Abstract
The plant MYB transcription factor family featured as highly conserved DNA-binding domains consisting of 1 to 4 imperfect repeats (R). Increasing evidence indicates that MYB genes participates in growth, differentiation, metabolism, and biotic and abiotic stress responses. However, the functions of MYB genes in the rubber tree remain to be deeply elucidated, especially R2R3-MYB gene family. In this study, molecular biology, bioinformatics, and qRT-PCR were used to identify and analyze HbR2R3-MYB gene family members in the rubber tree. A total of 132 members of the R2R3-MYB gene family were identified in the rubber tree based on genome-wide level. Most of the HbR2R3-MYBs were mapped to 17 rubber tree chromosomes except four genes. A phylogenetic analysis divided all the HbR2R3-MYBs into 20 subgroups with Arabidopsis thaliana. MEME analysis showed that the protein of HbR2R3-MYBs was characterized by 9 conserved motifs. Twenty-six representative R2R3 HbMYBs from different subgroups were selected for expression profiles analysis and the results revealed that the HbR2R3-MYBs members showed various expression patterns in different tissues, powdery mildew-infected and ethylene treatment, implying the diversity of their functions in rubber trees. These results provide fundamental knowledge for further studying the response of the HbR2R3-MYB family to stress and regulation latex flow in rubber tree.
1. Introduction
The MYB transcription factor (TF) superfamily is proteins with the largest members, functionally diverse, and represented in all eukaryotes [1]. The first plant MYB gene to be identified was ZmMYBC1 from corn (Zea mays L.), which is involved in flavonoid biosynthesis [2,3]. Plants encode large numbers of MYB genes relative to fungi and animals [4,5]. MYB protein family members share common characteristics and contain highly conserved DNA-binding domains consisting of 1 to 4 imperfect repeats (R), with each repeat forming a helix-turn-helix (HTH) motif that contains three regularly spaced tryptophan residues and consists of approximately about 50–55 amino acid residues [6]. Based on the number of conserved MYB domains, the MYB superfamily can be classified into four subfamilies, namely 1R-MYB, 2R-MYB, 3R-MYB, and 4R-MYB which containing one, two, three, and four MYB repeats, respectively. The MYB domain proteins of animals are mainly R1R2R3-MYB proteins, while R2R3-MYB proteins are more common in plants [7].
Large numbers of MYB genes have been identified in different plants, including 198 MYB members in Arabidopsis thaliana [8], 183 MYB members in Oryza sativa L., 279 MYB members in Vitis vinifera L., and 197 MYB members in Populus L. [9]. A total of 252 MYBs have been identified in Glycine max, including 244 R2R3-MYB (2R-MYB) genes, 6 R1R2R3-MYB (3R-MYB) genes, and 2 R0R1R2R3-MYB (4R-MYB) genes [10]. MYB transcription factors (TFs) play a key role in plant development, secondary metabolism, hormone signaling, disease resistance, and abiotic stress tolerance [11]. Some R2R3-MYB TFs are involved in the regulation of responses to environmental stresses such as drought, salt, and cold [12]. Expression analysis indicated that the MYB gene family has a broad expression profile during sweet orange development and plays an important role in developmental and stress responses [13]. OsMYB1 is an R2R3-type MYB TF that regulates rice root development and maintains phosphate homeostasis [14]. AtMYB62 is an Arabidopsis R2R3-MYB TF that localizes to the nucleus and negatively regulates Pi starvation-induced (PSI) genes. The overexpression of AtMYB62 alters root architecture and promotes the plant uptake of Pi [15].
Under environmental stress, relevant genes participate in hormone signal transduction mechanisms. Studies have shown that MYB genes can respond to biotic and abiotic stresses, such as those imposed by drought, fungi, calcium ion signals, salt and microorganisms and can participate in the interactions of various signaling pathways, such as the gibberellin A3 (GA3), jasmonic acid (JA), ethylene (ET), abscisic acid (ABA), and salicylic acid (SA) pathways. AtMYB44 is rapidly induced by methyl jasmonate (MeJA) in Arabidopsis [16]. AtMYB44 directly regulates WRKY70, which in turn regulates pathogenesis-related (PR) genes and is a negative regulator of the JA signaling pathway. AtMYB44 regulates the antagonism between two signaling pathways by activating SA-mediated defense and regulating the inhibition of JA-mediated defense by WRKY70 [17]. Western blot analysis indicated that FtMYB13, FtMYB14, and FtMYB15 can be degraded by the 26S proteasome in the JA signaling pathway [18].
Rubber tree [Hevea brasiliensis (Willd. Ex A. Juss) Müll. Arg (para rubber tree)] is an important crop for natural rubber (NR, cis-1,4-polyisoprene (cPIP)) production and carbocationic polymerization [19]. NR is referred to as latex when it is tapped from rubber tree bark. From a biological point of view, NR plays important roles in the responses to disease, insect infection, and high-temperature stress in rubber trees [20]. Although the NR biosynthesis pathway has been clearly elucidated, its regulatory mechanisms seem to be more complex. In rubber trees, the MADS-box TF HbMADS4 [21] and the HbWRKY1 TF negatively regulate HbSRPP [22]. Few MYB TF genes related to the NR biosynthesis pathway in rubber trees have been reported. However, studies were based on transcriptome data in rubber tree latex and their results had many repeat MYB sequences [23]. The growth and development of rubber trees are regulated in response to both biotic and abiotic stresses, such as powdery mildew infection and wind, cold, and drought stresses. Powdery mildew (Oidium heveae Steinm.) is one of the most important leaf diseases in rubber trees.
In this study, 132 R2R3-MYB subfamily members in the rubber tree were identified using bioinformatics methods. The physical and chemical properties, conserved motifs, three-dimensional structures, and chromosomal location of HbR2R3-MYB family genes were conducted. In addition, we analyzed the classification and expression patterns of HbR2R3-MYB in different tissues, abiotic stresses caused by mildew-infected, and ethephon (ETH) treatment. Our work will facilitate further research into the functional characterization of HbR2R3-MYBs in rubber trees.
2. Materials and methods
2.1. Plant Materials and Treatments
The rubber tree variety CATAS73397 (bred from a RRIM600×PR107 cross) was planted at the experimental farm of the Chinese Academy of Tropical Agricultural Sciences, Danzhou City, Hainan Province, China (19°51′51 N; 109°55′63 E). Samples of different tissues, including 10 g leaf, 10 g bark, 300 mL latex, and 5 g flowers were collected from three 15-year-old healthy tapping trees with branch shears and tapping knife in April used for tissue-specific expression analysis. Tissue culture seedlings of CATAS73397 grown in plastic pots in a chamber with clay and turfy soil (1:3) were used for the hormone Ethephon and powdery mildew treatments. Powdery mildew treatment was performed according to a previously described method [24]. The powdery mildew fungi were maintained on seedlings of a highly susceptible rubber tree clone RO/PB/2 under 23 °C, 16 h day light, and 80% relative humidity in green house. Inoculation was performed when the second unit leaf of CATAS73397 was fully expanded. Fresh powdery mildew spores from the infected seedlings of RO/PB/2 were inoculated to seedlings of CATAS73397 with a camelhair brush. Seedlings of CATAS73397 without inoculation were used as control, and kept in a separated illumination incubator to avoid infection by the powdery mildew. The inoculated and control seedlings were grown at 25 °C, 16 h day light, and 80% relative humidity in green house. Treatment with 1.5% (v/v) ETH was also performed according to a previously described method [25]. The harvested samples were immediately frozen in liquid nitrogen and stored at −80 °C for future analysis.
2.2. Identification of H. brasiliensis MYB Superfamily Genes
The H. brasiliensis nucleotide and protein sequences were downloaded from NCBI genome database. A Hidden Markov Model (HHM) search based on the rubber tree genome database was performed with the MYB DNA-binding domain PF00249 using HMMER 3.1 [26,27]. All the candidate HbMYBs were assessed by NCBI online analysis and DNAMAN software to remove redundant sequences. The analyses of the coding regions and the amino acid sequences of the rubber tree HbMYB genes were performed using the online analysis software NCBI ORFfinder.
2.3. Physicochemical Properties, Protein Architecture and Conserved Motif Analysis of MYB Proteins
NCBI conserved domain searches (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi? (accessed on 1 January 2021)) and SMART (http://smart.embl-heidelberg.de/smart/set_mode.cgi?NORMAL=1 (accessed on 1 January 2021)) were used to identify the architecture of HbMYBs. The physicochemical properties of the proteins were analyzed using the ProtParam tool (https://web.expasy.org/protparam/ (accessed on 1 January 2021)) [28] by uploading the amino acid sequences of the HbMYB members in H. brasiliensis. Sequence alignments were performed using DNAMAN version 8.0 and ClustalX [29]. Protein modeling was predicted using the SWISS-MODEL (https://swissmodel.expasy.org/ (accessed on 1 January 2021)) based on the 3D structure of the AtMYB66 (PDB number: 6kks) [30]. Conserved motifs shared by MYB proteins were analyzed using the Multiple Em for Motif Elicitation (MEME v5.0.5, http://meme-suite.org/tools/meme (accessed on 1 January 2022)) [31] online tool by uploading the amino acid sequences of the HbMYB family members. The physicochemical properties of the identified genes, such as their isoelectric point, protein molecular weight, instability index and grand average of hydropathicity, were analyzed by using ExPASy ProtParam online analysis software.
