chinese fir seedlings to shift their N uptake preference from NO3− to NH4+ [166].
In grassland ecosystems, allelochemicals exuded by toxic weeds may trigger a series of changes in soil enzyme activities, pH, nutrient availability and mycorrhizal fungal colonization [12,13,167]. In particular, the exudate-induced alteration of the soil microbial community heavily promotes the expansion of toxic weeds by supplying higher rhizosphere nutrients [14,45]. Compared with the soil free of toxic weed Stellera chamaejasme, the soil infested with S. chamaejasme exhibited lower nutrition, organic matter, fungal alpha diversity, and relative abundance of AMF, but a higher abundance of pathogenic fungi [13]. Moreover, S. chamaejasme root exudates were alkalescent (pH = 9.28) and had a negative effect on the rate of mycorrhiza infection and spore density of the AMF [167]. Together, allelochemicals exuded from S. chamaejasme might increase the soil pH, reduce the soil nutrient availability, damage the AMF of other plants and recruit more pathogenic fungi, thereby posing a great threat to grassland vegetation [167].
In forest ecosystems, the roots of most tree species are extensively infested with obligately soil-borne fungi and mycorrhizas that assist plants in nutrient acquisition, pathogen resistance and carbon transportation [168,169]. Several studies found the critical role of soil microorganisms in the maintenance of Eucalyptus plantations, which may mitigate the allelopathic effect of E. grandisis leachates [170]. Specifically, a lower content of total phenolics occurred in nonsterile soils than in sterile soils when both were exposed to the E. grandisis leachates [171]. In addition, root-associated fungi probably utilize Eucalyptus allelochemicals as a carbon source to decompose, ultimately alleviating the allelopathic effect of Eucalyptus. AMF colonized in Eucalyptus roots could better protect woody species from the allelopathic interference of Eucalyptus [172,173]. Allelochemicals of Chinese fir not only exert a direct phytotoxic effect on plant roots but also indirectly disturb the soil microbial community’s composition and structure. Compared with the first rotation plantation, allelochemicals of Chinese fir probably suppress beneficial mycorrhizal species while promoting harmful fungi in the second rotation plantation, resulting in the deterioration of the soil microbial community [174]. Interestingly, the hyphal network enables allelochemicals of Juglans nigra to extend their bioactive zone and promote the effectiveness of allelopathy, indicating the importance of AMF in the movement of allelochemicals [175,176]. In another example, the leaf litter of nonmycorrhizal willows (Salix glauca and Salix brachycarpa) cannot reduce AMF colonization of understory herbaceous plants, but transplanted ectomycorrhizal willows can suppress AMF colonization of herbaceous hosts through the interaction of leaf litter and ectomycorrhizal fungi [177]. In addition, some soil fungi function as a ’shield’ to protect plant roots from the attack of allelochemicals. E. urophylla root-associated fungi have the ability to partly offset the autotoxicity of phenolic acids [172].
On the other hand, allelochemicals are able to either promote or reduce the abundance and diversity of soil microbes. The leachate of Acacia dealbata can modify the soil microbial community’s assembly, leading particularly to a prominent decline in bacterial richness and diversity in pine forest soil [109]. Likewise, extracts of Eupatorium adenophorum, especially from its leaves, can reduce bacterial richness and diversity in soils, [178]. Additionally, root exudates of V. villosa can shift the soil microbial community’s composition, particularly increasing the abundance of Firmicutes and Actinobacteria while decreasing that of Proteobacteria and Acidobacteria [141]. In contrast, the litter of P. juliflora benefits the growth and reproduction of some soil microbes and can stimulate the soil microbial biomass carbon and soil metabolic quotient [179]. Similarly, the litter of Mikania micrantha can increase soil bacterial richness, yet decrease fungal richness, which enhances immediate nutrient availability and provides ecological advantages to M. micrantha [133]. Plus, allelochemicals may facilitate the reduction of soil pathogens. Aqueous root extracts of Diplotaxis tenuifolia can inhibit the activity of Phytophthora cinnamomi [180], illustrating that D. tenuifolia can be exploited for biological control in pathogen suppression. These studies indicate a shift in bacterial diversity, or a shift from fungal richness toward bacterial richness. However, there is a lack of data on the functional shifts’ impact on the affected grasslands and forests, which calls for further studies.
5.2. Below-Ground Chemical Interactions Drive Plant–Soil Feedback
Plant–soil feedbacks (PSFs) are interactions among plants, soil organisms, and abiotic soil conditions that influence plant performance, plant species diversity and community structure, ultimately driving ecosystem processes [8]. Allelochemical-mediated below-ground interactions may alter PSFs and their potential consequences for ecosystem functioning. Allelochemicals influence PSFs through the performance of interacting species and altered community composition resulting from changes in species distributions. Allelochemicals affect plant inputs into the soil subsystem via litter and rhizodeposits. Further, root-exuded and litter-decomposed allelochemicals modulate microbial succession. These interactive effects may cause specific PSFs where the match between the species identity of living roots and litter can modify decomposition and feed back to plant nutrition [7,8].
Allelochemical-mediated below-ground interactions drive plant–soil feedback in grasslands and forests (Figure 4). In grasslands, S. chamaejasme exudes allelochemicals that incur the change of soil pH and nutrient availability, which partly contributes to the inhibition of adjacent L. chinensis [165]. Similarly, spotted knapweed can reduce the total soil carbon and nitrogen content and alter the soil elemental composition via allelochemicals, subsequently impacting soil ecosystem function and impeding the native plant growth [181]. Allelochemicals of M. micrantha can enhance the abundance of soil ammonia-oxidizing bacteria and promote the N cycling process. This plant–soil feedback by which M. micrantha improves soil N transformation facilitates its invasion in natural environments [182]. In forests, litter of Robinia pseudoacacia through allelopathy decrease understory soil nutrient availability, especially of P, and then hinder the growth of Phytolacca americana. This negative plant–soil feedback might underlie the limiting factors in the invasion of exotic plants [183]. Likewise, Juniperus virginiana exudes allelochemicals into the soil that allow the collapse or transformation of soil microbial communities, followed by inhibiting the growth of certain grass species through negative plant–soil feedback [184].
Importantly, many of these plant–soil feedback are species-specific and are greatly affected by the identity of co-occurring plant species. The presence of co-occurring plant species can alter the direction of plant–soil feedback as a result of long-lasting effects on below-ground interactions and plant responses to subsequent allelochemicals (Figure 4). In successful mixed-species tree plantations, an appropriate species can enhance autotoxic species growth through below-ground chemical interactions. For example, the presence of larch and M. macclurei can improve the establishment and productivity of autotoxic Manchurian walnut and Chinese fir in their mixed-species plantations. This is due to the fact that root exudates of larch and M. macclurei can facilitate the growth of autotoxic species and increase the degradation of allelochemicals from autotoxic species [92,93]. Accordingly, the allelochemical context alters the consequences of the below-ground ecological interactions, resulting in positive plant–soil feedback in mixed-species plantations.
6. Challenges and Opportunities
The importance of allelopathy and allelochemicals cannot be overemphasized in grasslands and forests. Recent efforts have made considerable progress toward understanding allelopathy and allelochemicals in grasslands and forests. Nevertheless, the functional consequences of allelopathy for plant communities in natural and managed grasslands and forests remain unsolved.
