Genome-Wide Identification and Expression Analysis of the TCP Genes in Jatropha curcas L. Reveals Its Roles in Involvement of Leaf Shape
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Identification, Phylogeny, and Classification of TCP Proteins in Jatropha
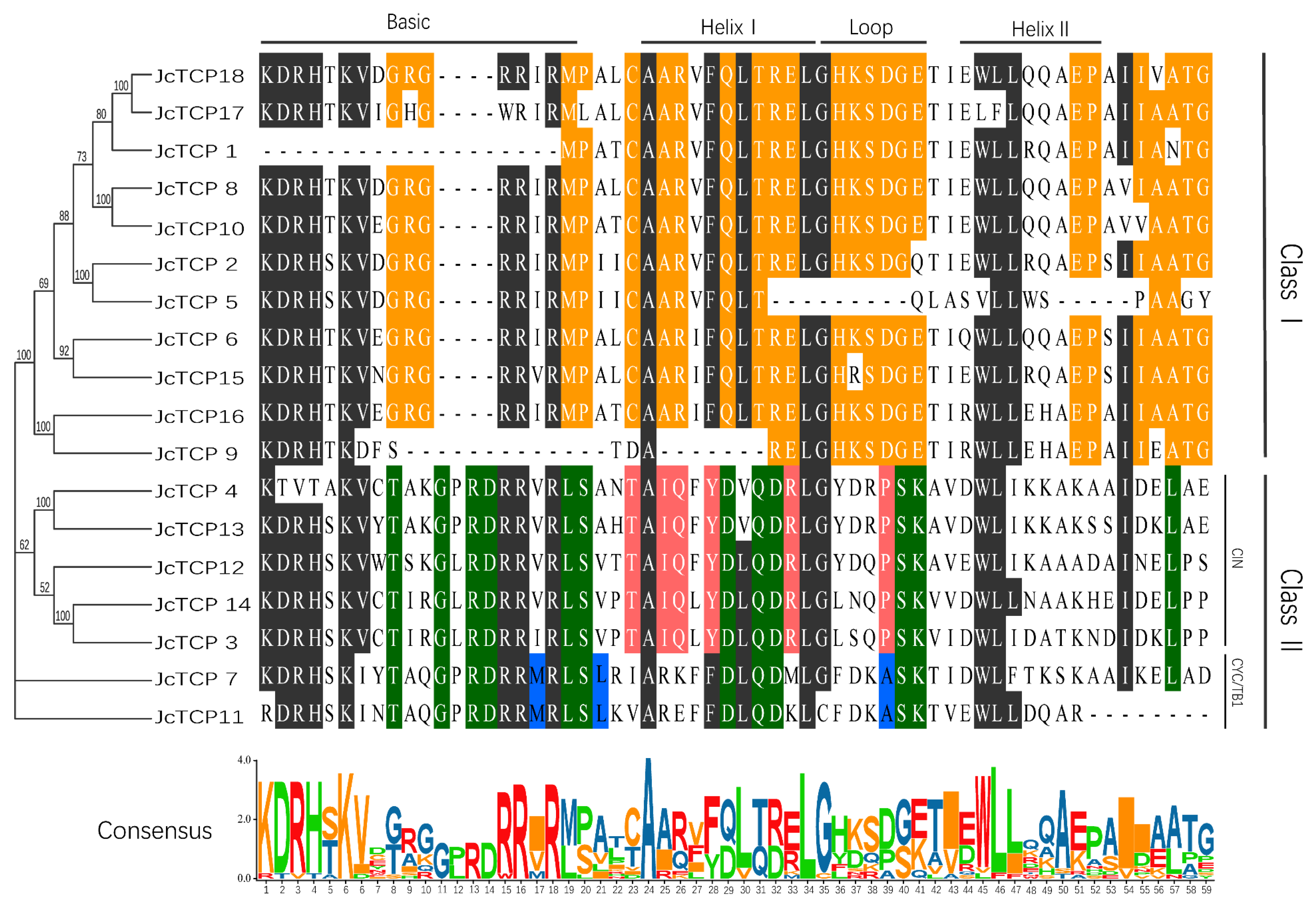
2.3. Sequence Alignment and Phylogenetic Analysis
2.4. Chromosomal Location, Duplication Analysis, and Prediction of the Cis-Regulatory Elements