2.4. Phylogenetic Tree Analysis
A total of 126 Arabidopsis R2R3-MYB protein sequences and 5 R1R2R3-MYB protein sequences were downloaded from the TAIR database (Table S1). The 131 Arabidopsis MYB protein sequences and the deduced amino acid sequences of the 132 HbR2R3-MYBs were then aligned using ClustalW. The first phylogenetic tree was constructed using the MYB subfamily proteins of Arabidopsis and the 132 HbR2R3-MYB proteins of rubber trees (Table S2). Phylogenetic analysis was conducted, and the results were viewed using MEGA version 11, based on the Maximum Parsimony method with a Poisson correction model and a bootstrap test with 1000 replicates [32].
2.5. Expression Analysis of HbR2R3-MYB Genes
A Total RNA Isolation System kit (OMEGA) was used for total RNA extraction from different tissues. First-strand cDNA was synthesized from 2 μg of total RNA with a RevertAidTM First Strand cDNA Synthesis Kit (Fermentas, Waltham, MA, USA) according to the manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) was performed on the CFX96TM Real-Time System (Bio-Rad, Hercules, CA, USA) using SYBR Premix Ex Taq (TaKaRa, Tokyo, Japan) in a total volume of 25 μL. Thirty-one specific primers (Table S3) were designed for the reactions, and Hb18S was used as the housekeeping gene. All treatments were 3 biological replicates per sample and 3 technique replicates, and expression levels were analyzed using the 2−ΔΔCT method (Table S4) [33]. All data were analyzed by one-way ANOVA, and multiple comparison analyses were performed with Tukey’s test at the p < 0.01 level. Those results were visualized by heatmaps using the bioinformatics online tool (http://www.bioinformatics.com.cn (accessed on 1 January 2021)).
3. Results
3.1. Identification, Physicochemical Properties and Chromosomal Location of the HbMYBs
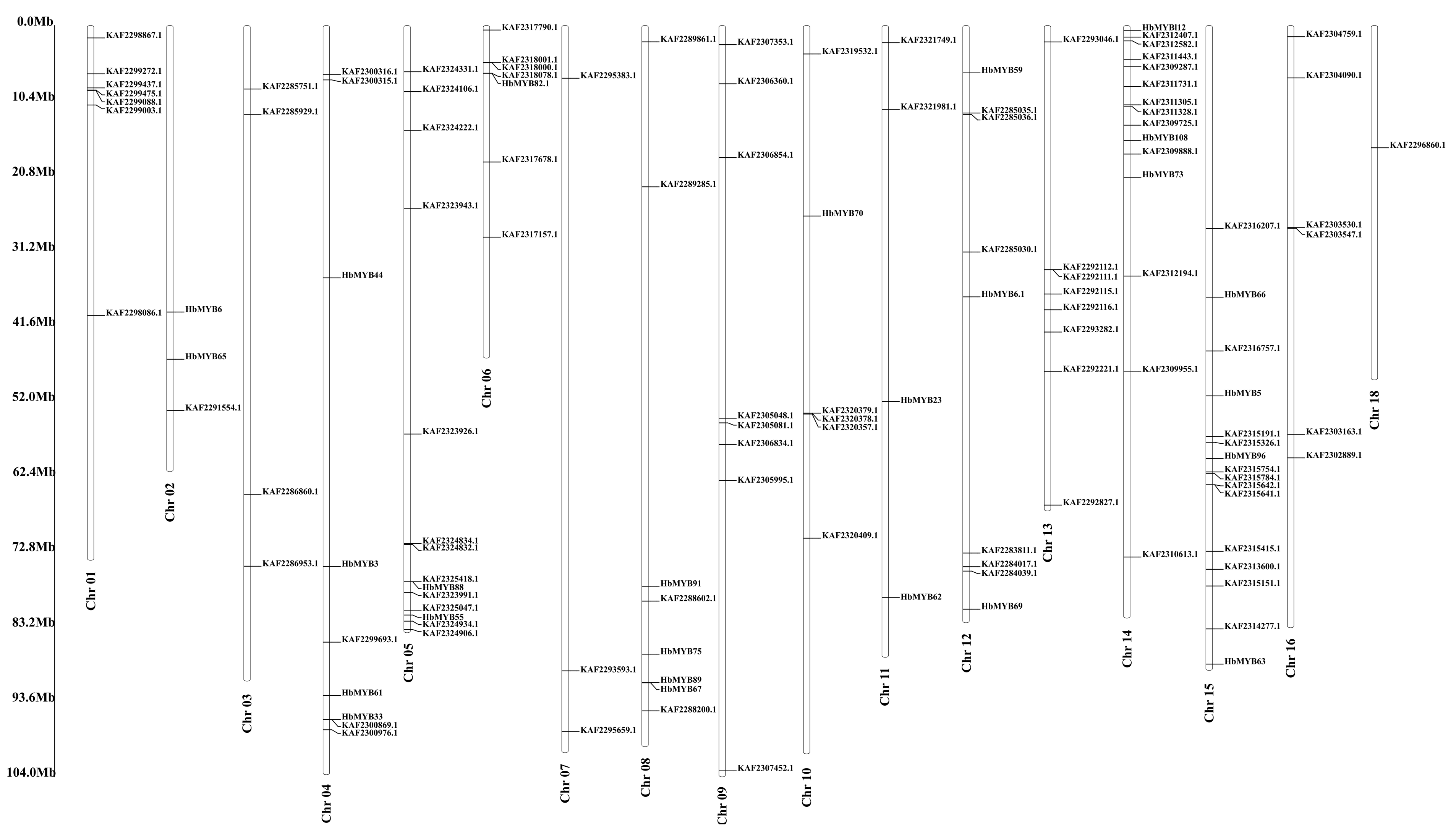
The rubber tree genome was searching for genes containing MYB or MYB-like domains using HMMER 3.1. After being confirmed by SMART and Pfam, a total of 132 unique R2R3-MYB family genes were identified in the rubber tree (Table S2). All the HbR2R3-MYBs were unevenly distributed across the 17 rubber tree chromosomes, except for KAF2283161.1, KAF2282816.1, KAF2282567.1, and KAF2282578.1. The largest number of HbMYB genes were mapped to chromosome 15 (Chr15), followed by Chr14 (14), and Chr05 (15). Interestingly, only one HbMYB was mapped to Chr17 and none to Chr18 (Figure 1, Supplement Table S1). To further understand the molecular characteristics of the HbMYB genes, a physicochemical properties analysis was performed. The amino acids (aa) length of HbMYB encoded proteins ranges from 110 to 1014 with isoelectric points range from 4.78 to 10.14, and theoretical molecular weight range from 12.65 kDa to 113.83 kDa, respectively (Table S2).
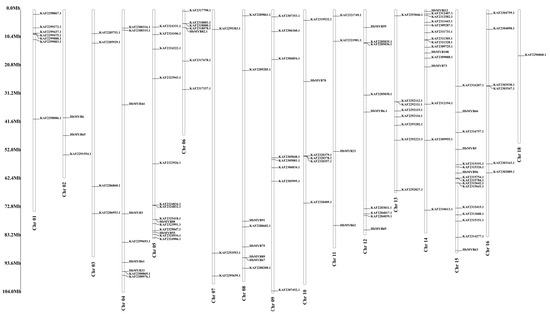
Figure 1.
Chromosomal location of HbMYBs.
3.2. Conserved Motifs of HbR2R3-MYB Proteins
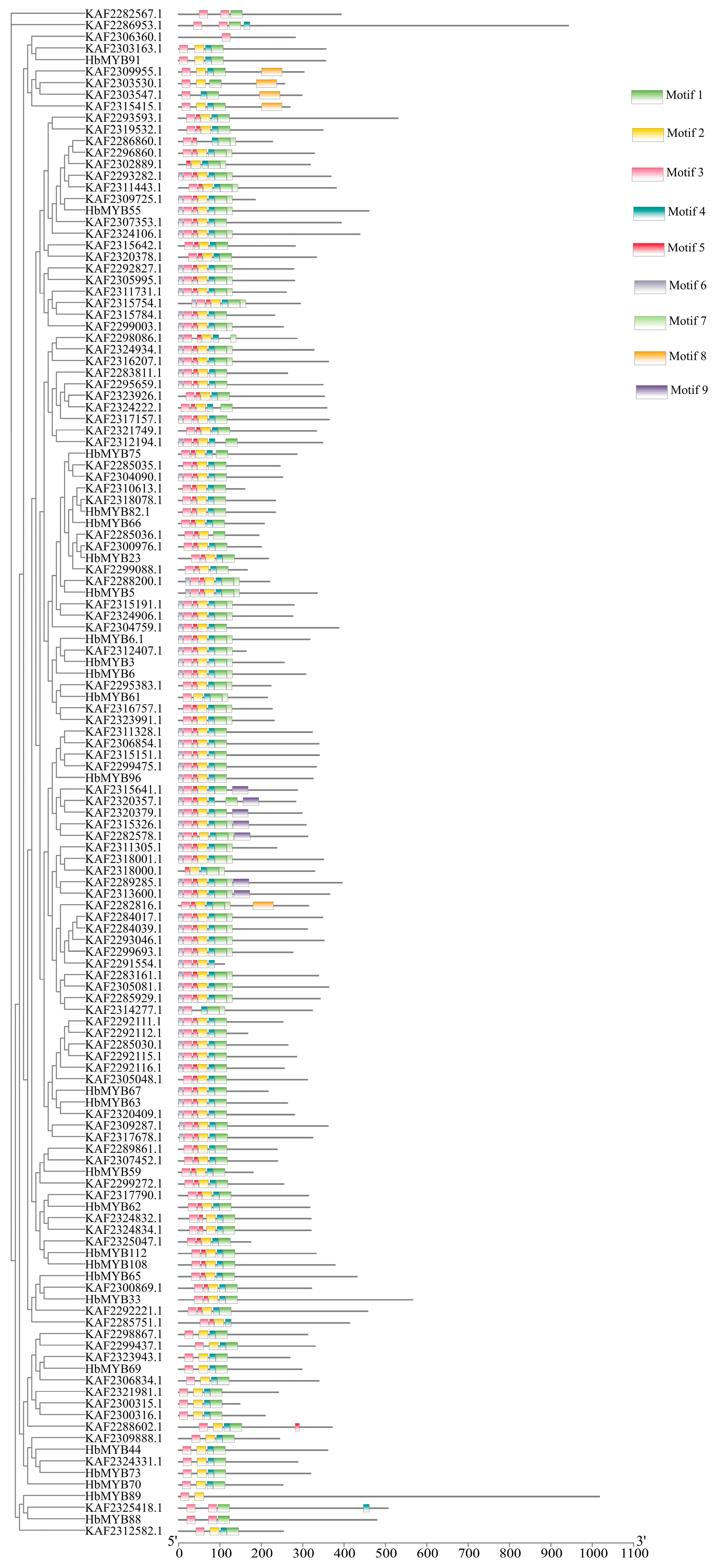
To investigate the systematics evolution and sequence features of the HbR2R3-MYB proteins, a phylogenetic tree and motif composition analysis were performed. As showed in Figure 2, nine conserved motifs were identified in these proteins by MEME analysis. Almost all HbR2R3-MYBs contains motif 1, 2, 3, and 4, which were part of the R2 or R3 (Figure 2). About half of the HbR2R3-MYB proteins contains motif 6 which did not belong to the conserved R2 or R3 domain. Motif 8 was present in five genes (KAF2309955.1, KAF2303530.1, KAF2303547.1, KAF2315415.1, and KAF2282816.1), while motif 5 was only present in one gene (KAF2288602.1). Additionally, motif 9 was present in all members of S9 and two genes (KAF2289285.1and KAF2313600.1) did not divide into any subgroups. Moti 7 existed simultaneously in 44 members of HbR2R3-MYBs from S4, S9, S11 S13, and S24. These conserved motifs may be associated with the specific biological functions of the different subgroups proteins.