To confirm whether plant–plant allelopathic interactions occur in a grassland or a forest, there are four steps: (1) to find and select ecologically relevant plant species through field observation and investigation; (2) to determine that the selected plant species can produce and release allelochemicals into the environment through appropriate pathways (volatilization, leaching, root exudation or/and residues decomposition); (3) to qualify and quantify allelochemicals and their migration and transformation in soil; (4) to verify the effect of allelochemicals at effective states and concentrations on neighboring plants. These steps and processes involve multiple biotic and abiotic factors, but the focus and central driver must be allelochemicals. However, identifying when and how plant species produce and release allelochemicals is challenging.
An increasing number of studies have shown that the production of allelochemicals depends on the identity of neighboring plants. Allelopathic plants are capable of discriminating between their neighboring competitors and collaborators, adjusting their production of allelochemicals accordingly [185]. In particular, allelopathic plants may detect competing neighbors and respond by increasing allelochemicals to inhibit them, thereby maximizing their own growth. Accordingly, allelopathic interference involves two inseparable processes of plant neighbor detection and allelochemical response via signaling interactions [185], and even intraspecific kin recognition [186,187]. Particularly intriguing is intraspecific kin recognition’s contribution to interspecific allelopathy and improving plant productivity [188]. (–)-Loliolide, jasmonic acid and several chemicals are responsible for these signaling interactions [161,189,190,191]. Importantly, these signaling chemicals are ubiquitous in plant species. Such plant neighbor detection and allelochemical response, as well as their underlying mechanisms, will provide a wealth of research opportunities in grasslands and forests.
In fact, plant species occurring in grasslands and forests can take advantage of both allelochemicals and signaling chemicals released by neighbors, regulating intraspecific and interspecific interactions. Allelochemicals and signaling chemicals synergistically interact to influence plant coexistence, diversity and community structure in grasslands and forests. Plant neighbor detection and allelochemical response have been found in several mixed-species tree plantations [90,91]. Interestingly, kin recognition could even help forests regenerate. A family of firs may grow faster than unrelated trees by tracing flows of nutrients and chemical signals between trees connected by underground fungi [186,192]. Therefore, revealing the intraspecific and interspecific interactions mediated by allelochemicals and signaling chemicals in grasslands and forests can not only broaden our insight into the key processes and mechanisms of the land surface, but also enhance our ability to predict terrestrial ecosystems’ responses to global changes.
Author Contributions
Conceptualization, C.-H.K.; investigation, Y.X. and X.C.; resources, Y.X., X.C. and L.D.; writing—original draft preparation, Y.X. and C.-H.K.; writing—review and editing, C.-H.K.; visualization, Y.X., L.D. and X.C.; supervision, C.-H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors sincerely thank the anonymous referees for their constructive comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dangal, S.R.; Tian, H.; Lu, C.; Pan, S.; Pederson, N.; Hessl, A. Synergistic effects of climate change and grazing on net primary production of Mongolian grasslands. Ecosphere 2016, 7, e1274. [Google Scholar] [CrossRef]
- De Frenne, P.; Lenoir, J.; Luoto, M.; Scheffers, B.R.; Zellweger, F.; Aalto, J.; Ashcroft, M.B.; Christiansen, D.M.; Decocq, G.; De Pauw, K. Forest microclimates and climate change: Importance, drivers and future research agenda. Global Change Biol. 2021, 27, 2279–2297. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, H.; Kumar, P.; Masroor, M.; Rahaman, M.H.; Rehman, S.; Ahmed, R.; Sahana, M. Forest vulnerability to climate change: A review for future research framework. Forests 2022, 13, 917. [Google Scholar]
- Lamarque, P.; Tappeiner, U.; Turner, C.; Steinbacher, M.; Bardgett, R.D.; Szukics, U.; Schermer, M.; Lavorel, S. Stakeholder perceptions of grassland ecosystem services in relation to knowledge on soil fertility and biodiversity. Reg. Environ. Chang. 2011, 11, 791–804. [Google Scholar] [CrossRef]
- Ninan, K.N.; Inoue, M. Valuing forest ecosystem services: What we know and what we don’t. Ecol. Econ. 2013, 93, 137–149. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Evol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Bennett, J.A.; Maherali, H.; Reinhart, K.O.; Lekberg, Y.; Hart, M.M.; Klironomos, J. Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 2017, 355, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.A.; Klironomos, J. Mechanisms of plant–soil feedback: Interactions among biotic and abiotic drivers. New Phytol. 2019, 222, 91–96. [Google Scholar] [CrossRef]
- Hierro, J.L.; Callaway, R.M. The ecological importance of allelopathy. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 25–45. [Google Scholar] [CrossRef]
- Meiners, S.J.; Kong, C.; Ladwig, L.M.; Pisula, N.L.; Lang, K.A. Developing an ecological context for allelopathy. Plant Ecol. 2012, 213, 1221–1227. [Google Scholar] [CrossRef]
- Kong, C.H.; Xuan, T.D.; Khanh, T.D.; Tran, H.; Trung, N.T. Allelochemicals and signaling chemicals in plants. Molecules 2019, 24, 2737. [Google Scholar] [CrossRef]
- Sun, G.; Luo, P.; Wu, N.; Qiu, P.F.; Gao, Y.H.; Chen, H.; Shi, F.S. Stellera chamaejasme L. increases soil N availability, turnover rates and microbial biomass in an alpine meadow ecosystem on the eastern Tibetan Plateau of China. Soil Biol. Biochem. 2009, 41, 86–91. [Google Scholar] [CrossRef]
- He, W.; Detheridge, A.; Liu, Y.; Wang, L.; Wei, H.; Griffith, G.W.; Scullion, J.; Wei, Y. Variation in soil fungal composition associated with the invasion of Stellera chamaejasme L. in Qinghai–Tibet plateau grassland. Microorganisms 2019, 7, 587. [Google Scholar] [CrossRef]
- Jin, H.; Guo, H.; Yang, X.; Xin, A.; Liu, H.; Qin, B. Effect of allelochemicals, soil enzyme activity and environmental factors from Stellera chamaejasme L. on rhizosphere bacterial communities in the northern Tibetan Plateau. Arch. Agron. Soil Sci. 2022, 68, 547–560. [Google Scholar] [CrossRef]
