Roots, Litter, and Seasonal Drought Together Inhibit Plant Growth in the Herbaceous Layer in a Subtropical Moist Forest of Southwestern China
Abstract
1. Introduction
2. Materials and Methods
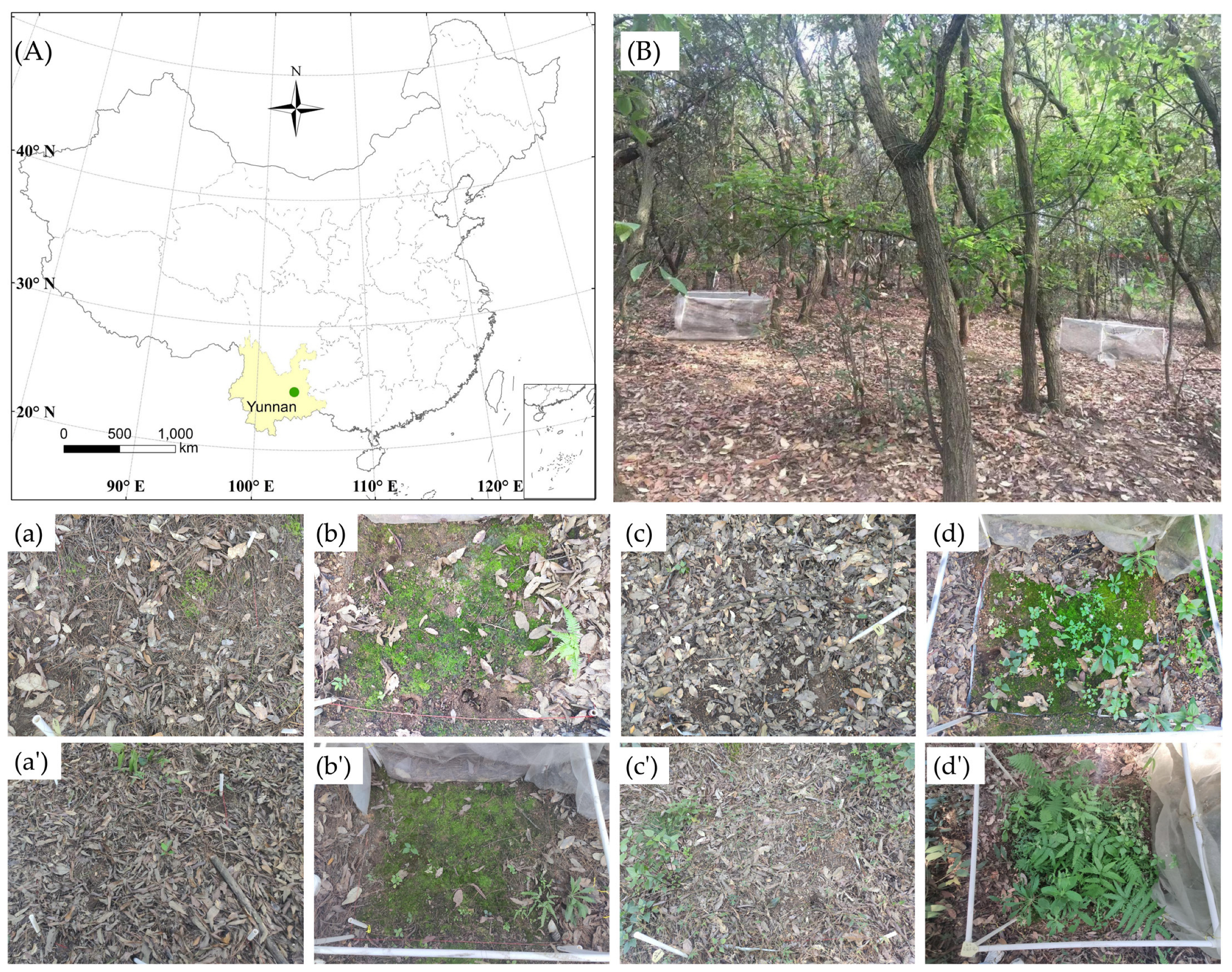
2.1. Study Sites
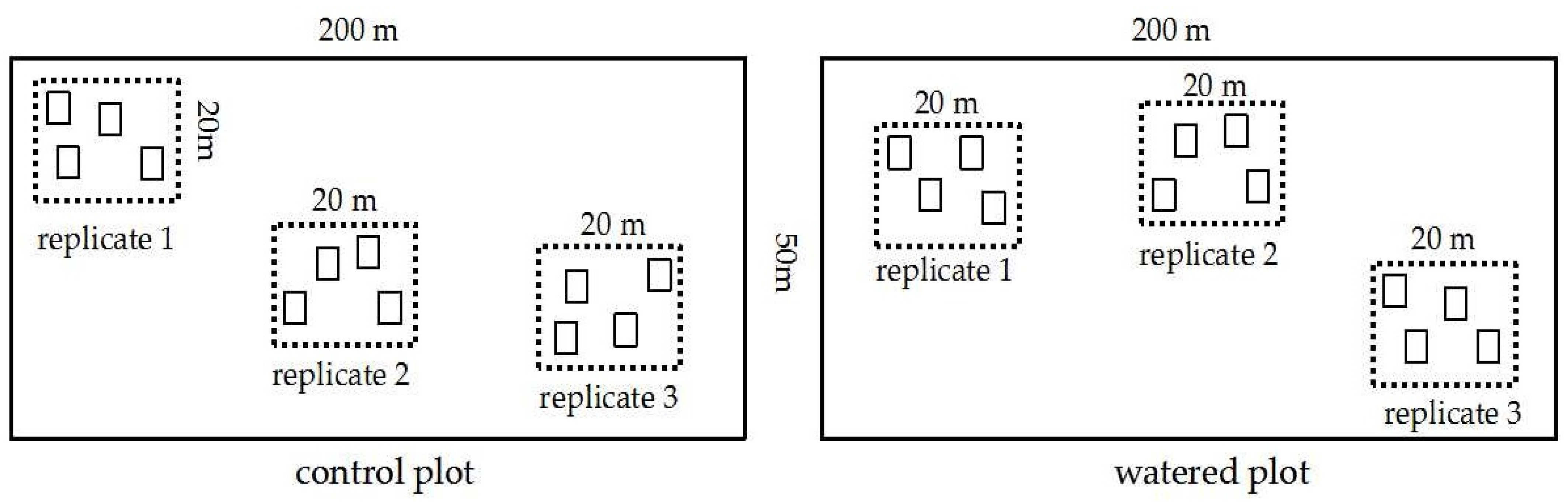
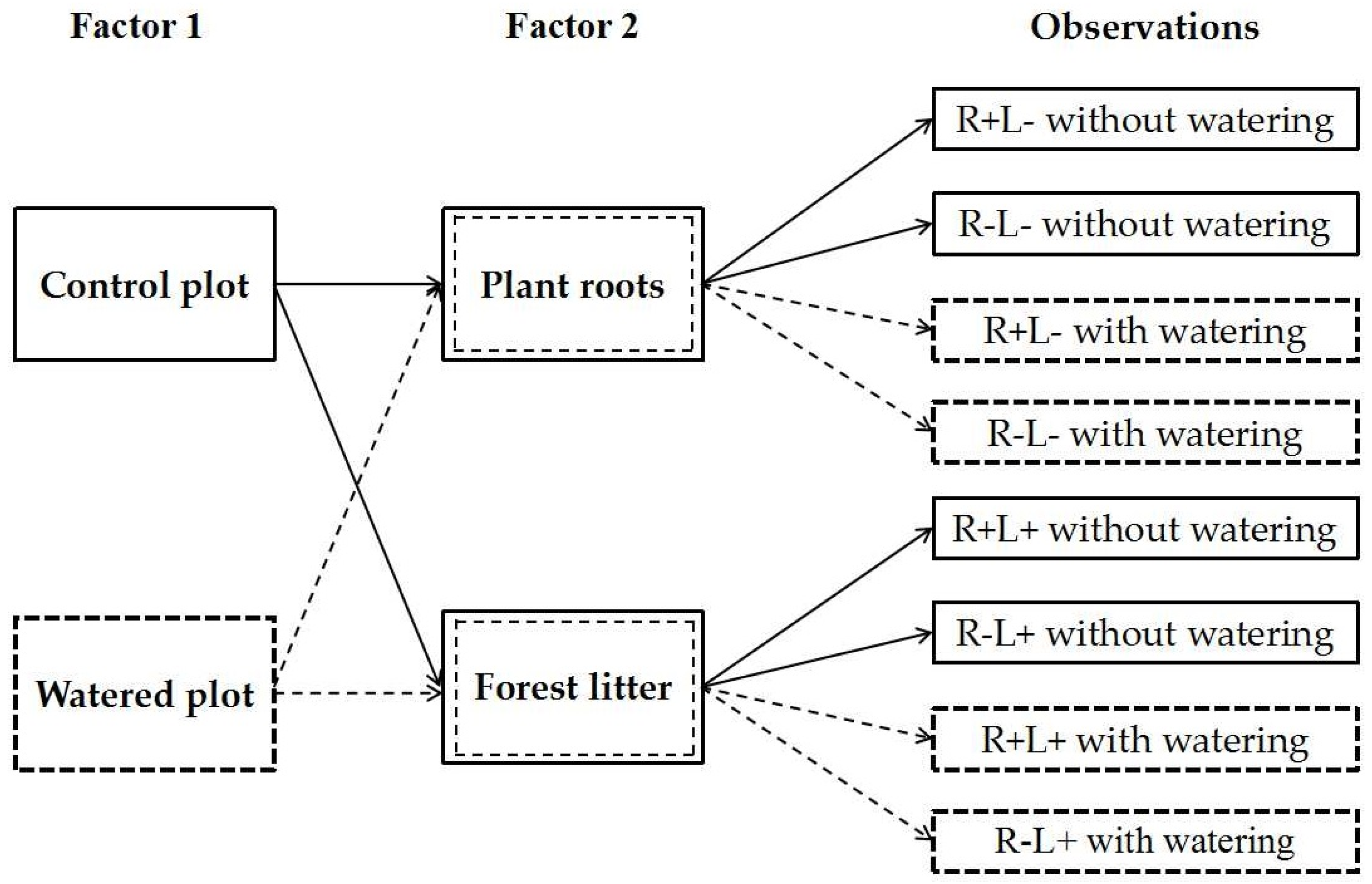
2.2. Experimental Design
2.3. Data Collection
2.4. Data Analysis
3. Results
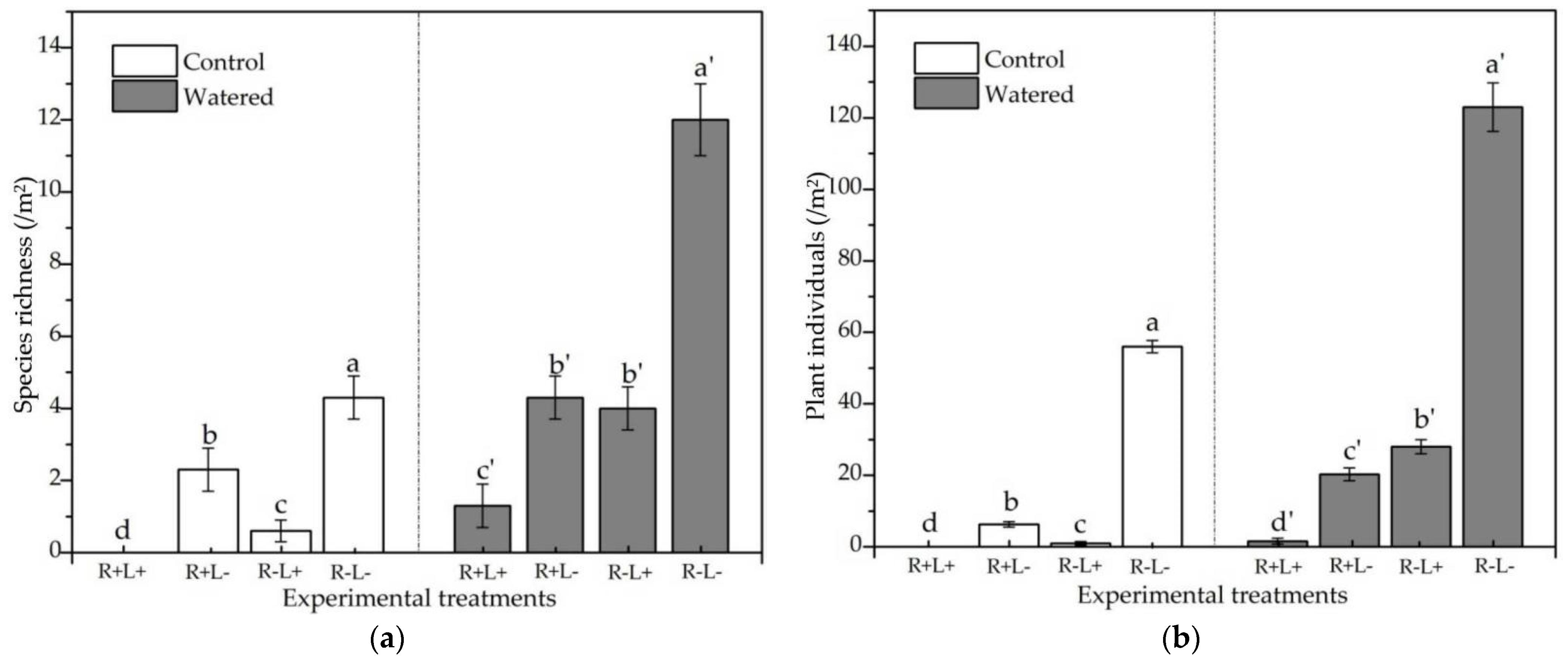
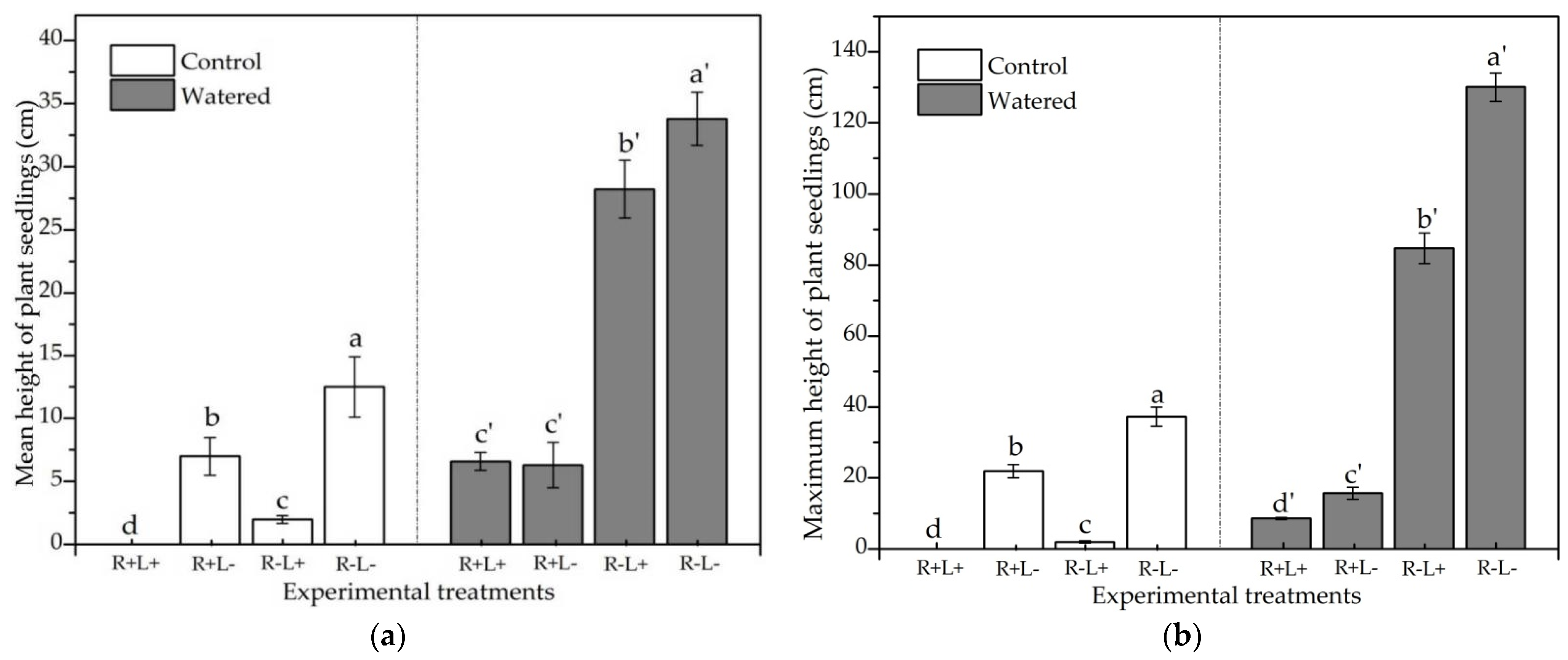
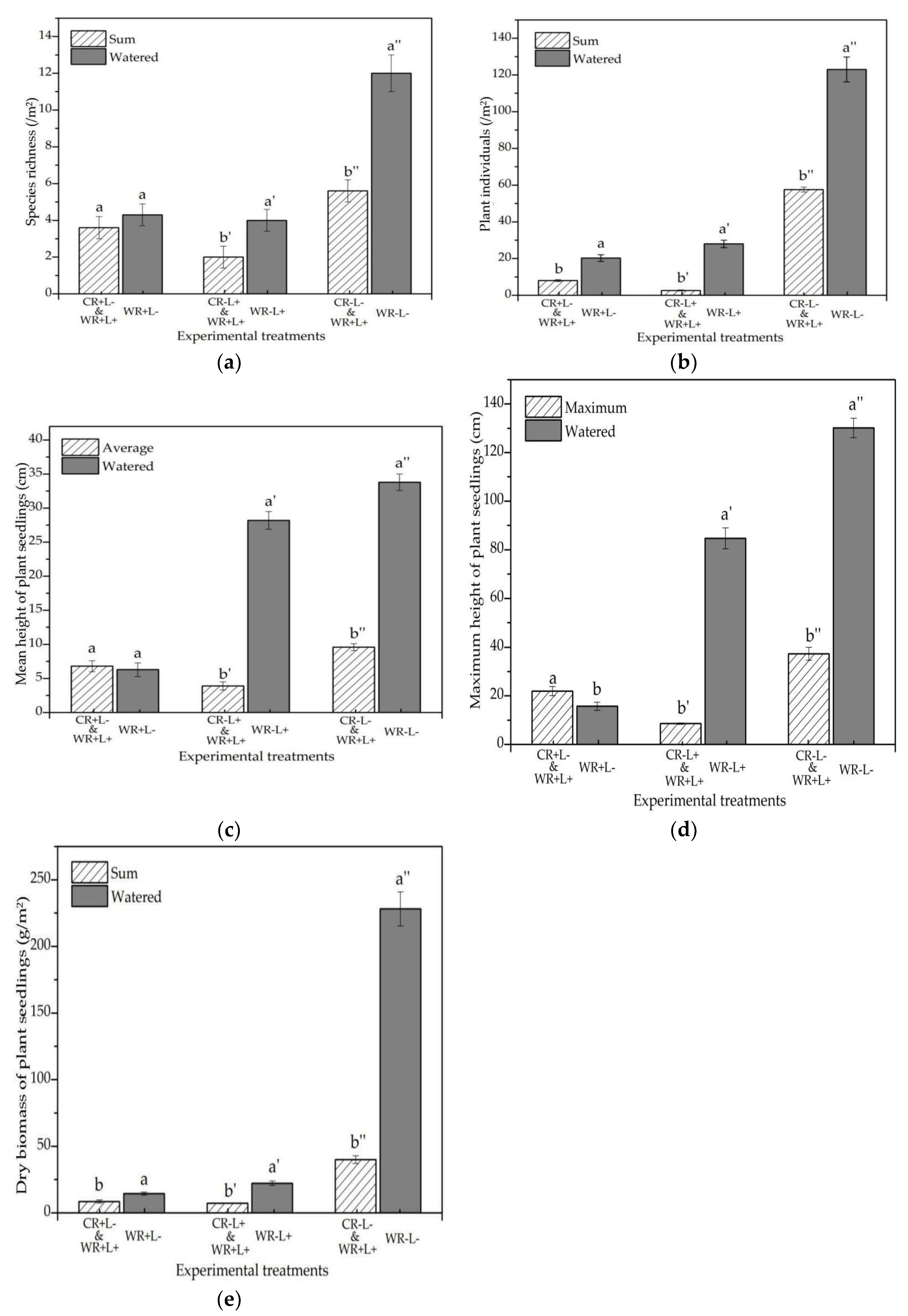
3.1. Species Richness and the Number of Plant Individuals
3.2. Mean and Maximum Plant Heights
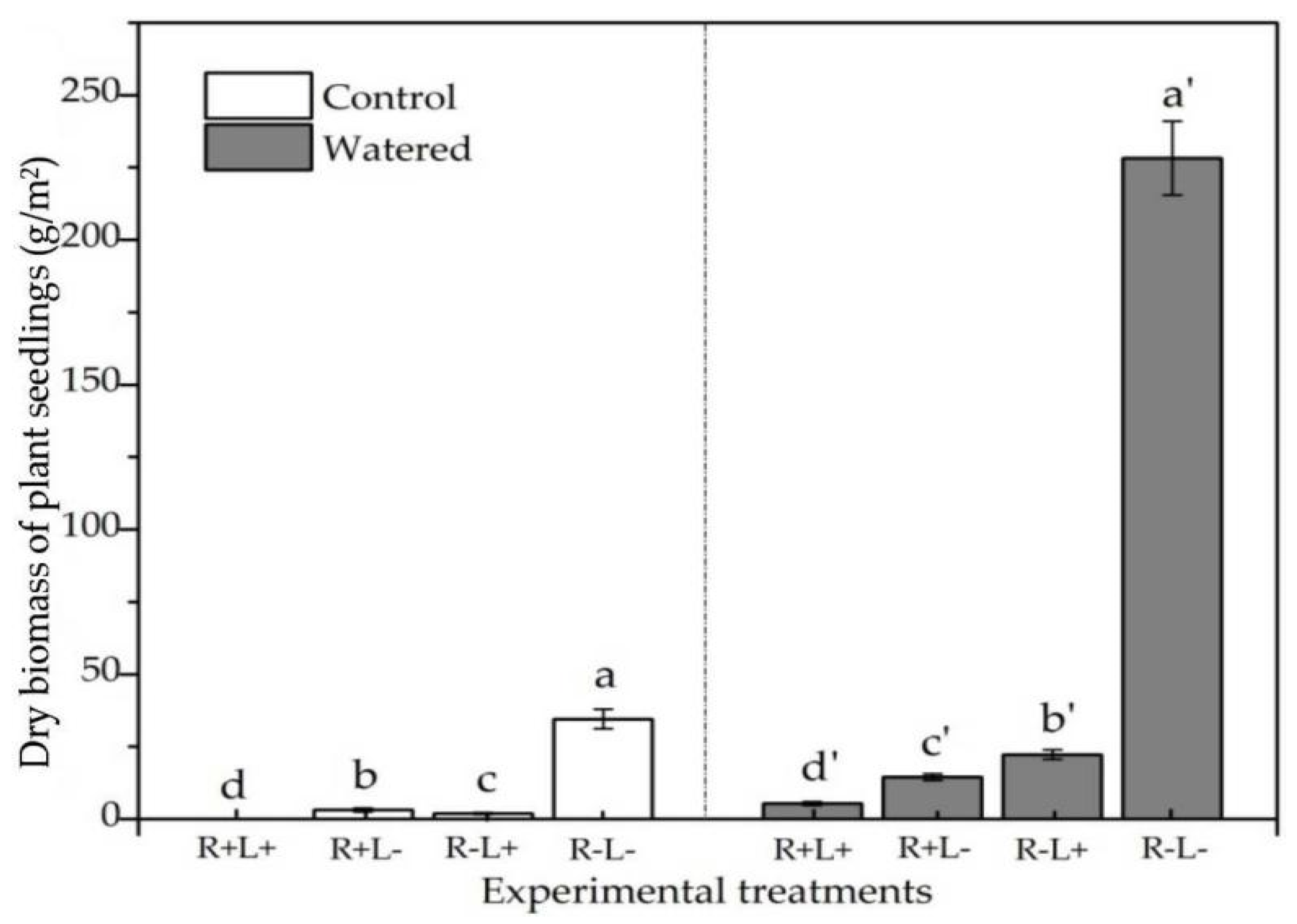
3.3. Plant Dry Biomass
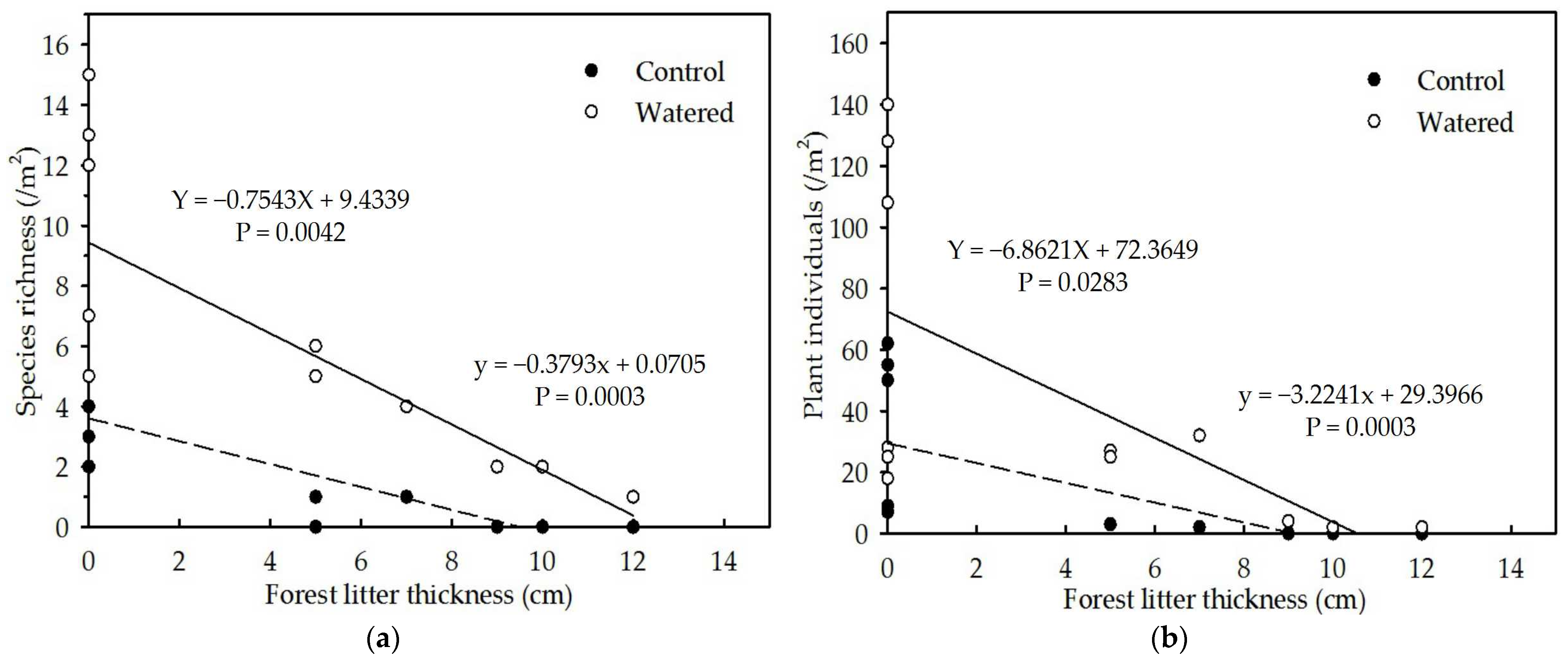
3.4. Analyses of the Controlled Environmental Factors
3.5. Synergistic Analyses
4. Discussion
4.1. Impacts of Forest Litter on Plant Diversity and Seedling Growth in the Herbaceous Layer
4.2. Impacts of Plant Roots on Plant Diversity and Seedling Growth in the Herbaceous Layer
4.3. Impacts of Seasonal Drought on Plant Diversity and Seedling Growth in the Herbaceous Layer
