Carbon Sink Limitation Determines the Formation of the Altitudinal Upper Limit of an Evergreen Oak in Eastern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Woody Tissue Sampling and NSC Measurements
2.3. Debudding and Defoliation Experiment
2.4. Measurement of Air Temperature
2.5. Data Analyses
2.5.1. Variations in NSC Concentration along Temperature Gradients
2.5.2. Responses of Growth and NSC Concentration of Seedlings to Different Treatments
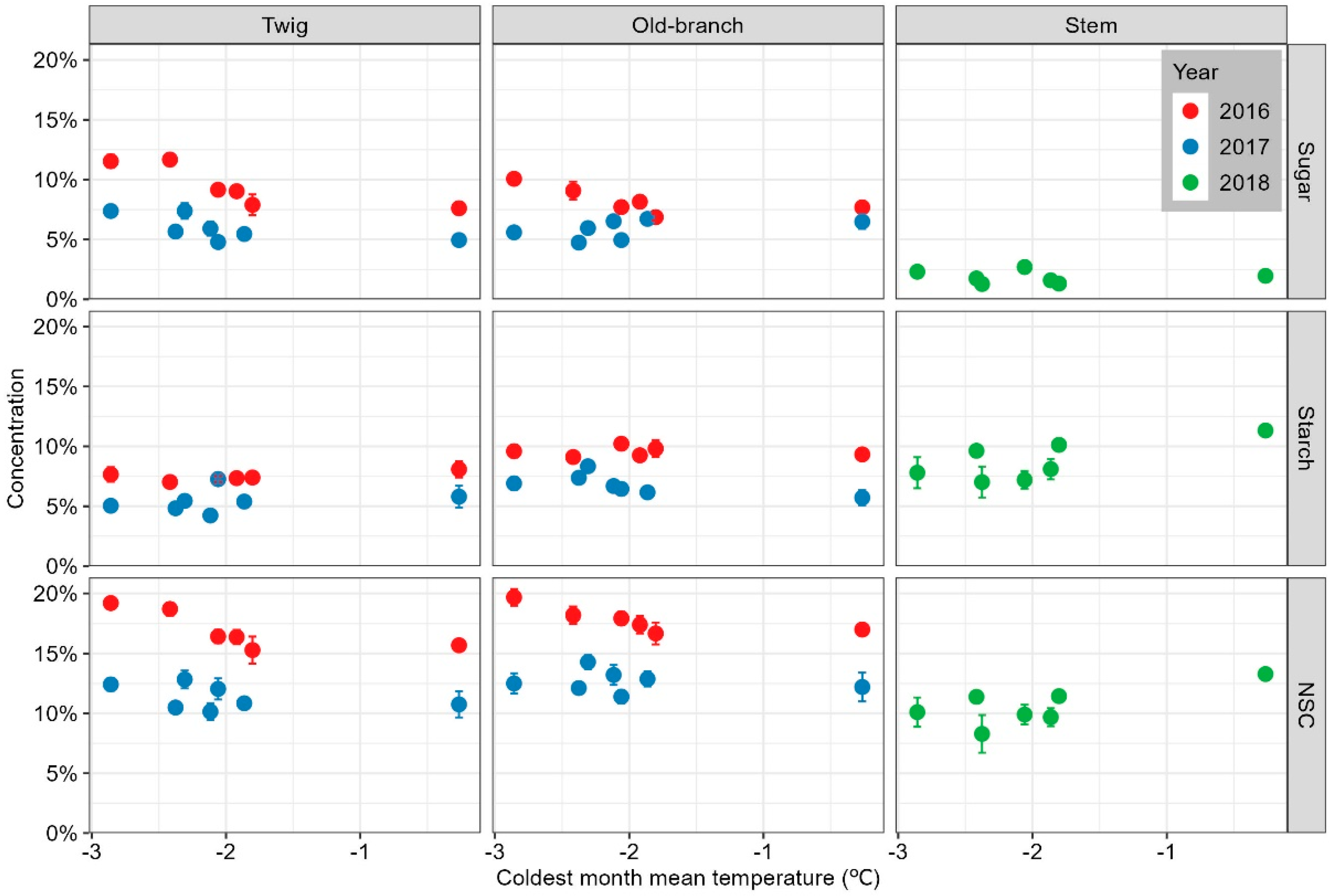
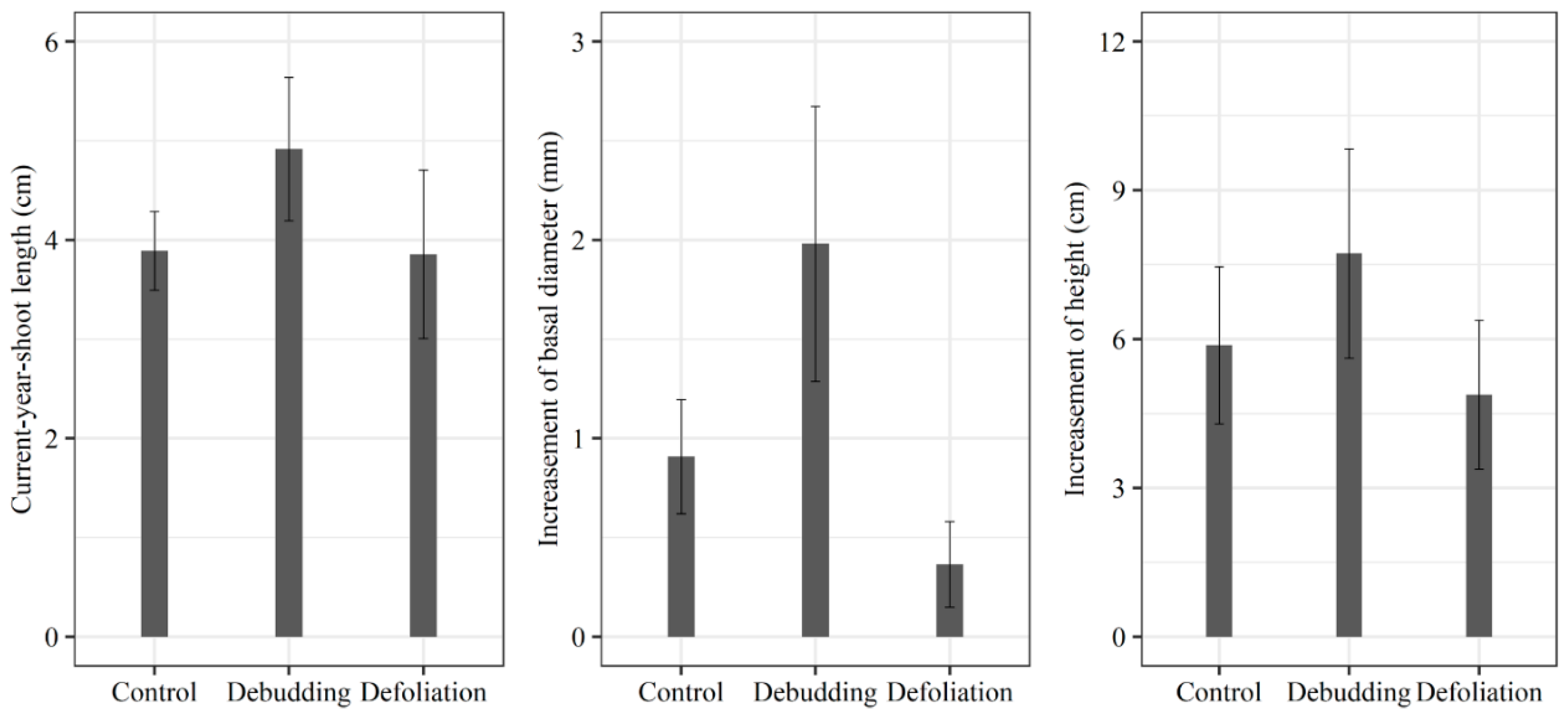
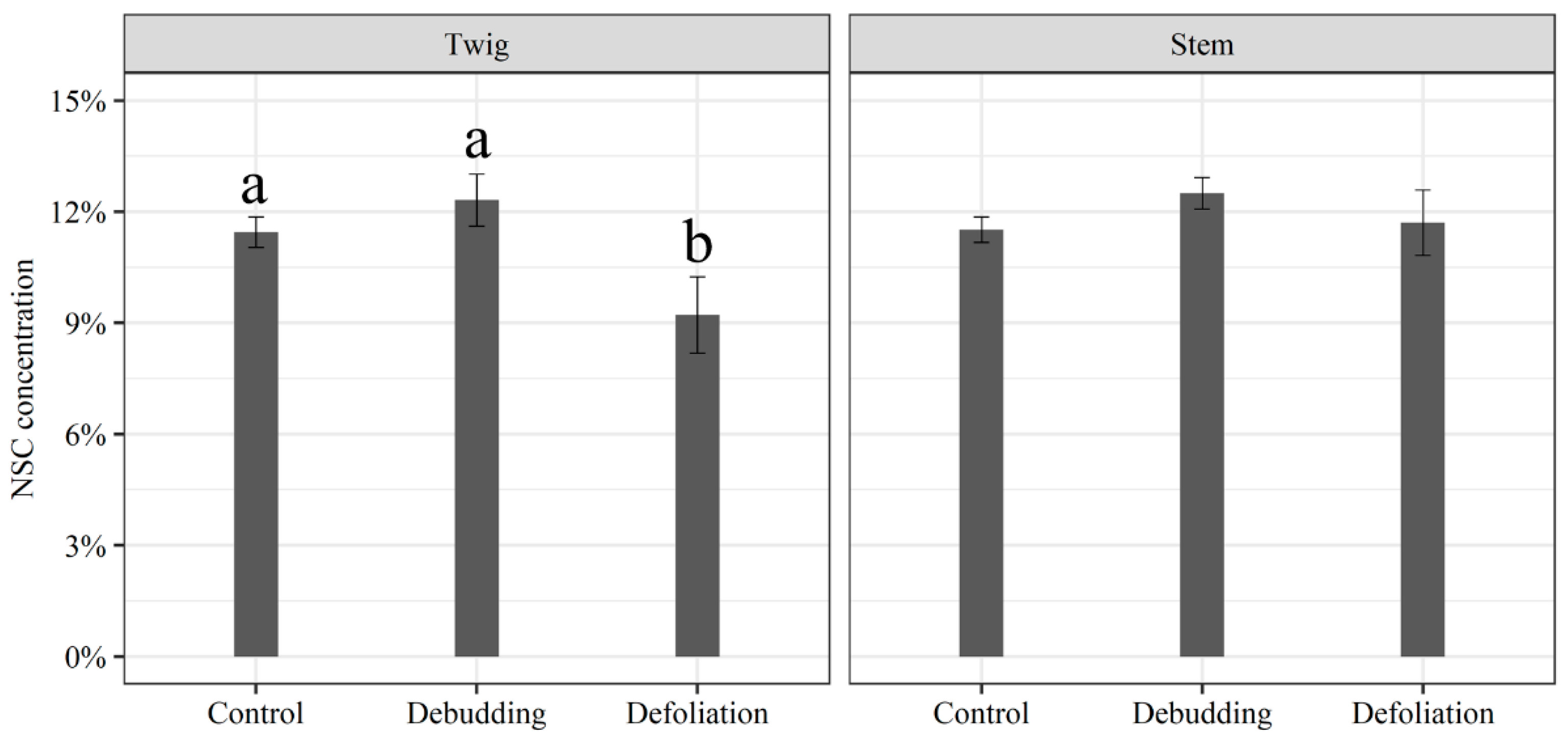
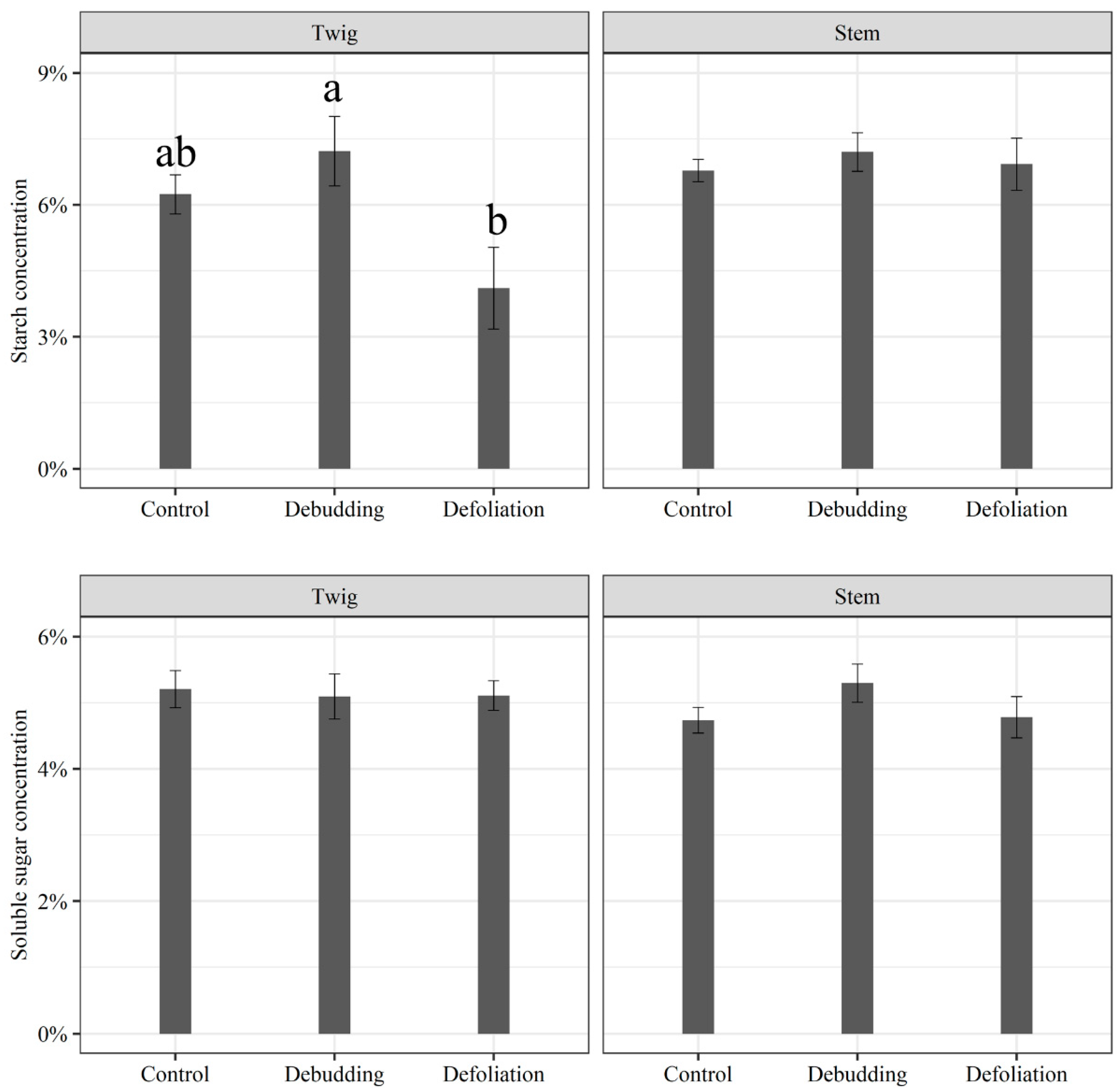
3. Results
3.1. NSC Variations in Cyclobalanopsis Gracilis Adults along the Temperature Gradient
3.2. Responses of Growth and NSC Concentration of Seedlings to Different Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Körner, C. Alpine Treelines: Functional Ecology of the Global High Elevation Tree Limits; Springer Science & Business Media: Basel, Switzerland, 2012. [Google Scholar]
- Stevens, G.C.; Fox, J.F. The Causes of Treeline. Annu. Rev. Ecol. Evol. Syst. 1991, 22, 177–191. [Google Scholar] [CrossRef]
- Wardle, P. Causes of alpine timberline: A review of the hypothesis. In Forest Development in Cold Climates; Plenum Press: New York, NY, USA, 1993; pp. 89–103. [Google Scholar]
- Körner, C. A Re-Assessment of High Elevation Treeline Positions and Their Explanation. Oecologia 1998, 115, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, A.; Piper, F.I.; Pfund, L.; Körner, C.; Hoch, G. Variation of mobile carbon reserves in trees at the alpine treeline ecotone is under environmental control. New Phytol. 2012, 195, 794–802. [Google Scholar] [CrossRef]
- Hoch, G.; Popp, M.; Körner, C. Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline. Oikos 2002, 98, 361–374. [Google Scholar] [CrossRef]
