Short-Term Effects of Anthropogenic Disturbances on Stand Structure, Soil Properties, and Vegetation Diversity in a Former Virgin Mixed Forest
Abstract
1. Introduction
2. Materials and Methods
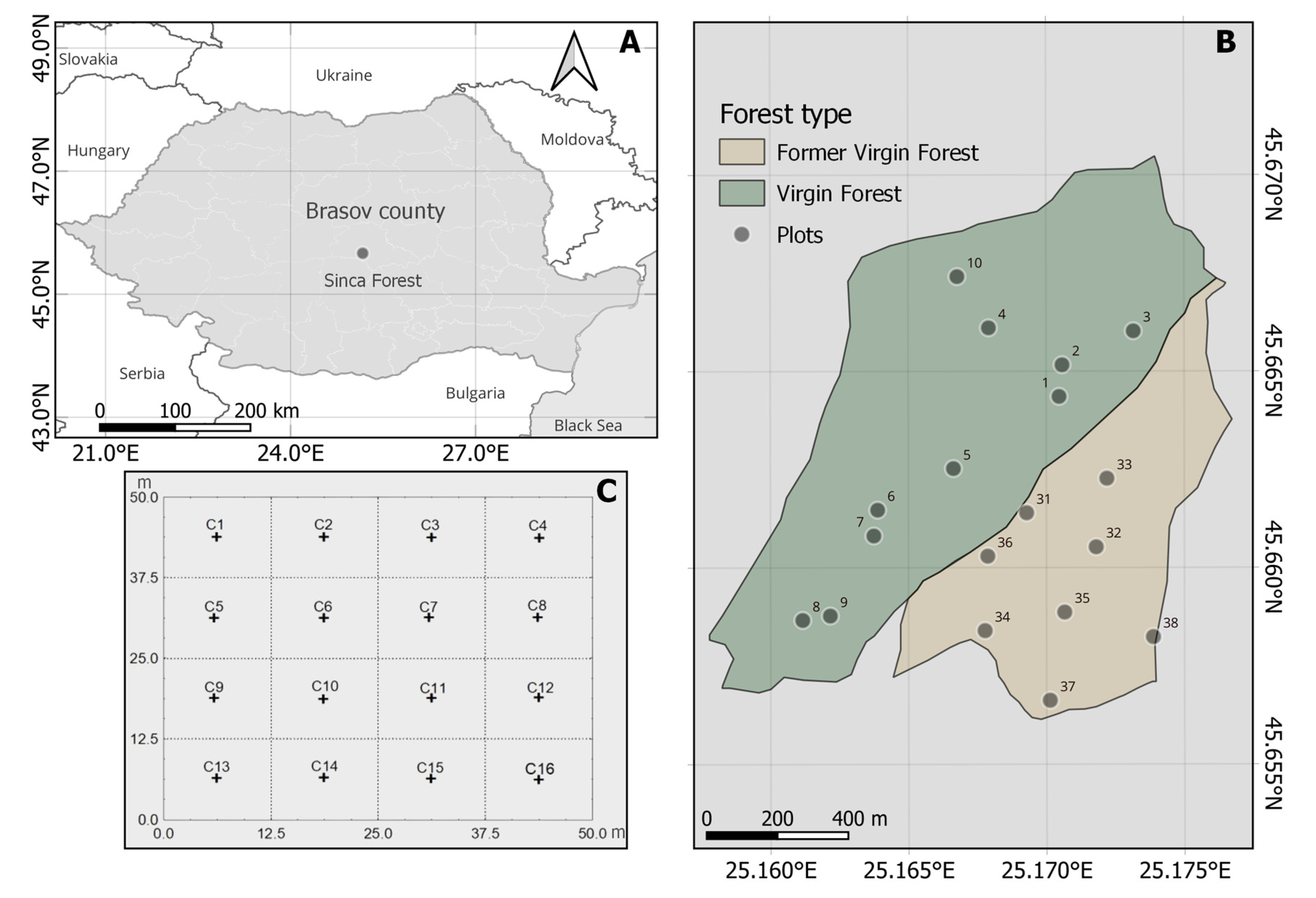
2.1. Study Site
2.2. Field Sampling
2.3. Data Analysis
3. Results
3.1. Structural Characteristics
3.2. Deadwood
3.3. Natural Regeneration and Light Conditions
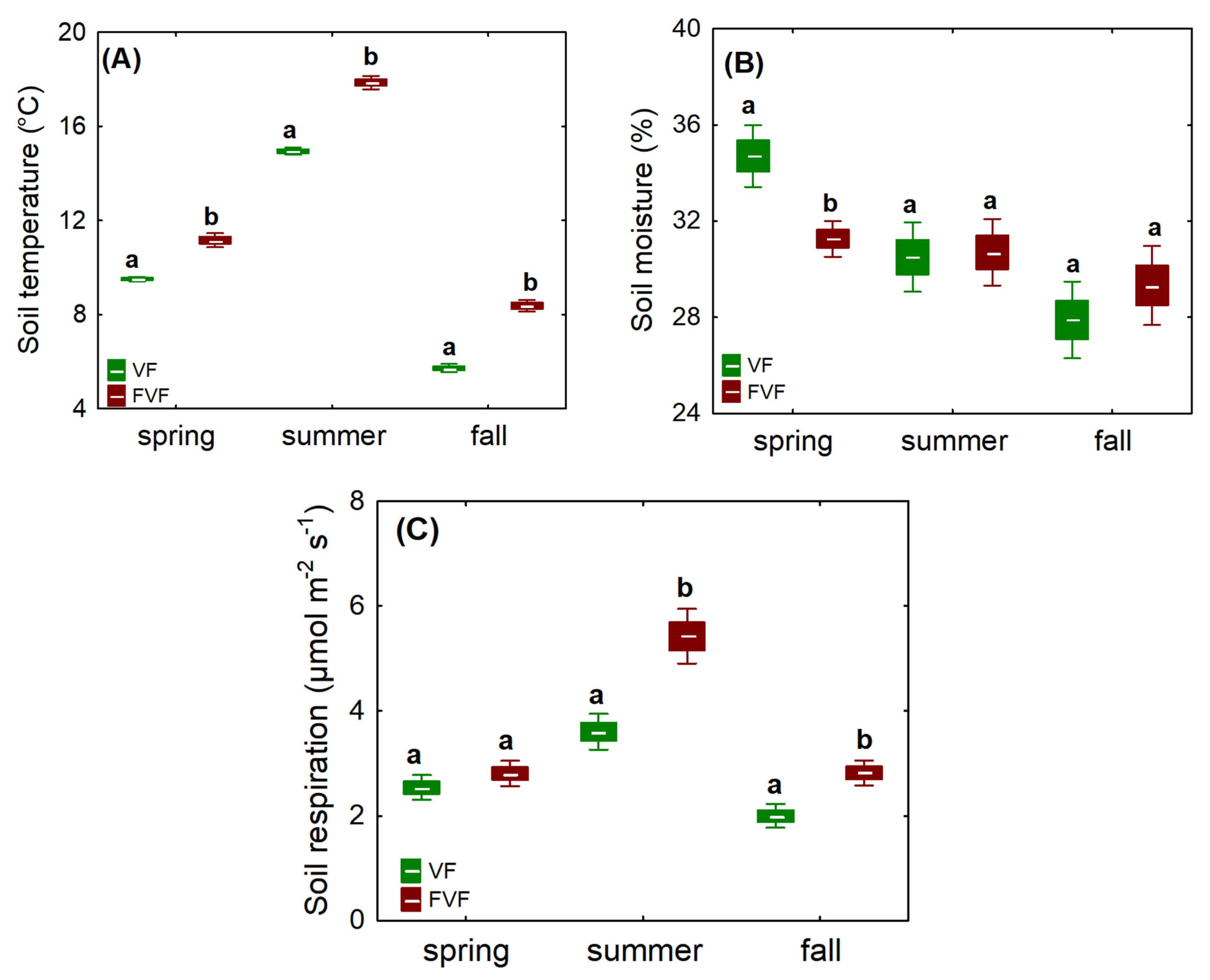
3.4. Soil Microclimate and Soil Respiration
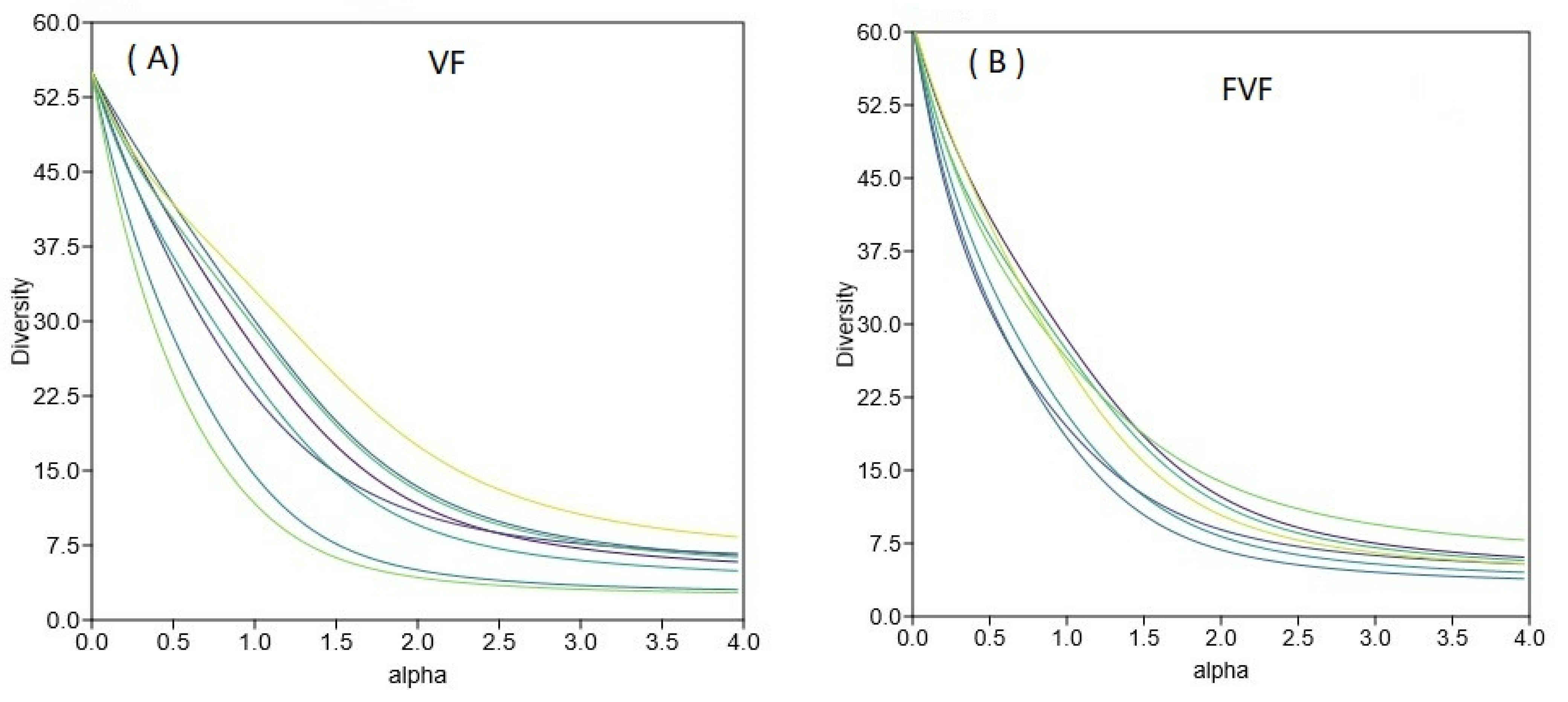
3.5. Ground Vegetation Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parviainen, J.; Little, D.; Doyle, M.; O’Sullivan, A.; Kettunen, M.; Korhonen, M. Metsäntutkimuslaitos Research in Forest Reserves and Natural Forests in European Countries: Country Reports for the COST Action E4: Forest Reserves Research Network. 1999, p. 304, ISBN 952-9844-31-X, ISSN 1237-8801. Available online: https://efi.int/sites/default/files/files/publication-bank/2018/proc16_net.pdf (accessed on 8 February 2023).