4.4. Synergistic Effect of Plant Roots, Forest litter, and Seasonal Drought on Plant Diversity and Seedling Growth in the Herbaceous Layer
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, G.; Mi, X.; Eiserhardt, W.L.; Jin, G.; Sang, W.; Lu, Z.; Wang, X.; Li, X.; Li, B.; Sun, I. Assembly of forest communities across East Asia–insights from phylogenetic community structure and species pool scaling. Sci. Rep. 2015, 5, 9337. [Google Scholar] [CrossRef]
- Zavala, M.A.; Zea, E. Mechanisms maintaining biodiversity in Mediterranean pine-oak forests: Insights from a spatial simulation model. Plant Ecol. 2004, 171, 197–207. [Google Scholar] [CrossRef]
- Liu, P.; Wang, W.; Bai, Z.; Guo, Z.; Ren, W.; Huang, J.; Xu, Y.; Yao, J.; Ding, Y.; Zang, R. Competition and facilitation co-regulate the spatial patterns of boreal tree species in Kanas of Xinjiang, northwest China. For. Ecol. Manag. 2020, 467, 118167. [Google Scholar] [CrossRef]
- Peter, B.R.; Peter, B.; Carlson, D.; Lee, E.F.; K.Friedman, S.; David, F.G. Influence of logging, fire, and forest type on biodiversity and productivity in southern boreal forests. Ecology 2001, 82, 2731–2748. [Google Scholar] [CrossRef]