- Shi, P.; Körner, C.; Hoch, G. A test of the growth-limitation theory for alpine tree line formation in evergreen and deciduous taxa of the eastern Himalayas. Funct. Ecol. 2008, 22, 213–220. [Google Scholar] [CrossRef]
- Hoch, G.; Körner, C. Growth, demography and carbon relations of Polylepis trees at the world’s highest treeline. Funct. Ecol. 2005, 19, 941–951. [Google Scholar] [CrossRef]
- Hoch, G.; Körner, C. Global patterns of mobile carbon stores in trees at the high-elevation tree line. Global Ecol. Biogeogr. 2012, 21, 861–871. [Google Scholar] [CrossRef]
- Fajardo, A.; Piper, F.I.; Hoch, G. Similar variation in carbon storage between deciduous and evergreen treeline species across elevational gradients. Ann. Bot.-Lond. 2013, 112, 623–631. [Google Scholar] [CrossRef]
- Körner, C.; Basler, D.; Hoch, G.; Kollas, C.; Lenz, A.; Randin, C.F.; Vitasse, Y.; Zimmermann, N.E. Where, why and how? Explaining the low-temperature range limits of temperate tree species. J. Ecol. 2016, 104, 1076–1088. [Google Scholar] [CrossRef]
- Lenz, A.; Vitasse, Y.; Hoch, G.; Körner, C. Growth and carbon relations of temperate deciduous tree species at their upper elevation range limit. J. Ecol. 2014, 102, 1537–1548. [Google Scholar] [CrossRef]
- Wiley, E.; Helliker, B. A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytol. 2012, 195, 285–289. [Google Scholar] [CrossRef]
- Sala, A.; Woodruff, D.R.; Meinzer, F.C. Carbon dynamics in trees: Feast or famine? Tree Physiol. 2012, 32, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Handa, I.T.; Körner, C.; Hättenschwiler, S. A test of the treeline carbon limitation hypothesis by in situ CO2 enrichment and defoliation. Ecology 2005, 86, 1288–1300. [Google Scholar] [CrossRef]