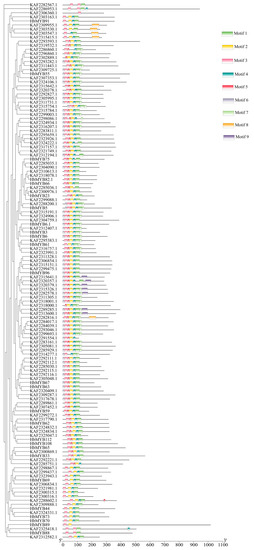
Figure 2.
Motif prediction of HbMYBs from rubber tree. The motifs of the 1R, 2R-MYB, and 3R-MYB subfamily proteins were analyzed using the MEME web server. Colored blocks represent 3 kinds of motifs. The length of the gray lines indicates the length of a sequence relative to that of all the other sequences. The position of each block indicates the location of a motif with a matching sequence. The results showed that there were 3 different motifs in the 132 HbMYBs, and the motifs of each sequence are visualized. Squares of different colors represent different motifs and the positions of the motifs.
3.3. Conserved Domains and Sequcence Analysis of HbR2R3-MYBs
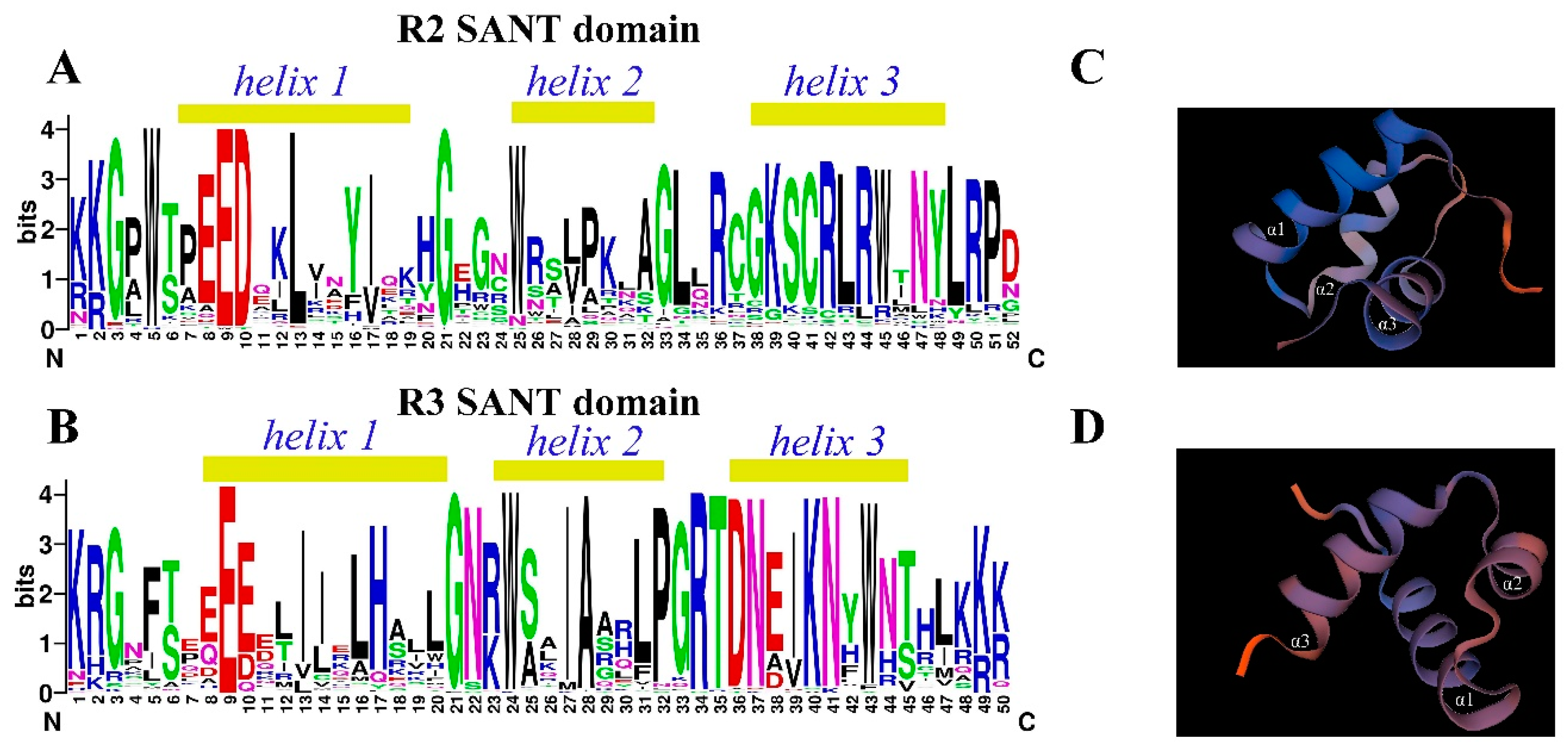
To further analyze the conserved domains of the 132 R2R3-MYB family members, online multisequence alignment and tertiary structure prediction of the R2 domain and R3 domain were performed by using the online tools CLUSTAL W and SWISS-MODEL based on the 3D structure of AtMYB66 (PDB number: 6kks). The R2 domains of 132 HbR2R3-MYBs harbored three highly conserved tryptophan residues (W) at positions 5, 25, and 45 (Figure 3A, Table S2). By contrast, the highly conserved tryptophan residues of the R3 domain were located at positions 24 and 43 (Figure 3B). Other highly conserved residues in addition to the tryptophan residues were confirmed in the R2 and R3 domains of HbR2R3-MYBs. The residues included Gly (G3), Glu (E9), Asp (D10), Leu (L13), Gly (G21), Lys(K39), Ser (S40), Cys (C41), Arg (R42), Leu (L43), Arg (R44), Asn (N47), Leu (L49), Arg (R50), and Pro (P51) in the R2 domains and Lys (K1), Arg (R2), Gly (G3), Glu (E9), Gly (G21), Asn (N22), Ala (A28), Pro (P32), Gly (G33), Arg (R34), Thr (T35), Asp (D36), Asn (N41), and Lys (K49) in the R3 domains (Figure 3A,B, Table S2). In addition, 3D protein structure prediction showed that the R2 and R3 domains of 26 HbR2R3-MYB proteins formed three α-helices (Figure 3C,D).
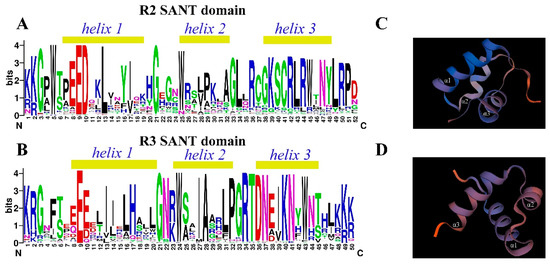
Figure 3.
Domains of HbR2R3-MYBs and 3D structure models of R2 and R3 MYB repeats. Sequence logos of R2 domain (A) and R3 domain (B). The bit score indicates the information content for each position in the sequence and the light yellow box above the sequence indicate the HTH structure. Predicted 3D structure models of the R2 (C) and R3 domains (D). The 3D structure of the R2 and R3 repeats domains was constructed using AtMYB66 (PDB: 6kks) as a template.