- Reich, P.B.; Frelich, L.E.; Voldseth, R.A.; Bakken, P.; Adair, E.C. Understorey diversity in southern boreal forests is regulated by productivity and its indirect impacts on resource availability and heterogeneity. J. Ecol. 2012, 100, 539–545. [Google Scholar] [CrossRef]
- Gao, Y.; Markkanen, T.; Thum, T.; Aurela, M.; Lohila, A.; Mammarella, I.; Kämäräinen, M.; Hagemann, S.; Aalto, T. Assessing various drought indicators in representing summer drought in boreal forests in Finland. Hydrol. Earth Syst. Sci. 2015, 12, 8091–8129. [Google Scholar] [CrossRef]
- Bernhardt-Römermann, M.; Baeten, L.; Craven, D.; De Frenne, P.; Hédl, R.; Lenoir, J.; Bert, D.; Brunet, J.; Chudomelová, M.; Decocq, G. Drivers of temporal changes in temperate forest plant diversity vary across spatial scales. Glob. Chang. Biol. 2015, 21, 3726–3737. [Google Scholar] [CrossRef]
- Dar, J.A.; Sundarapandian, S. Patterns of plant diversity in seven temperate forest types of Western Himalaya, India. J. Asia-Pac. Biodivers. 2016, 9, 280–292. [Google Scholar] [CrossRef]