- Soderquist, C.J. Juglone and allelopathy. J. Chem. Edu. 1973, 50, 782. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.H.; Muller, W.H.; Haines, B.L. Volatile growth inhibitors produced by aromatic shrubs. Science 1964, 143, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science 2003, 301, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Pollock, J.L.; Kogan, L.A.; Thorpe, A.S.; Holben, W.E. (±)-Catechin, a root exudate of the invasive Centaurea stoebe Lam. (spotted knapweed) exhibits bacteriostatic activity against multiple soil bacterial populations. J. Chem. Ecol. 2011, 37, 1044–1053. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Rinella, M. Comparing susceptibility of eastern and western US grasslands to competition and allelopathy from spotted knapweed [Centaurea stoebe L. subsp. micranthos (Gugler) Hayek]. Plant Ecol. 2011, 212, 821–828. [Google Scholar] [CrossRef]
- Aschehoug, E.T.; Callaway, R.M.; Newcombe, G.; Tharayil, N.; Chen, S. Fungal endophyte increases the allelopathic effects of an invasive forb. Oecologia 2014, 175, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Thorpe, A.S.; Thelen, G.C.; Diaconu, A.; Callaway, R.M. Root exudate is allelopathic in invaded community but not in native community: Field evidence for the novel weapons hypothesis. J. Ecol. 2009, 97, 641–645. [Google Scholar] [CrossRef]
- Barto, E.K.; Powell, J.R.; Cipollini, D. How novel are the chemical weapons of garlic mustard in North American forest understories? Biol. Invasions 2010, 12, 3465–3471. [Google Scholar] [CrossRef]
- Kim, Y.O.; Lee, E.J. Comparison of phenolic compounds and the effects of invasive and native species in East Asia: Support for the novel weapons hypothesis. Ecol. Res. 2011, 26, 87–94. [Google Scholar] [CrossRef]
- Pinzone, P.; Potts, D.; Pettibone, G.; Warren, R. Do novel weapons that degrade mycorrhizal mutualisms promote species invasion? Plant Ecol. 2018, 219, 539–548. [Google Scholar] [CrossRef]
- Blair, A.C.; Nissen, S.J.; Brunk, G.R.; Hufbauer, R.A. A lack of evidence for an ecological role of the putative allelochemical (±)-catechin in spotted knapweed invasion success. J. Chem. Ecol. 2006, 32, 2327–2331. [Google Scholar] [CrossRef]
- Duke, S.O.; Blair, A.C.; Dayan, F.E.; Johnson, R.D.; Meepagala, K.M.; Cook, D.; Bajsa, J. Is (−)-catechin a novel weapon of spotted knapweed (Centaurea stoebe)? J. Chem. Ecol. 2009, 35, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Yannelli, F.A.; Novoa, A.; Lorenzo, P.; Rodríguez, J.; Le Roux, J.J. No evidence for novel weapons: Biochemical recognition modulates early ontogenetic processes in native species and invasive acacias. Biol. Invasions 2020, 22, 549–562. [Google Scholar] [CrossRef]
- Renne, I.J.; Sinn, B.T.; Shook, G.W.; Sedlacko, D.M.; Dull, J.R.; Villarreal, D.; Hierro, J.L. Eavesdropping in plants: Delayed germination via biochemical recognition. J. Ecol. 2014, 102, 86–94. [Google Scholar] [CrossRef]
- Kalisz, S.; Kivlin, S.N.; Bialic-Murphy, L. Allelopathy is pervasive in invasive plants. Biol. Invasions 2021, 23, 367–371. [Google Scholar] [CrossRef]
- Ning, L.; Yu, F.H.; van Kleunen, M. Allelopathy of a native grassland community as a potential mechanism of resistance against invasion by introduced plants. Biol Invasions 2016, 18, 3481–3493. [Google Scholar] [CrossRef]
- Akiyama, T.; Kawamura, K. Grassland degradation in China: Methods of monitoring, management and restoration. Grassl. Sci. 2007, 53, 1–17. [Google Scholar] [CrossRef]
- Gang, C.; Zhou, W.; Chen, Y.; Wang, Z.; Sun, Z.; Li, J.; Qi, J.; Odeh, I. Quantitative assessment of the contributions of climate change and human activities on global grassland degradation. Environ. Earth. Sci. 2014, 72, 4273–4282. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Bullock, J.M.; Lavorel, S.; Manning, P.; Schaffner, U.; Ostle, N.; Chomel, M.; Durigan, G.; Fry, E.L.; Johnson, D.; et al. Combatting global grassland degradation. Nat. Rev. Earth Environ. 2021, 2, 720–735. [Google Scholar] [CrossRef]
- Li, X.F.; Wang, J.; Huang, D.; Wang, L.X.; Wang, K. Allelopathic potential of Artemisia frigida and successional changes of plant communities in the northern China steppe. Plant Soil 2011, 341, 383–398. [Google Scholar] [CrossRef]
- Zuo, Z.J.; Zhang, R.M.; Gao, P.J.; Wen, G.S.; Hou, P.; Gao, Y. Allelopathic effects of Artemisia frigida Willd. on growth of pasture grasses in Inner Mongolia, China. Biochem. Syst. Ecol. 2011, 39, 377–383. [Google Scholar]
- Guo, H.; Cui, H.; Jin, H.; Yan, Z.; Ding, L.; Qin, B. Potential allelochemicals in root zone soils of Stellera chamaejasme L. and variations at different geographical growing sites. Plant Growth Regul. 2015, 77, 335–342. [Google Scholar] [CrossRef]
- Holechek, J.L. Do most livestock losses to poisonous plants result from “poor” range management? J Range Manag. Arch. 2002, 55, 270–276. [Google Scholar] [CrossRef]
- Tokarnia, C.H.; Döbereiner, J.; Peixoto, P.V. Poisonous plants affecting livestock in Brazil. Toxicon 2002, 40, 1635–1660. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, S.; Liu, K.; Li, X.; Huang, D.; Wang, K. The allelopathic effect of Potentilla acaulis on the changes of plant community in grassland, northern China. Ecol. Res. 2015, 30, 41–47. [Google Scholar] [CrossRef]
- Goldschmidt, F.; Regoes, R.R.; Johnson, D.R. Metabolite toxicity slows local diversity loss during expansion of a microbial cross-feeding community. ISME J. 2018, 12, 136–144. [Google Scholar] [CrossRef]
- Humphries, T.; Florentine, S.K.; Dowling, K.; Turville, C.; Sinclair, S. Weed management for landscape scale restoration of global temperate grasslands. Land Degrad. Dev. 2021, 32, 1090–1102. [Google Scholar] [CrossRef]
- Ma, L.; Wu, H.; Bai, R.; Zhou, L.; Yuan, X.; Hou, D. Phytotoxic effects of Stellera chamaejasme L. root extract. Afri. J. Agric. Res. 2011, 6, 1170–1176. [Google Scholar]
- Wang, W.; Jia, T.; Qi, T.; Li, S.; Degen, A.A.; Han, J.; Bai, Y.; Zhang, T.; Qi, S.; Huang, M. Root exudates enhanced rhizobacteria complexity and microbial carbon metabolism of toxic plants. iScience 2022, 25, 105243. [Google Scholar] [CrossRef]