2.5. Expression of Jc TCPs in Different Organs and at Different Jc Developmental Stages
2.6. Analysis of TCP Gene Expression in Jn and Jc Leaves
3. Results
3.1. Identification of TCP Gene Family in Jatropha
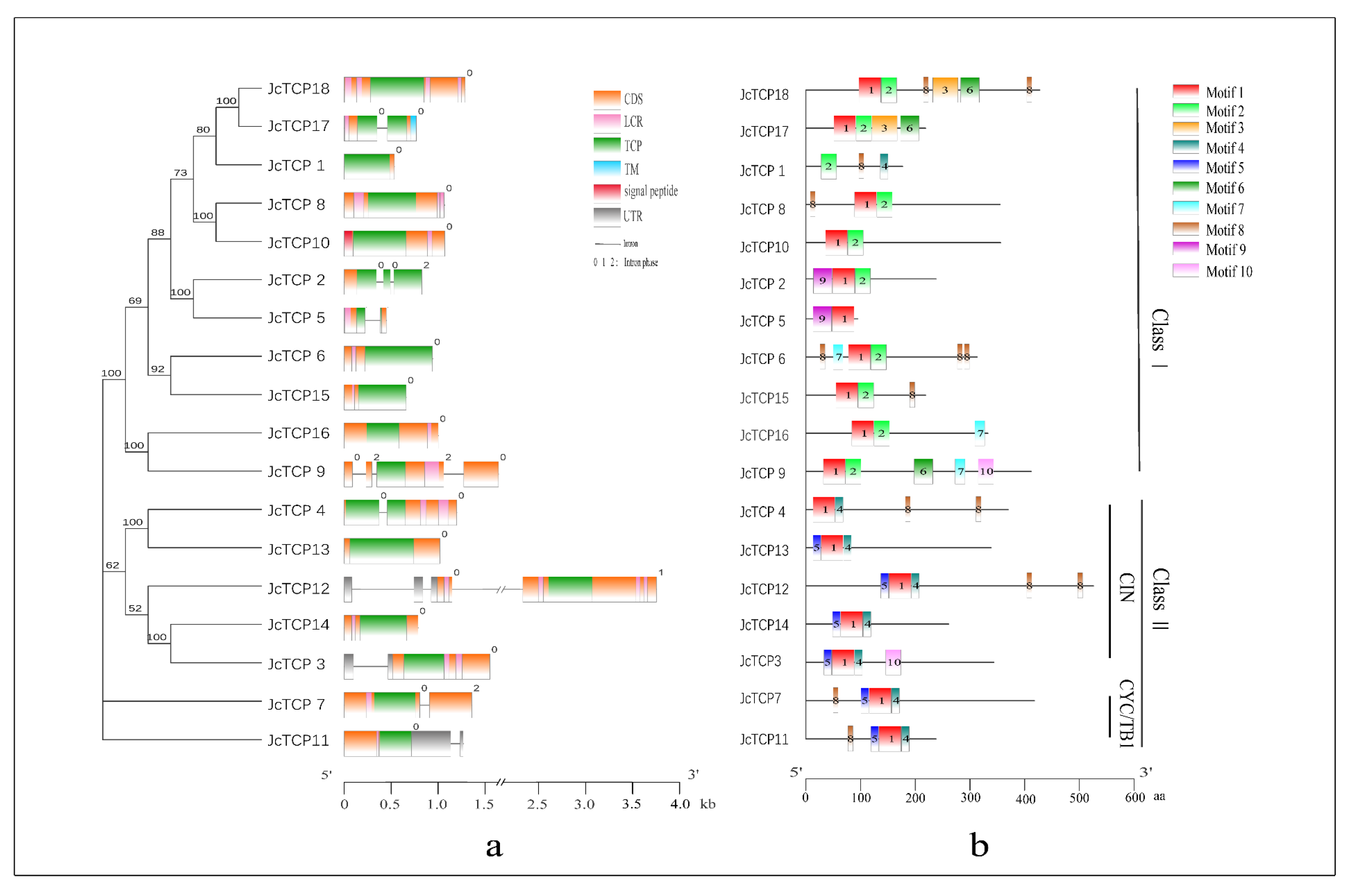