- Bedford, B.L.; Walbridge, M.R.; Aldous, A. Patterns in nutrient availability and plant diversity of temperate North American wetlands. Ecology 1999, 80, 2151–2169. [Google Scholar] [CrossRef]
- Wright, J.S. Plant diversity in tropical forests: A review of mechanisms of species coexistence. Oecologia 2002, 130, 1–14. [Google Scholar] [CrossRef]
- Corlett, R.T. Plant diversity in a changing world: Status, trends, and conservation needs. Plant Divers. 2016, 38, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fang, J.; Tang, Z.; Lin, X. Patterns, determinants and models of woody plant diversity in China. Proc. R. Soc. B Biol. Sci. 2011, 278, 2122–2132. [Google Scholar] [CrossRef] [PubMed]
- Veen, G.; Fry, E.L.; ten Hooven, F.C.; Kardol, P.; Morriën, E.; De Long, J.R. The role of plant litter in driving plant-soil feedbacks. Front. Environ. Sci. 2019, 7, 168. [Google Scholar] [CrossRef]
- Esmailzadeh, O.; Hosseini, S.; Tabari, M. Relationship between soil seed bank and above-ground vegetation of a mixed-deciduous temperate forest in northern Iran. J. Agric. Sci. Technol. 2011, 13, 411–424. Available online: https://jast.modares.ac.ir/article-23-9196-en.html (accessed on 27 August 2022).