- Jiang, Z.; Tanaka, T.; Sakamoto, T.; Kouno, I.; Duan, J.; Zhou, R. Biflavanones, diterpenes, and coumarins from the roots of Stellera chamaejasme L. Chem. Pharm. Bull. 2002, 50, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Guo, H.; Yang, J.; Liu, Q.; Jin, H.; Xu, R.; Cui, H.; Qin, B. Phytotoxic flavonoids from roots of Stellera chamaejasme L.(Thymelaeaceae). Phytochemistry 2014, 106, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, W.; Zuo, Z.; Li, R.; Wu, J.; Gao, Y. Inhibition effects of volatile organic compounds from Artemisia frigida Willd. on the pasture grass intake by lambs. Small Ruminant Res. 2014, 121, 248–254. [Google Scholar] [CrossRef]
- Lu, H.; Wang, S.S.; Zhou, Q.W.; Zhao, Y.N.; Zhao, B.Y. Damage and control of major poisonous plants in the western grasslands of China—A review. Rangel. J. 2012, 34, 329–339. [Google Scholar] [CrossRef]
- Zhigzhitzhapova, S.V.; Randalova, T.E.; Radnaeva, L.D.; Dylenova, E.P.; Chen, S.; Zhang, F. Chemical composition of essentials oils of Artemisia frigida Willd.(Asteraceae) grown in the North and Central Asia. J. Essent. Oil Bear Pl. 2017, 20, 915–926. [Google Scholar] [CrossRef]
- Zhang, R.M.; Zuo, Z.J.; Gao, P.J.; Hou, P.; Wen, G.S.; Gao, Y. Allelopathic effects of VOCs of Artemisia frigida Willd. on the regeneration of pasture grasses in Inner Mongolia. J. Arid Environ. 2012, 87, 212–218. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Yang, Q.; Wang, T.; Zhang, Z.; Liu, J.; Shi, M.; Ping, X. The impact of grazing intensity on the allelopathic effect of Artemisia frigida in a temperate grassland in northeasssrn China. Flora 2022, 288, 152005. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, R.; Yan, Z.; Jin, H.; Cui, H.; Lu, L.; Zhang, D.; Qin, B. Phytotoxic allelochemicals from roots and root exudates of Trifolium pratense. J. Agric. Food Chem. 2013, 61, 6321–6327. [Google Scholar] [CrossRef] [PubMed]
- Braun, R.C.; Patton, A.J.; Watkins, E.; Koch, P.L.; Anderson, N.P.; Bonos, S.A.; Brilman, L.A. Fine fescues: A review of the species, their improvement, production, establishment, and management. Crop Sci. 2020, 60, 1142–1187. [Google Scholar] [CrossRef]
- Tabaglio, V.; Marocco, A.; Schulz, M. Allelopathic cover crop of rye for integrated weed control in sustainable agroecosystems. Ital. J. Agron. 2013, 8, e5. [Google Scholar] [CrossRef]
- Schulz, M.; Marocco, A.; Tabaglio, V.; Macias, F.A.; Molinillo, J.M. Benzoxazinoids in rye allelopathy-from discovery to application in sustainable weed control and organic farming. J. Chem. Ecol. 2013, 39, 154–174. [Google Scholar] [CrossRef]
- Serajchi, M.; Schellenberg, M.P.; Lamb, E.G. The potential of seven native North American forage species to suppress weeds through allelopathy. Can. J. Plant Sci. 2017, 97, 881–890. [Google Scholar]
- Zhao, H.H.; Kong, C.H.; Xu, X.H. Herbicidal efficacy and ecological safety of an allelochemical-based benzothiazine derivative. Pest Manag. Sci. 2019, 75, 2690–2697. [Google Scholar] [CrossRef]
- Shang, Z.; Hou, Y.; Li, F.; Guo, C.; Jia, T.; Degen, A.A.; White, A.; Ding, L.; Long, R. Inhibitory action of allelochemicals from Artemisia nanschanica to control Pedicularis kansuensis, an annual weed of alpine grasslands. Aust. J. Bot. 2017, 65, 305–314. [Google Scholar] [CrossRef]
- Adhikari, L.; Mohseni-Moghadam, M.; Missaoui, A. Allelopathic effects of cereal rye on weed suppression and forage yield in Alfalfa. Amer. J. Plant Sci. 2018, 9, 685. [Google Scholar] [CrossRef]
- Chon, S.U.; Jennings, J.A.; Nelson, C.J. Alfalfa (Medicago sativa L.) autotoxicity: Current status. Allelopathy J. 2006, 18, 57–80. [Google Scholar]
- Ghimire, B.K.; Ghimire, B.; Yu, C.Y.; Chung, I. Allelopathic and autotoxic effects of Medicago sativa—Derived allelochemicals. Plants 2019, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Z.; Wang, Z.; Pang, W.; Zhang, L.; Wen, Z.; Zhao, Y.; Sun, J.; Wang, Z.; Yang, C. Effects of autotoxicity and allelopathy on seed germination and seedling growth in Medicago truncatula. Front. Plant Sci. 2022, 13, 908426. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, S.; Li, X.; Li, C.; Zhang, C.; Kang, W.; Yin, G. Effects of autotoxicity on alfalfa (Medicago sativa): Seed germination, oxidative damage and lipid peroxidation of seedlings. Agronomy 2021, 11, 1027. [Google Scholar] [CrossRef]
- Li, Q.; Song, Y.; Li, G.; Yu, P.; Wang, P.; Zhou, D. Grass-legume mixtures impact soil N, species recruitment, and productivity in temperate steppe grassland. Plant Soil 2015, 394, 271–285. [Google Scholar] [CrossRef]
- Mischkolz, J.M.; Schellenberg, M.P.; Lamb, E.G. Assembling productive communities of native grass and legume species: Finding the right mix. Appl. Veg. Sci. 2016, 19, 111–121. [Google Scholar] [CrossRef]
- Quan, X.; Qiao, Y.; Chen, M.; Duan, Z.; Shi, H. Comprehensive evaluation of the allelopathic potential of Elymus nutans. Ecol. Evol. 2021, 11, 12389–12400. [Google Scholar] [CrossRef]
- Rakoczy Trojanowska, M.; Święcicka, M.; Bakera, B.; Kowalczyk, M.; Stochmal, A.; Bolibok, L. Cocultivating rye with berseem clover affects benzoxazinoid production and expression of related genes. Crop Sci. 2020, 60, 3228–3246. [Google Scholar] [CrossRef]
- Tsubo, M.; Nishihara, E.; Nakamatsu, K.; Cheng, Y.; Shinoda, M. Plant volatiles inhibit restoration of plant species communities in dry grassland. Basic Appl. Ecol. 2012, 13, 76–84. [Google Scholar] [CrossRef]
- Yu, R.P.; Li, X.X.; Xiao, Z.H.; Lambers, H.; Li, L. Phosphorus facilitation and covariation of root traits in steppe species. New Phytol. 2020, 226, 1285–1298. [Google Scholar] [CrossRef]
- Wu, M.; Yao, L.; Ai, X.; Zhu, J.; Zhu, Q.; Wang, J.; Huang, X.; Hong, J. The reproductive characteristics of core germplasm in a native Metasequoia glyptostroboides population. Biodiver. Sci. 2020, 28, 303. [Google Scholar]
- Xu, L.; Yao, L.; Ai, X.; Guo, Q.; Wang, S.; Zhou, D.; Deng, C.; Ai, X. Litter autotoxicity limits natural regeneration of Metasequoia glyptostroboides. New Forest 2022. [Google Scholar] [CrossRef]
- Huang, X.; Chen, J.; Liu, J.; Li, J.; Wu, M.; Tong, B. Autotoxicity hinders the natural regeneration of Cinnamomum migao HW Li in Southwest China. Forests 2019, 10, 919. [Google Scholar] [CrossRef]
- Lang, T.; Wei, P.; Chen, X.; Fu, Y.; Tam, N.F.; Hu, Z.; Chen, Z.; Li, F.; Zhou, H. Microcosm study on allelopathic effects of leaf litter leachates and purified condensed tannins from Kandelia obovata on germination and growth of Aegiceras corniculatum. Forests 2021, 12, 1000. [Google Scholar] [CrossRef]
- Hane, E.N. Indirect effects of beech bark disease on sugar maple seedling survival. Can. J. Forest Res. 2003, 33, 807–813. [Google Scholar] [CrossRef]