- Susiluoto, S.; Hilasvuori, E.; Berninger, F. Testing the growth limitation hypothesis for subarctic Scots pine. J. Ecol. 2010, 98, 1186–1195. [Google Scholar] [CrossRef]
- Weber, R.; Gessler, A.; Hoch, G. High carbon storage in carbon-limited trees. New Phytol. 2019, 222, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Ohsawa, M.; Kira, T. Vertical vegetation zones along 30° N latitude in humid East Asia. Vegetatio 1996, 126, 135–149. [Google Scholar] [CrossRef]
- Ishizuka, K. The distribution of the evergreen broad-leaved trees in the Tohoku district, Japan and the Korean peninsula. Ecol. Rev. 1947, 10, 98–100. [Google Scholar]
- Ohsawa, M. An interpretation of latitudinal patterns of forest limits in south and east Asian mountains. J. Ecol. 1990, 78, 326–339. [Google Scholar] [CrossRef]
- Yim, Y.; Kira, T. Distribution of forest vegetation and climate in the Korean peninsula.: I. Distribution of some indices of thermal climate. Jpn. J. Ecol. 1975, 25, 77–88. [Google Scholar] [CrossRef]
- Song, K.; Da, L. Evergreen-deciduous broad-leaved forest ecotone in eastern China: Retrospect and new perspectives. In Vegetation Structure and Function at Multiple Spatial, Temporal and Conceptual Scales; Springer: Cham, Switzerland, 2016; pp. 129–147. [Google Scholar]
- Song, K.; Kohyama, T.S.; Da, L. Transition patterns across an evergreen—Deciduous broad-leaved forest ecotone: The effect of topographies. J. Veg. Sci. 2014, 25, 1257–1266. [Google Scholar] [CrossRef]
- Zhang, X.J.; Song, K.; Pan, Y.J.; Gao, Z.W.; Pu, F.G.; Lu, J.H.; Shang, K.K.; Da, L.J.; Cieraad, E. Responses of leaf traits to low temperature in an evergreen oak at its upper limit. Ecol. Res. 2020, 35, 900–911. [Google Scholar] [CrossRef]
- Fang, J.; Wang, Z.; Tang, Z. Atlas of Woody Plants in China: Distribution and Climate; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Shen, X. The study on the vegetation of the Tiantangzhai Mountains of the Dabie Mountains, Anhui Province. J. Wuhan Bot. Res. 1989, 7, 131–139. (In Chinese) [Google Scholar]