- Kaplan, J.O.; Krumhardt, K.M.; Zimmermann, N. The Prehistoric and Preindustrial Deforestation of Europe. Quat. Sci. Rev. 2009, 28, 3016–3034. [Google Scholar] [CrossRef]
- Stillhard, J.; Hobi, M.L.; Brang, P.; Brändli, U.-B.; Korol, M.; Pokynchereda, V.; Abegg, M. Structural Changes in a Primeval Beech Forest at the Landscape Scale. For. Ecol. Manag. 2022, 504, 119836. [Google Scholar] [CrossRef]
- Sabatini, F.M.; Bluhm, H.; Kun, Z.; Aksenov, D.; Atauri, J.A.; Buchwald, E.; Burrascano, S.; Cateau, E.; Diku, A.; Duarte, I.M.; et al. European Primary Forest Database v2.0. Sci. Data 2021, 8, 220. [Google Scholar] [CrossRef]
- Catalogul Padurilor Virgine. 2022. Available online: https://www.mmediu.ro/articol/catalogul-padurilor-virgine-si-cvasivirgine-din-romania/5550 (accessed on 8 February 2023).
- Commarmot, B.; Bachofen, H.; Bundziak, Y.; Bürgi, A.; Ramp, B.; Shparyk, Y.; Sukhariuk, D.; Viter, R.; Zingg, A. Structures of Virgin and Managed Beech Forests in Uholka (Ukraine) and Sihlwald (Switzerland): A Comparative Study. For. Snow Landsc. Res. 2005, 79, 45–56. [Google Scholar]
- Frankovič, M.; Janda, P.; Mikoláš, M.; Čada, V.; Kozák, D.; Pettit, J.L.; Nagel, T.A.; Buechling, A.; Matula, R.; Trotsiuk, V.; et al. Natural Dynamics of Temperate Mountain Beech-Dominated Primary Forests in Central Europe. For. Ecol. Manag. 2021, 479, 118522. [Google Scholar] [CrossRef]
- Veen, P.; Fanta, J.; Raev, I.; Biriş, I.-A.; de Smidt, J.; Maes, B. Virgin Forests in Romania and Bulgaria: Results of Two National Inventory Projects and Their Implications for Protection. Biodivers. Conserv. 2010, 19, 1805–1819. [Google Scholar] [CrossRef]
- Sabatini, F.M.; Burrascano, S.; Keeton, W.S.; Levers, C.; Lindner, M.; Pötzschner, F.; Verkerk, P.J.; Bauhus, J.; Buchwald, E.; Chaskovsky, O.; et al. Where Are Europe’s Last Primary Forests? Divers. Distrib. 2018, 24, 1426–1439. [Google Scholar] [CrossRef]
- Gibson, L.; Lee, T.M.; Koh, L.P.; Brook, B.W.; Gardner, T.A.; Barlow, J.; Peres, C.A.; Bradshaw, C.J.A.; Laurance, W.F.; Lovejoy, T.E.; et al. Primary Forests Are Irreplaceable for Sustaining Tropical Biodiversity. Nature 2011, 478, 378–381. [Google Scholar] [CrossRef]
- Višnjić, Ć.; Solaković, S.; Mekić, F.; Balić, B.; Vojniković, S.; Dautbašić, M.; Gurda, S.; Ioras, F.; Ratnasingam, J.; Abrudan, I.V. Comparison of Structure, Regeneration and Dead Wood in Virgin Forest Remnant and Managed Forest on Grmeč Mountain in Western Bosnia. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2013, 147, 913–922. [Google Scholar] [CrossRef]
- Keren, S.; Medarević, M.; Obradović, S.; Zlokapa, B. Five Decades of Structural and Compositional Changes in Managed and Unmanaged Montane Stands: A Case Study from South-East Europe. Forests 2018, 9, 479. [Google Scholar] [CrossRef]
- Knohl, A.; Schulze, E.-D.; Kolle, O.; Buchmann, N. Large Carbon Uptake by an Unmanaged 250-Year-Old Deciduous Forest in Central Germany. Agric. For. Meteorol. 2003, 118, 151–167. [Google Scholar] [CrossRef]
- Luyssaert, S.; Schulze, E.-D.; Börner, A.; Knohl, A.; Hessenmöller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-Growth Forests as Global Carbon Sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Glatthorn, J.; Feldmann, E.; Pichler, V.; Hauck, M.; Leuschner, C. Biomass Stock and Productivity of Primeval and Production Beech Forests: Greater Canopy Structural Diversity Promotes Productivity. Ecosystems 2018, 21, 704–722. [Google Scholar] [CrossRef]
- Watson, J.E.M. The Exceptional Value of Intact Forest Ecosystems. Nat. Ecol. Evol. 2018, 2, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J.F.; Van Pelt, R. Spatial Aspects of Structural Complexity in Old-Growth Forests. J. For. 2004, 102, 22–28. [Google Scholar]
- Thom, D.; Seidl, R. Natural Disturbance Impacts on Ecosystem Services and Biodiversity in Temperate and Boreal Forests. Biol. Rev. 2016, 91, 760–781. [Google Scholar] [CrossRef]
- Bauhus, J.; Puettmann, K.; Messier, C. Silviculture for Old-Growth Attributes. For. Ecol. Manag. 2009, 258, 525–537. [Google Scholar] [CrossRef]
- Keeton, W.S.; Chernyavskyy, M.; Gratzer, G.; Main-Knorn, M.; Shpylchak, M.; Bihun, Y. Structural Characteristics and Aboveground Biomass of Old-growth Spruce–Fir Stands in the Eastern Carpathian Mountains, Ukraine. Plant Biosyst.-Int. J. Deal. Asp. Plant Biol. 2010, 144, 148–159. [Google Scholar] [CrossRef]
- Diaci, J.; Rozenbergar, D.; Anic, I.; Mikac, S.; Saniga, M.; Kucbel, S.; Visnjic, C.; Ballian, D. Structural Dynamics and Synchronous Silver Fir Decline in Mixed Old-Growth Mountain Forests in Eastern and Southeastern Europe. Forestry 2011, 84, 479–491. [Google Scholar] [CrossRef]
- Matović, B.; Stjepanović, S.; Kneginjić, I.; Stojanović, D.; Kisin, B.; Koprivica, M. Comparison of Stand Structure in Managed and Virgin European Beech Forests in Serbia. Šumar. List 2018, 142, 57. [Google Scholar] [CrossRef]
- Wirth, C.; Messier, C.; Bergeron, Y.; Frank, D.; Kahl, A. Old-Growth Forest Definitions: A Pragmatic View. Old-Growth For. 2009, 207, 11–33. [Google Scholar] [CrossRef]
- Seidl, R.; Schelhaas, M.-J.; Rammer, W.; Verkerk, P.J. Increasing Forest Disturbances in Europe and Their Impact on Carbon Storage. Nat. Clim. Chang. 2014, 4, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Schelhaas, M.-J.; Nabuurs, G.-J.; Schuck, A. Natural Disturbances in the European Forests in the 19th and 20th Centuries. Global Change Biol. 2003, 9, 1620–1633. [Google Scholar] [CrossRef]