3.4. Phylogenetic Analysis of HbR2R3-MYBs
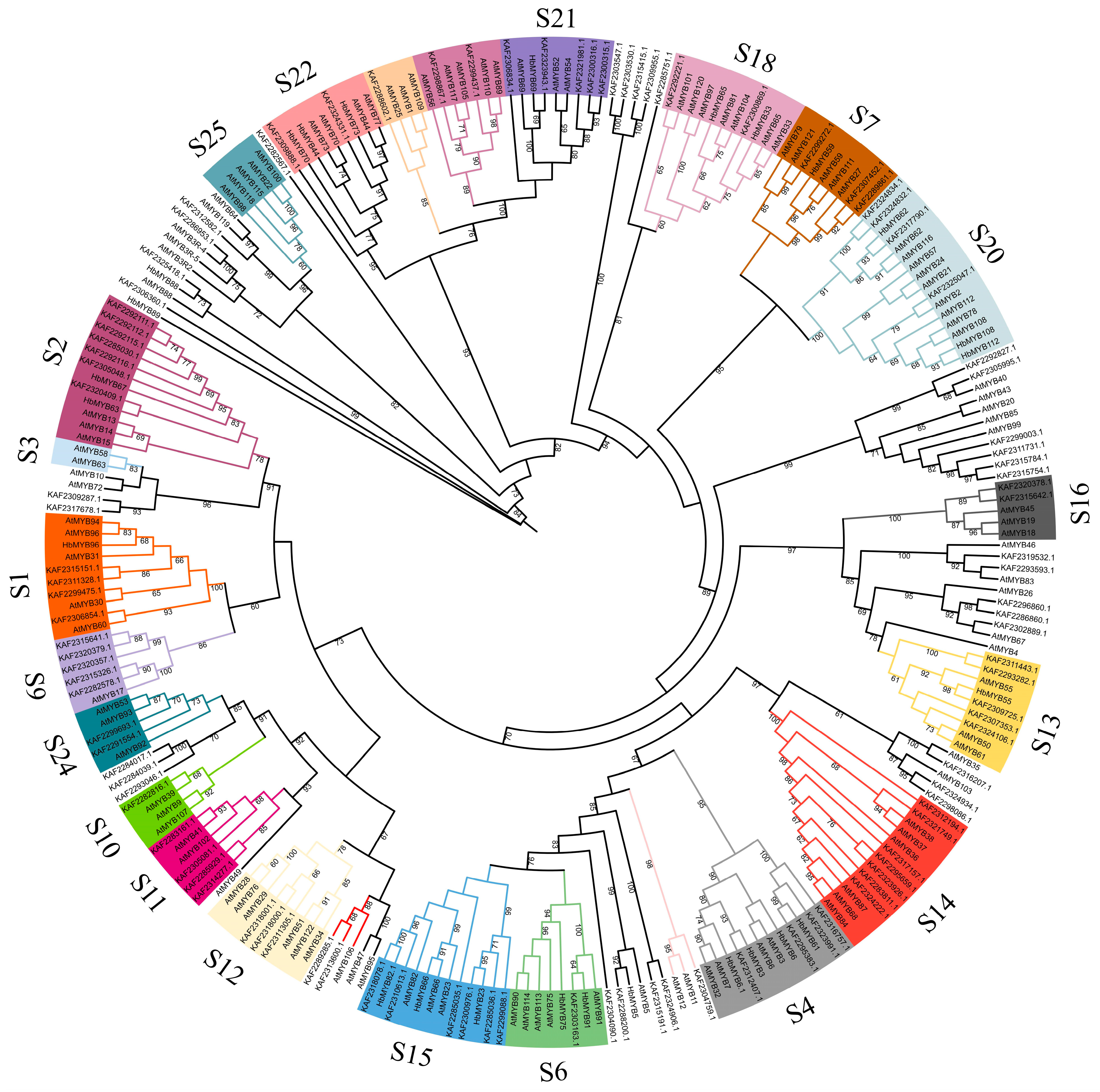
To infer the evolutionary relationship of the rubber tree R2R3-MYB family, the 132 rubber tree HbR2R3-MYBs, and the Arabidopsis AtMYB family members were clustered by using MEGA 11 and a phylogenetic tree was constructed (Figure 4). Based on the results and previous studies on the Arabidopsis AtMYB family, 132 HbR2R3-MYBs were divided into 20 subgroups (S1, S2, S3, S4, S6, S7, S9, S10, S11, S12, S13, S14, S15, S16, S18, S20, S21, S22, S24, and S25). Among the subgroups, the majority of R2R3-MYB members were found in the S2 and S15, each containing nine members, followed by S4 which consisted of eight members. In addition, two MYB subgroups (S3, and S25) did not exhibit any rubber tree HbR2R3-MYBs.
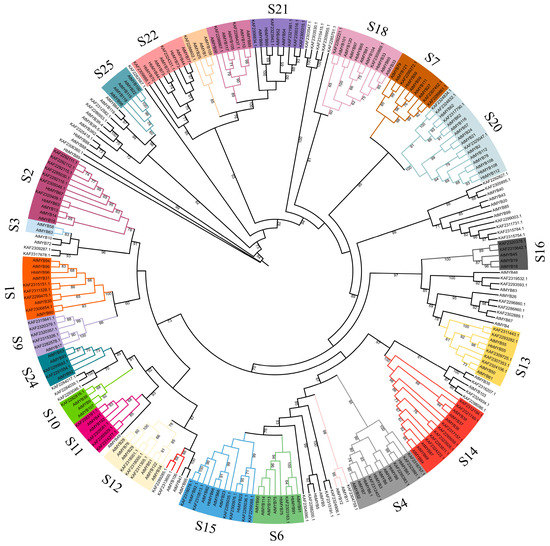
Figure 4.
Phylogenetic analysis of HbR2R3-MYBs and AtMYBs. The protein sequence of HbR2R3-MYBs and AtMYBs were aligned by ClustalW and the unrooted phylogenetic tree constructed by the neighbor-joining (NJ) method with 1000 bootstrap replicates.
3.5. Expression Profiles of HbR2R3-MYBs in Different Tissues
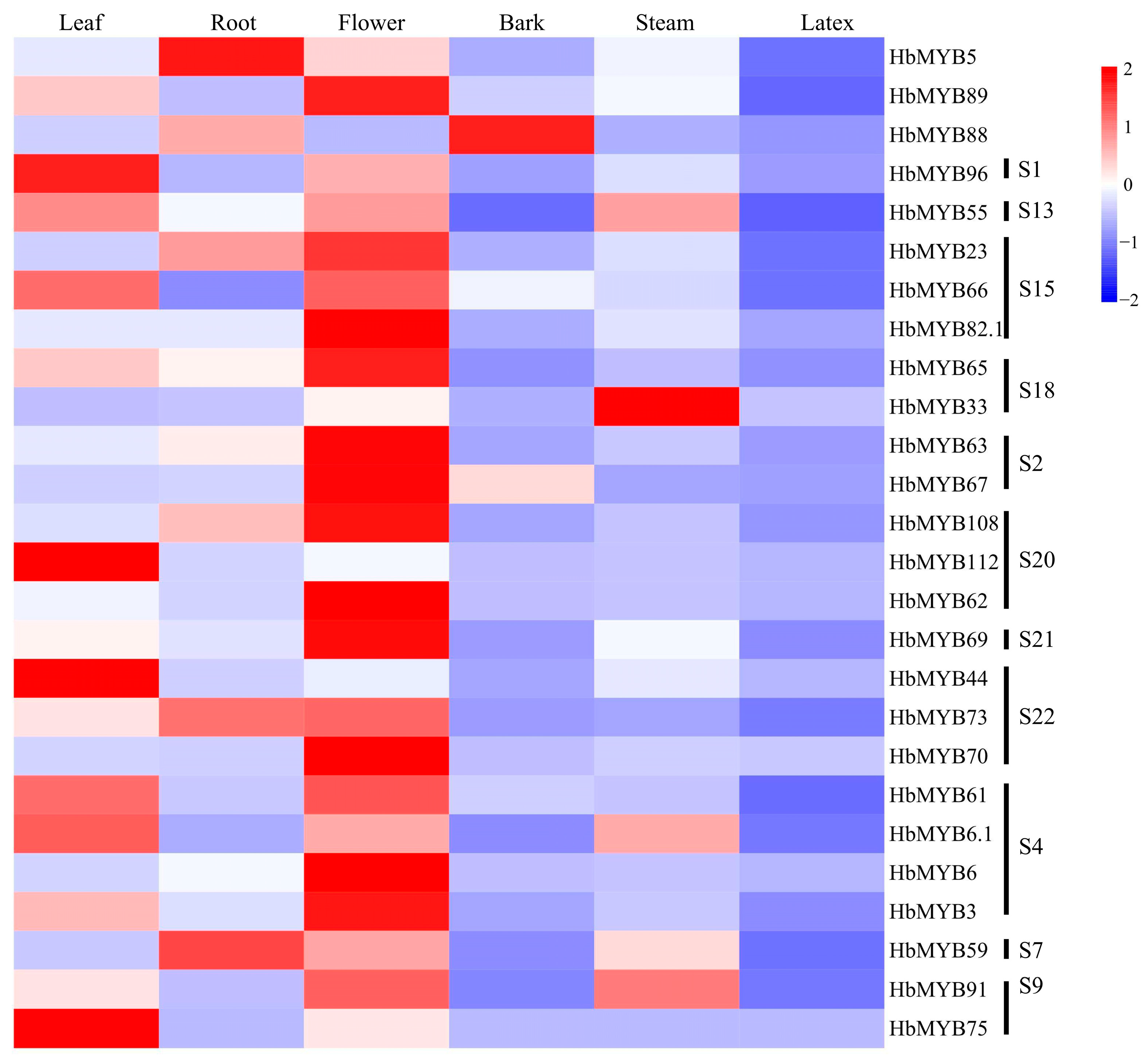
The expression of the 26 representative rubber tree R2R3-MYB genes in different tissues showed that the greatest number of the genes were expressed in flowers, followed by leaves, roots and stems, whereas the gene expression in bark and latex was lowest (Figure 5, Table S4). Thirteen genes have the highest expression in flowers, including HbMYB89, HbMYB23, HbMYB66, HbMYB82.1, HbMYB65, HbMYB63, HbMYB67, HbMYB108, HbMYB62, HbMYB69, HbMYB73, HbMYB70, HbMYB61, HbMYB6, HbMYB3, and HbMYB91, among which HbMYB70 showed the highest expression (Figure 5, Table S4). Six genes were highly expressed in leaves, including HbMYB96, HbMYB55, HbMYB112, HbMYB44, HbMYB6.1, and HbMYB75. HbMYB5 and HbMYB59 showed the highest expression level in roots, while HbMYB88 in barks and HbMYB33 in steams (Figure 5, Table S4). All the HbR2R3-MYBs showed the lowest expression level in latex. Interestingly, HbMYB75 was expressed in flowers and leaves and was not expressed in other tissues (Figure 5, Table S4). The gene HbMYB33 was expressed at the highest level in latex, while HbMYB73 in the barks. HbMYB70 had the highest expression in flowers and showed low expression level in other tissues (Figure 5, Table S4). These findings led us to draw the conclusion that HbR2R3-MYBs had significant functional differentiation in rubber tree tissues and majority of the HbR2R3-MYB genes may play specific roles in flower development.