- Deng, M.; Wei, H.; Yao, G. Evergreen plants and evergreen broad-leaved forests in Huoshan and Jinzhai counties of the Dabie Mountaion region. Acta Phytoecol. Geobot. Sin. 1985, 9, 142–149. (In Chinese) [Google Scholar]
- Buysse, J.; Merckx, R. An Improved Colorimetric Method to Quantify Sugar Content of Plant Tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Tichý, L. Field test of canopy cover estimation by hemispherical photographs taken with a smartphone. J. Veg. Sci. 2016, 27, 427–435. [Google Scholar] [CrossRef]
- Da, L.; Kang, M.; Song, K.; Shang, K.; Yang, Y.; Xia, A.; Qi, Y. Altitudinal zonation of human-disturbed vegetation on Mt. Tianmu, eastern China. Ecol. Res. 2009, 24, 1287–1299. [Google Scholar] [CrossRef]
- Sala, A.; Hoch, G. Height-related growth declines in ponderosa pine are not due to carbon limitation. Plant Cell Environ. 2009, 32, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Lacointe, A.; Deleens, E.; Ameglio, T.; Saint-Joanis, B.; Lelarge, C.; Vandame, M.; Song, G.C.; Daudet, F.A. Testing the branch autonomy theory: A 13C/14C double-labelling experiment on differentially shaded branches. Plant Cell Environ. 2004, 27, 1159–1168. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Leuzinger, S.; Philipson, C.D.; Tay, J.; Hector, A. Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat. Clim. Chang. 2014, 4, 710–714. [Google Scholar] [CrossRef]
- Walter, L. Temperature stress and survival ability of Mediterranean sclerophyllous plants. G. Bot. Ital. 2000, 134, 279–295. [Google Scholar] [CrossRef]
- Li, H.; Hoch, G.; Körner, C. Source/sink removal affects mobile carbohydrates in Pinus cembra at the Swiss treeline. Trees 2002, 16, 331–337. [Google Scholar] [CrossRef]