- Ulanova, N. The Effects of Windthrow on Forests at Different Spatial Scales: A Review. Forest Ecol. Manag. 2000, 135, 155–167. [Google Scholar] [CrossRef]
- Janda, P.; Trotsiuk, V.; Mikoláš, M.; Bače, R.; Nagel, T.A.; Seidl, R.; Seedre, M.; Morrissey, R.C.; Kucbel, S.; Jaloviar, P.; et al. The Historical Disturbance Regime of Mountain Norway Spruce Forests in the Western Carpathians and Its Influence on Current Forest Structure and Composition. For. Ecol. Manag. 2017, 388, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Hirschmugl, M.; Gallaun, H.; Dees, M.; Datta, P.; Deutscher, J.; Koutsias, N.; Schardt, M. Methods for Mapping Forest Disturbance and Degradation from Optical Earth Observation Data: A Review. Curr. For. Rep. 2017, 3, 32–45. [Google Scholar] [CrossRef]
- Svoboda, M.; Nagel, T. Gap Disturbance Regime in an Old-Growth Fagus-Abies Forest in the Dinaric Mountains, Bosnia-Herzegovina. Can. J. For. Res. 2008, 38, 2728–2737. [Google Scholar] [CrossRef]
- Petritan, A.M.; Bouriaud, O.; Frank, D.C.; Petritan, I.C. Dendroecological Reconstruction of Disturbance History of an Old-Growth Mixed Sessile Oak–Beech Forest. J. Veg. Sci. 2017, 28, 117–127. [Google Scholar] [CrossRef]
- Any Mary Petritan, Robert S Nuske, Ion Catalin Petritan, Nicu Constantin Tudose Gap Disturbance Patterns in an Old-Growth Sessile Oak (Quercus petraea L.)–European Beech (Fagus sylvatica L.) Forest Remnant in the Carpathian Mountains, Romania. For. Ecol. Manag. 2013, 308, 67–75. [CrossRef]
- Nagel, T.A.; Firm, D.; Rozman, A. Intermediate Disturbances Are a Key Driver of Long-Term Tree Demography across Old-Growth Temperate Forests. Ecol. Evol. 2021, 11, 16862–16873. [Google Scholar] [CrossRef]
- Willim, K.; Ammer, C.; Seidel, D.; Annighöfer, P.; Schmucker, J.; Schall, P.; Ehbrecht, M. Short—Term Dynamics of Structural Complexity in Differently Managed and Unmanaged European Beech Forests. Trees For. People 2022, 8, 100231. [Google Scholar] [CrossRef]
- Boncina, A. Comparison of Structure and Biodiversity in the Rajhenav Virgin Forest Remnant and Managed Forest in the Dinaric Region of Slovenia. Glob. Ecol. Biogeogr. 2000, 9, 201–211. [Google Scholar] [CrossRef]
- Keren, S.; Diaci, J.; Motta, R.; Govedar, Z. Stand Structural Complexity of Mixed Old-Growth and Adjacent Selection Forests in the Dinaric Mountains of Bosnia and Herzegovina. For. Ecol. Manag. 2017, 400, 531–541. [Google Scholar] [CrossRef]
- Tauber, F. Contributii la sintaxonomia Fagetelor Carpato Dacice (Symphyto- Fagenalia Subordo Novum). Trib. Bot. 1987, 27, 179–191. [Google Scholar]
- Vasile, D.; Petritan, A.M.; Tudose, N.C.; Toiu, F.L.; Scarlatescu, V.; Petritan, I.C. Structure and Spatial Distribution of Dead Wood in Two Temperate Old-Growth Mixed European Beech Forests. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 639–645. [Google Scholar] [CrossRef]
- Petritan, I.C.; Mihăilă, V.-V.; Bragă, C.I.; Boura, M.; Vasile, D.; Petritan, A.M. Litterfall Production and Leaf Area Index in a Virgin European Beech (Fagus sylvatica L.)–Silver Fir (Abies alba Mill.) Forest. Dendrobiology 2020, 83, 75–84. [Google Scholar] [CrossRef]
- Nicolescu, V.-N.; Ghinescu, M.N.; Mihăilescu, G. Surprinzătoarea simplitate a tratamentelor adecvate stejăretelor pure și amestecurilor cu stejar. Bucov. For. 2021, 21, 183–197. [Google Scholar] [CrossRef]
- Niculescu, V.N. The Practice of Silviculture; Aldus: Brasov, Romania, 2018. [Google Scholar]
- Keller, M. Swiss National Forest Inventory. In Manual of the Field Survey 2004–2007; Swiss Federal Research Institute WSL Birmendsdorf, CH: Zürich, Switzerland, 2011. [Google Scholar]
- Petritan, I.C.; Commarmot, B.; Hobi, M.L.; Petritan, A.M.; Bigler, C.; Abrudan, I.V.; Rigling, A. Structural Patterns of Beech and Silver Fir Suggest Stability and Resilience of the Virgin Forest Sinca in the Southern Carpathians, Romania. For. Ecol. Manag. 2015, 356, 184–195. [Google Scholar] [CrossRef]
- Cristea, V.; Denayer, S. De La Biodiversitate La OMG-Uri? Eikon: Cluj-Napoca, Romania, 2004; ISBN 973-7987-77-2. [Google Scholar]
- Van der Maarel, E.; Franklin, J. Vegetation Ecology; Wiley-Blackwell: Oxford, UK, 2013. [Google Scholar]
- Curiel Yuste, J.; Flores-Rentería, D.; García-Angulo, D.; Hereş, A.-M.; Braga, C.; Petritan, A.M.; Petritan, I. Cascading Effects Associated with Climate-Change-Induced Conifer Mortality in Mountain Temperate Forests Result in Hot-Spots of Soil CO2 Emissions. Soil Biol. Biochem. 2019, 133, 50–59. [Google Scholar] [CrossRef]
- Giurgiu, V.; Drăghiciu, D.; Decei, I. Metode Si Tabele de Productie; Ceres: Austin, TX, USA, 2004. [Google Scholar]
- Pretzsch, H. Strukturvielfalt Als Ergebnis Waldbaulichen Handelns. Allg. Forst-Und Jagdztg. 1996, 167, 213–221. [Google Scholar]
- Petritan, A.M.; Biris, I.A.; Merce, O.; Turcu, D.O.; Petritan, I.C. Structure and Diversity of a Natural Temperate Sessile Oak (Quercus petraea L.)—European Beech (Fagus sylvatica L.) Forest. For. Ecol. Manag. 2012, 280, 140–149. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Tóthmérész, B. Comparison of Different Methods for Diversity Ordering. J. Veg. Sci. 1995, 6, 283–290. [Google Scholar] [CrossRef]
- Andrade, E.R.; Jardim, J.G.; Santos, B.A.; Melo, F.P.L.; Talora, D.C.; Faria, D.; Cazetta, E. Effects of Habitat Loss on Taxonomic and Phylogenetic Diversity of Understory Rubiaceae in Atlantic Forest Landscapes. For. Ecol. Manag. 2015, 349, 73–84. [Google Scholar] [CrossRef]
- StatSoft, Inc. STATISTICA (Data Analysis Software System); Version 12; 2013. Available online: http://www.statsoft.com (accessed on 8 February 2023).