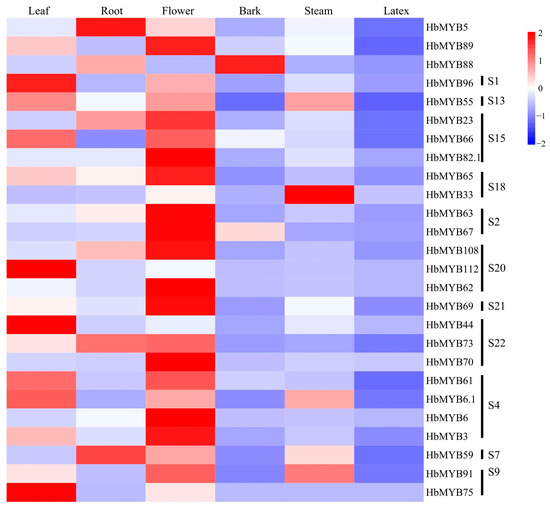
Figure 5.
Tissue expression profiles of HbR2R3-MYBs in different tissues. The color bar represents the gene expression level, with red indicating high expression level, white indicating no expression, and blue indicating low expression level of transcript abundance (the same in Figure 6 and Figure 7).
3.6. Expression Patterns of HbR2R3-MYBs in Response to Powdery Mildew Infection and Ethephon Treatment
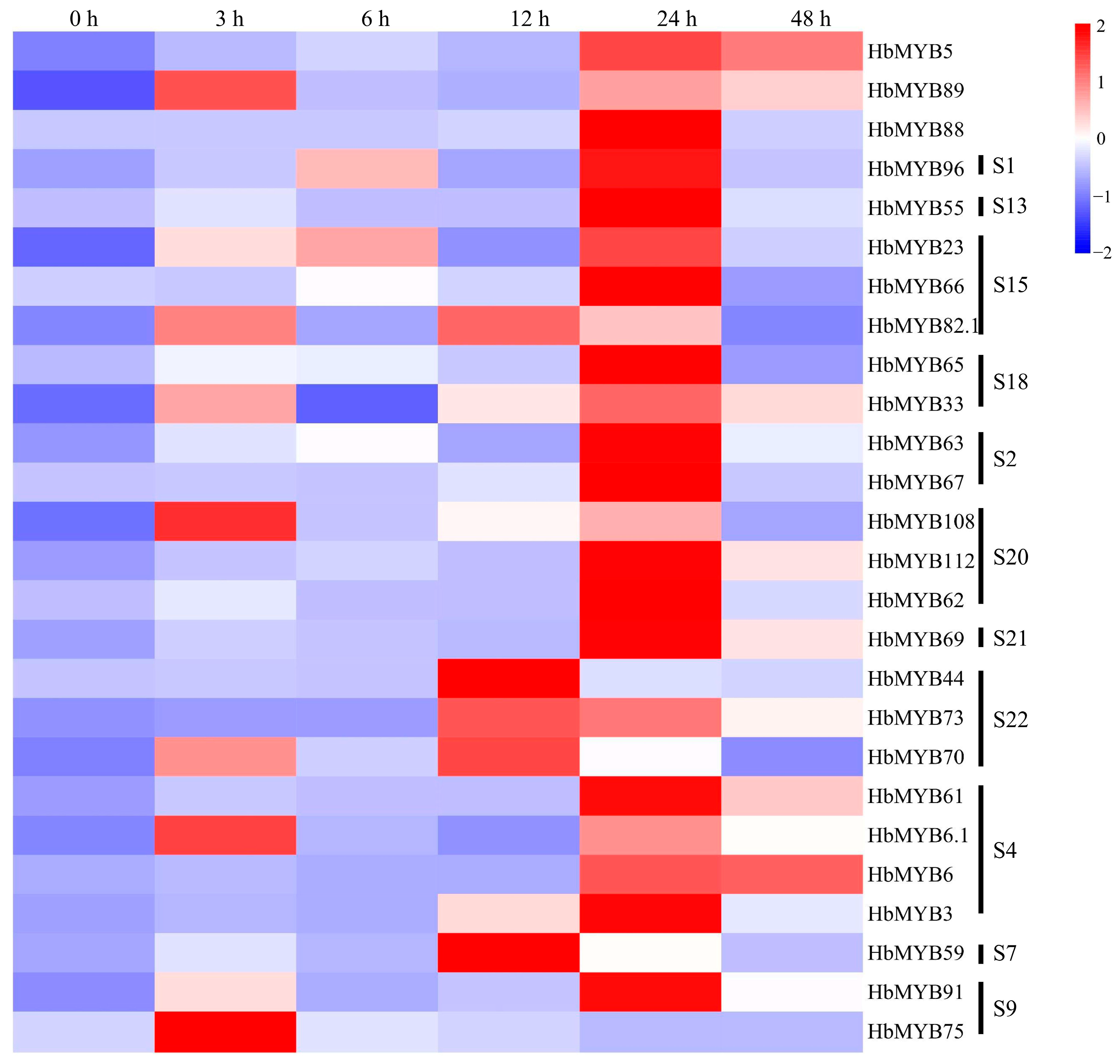
Leaves of the rubber tree variety CATAS73397 were treated with powdery mildew. After sampling, the expression patterns of each gene under powdery mildew treatment were analyzed by qRT-PCR. As shown in Figure 6 and Table S4, the expression levels of most genes were significantly increased after 3 h, 12 h, and 24 h. Compared to the levels detected at 0 h, the expression levels of HbMYB82.1, HbMYB108, HbMYB62, and HbMYB70 were significantly increased by 28–62 times at 3 h (Figure 6, Table S4). At 6 h, none of the genes showed a significant difference in expression compared to that recorded at 0 h (Figure 6, Table S4). At 12 h, the expression levels of HbMYB82.1, HbMYB59, HbMYB108, HbMYB70, HbMYB73, and HbMYB44 were significantly increased by 28–62 times relative to the levels detected before treatment (Figure 6, Table S4). At 24 h, the expression levels of HbMYB55, HbMYB23, HbMYB73, HbMYB108, HbMYB67, HbMYB88, and HbMYB62 were significantly increased by 24–243 times relative to those detected before treatment (Figure 6, Table S4). At 48 h, all of the genes but HbMYB65, HbMYB66, and HbMYB75 showed higher expression levels than those detected at 0 h (Figure 6, Table S4). The expression levels of HbMYB62, HbMYB44, and HbMYB88 were the most significantly increased among the 26 genes during powdery mildew infection (Figure 6, Table S4). HbMYB62 had the highest expression level at 24 h with 243 times higher than that detected before infection, and HbMYB44 showed the highest expression level at 12 h with 135 times higher than that detected before infection (Figure 6, Table S4). HbMYB88 gene expression was 135-fold higher at 24 h than before treatment (Figure 6, Table S4).
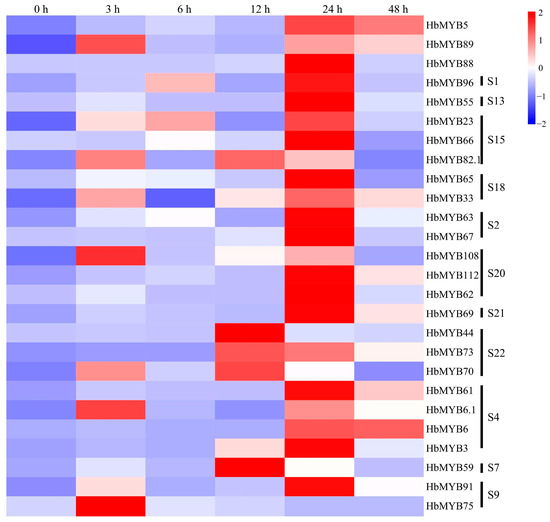
Figure 6.