- Baraza, E.; Zamora, R.; Hódar, J.A. Species-specific responses of tree saplings to herbivory in contrasting light environments: An experimental approach. Écoscience 2015, 17, 156–165. [Google Scholar] [CrossRef]
- Suchocka, M.; Swoczyna, T.; Kosno-Jończy, J.; Kalaji, H.M. Impact of heavy pruning on development and photosynthesis of Tilia cordata Mill. trees. PLoS ONE 2021, 16, e256465. [Google Scholar] [CrossRef] [PubMed]
- Palacio, S.; Hoch, G.; Sala, A.; Körner, C.; Millard, P. Does carbon storage limit tree growth? New Phytol. 2014, 201, 1096–1100. [Google Scholar] [CrossRef]
- Wiley, E.; Huepenbecker, S.; Casper, B.B.; Helliker, B.R. The effects of defoliation on carbon allocation: Can carbon limitation reduce growth in favour of storage? Tree Physiol. 2013, 33, 1216–1228. [Google Scholar] [CrossRef]
- Hartmann, H.; McDowell, N.G.; Trumbore, S. Allocation to carbon storage pools in Norway spruce saplings under drought and low CO2. Tree Physiol. 2015, 35, 243–252. [Google Scholar] [CrossRef]
- Huang, J.; Hammerbacher, A.; Weinhold, A.; Reichelt, M.; Gleixner, G.; Behrendt, T.; Dam, N.M.; Sala, A.; Gershenzon, J.; Trumbore, S.; et al. Eyes on the future—Evidence for trade-offs between growth, storage and defense in Norway spruce. New Phytol. 2019, 222, 144–158. [Google Scholar] [CrossRef]
- Saffell, B.J.; Meinzer, F.C.; Woodruff, D.R.; Shaw, D.C.; Voelker, S.L.; Lachenbruch, B.; Falk, K. Seasonal carbohydrate dynamics and growth in Douglas-fir trees experiencing chronic, fungal-mediated reduction in functional leaf area. Tree Physiol. 2014, 34, 218–228. [Google Scholar] [CrossRef]
- Ball, M.C.; Hodges, V.S.; Laughlin, G.P. Cold-induced photoinhibition limits regeneration of snow gum at tree-line. Funct. Ecol. 1991, 5, 663–668. [Google Scholar] [CrossRef]