- Cortez, J. Field decomposition of leaf litters: Relationships between decomposition rates and soil moisture, soil temperature and earthworm activity. Soil Biol. Biochem. 1998, 30, 783–793. [Google Scholar] [CrossRef]
- Erickson, H.E.; Helmer, E.; Brandeis, T.J.; Lugo, A.E. Controls on fallen leaf chemistry and forest floor element masses in native and novel forests across a tropical island. Ecosphere 2014, 5, 1–28. [Google Scholar] [CrossRef]
- Likasiri, C.; Duangdai, E.; Pongvuthithum, R. Mathematical model on the effects of global climate change and decreasing forest cover on seasonal rainfall in Northern Thailand. Ecol. Model. 2014, 272, 388–393. [Google Scholar] [CrossRef]
- Schuldt, B.; Buras, A.; Arend, M.; Vitasse, Y.; Beierkuhnlein, C.; Damm, A.; Gharun, M.; Grams, T.E.; Hauck, M.; Hajek, P. A first assessment of the impact of the extreme 2018 summer drought on Central European forests. Basic Appl. Ecol. 2020, 45, 86–103. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Derose, R.J.; Long, J.N. Drought-driven disturbance history characterizes a southern Rocky Mountain subalpine forest. Can. J. For. Res. 2012, 42, 1649–1660. [Google Scholar] [CrossRef]
- Canelles, Q.; Aquilué, N.; James, P.M.A.; Lawler, J.; Brotons, L. Global review on interactions between insect pests and other forest disturbances. Landsc. Ecol. 2021, 36, 945–972. [Google Scholar] [CrossRef]
- Rowland, L.; da Costa, A.C.; Oliveira, A.A.; Almeida, S.S.; Ferreira, L.V.; Malhi, Y.; Metcalfe, D.B.; Mencuccini, M.; Grace, J.; Meir, P. Shock and stabilisation following long-term drought in tropical forest from 15 years of litterfall dynamics. J. Ecol. 2018, 106, 1673–1682. [Google Scholar] [CrossRef]
- Barba, J.; Lloret, F.; Yuste, J.C. Effects of drought-induced forest die-off on litter decomposition. Plant Soil 2015, 402, 91–101. [Google Scholar] [CrossRef]
- Edwards, W.; Krockenberger, A. Seedling mortality due to drought and fire associated with the 2002 El Nino event in a tropical rain forest in north-east Queensland, Australia. Biotropica 2006, 38, 16–26. [Google Scholar] [CrossRef]
- Peñuelas, J.; Rico, L.; Ogaya, R.; Jump, A.S.; Terradas, J. Summer season and long-term drought increase the richness of bacteria and fungi in the foliar phyllosphere of Quercus ilex in a mixed Mediterranean forest. Plant Biol. 2012, 14, 565–575. [Google Scholar] [CrossRef]
- Peters, M.P.; Iverson, L.R.; Matthews, S.N. Long-term droughtiness and drought tolerance of eastern US forests over five decades. For. Ecol. Manag. 2015, 345, 56–64. [Google Scholar] [CrossRef]
- Huang, W.H.; Sui, Y.; Yang, X.G.; Dai, W.S.; Li, M.S. Characteristics and adaptation of seasonal drought in southern China under the background of climate change. III. Spatiotemporal characteristics of seasonal drought in southern China based on the percentage of precipitation anomalies. Chin. J. Appl. Ecol. 2013, 24, 397–406. [Google Scholar]
- Ahmed, K.; Shahid, S.; Chung, E.S.; Wang, X.J.; Harun, S.B. Climate change uncertainties in seasonal drought severity-area-frequency curves: Case of arid region of Pakistan. J. Hydrol. 2019, 570, 473–485. [Google Scholar] [CrossRef]
- Jain, M.K.; Jain, V.K.; Pandey, R.P. Impact of Climate Change on Extreme Drought Events in Ken River Basin, India. In Proceedings of the Asabe International Meeting Sponsored by Asabe, Dallas, TX, USA, 29 July–1 August 2012; p. 121340846. [Google Scholar] [CrossRef]
- Lin, C.; Wang, K.; Chen, R.; Zhang, J.; Zhang, B.; Lu, J.Z. Characteristics of erosive rainfall in Jianshan River Watershed of Yuxi City. Bull. Soil Water Conserv. 2018, 38, 235–240. [Google Scholar] [CrossRef]
- Wu, J.; Man, Y.; Sun, G.; Shang, L. Occurrence and health-risk assessment of trace metals in raw and boiled drinking water from rural areas of China. Water 2018, 10, 641. [Google Scholar] [CrossRef]
- Schielzeth, H.; Nakagawa, S. Nested by desing: Model fitting and interpretation in a mixed model era. Methods Ecol. Evol. 2013, 4, 14–24. [Google Scholar] [CrossRef]
- Cabin, R.J.; Weller, S.G.; Lorence, D.H.; Cordell, S.; Hadway, L.J. Effects of microsite, water, weeding, and direct seeding on the regeneration of native and alien species within a Hawaiian dry forest preserve. Biol. Conserv. 2002, 104, 181–190. [Google Scholar] [CrossRef]
- Cola, V.D.; Broennimann, O.; Petipierre, B.; Breiner, F.T.; D’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A.; et al. Ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Han, A.R.; Kim, H.J.; Jung, J.B.; Park, P.S. Seed germination and initial seedling survival of the subalpine tree species, Picea jezoensis, on different forest floor substrates under elevated temperature. For. Ecol. Manag. 2018, 429, 579–588. [Google Scholar] [CrossRef]
- Jourgholami, M.; Fathi, K.; Labelle, E.R. Effects of litter and straw mulch amendments on compacted soil properties and Caucasian alder (Alnus subcordata) growth. New For. 2020, 51, 349–365. [Google Scholar] [CrossRef]
- Dickinson, C.H. Decomposition of Litter in Soil. Biology of Plant Litter Decomposition (2); Dickinson, C.H., Pugh, G.J.F., Eds.; Academic Press Inc.: London, UK, 2012; pp. 633–658. [Google Scholar]
- Kostel-Hughes, F.; Young, T.P.; Wehr, J.D. Effects of leaf litter depth on the emergence and seedling growth of deciduous forest tree species in relation toseed size1. J. Torrey Bot. Soc. 2005, 132, 50–61. [Google Scholar] [CrossRef]
- Seiwa, K.; Watanabe, A.; Saitoh, T.; Kannu, H.; Akasaka, S. Effects of burying depth and seed size on seedling establishment of Japanese chestnuts, Castanea crenata. For. Ecol. Manag. 2002, 164, 149–156. [Google Scholar] [CrossRef]
- Lodge, D.J.; Scatena, F.; Asbury, C.; Sanchez, M. Fine litterfall and related nutrient inputs resulting from Hurricane Hugo in subtropical wet and lower montane rain forests of Puerto Rico. Biotropica 1991, 23, 336–342. [Google Scholar] [CrossRef]
- Bellingham, P.J.; Morse, C.W.; Buxton, R.P.; Bonner, K.I.; Mason, N.W.; Wardle, D.A. Litterfall, nutrient concentrations and decomposability of litter in a New Zealand temperate montane rain forest. N. Z. J. Ecol. 2013, 37, 162–173. Available online: https://www.jstor.org/stable/24060777 (accessed on 1 May 2013).