- Alessandrini, A.; Biondi, F.; Di Filippo, A.; Ziaco, E.; Piovesan, G. Tree Size Distribution at Increasing Spatial Scales Converges to the Rotated Sigmoid Curve in Two Old-Growth Beech Stands of the Italian Apennines. For. Ecol. Manag. 2011, 262, 1950–1962. [Google Scholar] [CrossRef]
- Šamonil, P.; Antolík, L.; Svoboda, M.; Adam, D. Dynamics of Windthrow Events in a Natural Fir-Beech Forest in the Carpathian Mountains. For. Ecol. Manag. 2009, 257, 1148–1156. [Google Scholar] [CrossRef]
- Čavlović, J.; Andabaka, M.; Božić, M.; Teslak, K.; Beljan, K. Current Status and Recent Stand Structure Dynamics in Mixed Silver Fir—European Beech Forests in Croatian Dinarides: Are There Differences between Managed and Unmanaged Forests? Sustainability 2021, 13, 9179. [Google Scholar] [CrossRef]
- Mason, W.L.; Diaci, J.; Carvalho, J.; Valkonen, S. Continuous Cover Forestry in Europe: Usage and the Knowledge Gaps and Challenges to Wider Adoption. For. Int. J. For. Res. 2022, 95, 450. [Google Scholar] [CrossRef]
- Vandekerkhove, K.; Vanhellemont, M.; Vrška, T.; Meyer, P.; Tabaku, V.; Thomaes, A.; Leyman, A.; De Keersmaeker, L.; Verheyen, K. Very Large Trees in a Lowland Old-Growth Beech (Fagus sylvatica L.) Forest: Density, Size, Growth and Spatial Patterns in Comparison to Reference Sites in Europe. For. Ecol. Manag. 2018, 417, 1–17. [Google Scholar] [CrossRef]
- Šamonil, P.; Vrška, T. Trends and Cyclical Changes in Natural Fir-Beech Forests at the North-Western Edge of the Carpathians. Folia Geobot. 2007, 42, 337–361. [Google Scholar] [CrossRef]
- Keren, S.; Motta, R.; Govedar, Z.; Lucic, R.; Medarevic, M.; Diaci, J. Comparative Structural Dynamics of the Janj Mixed Old-Growth Mountain Forest in Bosnia and Herzegovina: Are Conifers in a Long-Term Decline? Forests 2014, 5, 1243–1266. [Google Scholar] [CrossRef]
- Kulla, L.; Roessiger, J.; Bošeľa, M.; Kucbel, S.; Murgaš, V.; Vencurik, J.; Pittner, J.; Jaloviar, P.; Šumichrast, L.; Saniga, M. Changing Patterns of Natural Dynamics in Old-Growth European Beech (Fagus sylvatica L.) Forests Can Inspire Forest Management in Central Europe. For. Ecol. Manag. 2023, 529, 120633. [Google Scholar] [CrossRef]
- Debeljak, M. Coarse Woody Debris in Virgin and Managed Forest. Ecol. Indic. 2006, 6, 733–742. [Google Scholar] [CrossRef]
- Radu, S. The Ecological Role of Deadwood in Natural Forests. In Nature Conservation; Environmental Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2006; pp. 137–141. [Google Scholar] [CrossRef]
- Öder, V.; Petritan, A.M.; Schellenberg, J.; Bergmeier, E.; Walentowski, H. Patterns and Drivers of Deadwood Quantity and Variation in Mid-Latitude Deciduous Forests. For. Ecol. Manag. 2021, 487, 118977. [Google Scholar] [CrossRef]
- Spînu, A.P.; Asbeck, T.; Bauhus, J. Combined Retention of Large Living and Dead Trees Can Improve Provision of Tree-Related Microhabitats in Central European Montane Forests. Eur. J. For. Res. 2022, 141, 1105–1120. [Google Scholar] [CrossRef]
- Keren, S.; Diaci, J. Comparing the Quantity and Structure of Deadwood in Selection Managed and Old-Growth Forests in South-East Europe. Forests 2018, 9, 76. [Google Scholar] [CrossRef]
- Christensen, M.; Hahn, K.; Mountford, E.P.; Ódor, P.; Standovár, T.; Rozenbergar, D.; Diaci, J.; Wijdeven, S.; Meyer, P.; Winter, S.; et al. Dead Wood in European Beech (Fagus sylvatica) Forest Reserves. For. Ecol. Manag. 2005, 210, 267–282. [Google Scholar] [CrossRef]
- Diaci, J.; Adamic, T.; Fidej, G.; Roženbergar, D. Toward a Beech-Dominated Alternative Stable State in Dinaric Mixed Montane Forests: A Long-Term Study of the Pecka Old-Growth Forest. Front. For. Glob. Change 2022, 5, 937404. [Google Scholar] [CrossRef]
- Chivulescu, Ș.; Pitar, D.; Apostol, B.; Leca, Ș.; Badea, O. Importance of Dead Wood in Virgin Forest Ecosystem Functioning in Southern Carpathians. Forests 2022, 13, 409. [Google Scholar] [CrossRef]
- Dynamics of Dead Wood Decay in Swiss Forests|Forest Ecosystems. Available online: https://forestecosyst.springeropen.com/articles/10.1186/s40663-020-00248-x (accessed on 6 February 2023).