- Collin, A.; Messier, C.; Kembel, S.W.; Bélanger, N. Low light availability associated with American beech is the main factor for reduced sugar maple seedling survival and growth rates in a hardwood forest of Southern Quebec. Forests 2017, 8, 413. [Google Scholar] [CrossRef]
- Taylor, K.; Rowland, A.P.; Jones, H.E. Molinia caerulea (L.) Moench. J. Ecol. 2001, 89, 126–144. [Google Scholar] [CrossRef]
- Fernandez, M.; Malagoli, P.; Gallet, C.; Fernandez, C.; Vernay, A.; Ameglio, T.; Balandier, P. Investigating the role of root exudates in the interaction between oak seedlings and purple moor grass in temperate forest. Forest Ecol. Manag. 2021, 491, 119175. [Google Scholar] [CrossRef]
- Nilsen, E.T.; Huebner, C.D.; Carr, D.E.; Bao, Z. Interaction between Ailanthus altissima and native Robinia pseudoacacia in early succession: Implications for forest management. Forests 2018, 9, 221. [Google Scholar] [CrossRef]
- Demeter, A.; Saláta, D.; Tormáné Kovács, E.; Szirmai, O.; Trenyik, P.; Meinhardt, S.; Rusvai, K.; Verbényiné Neumann, K.; Schermann, B.; Szegleti, Z. Effects of the Invasive tree species Ailanthus altissima on the floral diversity and soil properties in the Pannonian region. Land 2021, 10, 1155. [Google Scholar] [CrossRef]
- Stone, R. Nursing China’s ailing forests back to health. Science 2009, 325, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.A. Mitigating biodiversity concerns in Eucalyptus plantations located in South China. J. Biosci. Med. 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Sasikumar, K.; Vijayalakshmi, C.; Parthiban, K.T. Allelopathic effects of four Eucalyptus species on redgram (Cajanus cajan L.). J. Trop. Agric. 2006, 39, 134–138. [Google Scholar]
- Ahmed, R.; Hoque, A.; Hossain, M.K. Allelopathic effects of leaf litters of Eucalyptus camaldulensis on some forest and agricultural crops. J. Forestry Res. 2008, 19, 19–24. [Google Scholar] [CrossRef]
- Chapuis-Lardy, L.; Contour-Ansel, D.; Bernhard-Reversat, F. High-performance liquid chromatography of water-soluble phenolics in leaf litter of three Eucalyptus hybrids (Congo). Plant Sci. 2002, 163, 217–222. [Google Scholar] [CrossRef]
- Song, Q.; Qin, F.; He, H.; Wang, H.; Yu, S. Allelopathic potential of rain leachates from Eucalyptus urophylla on four tree species. Agroforest Syst. 2019, 93, 1307–1318. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Chen, Y.; Zhao, J.; Wan, S.; Lin, Y.; Fu, S. Effects of Eucalyptus litter and roots on the establishment of native tree species in Eucalyptus plantations in South China. Forest Ecol. Manag. 2016, 375, 76–83. [Google Scholar] [CrossRef]
- Qin, F.; Liu, S.; Yu, S. Effects of allelopathy and competition for water and nutrients on survival and growth of tree species in Eucalyptus urophylla plantations. Forest Ecol. Manag. 2018, 424, 387–395. [Google Scholar] [CrossRef]
- Chen, L.; Wang, S. Allelopathic behaviour of Chinese fir from plantations of different ages. Forestry 2013, 86, 225–230. [Google Scholar] [CrossRef]
- Chen, L.; Wang, S.; Wang, P.; Kong, C. Autoinhibition and soil allelochemical (cyclic dipeptide) levels in replanted Chinese fir (Cunninghamia lanceolata) plantations. Plant Soil 2014, 374, 793–801. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J.; Cowie, A.L.; Vanclay, J.K. Mixed-species plantations of Eucalyptus with nitrogen-fixing trees: A review. Forest Ecol. Manag. 2006, 233, 211–230. [Google Scholar] [CrossRef]
- Yang, L.X.; Wang, P.; Kong, C.H. Effect of larch (Larix gmelini Rupr.) root exudates on Manchurian walnut (Juglans mandshurica Maxim.) growth and soil juglone in a mixed-species plantation. Plant Soil 2010, 329, 249–258. [Google Scholar] [CrossRef]
- Xia, Z.C.; Kong, C.H.; Chen, L.C.; Wang, P.; Wang, S.L. A broadleaf species enhances an autotoxic conifers growth through belowground chemical interactions. Ecology 2016, 97, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Hashoum, H.; Santonja, M.; Gauquelin, T.; Saatkamp, A.; Gavinet, J.; Greff, S.; Lecareux, C.; Fernandez, C.; Bousquet-Mélou, A. Biotic interactions in a Mediterranean oak forest: Role of allelopathy along phenological development of woody species. Eur. J. Forest Res. 2017, 136, 699–710. [Google Scholar] [CrossRef]
- Khaled, A.; Sleiman, M.; Goupil, P.; Richard, C. Phytotoxic effect of Macerates and Mulches from Cupressus leylandii leaves on clover and cress: Role of chemical composition. Forests 2020, 11, 1177. [Google Scholar] [CrossRef]
- Lorenzo, P.; Palomera-Pérez, A.; Reigosa, M.J.; González, L. Allelopathic interference of invasive Acacia dealbata link on the physiological parameters of native understory species. Plant Ecol. 2011, 212, 403–412. [Google Scholar] [CrossRef]
- Constán-Nava, S.; Soliveres, S.; Torices, R.; Serra, L.; Bonet, A. Direct and indirect effects of invasion by the alien tree Ailanthus altissima on riparian plant communities and ecosystem multifunctionality. Biol. Invasions 2015, 17, 1095–1108. [Google Scholar] [CrossRef]
- Warren, R.J.; Labatore, A.; Candeias, M. Allelopathic invasive tree (Rhamnus cathartica) alters native plant communities. Plant Ecol. 2017, 218, 1233–1241. [Google Scholar] [CrossRef]
- de Las Heras, P.; Medina-Villar, S.; Pérez-Corona, M.E.; Vázquez-de-Aldana, B.R. Leaf litter age regulates the effect of native and exotic tree species on understory herbaceous vegetation of riparian forests. Basic Appl. Ecol. 2020, 48, 11–25. [Google Scholar] [CrossRef]
- Huang, W.; Hu, H.; Hu, T.; Chen, H.; Wang, Q.; Chen, G.; Tu, L. Impact of aqueous extracts of Cinnamomum septentrionale leaf litter on the growth and photosynthetic characteristics of Eucalyptus grandis seedlings. New Forest 2015, 46, 561–576. [Google Scholar] [CrossRef]
- Muturi, G.M.; Poorter, L.; Bala, P.; Mohren, G.M.J. Unleached Prosopis litter inhibits germination but leached stimulates seedling growth of dry woodland species. J. Arid Environ. 2017, 138, 44–50. [Google Scholar] [CrossRef]
- Orr, S.P.; Rudgers, J.A.; Clay, K. Invasive plants can inhibit native tree seedlings: Testing potential allelopathic mechanisms. Plant Ecol. 2005, 181, 153–165. [Google Scholar] [CrossRef]
- Sayer, E.J. Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol. Rev. 2006, 81, 1–31. [Google Scholar] [CrossRef]
- Meiners, S.J. Functional correlates of allelopathic potential in a successional plant community. Plant Ecol. 2014, 215, 661–672. [Google Scholar] [CrossRef]