- Yang, G.L.; Li, J.Q.; Zuo, M.; Sun, K.; Hu, J. Litter production variation and nutrient return of Pinus yunnanensis forest in Mopan Mountains in Central Yunnan Plateau. Ecol. Environ. Sci. 2019, 28, 2158–2164. [Google Scholar] [CrossRef]
- Condit, R.; Engelbrecht, B.M.; Pino, D.; Pérez, R.; Turner, B.L. Species distributions in response to individual soil nutrients and seasonal drought across a community of tropical trees. Proc. Natl. Acad. Sci. USA 2013, 110, 5064–5068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zou, J.; Meng, D.; Dang, S.; Zhou, J.; Osborne, B.; Ren, Y.; Liang, T.; Yu, K. Effect of soil microorganisms and labile C availability on soil respiration in response to litter inputs in forest ecosystems: A meta-analysis. Ecol. Evol. 2020, 10, 13602–13612. [Google Scholar] [CrossRef]
- Wu, B.; Guo, Y.; He, M.; Han, X.; Zang, L.; Liu, Q.; Chen, D.; Xia, T.; Shen, K.; Kang, L.; et al. AM fungi endow greater plant biomass and soil nutrients under interspecific competition rather than nutrient releases for litter. Forests 2021, 12, 1704. [Google Scholar] [CrossRef]
- Huo, X.; Wang, D.; Bing, D.; Li, Y.; Kang, H.; Yang, H.; Wei, G.; Chao, Z. Appropriate removal of forest litter is beneficial to Pinus tabuliformis Carr. regeneration in a pine and oak mixed forest in the Qinling Mountains, China. Forests 2019, 10, 735. [Google Scholar] [CrossRef]
- Jin, M.; Wang, Z.; He, Z.; Jiang, L.; Liu, J.; Lan, Y.; Shi, Y.; Shen, C. Allelopathic effect of castanopsis kawakamii forest litter on seed germination of small Philippine acacia (Acacia confusa). Appl. Ecol. Environ. Res. 2019, 17, 15103–15116. [Google Scholar] [CrossRef]
- Wu, C.; Chen, Y.; Grenier-Héon, D. Effects of allelopathy and availability of nutrients and water resources on the survival and growth of plant species in a natural Dacrydium forest. Can. J. For. Res. 2022, 52, 100–108. [Google Scholar] [CrossRef]
- Ruan, H.H.; Zou, X.M.; Scatena, F.N.; Zimmerman, J.K. Asynchronous fluctuation of soil microbial biomass and plant litterfall in a tropical wet forest. Plant Soil 2004, 260, 147–154. [Google Scholar] [CrossRef]
- Sayer, E.J.; Heard, M.S.; Grant, H.K.; Marthews, T.R.; Tanner, E.V. Soil carbon release enhanced by increased tropical forest litterfall. Nat. Clim. Chang. 2011, 1, 304–307. [Google Scholar] [CrossRef]
- Jia, S.X.; Liu, S.F.; Lin, W.S.; Li, X.J.; Yang, L.M.; Sun, S.Y.; Hui, D.F.; Guo, J.F.; Zou, X.M.; Yang, Y.S. Tree roots exert greater influence on soil microbial necromass carbon than above-ground litter in subtropical natural and plantation forests. Soil Biol. Biochem. 2022, 173, 108811. [Google Scholar] [CrossRef]
- Allison, S.D.; Treseder, K.K. Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob. Chang. Biol. 2008, 14, 2898–2909. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Jin, Y. Stoichiometric responses of soil microflora to nutrient additions for two temperate forest soils. Biol. Fertil. Soils 2017, 53, 397–406. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.; He, T.; Liu, L.; Wu, J. Response of organic carbon mineralization and microbial community to leaf litter and nutrient additions in subtropical forest soils. Soil Biol. Biochem. 2014, 71, 13–20. [Google Scholar] [CrossRef]
- Peña-Claros, M.; Poorter, L.; Alarcón, A.; Blate, G.; Choque, U.; Fredericksen, T.S.; Justiniano, M.J.; Leaño, C.; Licona, J.C.; Pariona, W. Soil effects on forest structure and diversity in a moist and a dry tropical forest. Biotropica 2012, 44, 276–283. [Google Scholar] [CrossRef]
- Niinemets, Ü. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Bever, J.D.; Dickie, I.A.; Facelli, E.; Facelli, J.M.; Klironomos, J.; Moora, M.; Rillig, M.C.; Stock, W.D.; Tibbett, M.; Zobel, M. Rooting theories of plant community ecology in microbial interactions. Trends Ecol. Evol. 2010, 25, 468–478. [Google Scholar] [CrossRef]
- Hartmann, H.; Adams, H.D.; Hammond, W.M.; Hoch, G.; Landhäusser, S.M.; Wiley, E.; Zaehle, S. Identifying differences in carbohydrate dynamics of seedlings and mature trees to improve carbon allocation in models for trees and forests. Environ. Exp. Bot. 2018, 152, 7–18. [Google Scholar] [CrossRef]
- Landhäusser, S.M.; Lieffers, V.J. Defoliation increases risk of carbon starvation in root systems of mature aspen. Trees 2012, 26, 653–661. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, J.; Song, H.; Yang, K. Resorption-related nitrogen changes in the leaves and roots of Larix kaempferi seedlings under nutrient-sufficient and nutrient-starvation conditions. J. Plant Ecol. 2019, 12, 615–623. [Google Scholar] [CrossRef]
- Wiley, E.; Hoch, G.; Landhäusser, S.M. Dying piece by piece: Carbohydrate dynamics in aspen (Populus tremuloides) seedlings under severe carbon stress. J. Exp. Bot. 2017, 68, 5221–5232. [Google Scholar] [CrossRef]
- Lu, D.; Wang, G.G.; Yan, Q.; Gao, T.; Zhu, J. Effects of gap size and within-gap position on seedling growth and biomass allocation: Is the gap partitioning hypothesis applicable to the temperate secondary forest ecosystems in Northeast China? For. Ecol. Manag. 2018, 429, 351–362. [Google Scholar] [CrossRef]
- Bentos, T.V.; Nascimento, H.E.; Williamson, G.B. Tree seedling recruitment in Amazon secondary forest: Importance of topography and gap micro-site conditions. For. Ecol. Manag. 2013, 287, 140–146. [Google Scholar] [CrossRef]
- Eichhorn, M.P.; Nilus, R.; Compton, S.G.; Hartley, S.E.; Burslem, D.F. Herbivory of tropical rain forest tree seedlings correlates with future mortality. Ecology 2010, 91, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Wang, N.; Kong, C.; Wang, P.; Meiners, S.J. Root exudate signals in plant–plant interactions. Plant Cell Environ. 2021, 44, 1044–1058. [Google Scholar] [CrossRef]
- Nakayama, M.; Tateno, R. Solar radiation strongly influences the quantity of forest tree root exudates. Trees 2018, 32, 871–879. [Google Scholar] [CrossRef]
- Coomes, D.A.; Grubb, P.J. Impacts of root competition in forests and woodlands: A theorectical framework and review of experiments. Ecol. Monogr. 2000, 70, 171–207. [Google Scholar] [CrossRef]
- Rudgers, J.A.; Orr, S. Non-native grass alters growth of native tree species via leaf and soil microbes. J. Ecol. 2009, 97, 247–255. [Google Scholar] [CrossRef]