- Vrška, T.; Adam, D.; Hort, L.; Kolář, T.; Janík, D. European Beech (Fagus sylvatica L.) and Silver Fir (Abies alba Mill.) Rotation in the Carpathians—A Developmental Cycle or a Linear Trend Induced by Man? For. Ecol. Manag. 2009, 258, 347–356. [Google Scholar] [CrossRef]
- Horvat, V.; García De Vicuña, J.; Biurrun, I.; García-Mijangos, I. Managed and Unmanaged Silver Fir-Beech Forests Show Similar Structural Features in the Western Pyrenees. iFor.—Biogeosci. For. 2018, 11, 698. [Google Scholar] [CrossRef]
- Abrudan, I.V. Natural and Semi-Natural Mixed Stands in the Romanian Carpathians. In Forests in Sustainable Mountain Development. A State of Knowledge Report for 2000; Price, M.F., Butt, N., Eds.; CABI Publishing: Wallingford, UK, 2000; pp. 208–209. [Google Scholar]
- Senn, J.; Suter, W. Ungulate Browsing on Silver Fir (Abies alba) in the Swiss Alps: Beliefs in Search of Supporting Data. For. Ecol. Manag. 2003, 181, 151–164. [Google Scholar] [CrossRef]
- Schulze, E.D.; Bouriaud, O.B.; Wäldchen, J.; Eisenhauer, N.; Walentowski, H.; Seele, C.; Heinze, E.; Pruschitzki, U.P.; Dănilă, G.; Marin, G.; et al. Ungulate Browsing Causes Species Loss in Deciduous Forests Independent of Community Dynamics and Silvicultural Management in Central and Southeastern Europe. Ann. For. Res. 2014, 57, 267–288. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Andrews, J.A. Soil Respiration and the Global Carbon Cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, J.; Dang, S.; Osborne, B.; Ren, Y.; Ju, X. Topography Modifies the Effect of Land-Use Change on Soil Respiration: A Meta-Analysis. Ecosphere 2021, 12, e03845. [Google Scholar] [CrossRef]