- Da Silva, E.R.; Da Silveira, L.H.R.; Overbeck, G.E.; Soares, G.L.G. Inhibitory effects of Eucalyptus saligna leaf litter on grassland species: Physical versus chemical factors. Plant Ecol. Divers. 2018, 11, 55–67. [Google Scholar] [CrossRef]
- Bonanomi, G.; Incerti, G.; Barile, E.; Capodilupo, M.; Antignani, V.; Mingo, A.; Lanzotti, V.; Scala, F.; Mazzoleni, S. Phytotoxicity, not nitrogen immobilization, explains plant litter inhibitory effects: Evidence from solid-state13C NMR spectroscopy. New Phytol. 2011, 191, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.A.; Parker, I.M.; Gilbert, G.S. Allelopathy: A tool for weed management in forest restoration. Plant Ecol. 2012, 213, 1975–1989. [Google Scholar] [CrossRef]
- Matuda, Y.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathy and allelopathic substances of fossil tree species Metasequoia glyptostroboides. Agronomy 2022, 12, 83. [Google Scholar] [CrossRef]
- Lorenzo, P.; Pereira, C.S.; Rodríguez-Echeverría, S. Differential impact on soil microbes of allelopathic compounds released by the invasive Acacia dealbata Link. Soil Biol. Biochem. 2013, 57, 156–163. [Google Scholar] [CrossRef]
- Bao, Z.; Nilsen, E.T. Interactions between seedlings of the invasive tree Ailanthus altissima and the native tree Robinia pseudoacacia under low nutrient conditions. J. Plant Interact. 2015, 10, 173–184. [Google Scholar] [CrossRef]
- Metlen, K.L.; Aschehoug, E.T.; Callaway, R.M. Competitive outcomes between two exotic invaders are modified by direct and indirect effects of a native conifer. Oikos 2013, 122, 632–640. [Google Scholar] [CrossRef]
- Kaur, R.; Callaway, R.M.; Inderjit. Soils and the conditional allelopathic effects of a tropical invader. Soil Biol. Biochem. 2014, 78, 316–325. [Google Scholar] [CrossRef]
- Kaur, R.; Gonzáles, W.L.; Llambi, L.D.; Soriano, P.J.; Callaway, R.M.; Rout, M.E.; Gallaher, T.J. Community impacts of Prosopis Juliflora invasion: Biogeographic and congeneric comparisons. PLoS ONE 2012, 7, e44966. [Google Scholar] [CrossRef] [PubMed]
- Gunarathne, R.; Perera, G. Does the invasion of Prosopis juliflora cause the die-back of the native Manilkara hexandra in seasonally dry tropical forests of Sri Lanka. Trop. Ecol. 2016, 57, 475–488. [Google Scholar]
- Nuzzo, V. Invasion pattern of herb garlic mustard (Alliaria petiolata) in high quality forests. Biol. Invasions 1999, 1, 169–179. [Google Scholar] [CrossRef]
- Callaway, R.M.; Cipollini, D.; Barto, K.; Thelen, G.C.; Hallett, S.G.; Prati, D.; Stinson, K.; Klironomos, J. Novel weapons: Invasive plant suppresses fungal mutualists in America but not in its native Europe. Ecology 2008, 89, 1043–1055. [Google Scholar] [CrossRef]
- Hale, A.N.; Lapointe, L.; Kalisz, S. Invader disruption of belowground plant mutualisms reduces carbon acquisition and alters allocation patterns in a native forest herb. New Phytol. 2016, 209, 542–549. [Google Scholar] [CrossRef]
- Lankau, R.A.; Nuzzo, V.; Spyreas, G.; Davis, A.S. Evolutionary limits ameliorate the negative impact of an invasive plant. Proc. Natl. Acad. Sci. USA 2009, 106, 15362–15367. [Google Scholar] [CrossRef]
- Barto, E.K.; Antunes, P.M.; Stinson, K.; Koch, A.M.; Klironomos, J.N.; Cipollini, D. Differences in arbuscular mycorrhizal fungal communities associated with sugar maple seedlings in and outside of invaded garlic mustard forest patches. Biol. Invasions 2011, 13, 2755–2762. [Google Scholar] [CrossRef]
- Lankau, R.A. Coevolution between invasive and native plants driven by chemical competition and soil biota. Proc. Natl. Acad. Sci. USA 2012, 109, 11240–11245. [Google Scholar] [CrossRef]
- Huang, F.; Lankau, R.; Peng, S. Coexistence via coevolution driven by reduced allelochemical effects and increased tolerance to competition between invasive and native plants. New Phytol. 2018, 218, 357–369. [Google Scholar] [CrossRef]
- Liu, J.G.; Liao, H.X.; Chen, B.M.; Peng, S.L. Do the phenolic acids in forest soil resist the exotic plant invasion. Allelopathy J. 2017, 41, 167–175. [Google Scholar] [CrossRef]
- Takemura, T.; Kamo, T.; Sakuno, E.; Hiradate, S.; Fujii, Y. Discovery of coumarin as the predominant allelochemical in Gliricidia sepium. J. Trop. For. Sci. 2013, 25, 268–272. [Google Scholar]
- Yan, Z.; Wang, D.; Cui, H.; Zhang, D.; Sun, Y.; Jin, H.; Li, X.; Yang, X.; Guo, H.; He, X. Phytotoxicity mechanisms of two coumarin allelochemicals from Stellera chamaejasme in lettuce seedlings. Acta Physiol. Plant 2016, 38, 1–10. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, S.; Shi, H.; Zhao, K.; Wang, J.; Liu, Y.; Liu, X.; Wang, W. Physiological and biochemical mechanisms mediated by allelochemical isoliquiritigenin on the growth of lettuce seedlings. Plants 2020, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ye, Y.; Huang, H.; Dong, L. Kaempferol-3-O-β-D-glucoside, a potential allelochemical isolated from Solidago Canadensis. Allelopathy J. 2011, 28, 259–266. [Google Scholar]
- Perry, L.G.; Johnson, C.; Alford, É.R.; Vivanco, J.M.; Paschke, M.W. Screening of grassland plants for restoration after spotted knapweed invasion. Restor. Ecol. 2005, 13, 725–735. [Google Scholar] [CrossRef]
- Weir, T.L.; Bais, H.P.; Stull, V.J.; Callaway, R.M.; Thelen, G.C.; Ridenour, W.M.; Bhamidi, S.; Stermitz, F.R.; Vivanco, J.M. Oxalate contributes to the resistance of Gaillardia grandiflora and Lupinus sericeus to a phytotoxin produced by Centaurea maculosa. Planta 2006, 223, 785–795. [Google Scholar] [CrossRef]
- Willis, R.J. Juglans spp., juglone and allelopathy. Allelopathy J. 2000, 7, 1–5. [Google Scholar]
- Strugstad, M.; Despotovski, S. A summary of extraction, synthesis, properties, and potential uses of juglone: A literature review. J. Ecosyst. Manag. 2012, 13, 72–86. [Google Scholar] [CrossRef]
- Bai, L.; Wang, W.; Hua, J.; Guo, Z.; Luo, S. Defensive functions of volatile organic compounds and essential oils from northern white-cedar in China. BMC Plant Biol. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Santonja, M.; Bousquet Mélou, A.; Greff, S.; Ormeño, E.; Fernandez, C. Allelopathic effects of volatile organic compounds released from Pinus halepensis needles and roots. Ecol. Evol. 2019, 9, 8201–8213. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Le Roux, J.J.; Zhao, M.; Li, W. Mikania sesquiterpene lactones enhance soil bacterial diversity and fungal and bacterial activities. Biol. Invasions 2022, 25, 1–14. [Google Scholar] [CrossRef]