Expression analysis of HbR2R3-MYBs in rubber trees subjected to powdery mildew infection.
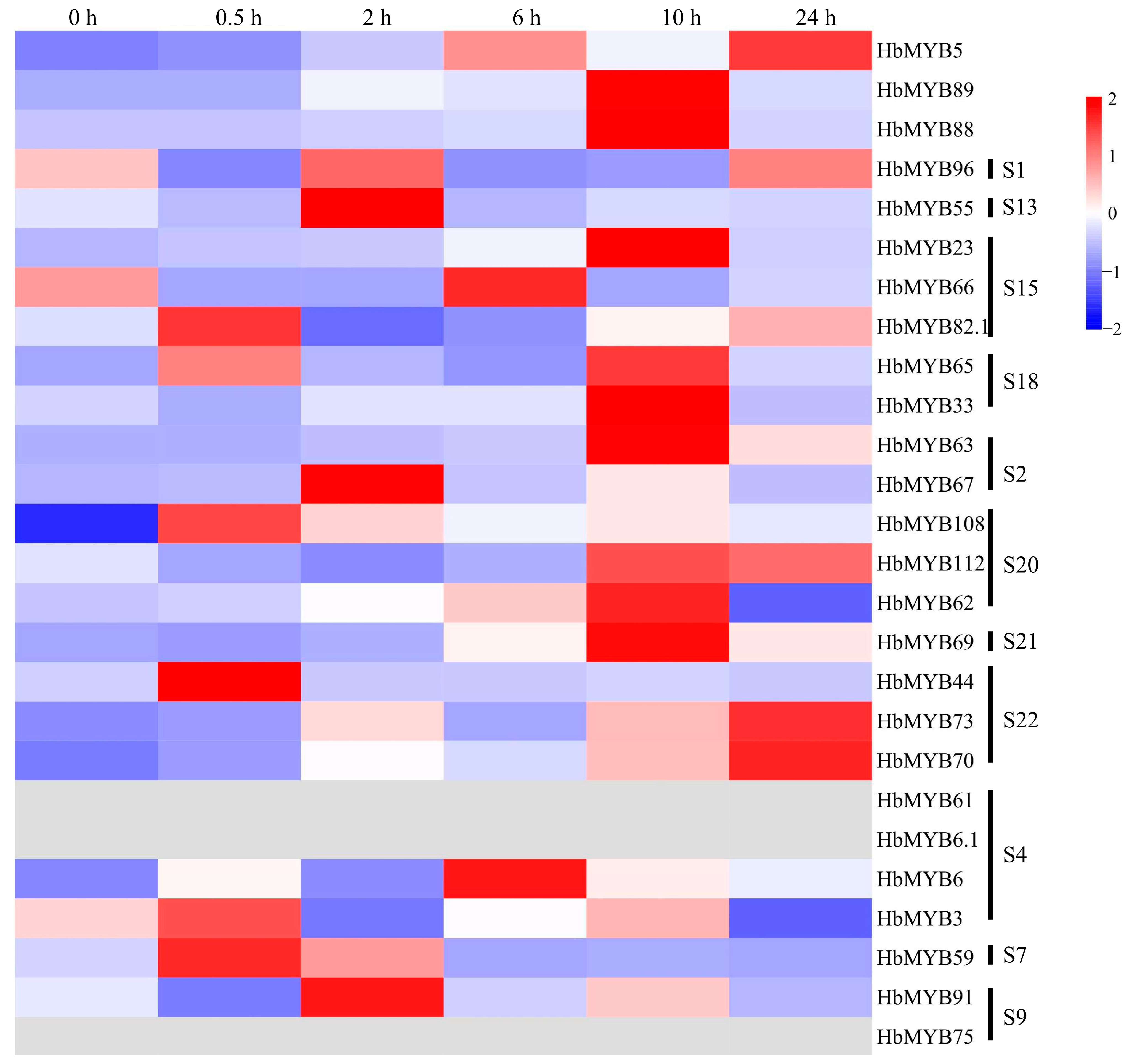
Figure 7 and Table S4 shows that there were four genes with significant differences in expression after ETH treatment, whose expression was 35–250 times higher than at 0 h. The expression of the HbMYB63 gene at 6 h was 19 times higher than that at 0 h, and its expression level was increased significantly at 10 h, to 272 times that at 0 h. At 24 h, HbMYB63 expression decreased, but it was still 94 times higher than that at 0 h (Figure 7, Table S4). The expression level of the HbMYB23 gene at 10 h was 37 times higher than that at 0 h, and the expression level of the HbMYB44 gene was significantly increased at 0.5 h to 42 times that at 0 h, after which it decreased significantly to a level close to that at 0 h (Figure 7, Table S4). The expression of the HbMYB69 gene increased significantly beginning at 6 h and peaked at 10 h, when it was 55 times higher than that at 0 h (Figure 7, Table S4).
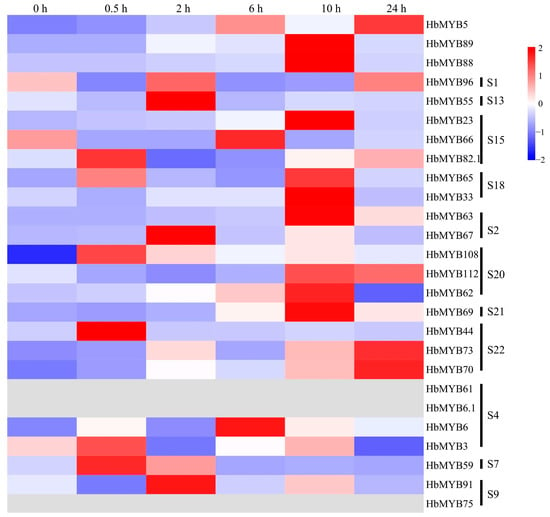
Figure 7.
Expression analysis of HbR2R3-MYBs in rubber trees under ETH treatment.
4. Discussion
The MYB family is a versatile family that exists in all eukaryotes [34]. To date, a large number of MYB genes have been identified in different plants, and MYB TFs have been shown to play key roles in plant development, secondary metabolism, hormone signaling, disease resistance, and abiotic stress tolerance [11]. Previously, a total of 44 members of laticifer MYB were identified, and many sequences were same gene [23]. The new results of Hevea genome analysis [27] and chromosomal location [35] provide new sights for the thoroughly analysis of HbMYB genes. In this study, 132 HbR2R3-MYBs were identified and the expression of 26 representative HbR2R3-MYBs from different MYB subgroups was analyzed. Members of the HbR2R3-MYB family genes are ubiquitously expressed in the cells of all examined organs of the trees, and it is presumed that they play different roles in different plant parts. In Arabidopsis, the S1 subgroup is associated with programmed cell death, drought stress, and disease resistance. The S3, S10, and S22 subfamilies are involved in stress responses, and the S15 and S21 subfamilies are involved in stress responses and cell wall biosynthesis. The S2, S6, and S18 subfamilies are involved in ABA-mediated signal transduction, flavonoid biosynthesis, and abiotic stress [36]. The S4 subgroup encodes transcriptional repressors. The S18 and S20 subfamilies are involved in pollen and anther development and stress [37]. The S25 subgroup is involved in cell differentiation and embryogenesis [38]. The members of a given subgroup may have similar functions. The interaction of AtMYB44 from S22 subgroup with the PYR1-LIKE8 (PYL8) ABA receptor promotes AtMYB44 TF binding to the MBSI motif in the promoter region of the downstream target gene, thereby affecting ABA response-related gene expression and mediating leaf senescence, and the response to stress and mechanical damage [39]. Treated with ABA solution, the expression of AtMYB44 was shown to be upregulated in the stomata and ducts. A yeast two-hybrid assay verified the interaction between AtMYB44 and the ABA receptor RCAR1 [40]. AtMYB2 activates the ABA-induced protein expression [41], and the expression of AtMYB15 is induced by ABA and drought stress [42]. JA-mediated stamen development and seed germination are regulated by bHLH-MYB complexes [43]. In the ET signal transduction pathway, AtMYB44 induces the expression of ETHYLENE INSENSITIVE2 (EIN2), while EIN2 is a central component of the ET signaling pathway [44], which regulates resistance to aphids and moths by regulating EIN2 expression. This regulatory process indicates that AtMYB44 regulates immune responses and induced resistance, which is an important defense mechanism of plants against herbivores [45]. The apple TFs MdMYB1 and MdMYB6 play a role in the regulation of SA and ET in transgenic Nicotiana benthamiana via the MdAAT2 promoter [46]. The regulation of hormones in plants is a complex process and is often accomplished through interactions. The role of AtMYB44 in plant stress resistance is carried out by regulating the expression of WRKY70 to regulate JA and SA signals [17]. ABA and gibberellin (GA) play antagonistic roles in plant seed germination and seedling development. GA inhibitors upregulate the expression of MYB44 to inhibit seed germination [47]. We also found HbMYB44, belongs to S22 subgroup, play important roles in modulating multiple phytohormone signaling and abiotic stress responses [48]. These suggests systematically analyzed the structure of HbR2R3-MYBs provides efficient guidelines for furthering functional verification.