- Peng, Y.; Thomas, S.; Tian, D. Forest Management and Soil Respiration: Implications for Carbon Sequestration. Environ. Rev. 2008, 16, 93–111. [Google Scholar] [CrossRef]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How Strongly Can Forest Management Influence Soil Carbon Sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Vasile, D.; Lazăr, G.; Cojocariu, D.; Enescu, R.; Crișan, V.; Scărlătescu, V.; Ienășoiu, G.; Petrițan, A.M. Comparative phytosociology study between a virgin mixed forest and a managed forest from the Șinca area. Rev. Silvic. Cineg. 2017, 22, 78–85. [Google Scholar]
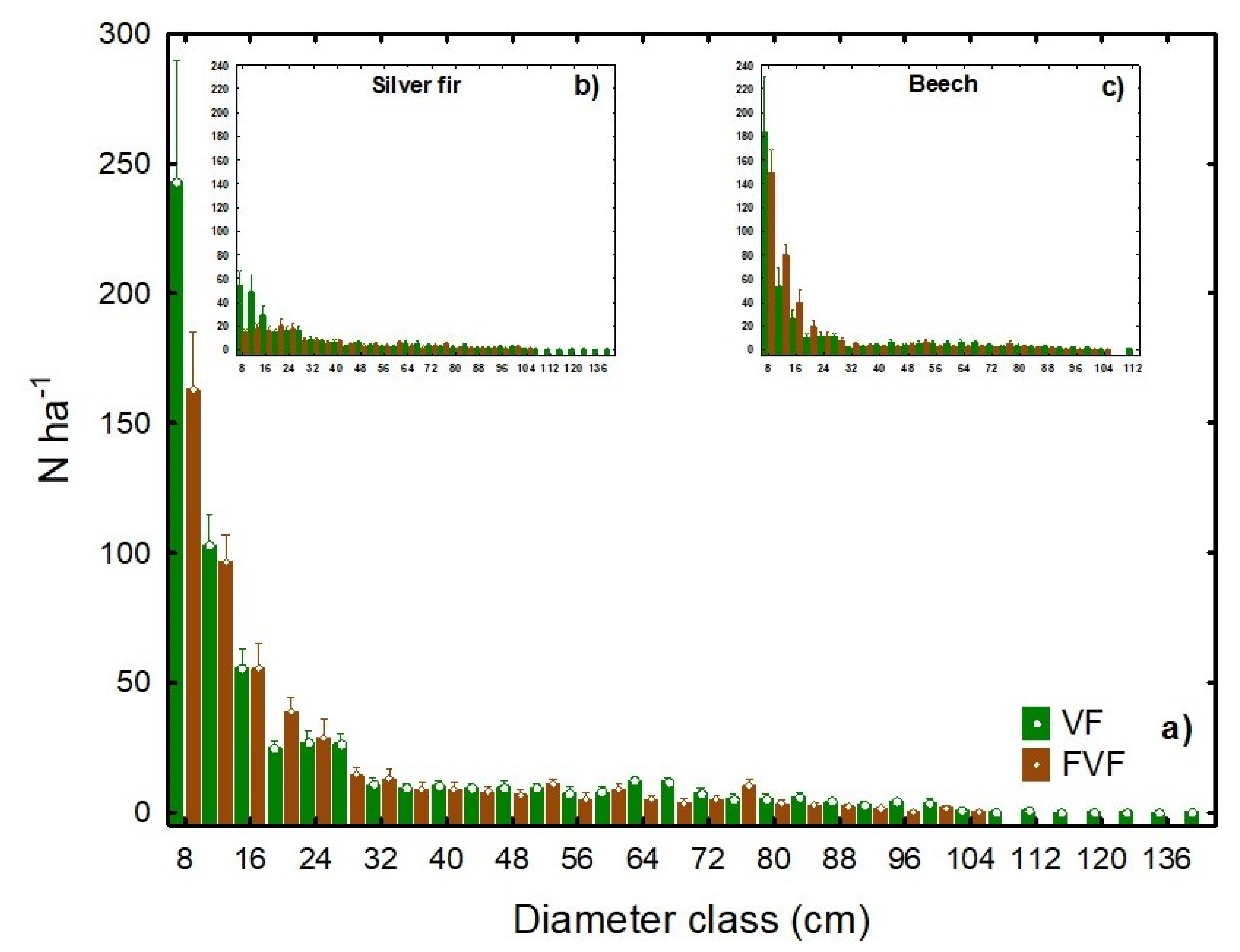
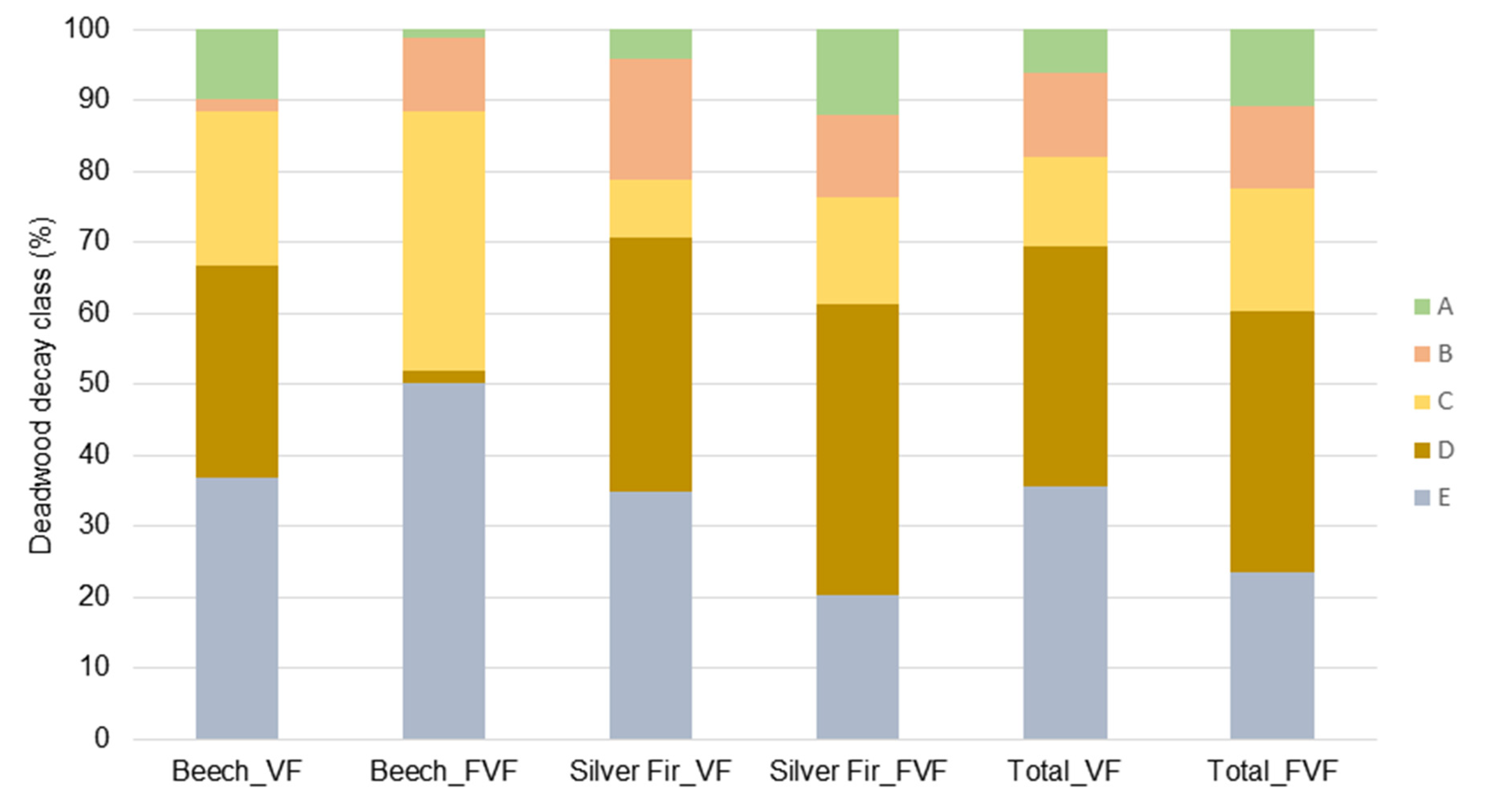
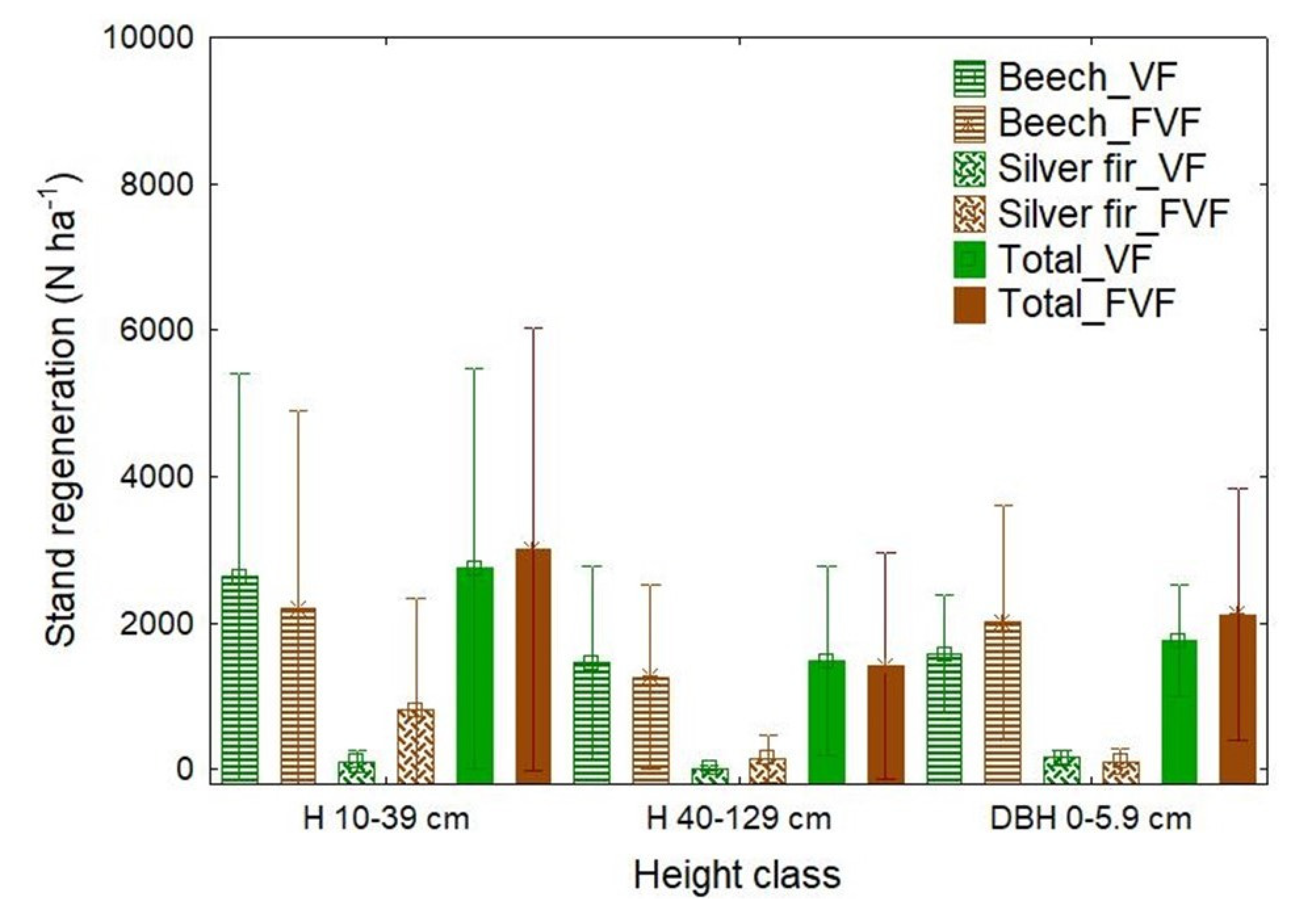

| Stand Characteristics/ Statistical Parameters | Virgin Forest | Former Virgin Forest | ||
|---|---|---|---|---|
| Mean ± Std. Error (Min–Max) | CV% | Mean ± Std. Error (Min–Max) | CV% | |
| No. of trees per ha | 650 ± 46.2 a (492–932) | 22 | 507 ± 53.0 a (320–776) | 29 |
| No. of trees per ha with dbh ≥ 80 cm | 28.4 ± 4.6 a (12–48) | 52 | 11 ± 1.8 b (4–16) | 46 |
| No. of trees per ha with height ≥ 40 m | 14.8 ± 4.0 a (0–40) | 85 | 15.5 ± 3.7 a (4–36) | 68 |
| Basal area (m2 ha−1) | 54.5 ± 3.2 a (44.7–77.3) | 18 | 35.0 ± 3.6 b (16.6–48.8) | 28 |
| Volume (m3 ha−1) | 893.1 ± 55.7 a (655.2–1236.6) | 19 | 510.0 ± 60.2 b (209.7–725.7) | 33 |
| hdom (m) | 38.4 ± 0.9 a (35.4–43.1) | 7 | 32.4 ± 1.7 b (24.3–36.1) | 11 |
| ddom (cm) | 75.9 ± 2.9 a (67.6–90.4) | 9 | 54.2 ± 3.8 b (37.2–66.3 | 18 |
| A index | 1.53 ± 0.04 a (1.25–1.66) | 9 | 1.58 ± 0.04 a (1.36–1.71) | 8 |
| Species | Beech | Silver Fir | Undefined | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Forest Type | Former Virgin | Virgin | Former Virgin | Virgin | Former Virgin | Virgin | Former Virgin | Virgin |
| Lying deadwood (m3 ha−1) | 10 ± 5.9 (0–45.7) | 60.2 ± 13.52 (8.6–144.3) | 57 ± 28 (0.5–182.5) | 114.4 ± 18.1 (12.8–214.9) | 0.3 ± 0.1 (0–3.5) | 0.6 ± 0.4 (0–3.5) | 67.2 ± 33.7 (1–228) | 174.2 ± 21.4 (71.8–277.8) |