- Braine, J.W.; Curcio, G.R.; Wachowicz, C.M.; Hansel, F.A. Allelopathic effects of Araucaria angustifolia needle extracts in the growth of Lactuca sativa seeds. J. Forest Res. Jpn. 2012, 17, 440–445. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kimura, F.; Ohno, O.; Suenaga, K. Involvement of allelopathy in inhibition of understory growth in red pine forests. J. Plant Physiol. 2017, 218, 66–73. [Google Scholar] [CrossRef]
- Wang, C.; Chen, H.; Li, T.; Weng, J.; Jhan, Y.; Lin, S.; Chou, C. The role of pentacyclic triterpenoids in the allelopathic effects of Alstonia scholaris. J. Chem. Ecol. 2014, 40, 90–98. [Google Scholar] [CrossRef]
- Hagan, D.L.; Jose, S.; Lin, C. Allelopathic exudates of cogongrass (Imperata cylindrica): Implications for the performance of native pine savanna plant species in the southeastern US. J. Chem. Ecol. 2013, 39, 312–322. [Google Scholar] [CrossRef]
- Bertin, C.; Weston, L.A.; Huang, T.; Jander, G.; Owens, T.; Meinwald, J.; Schroeder, F.C. Grass roots chemistry: meta-Tyrosine, an herbicidal nonprotein amino acid. Proc. Natl. Acad. Sci. USA 2007, 104, 16964–16969. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy and allelochemicals of Leucaena leucocephala as an invasive plant species. Plants 2022, 11, 1672. [Google Scholar] [CrossRef]
- Nakajima, N.; Hiradate, S.; Fujii, Y. Plant growth inhibitory activity of L-canavanine and its mode of action. J. Chem. Ecol. 2001, 27, 19–31. [Google Scholar] [CrossRef]
- Mardani-Korrani, H.; Nakayasu, M.; Yamazaki, S.; Aoki, Y.; Kaida, R.; Motobayashi, T.; Kobayashi, M.; Ohkama-Ohtsu, N.; Oikawa, Y.; Sugiyama, A. L-Canavanine, a root exudate from hairy vetch (Vicia villosa) drastically affecting the soil microbial community and metabolite pathways. Front. Microbiol. 2021, 12, 701796. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.H.; Chen, L.C.; Xu, X.H.; Wang, P.; Wang, S.L. Allelochemicals and activities in a replanted Chinese fir (Cunninghamia lanceolata (Lamb.) Hook) tree ecosystem. J. Agric. Food Chem. 2008, 56, 11734–11739. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Shudo, K. Chemistry of biologically active benzoxazinoids. Phytochemistry 1996, 43, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, H.M. Hydroxamic acids derived from 2-hydroxy-2H-1,4- benzoxazin-3(4H)-one: Key defense chemicals of cereals. J. Agric. Food Chem. 2009, 57, 1677–1696. [Google Scholar] [CrossRef]
- Lankau, R.A.; Strauss, S.Y. Mutual feedbacks maintain both genetic and species diversity in a plant community. Science 2007, 317, 1561–1563. [Google Scholar] [CrossRef]
- Oburger, E.; Schmidt, H. New methods to unravel rhizosphere processes. Trends Plant Sci. 2016, 21, 243–255. [Google Scholar] [CrossRef]
- Phillips, R.P.; Erlitz, Y.; Bier, R.; Bernhardt, E.S. New approach for capturing soluble root exudates in forest soils. Funct. Ecol. 2008, 22, 990–999. [Google Scholar] [CrossRef]
- Qiao, B.; Nie, S.; Li, Q.; Majeed, Z.; Cheng, J.; Yuan, Z.; Li, C.; Zhao, C. Quick and in situ detection of different polar allelochemicals in Taxus soil by microdialysis combined with UPLC-MS/MS. J. Agric. Food Chem. 2022, 70, 16435–16445. [Google Scholar] [CrossRef]
- Sosa, T.; Valares, C.; Alías, J.C.; Chaves Lobón, N. Persistence of flavonoids in Cistus ladanifer soils. Plant Soil 2010, 337, 51–63. [Google Scholar] [CrossRef]
- Del Valle, I.; Webster, T.M.; Cheng, H.; Thies, J.E.; Kessler, A.; Miller, M.K.; Ball, Z.T.; MacKenzie, K.R.; Masiello, C.A.; Silberg, J.J. Soil organic matter attenuates the efficacy of flavonoid-based plant-microbe communication. Sci. Adv. 2020, 6, x8254. [Google Scholar] [CrossRef]
- Hoang, L.; Joo, G.; Kim, W.; Jeon, S.; Choi, S.; Kim, J.; Rhee, I.; Hur, J.; Song, K. Growth inhibitors of lettuce seedlings from Bacillus cereus EJ-121. Plant Growth Regul. 2005, 47, 149–154. [Google Scholar] [CrossRef]
- Tuyen, P.T.; Xuan, T.D.; Tu Anh, T.T.; Mai Van, T.; Ahmad, A.; Elzaawely, A.A.; Khanh, T.D. Weed suppressing potential and isolation of potent plant growth inhibitors from Castanea crenata Sieb. et Zucc. Molecules 2018, 23, 345. [Google Scholar] [CrossRef]
- Ida, N.; Iwasaki, A.; Teruya, T.; Suenaga, K.; Kato-Noguchi, H. Tree fern Cyathea lepifera may survive by its phytotoxic property. Plants 2019, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Reigosa, M.J.; Pazos-Malvido, E. Phytotoxic effects of 21 plant secondary metabolites on Arabidopsis thaliana germination and root growth. J. Chem. Ecol. 2007, 33, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Hiradate, S.; Ohse, K.; Furubayashi, A.; Fujii, Y. Quantitative evaluation of allelopathic potentials in soils: Total activity approach. Weed Sci. 2010, 58, 258–264. [Google Scholar] [CrossRef]
- Kang, G.; Mishyna, M.; Appiah, K.S.; Yamada, M.; Takano, A.; Prokhorov, V.; Fujii, Y. Screening for plant volatile emissions with allelopathic activity and the identification of L-Fenchone and 1, 8-Cineole from star anise (Illicium verum) leaves. Plants 2019, 8, 457. [Google Scholar] [CrossRef] [PubMed]
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Castellano, D.; Simonet, A.M.; Molinillo, J.M. Structure—activity relationships (SAR) studies of benzoxazinones, their degradation products and analogues. Phytotoxicity on standard target species (STS). J. Agric. Food Chem. 2005, 53, 538–548. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li, L.L.; Meiners, S.J.; Kong, C.H. Root placement patterns in allelopathic plant -plant interactions. New Phytol. 2023, 237, 563–575. [Google Scholar] [CrossRef]
- Semchenko, M.; John, E.A.; Hutchings, M.J. Effects of physical connection and genetic identity of neighbouring ramets on root-placement patterns in two clonal species. New Phytol. 2007, 176, 644–654. [Google Scholar] [CrossRef]
- Semchenko, M.; Saar, S.; Lepik, A. Plant root exudates mediate neighbour recognition and trigger complex behavioural changes. New Phytol. 2014, 204, 631–637. [Google Scholar] [CrossRef]
- Wang, N.Q.; Kong, C.H.; Wang, P.; Meiners, S.J. Root exudate signals in plant–plant interactions. Plant Cell Environ. 2021, 44, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, M.; An, M.; Pratley, J.E.; Luckett, D.J.; Lemerle, D.; Coombes, N. The seedling root response of annual ryegrass (Lolium rigidum) to neighbouring seedlings of a highly-allelopathic canola (Brassica napus). Flora 2016, 219, 18–24. [Google Scholar] [CrossRef]
- van Dam, N.M.; Bouwmeester, H.J. Metabolomics in the rhizosphere: Tapping into belowground chemical communication. Trends Plant Sci. 2016, 21, 256–265. [Google Scholar] [CrossRef]