According to the tissue expression analysis, the greatest number of the genes were expressed in flowers, followed by leaves, roots, and stems, and the lowest gene expression was found in bark and latex. Li et al. also found that in grape the VvMYBB1 gene and the VvMYBA3 gene were strongly expressed in flowers [49]. These findings suggest that most MYB family members play an important role in flower development. MYB genes were found to be involved in plant hormone response processes in a previous study [50]. Multiple MYB genes were found to be involved in growth hormone, ethylene, and cytokinin responses in Arabidopsis [51]. AtMYB77 regulates auxin signaling by interacting with auxin response factors and regulating the expression of auxin-inducible genes to control lateral root growth and development [52]. After the ETH treatment of rubber trees in the study, the differences in the expression of four genes were significant. These results suggest that these four genes are involved in the ET signaling pathway in rubber trees. HbMYB63 and HbMYB67 and Arabidopsis AtMYB15, AtMYB13, and AtMYB14 clustered into the S2 subgroup. The overexpression of AtMYB15 and GsMYB15 in Arabidopsis increases salt stress [53]. AtMYB15 is also involved in cold stress tolerance [54]. MYB genes are also involved in the process of disease resistance response in plants. AtMYB44 mediates stomatal opening to increase resistance to Pseudomonas syringae pv. tomato DC3000, Pst [55,56]. In addition, AtMYB44 promotes the disease resistance of Arabidopsis and flowering [57]. When powdery mildew was applied to the leaves of rubber tree, the results showed that the expression levels of 14 genes were significantly changed. It indicates that these genes are involved in the resistance of rubber trees to powdery mildew at different times after powdery mildew treatment of rubber tree leaves, especially HbMYB62 plays an important role in the resistance of rubber trees to powdery mildew. AtMYB62 is an R2R3-MYB TF that localizes to the nucleus and is a negative regulator of PSI genes. The overexpression of AtMYB62 can change the response of plants to phosphorus starvation, alter root structure, and promote the plant absorption of Pi [15]. GmMYB62 interacts with soybean 14-3-3 proteins and GmMYB176, and 14-3-3 proteins act as a scaffold for GmMYB62 and GmMYB176, regulating their localization in soybean cells [58,59]. MYB gene structure is conserved, and they can combine with downstream MBSI(T/C)AAC(T/G)G and MBSIIG(G/T)T(A/T)G(G/T)T components in gene promoter regions [60]. The biosynthetic pathway of natural rubber requires the participation of HblMYB19 and HblMYB44 [23]. For example, MYB44 is a substrate for mitogen-activated protein kinase (MPK3) and an early response factor in plant defense [61] involved in the molecular mechanism of leaf stomatal resistance mediated by intergenic spacer regions (ISRs) [55]. Further studies of HbMYB62, HbMYB63, HbMYB73, and HbMYB88 will help to elucidate the mechanisms of rubber biosynthesis and stress regulation.
5. Conclusions
A total of 132 HbMYB family members were identified in rubber trees and characterized 9 unique motifs. Expression analysis revealed that the members of the HbR2R3-MYB family were mainly expressed in flowers. Based on all of the information, HbMYB62, HbMYB63, HbMYB73, and HbMYB88 are suggested as targets for further research. These results provide fundamental knowledge for further study of HbMYB family in transcriptional regulation in biological processes in rubber tree.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14040710/s1, Table S1: Sequences of primers designed for qRT-PCR analysis of 26 selected MYB superfamily genes. Table S2: Protein Structure of 132 HbR2R3-MYBs. Table S3: List of 131 MYB proteins/genes including 126 R1R2R3-MYBs and 5 R2R3-MYBs in Arabidopsis. Table S4: qRT-PCR analysis of 26 selected HbR2R3-MYB superfamily genes in rubber tree.
Author Contributions
Conceptualization, L.W. and M.W.; methodology, M.L., H.Y., S.F., B.G. and L.D.; software, M.L. and H.Y.; writing—original draft preparation, M.L.; writing—review and editing, L.W., H.Y. and M.W.; supervision, L.W. and M.W.; funding acquisition, L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Hainan Provincial Natural Science Foundation of China (321CXTD445) and the National Natural Science Foundation of China (Grant No. 31570591).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in [insert article or supplementary material here].
Conflicts of Interest
The authors declare no competing interest.
References
- Ambawat, S.; Sharma, P.; Yadav, N.R.; Yadav, R.C. MYB transcription factor genes as regulators for plant responses: An overview. Physiol. Mol. Biol. Plants 2013, 19, 307–321. [Google Scholar] [CrossRef]
- Paz-Ares, J.; Ghosal, D.; Wienand, U.; Petersont, P.A.; Saedler, H. The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J. 1987, 6, 3553–3558. [Google Scholar] [CrossRef] [PubMed]
- Heendeniya, R.G.; Gruber, M.Y.; Lei, Y.; Yu, P. Biodegradation Profiles of Proanthocyanidin-Accumulating Alfalfa Plants Coexpressing Lc-bHLH and C1-MYB Transcriptive Flavanoid Regulatory Genes. J. Agric. Food Chem. 2019, 67, 4793–4799. [Google Scholar] [CrossRef] [PubMed]
- Kranz, H.D.; Denekamp, M.; Greco, R.; Jin, H.; Leyva, A.; Meissner, R.C.; Petroni, K.; Urzainqui, A.; Bevan, M.; Martin, C.; et al. Towards functional characterisation of the members of the R2R3-MYB gene family from Arabidopsis thaliana. Plant J. 1998, 16, 263–276. [Google Scholar] [CrossRef]
- Riechmann, J.L.; Ratcliffe, O.J. A genomic perspective on plant transcription factors. Curr. Opin. Plant Biol. 2000, 3, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Kanei-Ishii, C.; Sarai, A.; Sawazaki, T.; Nakagoshi, H.; He, D.N.; Ogata, K.; Nishimura, Y.; Ishii, S. The tryptophan cluster: A hypothetical structure of the DNA-binding domain of the myb protooncogene product. J. Biol. Chem. 1990, 265, 19990–19995. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; He, K.; Liu, M.; Li, J.; Gao, Z.; Lin, Z.; Zhang, Y.; Wang, X.; Qiu, X.; et al. The MYB transcription factor superfamily of Arabidopsis: Expression analysis and phylogenetic comparison with the rice MYB family. Plant Mol. Biol. 2006, 60, 107–124. [Google Scholar]
- Wilkins, O.; Nahal, H.; Foong, J.; Provart, N.J.; Campbell, M.M. Expansion and diversification of the Populus R2R3-MYB family of transcription factors. Plant Physiol. 2009, 149, 981–993. [Google Scholar] [CrossRef]
- Du, H.; Yang, S.S.; Liang, Z.; Feng, B.R.; Liu, L.; Huang, Y.B.; Tang, Y.X. Genome-wide analysis of the MYB transcription factor superfamily in soybean. BMC Plant Biol. 2012, 12, 106. [Google Scholar] [CrossRef]
- Allan, A.C.; Hellens, R.P.; Laing, W.A. MYB transcription factors that colour our fruit. Trends Plant Sci. 2008, 13, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Cominelli, E.; Tonelli, C. A new role for plant R2R3-MYB transcription factors in cell cycle regulation. Cell Res. 2009, 19, 1231–1232. [Google Scholar] [CrossRef]
- Hou, X.J.; Li, S.B.; Liu, S.R.; Hu, C.G.; Zhang, J.Z. Genome-wide classification and evolutionary and expression analyses of citrus MYB transcription factor families in sweet orange. PLoS ONE 2014, 9, e112375. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Zhang, J.; Li, H.; Meng, D.; Li, R.; Dai, X.; Wang, S.; Liu, W.; Qu, H.; Xu, G. Maintenance of phosphate homeostasis and root development are coordinately regulated by MYB1, an R2R3-type MYB transcription factor in rice. J. Exp. Bot. 2017, 68, 3603–3615. [Google Scholar] [CrossRef] [PubMed]
- Devaiah, B.N.; Madhuvanthi, R.; Karthikeyan, A.S.; Raghothama, K.G. Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Mol. Plant 2009, 2, 43–58. [Google Scholar] [CrossRef]
- Jung, C.; Seo, J.S.; Han, S.W.; Koo, Y.J.; Kim, C.H.; Song, S.I.; Nahm, B.H.; Choi, Y.D.; Cheong, J.J. Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol. 2008, 146, 623–635. [Google Scholar] [CrossRef]
- Shim, J.S.; Choi, Y.D. Direct regulation of WRKY70 by AtMYB44 in plant defense responses. Plant Signal. Behav. 2013, 8, e20783. [Google Scholar]
- Zhang, K.; Logacheva, M.D.; Meng, Y.; Hu, J.; Wan, D.; Li, L.; Janovska, D.; Wang, Z.; Georgiev, M.I.; Yu, Z.; et al. Jasmonate-responsive MYB factors spatially repress rutin biosynthesis in Fagopyrum tataricum. J. Exp. Bot. 2018, 69, 1955–1966. [Google Scholar] [CrossRef]
- Puskas, J.E.; Gautriaud, E.; Deffieux, A.; Kennedy, J.P. Natural rubber biosynthesis—A living carbocationic polymerization? Prog. Polym. Sci. 2006, 31, 533–548. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Yeh, S. Isoprene Emission from Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 407–436. [Google Scholar] [CrossRef]