| Standing deadwood (m3 ha−1) | 5.6 ± 4.1 (0–34.4) | 29.44 ± 10.9 (0.7–117.8) | 37.8 ± 8.5 (2.7–65.2) | 41.8 ± 11.6 (2.9–101.7) | 1.5 ± 0.6 (0–4.9) | 0.0 | 45 ± 9.9 (8.5–80.9) | 70.3 ± 14.6 (3.6–145.6) |
| Total deadwood volume (m3 ha−1) | 15.6 ± 6.2 (0.3–45.7) | 89.6 ± 22.2 (10.9–262.2) | 94.8 ± 30.5 (4.6–230) | 154.3 ± 24.5 (15.8–248.1) | 1.8 ± 0.6 (0–5) | 0.6 ± 0.4 (0–3.5) | 112.3 ± 34.7 (10.9–276.2) | 244.2 ± 31.3 (75.5–400.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braga, C.I.; Crisan, V.E.; Petritan, I.C.; Scarlatescu, V.; Vasile, D.; Lazar, G.; Petritan, A.M. Short-Term Effects of Anthropogenic Disturbances on Stand Structure, Soil Properties, and Vegetation Diversity in a Former Virgin Mixed Forest. Forests 2023, 14, 742. https://doi.org/10.3390/f14040742
Braga CI, Crisan VE, Petritan IC, Scarlatescu V, Vasile D, Lazar G, Petritan AM. Short-Term Effects of Anthropogenic Disturbances on Stand Structure, Soil Properties, and Vegetation Diversity in a Former Virgin Mixed Forest. Forests. 2023; 14(4):742. https://doi.org/10.3390/f14040742
Chicago/Turabian StyleBraga, Cosmin Ion, Vlad Emil Crisan, Ion Catalin Petritan, Virgil Scarlatescu, Diana Vasile, Gabriel Lazar, and Any Mary Petritan. 2023. "Short-Term Effects of Anthropogenic Disturbances on Stand Structure, Soil Properties, and Vegetation Diversity in a Former Virgin Mixed Forest" Forests 14, no. 4: 742. https://doi.org/10.3390/f14040742
APA StyleBraga, C. I., Crisan, V. E., Petritan, I. C., Scarlatescu, V., Vasile, D., Lazar, G., & Petritan, A. M. (2023). Short-Term Effects of Anthropogenic Disturbances on Stand Structure, Soil Properties, and Vegetation Diversity in a Former Virgin Mixed Forest. Forests, 14(4), 742. https://doi.org/10.3390/f14040742