- Wu, F.; Wang, X.; Xue, C. Effect of cinnamic acid on soil microbial characteristics in the cucumber rhizosphere. Eur. J. Soil Biol. 2009, 45, 356–362. [Google Scholar] [CrossRef]
- Buee, M.; Rossignol, M.; Jauneau, A.; Ranjeva, R.; Bécard, G. The pre-symbiotic growth of arbuscular mycorrhizal fungi is induced by a branching factor partially purified from plant root exudates. Mol. Plant Microbe. Interact. 2000, 13, 693–698. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, H.; Chen, G.; Yuan, S.; Lan, T.; Zeng, J. Allelochemical-driven N preference switch from NO3− to NH4+ affecting plant growth of Cunninghamia lanceolata (lamb.) hook. Plant Soil 2020, 451, 419–434. [Google Scholar] [CrossRef]
- Zhu, X.; Li, X.; Xing, F.; Chen, C.; Huang, G.; Gao, Y. Interaction between root exudates of the poisonous plant Stellera chamaejasme L. and arbuscular mycorrhizal fungi on the growth of Leymus Chinensis (Trin.) Tzvel. Microorganisms 2020, 8, 364. [Google Scholar] [CrossRef]
- Brundrett, M.C. Mycorrhizal associations and other means of nutrition of vascular plants: Understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 2009, 320, 37–77. [Google Scholar] [CrossRef]
- Garcia, K.; Zimmermann, S.D. The role of mycorrhizal associations in plant potassium nutrition. Front. Plant Sci. 2014, 5, 337. [Google Scholar] [CrossRef] [PubMed]
- Merilä, P.; Malmivaara-Lämsä, M.; Spetz, P.; Stark, S.; Vierikko, K.; Derome, J.; Fritze, H. Soil organic matter quality as a link between microbial community structure and vegetation composition along a successional gradient in a boreal forest. Appl. Soil Ecol. 2010, 46, 259–267. [Google Scholar] [CrossRef]
- Lu, F.; Zheng, L.; Chen, Y.; Li, D.; Zeng, R.; Li, H. Soil microorganisms alleviate the allelopathic effect of Eucalyptus grandis× E. urophylla leachates on Brassica chinensis. J. Forestry Res. 2017, 28, 1203–1207. [Google Scholar] [CrossRef]
- Liu, S.; Qin, F.; Yu, S. Eucalyptus urophylla root-associated fungi can counteract the negative influence of phenolic acid allelochemicals. Appl. Soil Ecol. 2018, 127, 1–7. [Google Scholar] [CrossRef]
- Qin, F.; Yu, S. Arbuscular mycorrhizal fungi protect native woody species from novel weapons. Plant Soil 2019, 440, 39–52. [Google Scholar] [CrossRef]
- Xia, Z.C.; Kong, C.H.; Chen, L.C.; Wang, S.L. Allelochemical-mediated soil microbial community in long-term monospecific Chinese fir forest plantations. Appl. Soil Ecol. 2015, 96, 52–59. [Google Scholar] [CrossRef]
- Achatz, M.; Rillig, M.C. Arbuscular mycorrhizal fungal hyphae enhance transport of the allelochemical juglone in the field. Soil Boil. Biochem. 2014, 78, 76–82. [Google Scholar] [CrossRef]
- Achatz, M.; Morris, E.K.; Müller, F.; Hilker, M.; Rillig, M.C. Soil hypha-mediated movement of allelochemicals: Arbuscular mycorrhizae extend the bioactive zone of juglone. Funct. Ecol. 2014, 28, 1020–1029. [Google Scholar] [CrossRef]
- Becklin, K.M.; Pallo, M.L.; Galen, C. Willows indirectly reduce arbuscular mycorrhizal fungal colonization in understorey communities. J. Ecol. 2012, 100, 343–351. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Y.; Feng, Y.; Ma, K. Response of soil bacterial communities to secondary compounds released from Eupatorium adenophorum. Biol. Invasions 2017, 19, 1471–1481. [Google Scholar] [CrossRef]
- Mahdhi, M.; Tounekti, T.; Khemira, H. Effects of Prosopis juliflora on germination, plant growth of Sorghum bicolor, mycorrhiza and soil microbial properties. Allelopathy J. 2019, 46, 121–132. [Google Scholar] [CrossRef]
- Rodríguez-Romero, M.; Godoy-Cancho, B.; Calha, I.M.; Passarinho, J.A.; Moreira, A.C. Allelopathic effects of three herb species on phytophthora cinnamomi, a pathogen causing severe oak decline in mediterranean wood pastures. Forests 2021, 12, 285. [Google Scholar] [CrossRef]
- Singh, J.P.; Kuang, Y.; Ploughe, L.; Coghill, M.; Fraser, L.H. Spotted knapweed (Centaurea stoebe) creates a soil legacy effect by modulating soil elemental composition in a semi-arid grassland ecosystem. J. Environ. Manag. 2022, 317, 115391. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.B.; Chen, B.M.; Liao, H.X.; Su, J.Q.; Peng, S.L. Leaf leachates have the potential to influence soil nitrification via changes in ammonia—oxidizing archaea and bacteria populations. Eur. J. Soil Sci. 2020, 71, 119–131. [Google Scholar] [CrossRef]
- Chen, P.; Hou, Y.; Zhuge, Y.; Wei, W.; Huang, Q. The effects of soils from different forest types on the growth of the invasive plant Phytolacca americana. Forests 2019, 10, 492. [Google Scholar] [CrossRef]
- Bennion, L.D.; Ward, D. Plant–soil feedback from eastern redcedar (Juniperus virginiana) inhibits the growth of grasses in encroaching range. Ecol. Evol. 2022, 12, e9400. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.H.; Zhang, S.Z.; Li, Y.H.; Xia, Z.C.; Yang, X.F.; Meiners, S.J.; Wang, P. Plant neighbor detection and allelochemical response are driven by root-secreted signaling chemicals. Nat. Commun. 2018, 9, 3867. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, E. Do plants favor their kin? Science 2019, 363, 15–16. [Google Scholar] [CrossRef]
- Yang, X.F.; Li, L.L.; Xu, Y.; Kong, C.H. Kin recognition in rice (Oryza sativa L.) lines. New Phytol. 2018, 220, 567–578. [Google Scholar] [CrossRef]
- Xu, Y.; Cheng, H.F.; Kong, C.H.; Meiners, S.J. Intra-specific kin recognition contributes to inter-specific allelopathy: A case study of allelopathic rice interference with paddy weeds. Plant Cell Environ. 2021, 44, 3709–3721. [Google Scholar] [CrossRef]
- Li, L.L.; Zhao, H.H.; Kong, C.H. (–)-Loliolide, the most ubiquitous lactone, is involved in barnyardgrass-induced rice allelopathy. J. Exp. Bot. 2020, 71, 1540–1550. [Google Scholar] [CrossRef]
- Li, F.L.; Chen, X.; Luo, H.M.; Meiners, S.J.; Kong, C.H. Root-secreted (–)-loliolide modulates both belowground defense and aboveground flowering in Arabidopsis and tobacco. J. Exp. Bot. 2023, 74, 964–975. [Google Scholar] [CrossRef]
- Li, L.L.; Li, Z.; Lou, Y.G.; Meiners, S.J.; Kong, C.H. (–)-Loliolide is a general signal of plant stress that activates jasmonate-related responses. New Phytol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Asay, A.K.; Simard, S.W.; Dudley, S.A. Altering neighborhood relatedness and species composition affects interior Douglas-fir size and morphological traits with context-dependent responses. Front. Ecol. Evol. 2020, 8, 578524. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).