- Richardson, S.J.; Bonner, K.I.; Bickford, C.P. Cold tolerance of photosynthesis as a determinant of tree species regeneration patterns in an evergreen temperate forest. Plant Ecol. 2013, 214, 787–798. [Google Scholar] [CrossRef]
- Sakai, A. Freezing resistance of broad-leaved evergreen trees in the warm-temperate zone. Low Temp. Sci. Ser. B Biol. Sci. 1981, 38, 1–14. [Google Scholar]
- Sakai, A. Freezing tolerance of evergreen and deciduous broad-leaved trees in Japan with reference to tree regions. Low Temp. Sci. Ser. B Biol. Sci. 1979, 36, 1–19. [Google Scholar]
- Sakai, A. Freezing resistance of evergreen and deciduous broad-leaf trees in Japan with special reference to their distributions. Jpn. J. Ecol. (Jpn.) 1975, 25, 101–111. [Google Scholar]
- Wachendorf, M.; Schloz, M.; Küppers, M.; Güney, A.; Veste, M. Wintertime photosynthesis and spring recovery of Ilex aquifolium L. Ifor.—Biogeosci. For. 2019, 12, 389–396. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, K.; Goldstein, G. Winter photosynthesis of evergreen broadleaf trees from a montane cloud forest in subtropical China. In Photosynthesis Research for Food, Fuel and the Future; Springer: Berlin/Heidelberg, Germany, 2013; pp. 812–817. [Google Scholar]
- Zhu, W.; Cao, M.; Wang, S.; Xiao, W.; Li, M. Seasonal dynamics of mobile carbon supply in Quercus aquifolioides at the upper elevational limit. PLoS ONE 2012, 7, e34213. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, H.; Körner, C. Explaining the exceptional 4,270 m high elevation limit of an evergreen oak in the south-eastern Himalaya. Tree Physiol. 2020, 40, 1327–1342. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Peñuelas, J. Effects of winter cold stress on photosynthesis and photochemical efficiency of PSII of the Mediterranean Cistus albidus L. and Quercus ilex L. Plant Ecol. 2005, 175, 179–191. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Yamagishi, Y.; Oribe, Y.; Funada, R. Regulation of cambial activity in relation to environmental conditions: Understanding the role of temperature in wood formation of trees. Physiol. Plant. 2013, 147, 46–54. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, X.; Morris, H.; Jansen, S. Phylogeny Best Explains Latitudinal Patterns of Xylem Tissue Fractions for Woody Angiosperm Species across China. Front. Plant Sci. 2019, 10, 556. [Google Scholar] [CrossRef]


| Sample Campaign | Date | Sample Organs | Transects/Elevation (m a.s.l.) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TTZ/ 740 | MZL/ 837 | TTZ/ 922 | MZL/ 960 | TTZ/ 1016 | TTZ/ 1057 | TTZ/ 1096 | MZL/ 1120 | TTZ/ 1148 | TTZ/ 1200 | |||
| 1 | 2016/10 | branch | ||||||||||
| 2 | 2017/10 | branch | ||||||||||
| 3 | 2018/10 | trunk | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Song, K.; Cieraad, E. Carbon Sink Limitation Determines the Formation of the Altitudinal Upper Limit of an Evergreen Oak in Eastern China. Forests 2023, 14, 597. https://doi.org/10.3390/f14030597
Zhang X, Song K, Cieraad E. Carbon Sink Limitation Determines the Formation of the Altitudinal Upper Limit of an Evergreen Oak in Eastern China. Forests. 2023; 14(3):597. https://doi.org/10.3390/f14030597
Chicago/Turabian StyleZhang, Xijin, Kun Song, and Ellen Cieraad. 2023. "Carbon Sink Limitation Determines the Formation of the Altitudinal Upper Limit of an Evergreen Oak in Eastern China" Forests 14, no. 3: 597. https://doi.org/10.3390/f14030597
APA StyleZhang, X., Song, K., & Cieraad, E. (2023). Carbon Sink Limitation Determines the Formation of the Altitudinal Upper Limit of an Evergreen Oak in Eastern China. Forests, 14(3), 597. https://doi.org/10.3390/f14030597