- Li, H.L.; Wei, L.R.; Guo, D.; Wang, Y.; Zhu, J.H.; Chen, X.T.; Peng, S.Q. HbMADS4, a MADS-box Transcription Factor from Hevea brasiliensis, Negatively Regulates HbSRPP. Front. Plant Sci. 2016, 7, 1709. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, D.; Li, H.L.; Peng, S.Q. Characterization of HbWRKY1, a WRKY transcription factor from Hevea brasiliensis that negatively regulates HbSRPP. Plant Physiol. Biochem. 2013, 71, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhan, D.F.; Li, H.L.; Guo, D.; Zhu, J.H.; Peng, S.Q. Transcriptome-Wide Identification and Characterization of MYB Transcription Factor Genes in the Laticifer Cells of Hevea brasiliensis. Front. Plant Sci. 2017, 8, 1974. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.; Wang, M.; Zhang, Y. Effects of powdery mildew infection on chloroplast and mitochondrial functions in rubber tree. Trop. Plant Pathol. 2014, 39, 242–250. [Google Scholar] [CrossRef]
- Qin, B.; Zheng, F.; Zhang, Y. Molecular cloning and characterization of a Mlo gene in rubber tree (Hevea brasiliensis). J. Plant Physiol. 2015, 175, 78–85. [Google Scholar] [CrossRef]
- Blanco, E.; Curci, P.L.; Manconi, A.; Sarli, A.; Zuluaga, D.L.; Sonnante, G. R2R3-MYBs in Durum Wheat: Genome-Wide Identification, Poaceae-Specific Clusters, Expression, and Regulatory Dynamics Under Abiotic Stresses. Front. Plant Sci. 2022, 13, 896945. [Google Scholar] [CrossRef]
- Tang, C.; Yang, M.; Fang, Y.; Luo, Y.; Gao, S.; Xiao, X.; An, Z.; Zhou, B.; Zhang, B.; Tan, X.; et al. The rubber tree genome reveals new insights into rubber production and species adaptation. Nat. Plants 2016, 2, 16073. [Google Scholar] [CrossRef]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; de Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E.; et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple Sequence Alignment Using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2003, 2, 2.3.1–2.3.22. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Sheard, L.B.; Tan, X.; Mao, H.; Withers, J.; Ben-Nissan, G.; Hinds, T.R.; Kobayashi, Y.; Hsu, F.F.; Sharon, M.; Browse, J.; et al. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 2010, 468, 400–405. [Google Scholar] [CrossRef]
- Cheng, H.; Song, X.; Hu, Y.; Wu, T.; Yang, Q.; An, Z.; Feng, S.; Deng, Z.; Wu, W.; Zeng, X.; et al. Chromosome-level wild Hevea brasiliensis genome provides new tools for genomic-assisted breeding and valuable loci to elevate rubber yield. Plant Biotechnol. J. 2023. [Google Scholar] [CrossRef]
- Stracke, R.; Werber, M.; Weisshaar, B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 2001, 4, 447–456. [Google Scholar] [CrossRef]
- Millar, A.A.; Gubler, F. The Arabidopsis GAMYB-like genes, MYB33 and MYB65, are microRNA-regulated genes that redundantly facilitate anther development. Plant Cell 2005, 17, 705–721. [Google Scholar] [CrossRef]
- Wang, X.; Niu, Q.W.; Teng, C.; Li, C.; Mu, J.; Chua, N.H.; Zuo, J. Overexpression of PGA37/MYB118 and MYB115 promotes vegetative-to-embryonic transition in Arabidopsis. Cell Res. 2009, 19, 224–235. [Google Scholar] [CrossRef]
- Jaradat, M.R.; Feurtado, J.A.; Huang, D.; Lu, Y.; Cutler, A.J. Multiple roles of the transcription factor AtMYBR1/AtMYB44 in ABA signaling, stress responses, and leaf senescence. BMC Plant Biol. 2013, 13, 192. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Zhang, L.; Wang, X.; Zhao, Z.; Tao, Z.; Wang, J.; Wang, J.; Lin, M.; Li, X.; et al. Arabidopsis ABA receptor RCAR1/PYL9 interacts with an R2R3-type MYB transcription factor, AtMYB44. Int. J. Mol. Sci. 2014, 15, 8473–8490. [Google Scholar] [CrossRef]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef]
- Ding, Z.; Li, S.; An, X.; Liu, X.; Qin, H.; Wang, D. Transgenic expression of MYB15 confers enhanced sensitivity to abscisic acid and improved drought tolerance in Arabidopsis thaliana. J. Genet. Genom. 2009, 36, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Huang, H.; Song, S.; Xie, D. Regulation of Jasmonate-Mediated Stamen Development and Seed Production by a bHLH-MYB Complex in Arabidopsis. Plant Cell 2015, 27, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, L.; Jia, Z.; Lu, B.; Shi, H.; Shao, W.; Dong, H. Transcription factor AtMYB44 regulates induced expression of the ETHYLENE INSENSITIVE2 gene in Arabidopsis responding to a harpin protein. Mol. Plant-Microbe Interact. MPMI 2011, 24, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.B.; Li, X.J.; Sun, W.W.; Li, L.; Gao, R.; Zhu, Q.; Tian, S.M.; Fu, M.Q.; Yu, H.L.; Tang, X.M.; et al. AtMYB44 regulates resistance to the green peach aphid and diamondback moth by activating EIN2-affected defences in Arabidopsis. Plant Biol. 2013, 15, 841–850. [Google Scholar] [CrossRef]
- Li, P.C.; Yu, S.W.; Shen, J.; Li, Q.Q.; Li, D.P.; Li, D.Q.; Zheng, C.C.; Shu, H.R. The transcriptional response of apple alcohol acyltransferase (MdAAT2) to salicylic acid and ethylene is mediated through two apple MYB TFs in transgenic tobacco. Plant Mol. Biol. 2014, 85, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.C.; Hoang, M.H.; Kim, H.S.; Lee, K.; Liu, X.M.; Kim, S.H.; Bahk, S.; Park, H.C.; Chung, W.S. Phosphorylation of the transcriptional regulator MYB44 by mitogen activated protein kinase regulates Arabidopsis seed germination. Biochem. Biophys. Res. Commun. 2012, 423, 703–708. [Google Scholar] [CrossRef]
- Qin, B.; Fan, S.L.; Yu, H.Y.; Lu, Y.X.; Wang, L.F. HbMYB44, a Rubber Tree MYB Transcription Factor with Versatile Functions in Modulating Multiple Phytohormone Signaling and Abiotic Stress Responses. Front. Plant Sci. 2022, 13, 893896. [Google Scholar] [CrossRef]
- Li, G.; Quan, R.; Jing, P.; Wang, M.; Xu, W.; Hu, H. Cloning, Subcellular Location and Expression Analysis of Grape MYB Gene. Res. Sq. 2021, 1–16. [Google Scholar]
- Jiang, C.K.; Rao, G.Y. Insights into the Diversification and Evolution of R2R3-MYB Transcription Factors in Plants. Plant Physiol. 2020, 183, 637–655. [Google Scholar] [CrossRef]
- Abe, H.; Yamaguchi-Shinozaki, K.; Urao, T.; Lwasaki, T.; Hosokawa, D.; Shinozaki, K. Role of Arabidopsis MYC and MYB Homologs in Droughtand Abscisic Acid-Regulated Gene Expression. Plant Cell 1997, 9, 1859–1868. [Google Scholar] [PubMed]
- Shin, R.; Burch, A.Y.; Huppert, K.A.; Tiwari, S.B.; Murphy, A.S.; Guilfoyle, T.J.; Schachtman, D.P. The Arabidopsis transcription factor MYB77 modulates auxin signal transduction. Plant Cell 2007, 19, 2440–2453. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.J.; Wang, Y.Y.; Zhang, Y.X.; Guo, W.; Jiao, Y.Q.; Zhou, X.A. Overexpression of the Wild Soybean R2R3-MYB Transcription Factor GsMYB15 Enhances Resistance to Salt Stress and Helicoverpa Armigera in Transgenic Arabidopsis. Int. J. Mol. Sci. 2018, 19, 3958. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Hao, Y.; Kapoor, A.; Dong, C.H.; Fujii, H.; Zheng, X.; Zhu, J.K. A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J. Biol. Chem. 2006, 281, 37636–37645. [Google Scholar] [CrossRef]
- Hieno, A.; Naznin, H.A.; Hyakumachi, M.; Higuchi-Takeuchi, M.; Matsui, M.; Yamamoto, Y.Y. Possible Involvement of MYB44-Mediated Stomatal Regulation in Systemic Resistance Induced by Penicillium simplicissimum GP17-2 in Arabidopsis. Microbes Environ. 2016, 31, 154–159. [Google Scholar] [CrossRef]
- Liu, R.; Lu, B.; Wang, X.; Zhang, C.; Zhang, S.; Qian, J.; Chen, L.; Shi, H.; Dong, H. Thirty-seven transcription factor genes differentially respond to a harpin protein and affect resistance to the green peach aphid in Arabidopsis. J. Biosci. 2010, 35, 435–450. [Google Scholar] [CrossRef]
- Park, S.Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.F.; et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef]
- Li, X.; Dhaubhadel, S. 14-3-3 proteins act as scaffolds for GmMYB62 and GmMYB176 and regulate their intracellular localization in soybean. Plant Signal. Behav. 2012, 7, 965–968. [Google Scholar] [CrossRef]
- Kirik, V.; Kolle, K.; Misera, S.; Baumlein, H. Two novel MYB homologues with changed expression in late embryogenesis-defective Arabidopsis mutants. Plant Mol. Biol. 1998, 37, 819–827. [Google Scholar] [CrossRef]
- Romero, I.; Fuertes, A.; Benito, M.J.; Malpica, J.M.; Leyva, A.; Paz-Ares, J. More than 80 R2R3-MYB regulatory genes in the genome of Arabidopsis thaliana. Plant J. 1998, 14, 273–284. [Google Scholar] [CrossRef]
- Persak, H.; Pitzschke, A. Tight interconnection and multi-level control of Arabidopsis MYB44 in MAPK cascade signalling. PLoS ONE 2013, 8, e57547. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).