- Su, X.; Wang, M.; Huang, Z.; Fu, S.; Chen, H.Y. Forest understorey vegetation: Colonization and the availability and heterogeneity of resources. Forests 2019, 10, 944. [Google Scholar] [CrossRef]
- Onwuka, B.; Mang, B. Effects of soil temperature on some soil properties and plant growth. Adv. Plants Agric. Res. 2018, 8, 34–37. [Google Scholar] [CrossRef]
- Smith, L.M.; Reynolds, H.L. Plant–soil feedbacks shift from negative to positive with decreasing light in forest understory species. Ecology 2015, 96, 2523–2532. [Google Scholar] [CrossRef]
- Stella, J.C.; Battles, J.J. How do riparian woody seedlings survive seasonal drought? Oecologia 2010, 164, 579–590. [Google Scholar] [CrossRef]
- Esquivel-Muelbert, A.; Baker, T.R.; Dexter, K.G.; Lewis, S.L.; Ter Steege, H.; Lopez-Gonzalez, G.; Monteagudo Mendoza, A.; Brienen, R.; Feldpausch, T.R.; Pitman, N. Seasonal drought limits tree species across the Neotropics. Ecography 2017, 40, 618–629. [Google Scholar] [CrossRef]
- Jucker, T.; Bongalov, B.; Burslem, D.F.; Nilus, R.; Dalponte, M.; Lewis, S.L.; Phillips, O.L.; Qie, L.; Coomes, D.A. Topography shapes the structure, composition and function of tropical forest landscapes. Ecol. Lett. 2018, 21, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Rivera, L.W.; Aide, T.M. Forest recovery in the karst region of Puerto Rico. For. Ecol. Manag. 1998, 108, 63–75. [Google Scholar] [CrossRef]
- Tsai, C.J.; Lin, T.C.; Hwong, J.L.; Lin, N.H.; Wang, C.P.; Hamburg, S. Typhoon impacts on stream water chemistry in a plantation and an adjacent natural forest in central Taiwan. J. Hydrol. 2009, 378, 290–298. [Google Scholar] [CrossRef]
- Dale, V.H.; Joyce, L.A.; McNulty, S.; Neilson, R.P.; Ayres, M.P.; Flannigan, M.D.; Hanson, P.J.; Irland, L.C.; Lugo, A.E.; Peterson, C.J. Climate change and forest disturbances: Climate change can affect forests by altering the frequency, intensity, duration, and timing of fire, drought, introduced species, insect and pathogen outbreaks, hurricanes, windstorms, ice storms, or landslides. Bioscience 2001, 51, 723–734. [Google Scholar] [CrossRef]
- Vara-Vela, A.L.; Herdies, D.L.; Alvim, D.S.; Vendrasco, D.P.; Fernandez, J.P.R. A new predictive framework for Amazon forest fire smoke dispersion over South America. Bull. Am. Meteorol. Soc. 2021, 102, 1700–1713. [Google Scholar] [CrossRef]
- Frizano, J.; Johnson, A.H.; Vann, D.R.; Scatena, F.N. Soil phosphorus fractionation during forest development on landslide scars in the Luquillo Mountains, Puerto Rico. Biotropica 2002, 34, 17–26. [Google Scholar] [CrossRef]
- Boose, E.R.; Serrano, M.I.; Foster, D.R. Landscape and regional impacts of hurricanes in Puerto Rico. Ecol. Monogr. 2004, 74, 335–352. [Google Scholar] [CrossRef]
- Helmer, E.H. Forest conservation and land development in Puerto Rico. Landsc. Ecol. 2004, 19, 29–40. [Google Scholar] [CrossRef]
- Mage, S.M.; Porder, S. Parent material and topography determine soil phosphorus status in the Luquillo Mountains of Puerto Rico. Ecosystems 2013, 16, 284–294. [Google Scholar] [CrossRef]
- Hyndman, D.; Hyndman, D. Natural Hazards and Disasters; Cengage Learning: Stanford, CA, USA, 2016; pp. 247–317. [Google Scholar]
- Clague, J.; Bobrowsky, P. International year of planet earth 8. natural hazards in Canada. Geosci. Can. 2010, 37, 17–37. Available online: https://id.erudit.org/iderudit/geocan37_1ser01 (accessed on 1 January 2010).
- Agrawal, N. Natural Disasters and Risk Management in Canada; Springer: Berlin/Heidelberg, Germany, 2018; pp. 81–191. [Google Scholar]
- Babst, F.; Carrer, M.; Poulter, B.; Urbinati, C.; Neuwirth, B.; Frank, D. 500 years of regional forest growth variability and links to climatic extreme events in Europe. Environ. Res. Lett. 2012, 7, 045705. [Google Scholar] [CrossRef]
- Abbas, S.; Nichol, J.; Qamer, F.; Xu, J. Characterization of drought development through remote sensing: A case study in central Yunnan, China. Remote Sens. 2014, 6, 4998–5018. [Google Scholar] [CrossRef]
- Wang, J.; Meng, Y. An analysis of the drought in Yunnan, China, from a perspective of society drought severity. Nat. Hazards 2013, 67, 431–458. [Google Scholar] [CrossRef]
- Liu, B.X.; Lund, J.R.; Liu, L.J.; Liao, S.L.; Li, G.; Cheng, C.T. Climate change impacts on hydropower in Yunnan, China. Water 2020, 12, 197. [Google Scholar] [CrossRef]
- Turner, A.G.; Annamalai, H. Climate change and the south Asian summer monsoon. Nat. Clim. Chang. 2012, 2, 587–595. [Google Scholar] [CrossRef]
- Syed, F.S.; Iqbal, W.; Syed, A.; Rasul, G. Uncertainties in the regional climate models simulations of South-Asian summer monsoon and climate change. Clim. Dyn. 2014, 42, 2079–2097. [Google Scholar] [CrossRef]
- Yang, B.; Qin, C.; Bruning, A.; Osborn, T.J.; Stenseth, N.C. Long-term decrease in Asian monsoon rainfall and abrupt climate change events over the past 6,700 years. Proc. Natl. Acad. Sci. USA 2021, 118, e2102007118. [Google Scholar] [CrossRef]
- Padrón, R.S.; Gudmundsson, L.; Decharme, B.; Ducharne, A.; Seneviratne, S.I. Observed changes in dry-season water availability attributed to human-induced climate change. Nat. Geosci. 2020, 13, 477–481. [Google Scholar] [CrossRef]
- Chang, H.I.; Castro, C.L.; Carrillo, C.M.; Dominguez, F. The more extreme nature of U.S. warm season climate in the recent observational record and two “well-performing” dynamically downscaled CMIP3 models. J. Geophys. Res. Atmos. 2015, 120, 8244–8263. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Liu, Y.Q.; O’Brien, J.J.; Elliott, K.J.; Starr, G.; Miniat, C.F.; Hiers, J.K. Future climate and fire interactions in the southeastern region of the United States. For. Ecol. Manag. 2014, 327, 316–326. [Google Scholar] [CrossRef]
- Jactel, H.; Koricheva, J.; Castagneyrol, B. Responses of forest insect pests to climate change: Not so simple. Curr. Opin. Insect Sci. 2019, 35, 103–108. [Google Scholar] [CrossRef]
- Kane, J.M.; Varner, J.M.; Metz, M.R.; Van Mantgem, P.J. Characterizing interactions between fire and other disturbances and their impacts on tree mortality in western U.S. Forests. For. Ecol. Manag. 2017, 405, 188–199. [Google Scholar] [CrossRef]
- Mahon, C.L.; Holloway, G.L.; Bayne, E.M.; Toms, J.D. Additive and interactive cumulative effects on boreal landbirds: Winners and losers in a multi-stressor landscape. Ecol. Appl. 2019, 29, e01895. [Google Scholar] [CrossRef] [PubMed]
| Experimental Plot | Treatment | Scientific Name | Family | Individuals (/m2; ±SE) |
|---|---|---|---|---|
| Control plot | R+L+ | --- | --- | --- |
| R+L− | Nephrolepis cordifolia (L.) C. Presl | Nephrolepidaceae | 5 (2) | |
| Ageratina denophora (Spreng.) R.M. King et H.Rob. | Asteraceae | 1 (1) | ||
| Arthraxon hispidus (Trin.) Makino | Gramineae | 1 (1) | ||
| R−L+ | A. adenophora | Asteraceae | 1 (1) | |
| R−L− | A. adenophora | Asteraceae | 29 (2) | |
| N. cordifolia | Nephrolepidaceae | 17 (3) | ||
| Erigeron acris L. | Asteraceae | 8 (3) | ||
| Hedyotis diffusa Willd | Rubiaceae | 2 (1) | ||
| Senecio scandens Buch.-Ham. Ex D. Don | Asteraceae | 1 (1) | ||
| Watered plot | R+L+ | Cinnamomum camphora (L.) Presl | Lauraceae | 1 (1) |
| Peracarpa carnosa (Wall.) Hook. F. et Thoms. | Campanulaceae | 1 (1) | ||
| R+L− | A. adenophora | Asteraceae | 13 (2) | |
| Cibotium barometz (L.) J. Sm. | Dicksoniaceae | 3 (1) | ||
| Pteris ensiformis Burm. | Pteridaceae | 2 (1) | ||
| Crassocephalum crepidioides (Benth.) S. Moore | Asteraceae | 2 (1) | ||
| E. acris | Asteraceae | 2 (1) | ||
| R−L+ | A. hispidus | Gramineae | 10 (2) | |
| A. adenophora | Asteraceae | 9 (1) | ||
| Youngia japonica (L.) DC | Asteraceae | 8 (1) | ||
| P. carnosa | Campanulaceae | 1 (1) | ||
| Broussonetia papyrifera (Linnaeus) L’Heritier ex Ventenat | Moraceae | 1 (1) | ||
| R−L− | N. cordifolia | Nephrolepidaceae | 40 (3) | |
| Cyclosorus interruptus (Willd.) H. Ito | Thelypteridaceae | 21 (3) | ||
| A. adenophora | Asteraceae | 13 (2) | ||
| A. hispidus | Gramineae | 12 (3) | ||
| P. ensiformis | Pteridaceae | 10 (2) | ||
| Lespedeza bicolor Turcz. | Fabaceae | 9 (2) | ||
| E. acris | Asteraceae | 6 (2) | ||
| C. barometz | Dicksoniaceae | 4 (1) | ||
| Laggera pterodonta (DC.) Benth. | Asteraceae | 3 (1) | ||
| Y. japonica | Asteraceae | 3 (1) | ||
| Buddleja asiatica Lour. | Scrophulariaceae | 3 (2) | ||
| Solanum nigrum L. | Solanaceae | 3 (2) | ||
| Phytolacca americana L. | Phytolaccaceae | 2 (1) |
| Experimental Plot | Treatment | Scientific Name | Mean Height (cm; ±SE) | Maximum Height (cm; ±SE) |
|---|---|---|---|---|
| Control plot | R+L+ | --- | --- | --- |
| R+L− | --- | 10.5 (1.0) | 22.0 (1.9) | |
| Nephrolepis cordifolia (L.) C. Presl | 7.9 (0.4) | 7.9 (0.4) | ||
| Ageratina denophora (Spreng.) R.M. King et H.Rob. | 4.6 (0.6) | 4.6 (0.6) | ||
| R−L+ | Arthraxon hispidus (Trin.) Makino | 2.0 (0.3) | 2.0 (0.3) | |
| R−L− | A. adenophora | 7.6 (0.7) | 37.3 (2.8) | |
| A. adenophora | 5.6 (0.3) | 8.7 (0.9) | ||
| N. cordifolia | 10.8 (0.9) | 27.6 (3.1) | ||
| Erigeron acris L. | 25.5 (2.9) | 29.3 (2.0) | ||
| Hedyotis diffusa Willd | 34.6 (1.2) | 34.6 (1.2) | ||
| Watered plot | R+L+ | Senecio scandens Buch.-Ham. Ex D. Don | 8.7 (0.3) | 8.7 (0.3) |
| Cinnamomum camphora (L.) Presl | 5.9 (0.2) | 5.9 (0.2) | ||
| R+L− | Peracarpa carnosa (Wall.) Hook. F. et Thoms. | 9.4 (1.6) | 15.8 (1.8) | |
| A. adenophora | 2.5 (0.4) | 4.6 (0.7) | ||
| Cibotium barometz (L.) J. Sm. | 4.7 (0.3) | 6.6 (0.6) | ||
| Pteris ensiformis Burm. | 5.8 (0.9) | 12.2 (0.8) | ||
| Crassocephalum crepidioides (Benth.) S. Moore | 1.7 (0.6) | 3.2 (0.2) | ||
| R−L+ | E. acris | 64.9 (7.2) | 84.7 (4.4) | |
| A. hispidus | 36.9 (2.8) | 41.3 (3.1) | ||
| A. adenophora | 1.7 (0.4) | 3.6 (0.4) | ||
| 8.6 (0.8) | 8.6 (0.8) | |||
| P. carnosa | 9.6 (0.4) | 9.6 (0.4) | ||
| R−L− | Broussonetia papyrifera (Linnaeus) L’Heritier ex Ventenat | 6.6 (0.2) | 14.8 (3.0) | |
| N. cordifolia | 31.2 (3.0) | 73.8 (6.0) | ||
| Cyclosorus interruptus (Willd.) H. Ito | 30.4 (2.8) | 47.5 (2.6) | ||
| A. adenophora | 43.6 (5.3) | 89.6 (3.9) | ||
| A. hispidus | 11.7 (2.0) | 31.0 (3.5) | ||
| P. ensiformis | 5.1 (0.7) | 6.6 (1.9) | ||
| Lespedeza bicolor Turcz. | 18.7 (2.4) | 83.8 (5.7) | ||
| E. acris | 32.77 (2.9) | 73.9 (4.0) | ||
| C. barometz | 3.6 (0.6) | 10.5 (1.2) | ||
| Laggera pterodonta (DC.) Benth. | 20.6 (2.7) | 66.4 (4.1) | ||
| 33.6 (3.2) | 51.7 (5.4) | |||
| Buddleja asiatica Lour. | 102.2 (20.5) | 130.2 (4.1) | ||
| Solanum nigrum L. | 10.2 (1.9) | 22.8 (1.6) |
| Treatment | Species Richness | The Number of Plant Individuals | Mean Height of Plant Seedlings | Maximum Height of Plant Seedlings | Dry Biomass of Plant Seedlings |
|---|---|---|---|---|---|
| Water | 6.06 * | 82.93 * | 15.26 * | 35.00 * | 9.03 * |
| Roots | −4.33 ** | −5.13 * | −8.83 ** | −11.66 ** | −1.77 ** |
| Litter | 5.00 ** | 20.20 * | 7.82 * | 24.19 * | 2.07 * |
| Water + Roots | −5.53 *** | −1.72 *** | −5.19 *** | −12.54 *** | −14.87 *** |
| Water + Litter | 6.67 *** | 35.57 *** | 15.21 *** | 40.43 *** | 20.30 *** |
| Roots + Litter | −8.07 *** | −75.92 *** | −27.16 *** | −64.33 *** | −32.53 *** |
| Water + Roots + Litter | −11.33 *** | −89.13 *** | −39.77 *** | −94.67 *** | −173.30 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Li, Y.; Kong, L.; Lodge, D.J.; Hogan, J.A.; Wang, C. Roots, Litter, and Seasonal Drought Together Inhibit Plant Growth in the Herbaceous Layer in a Subtropical Moist Forest of Southwestern China. Forests 2023, 14, 712. https://doi.org/10.3390/f14040712
Liu X, Li Y, Kong L, Lodge DJ, Hogan JA, Wang C. Roots, Litter, and Seasonal Drought Together Inhibit Plant Growth in the Herbaceous Layer in a Subtropical Moist Forest of Southwestern China. Forests. 2023; 14(4):712. https://doi.org/10.3390/f14040712
Chicago/Turabian StyleLiu, Xianbin, Yun Li, Lingqian Kong, D. Jean Lodge, J. Aaron Hogan, and Chao Wang. 2023. "Roots, Litter, and Seasonal Drought Together Inhibit Plant Growth in the Herbaceous Layer in a Subtropical Moist Forest of Southwestern China" Forests 14, no. 4: 712. https://doi.org/10.3390/f14040712
APA StyleLiu, X., Li, Y., Kong, L., Lodge, D. J., Hogan, J. A., & Wang, C. (2023). Roots, Litter, and Seasonal Drought Together Inhibit Plant Growth in the Herbaceous Layer in a Subtropical Moist Forest of Southwestern China. Forests, 14(4), 712. https://doi.org/10.3390/f14040712