3.2. Conserved Domains and Motif Analysis
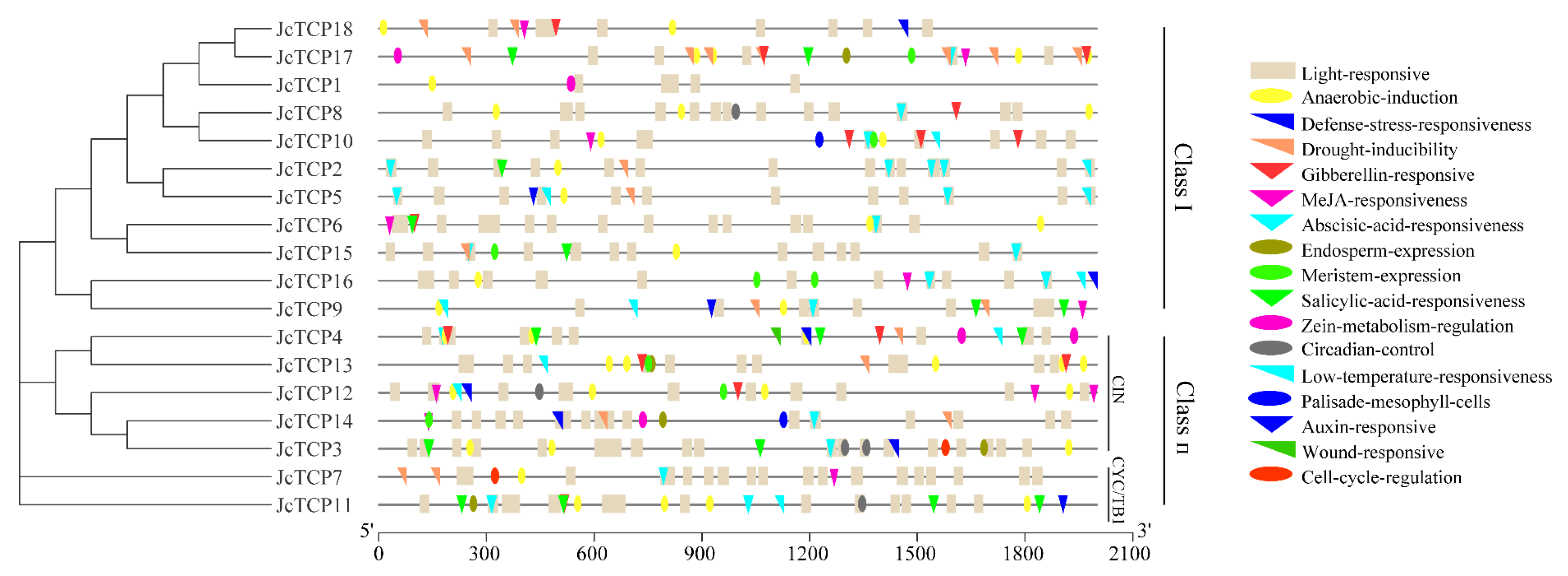
3.3. Analysis of Genetic Structure Characteristics
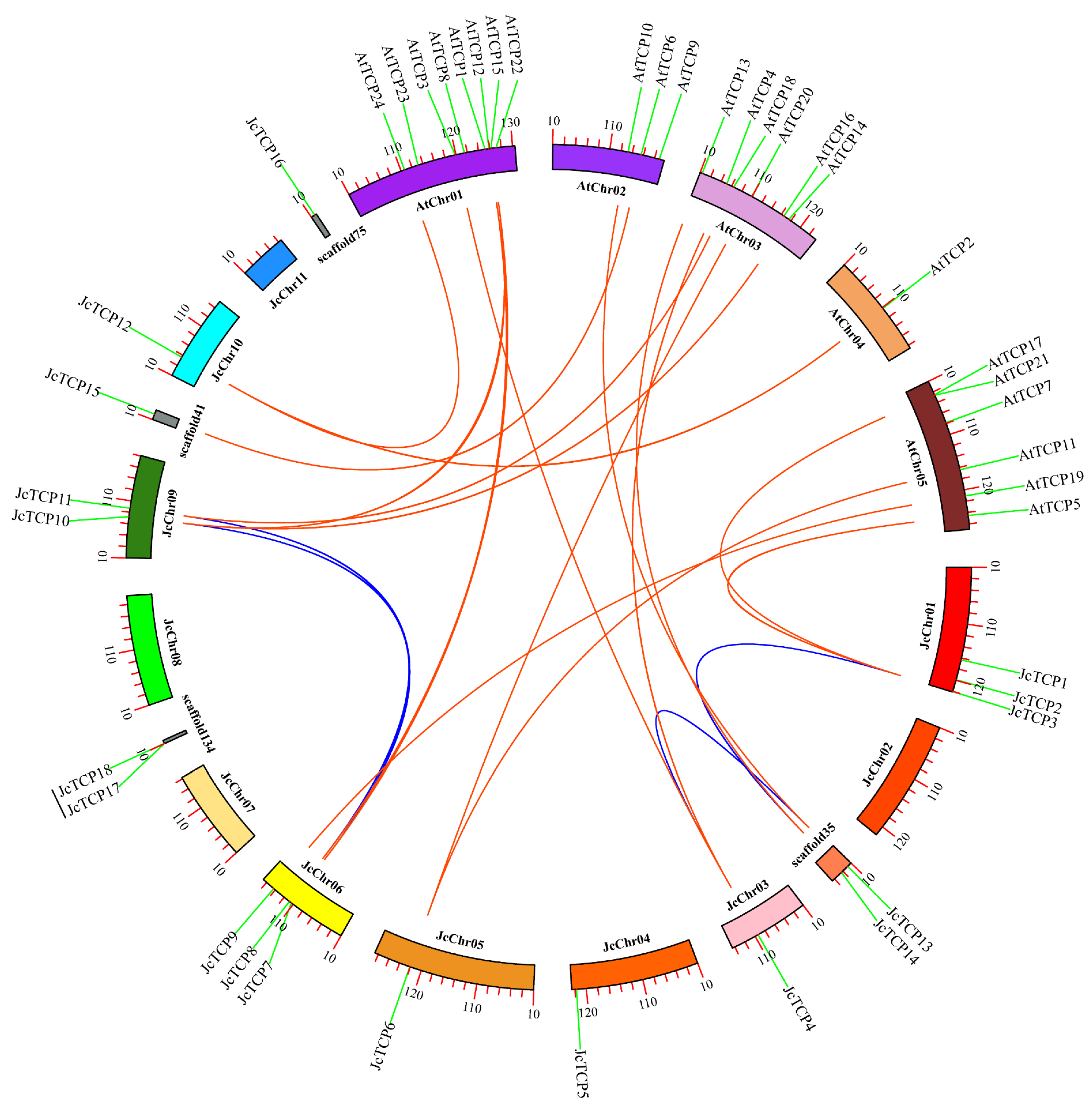
3.4. Chromosomal Localization and Collinear Analysis
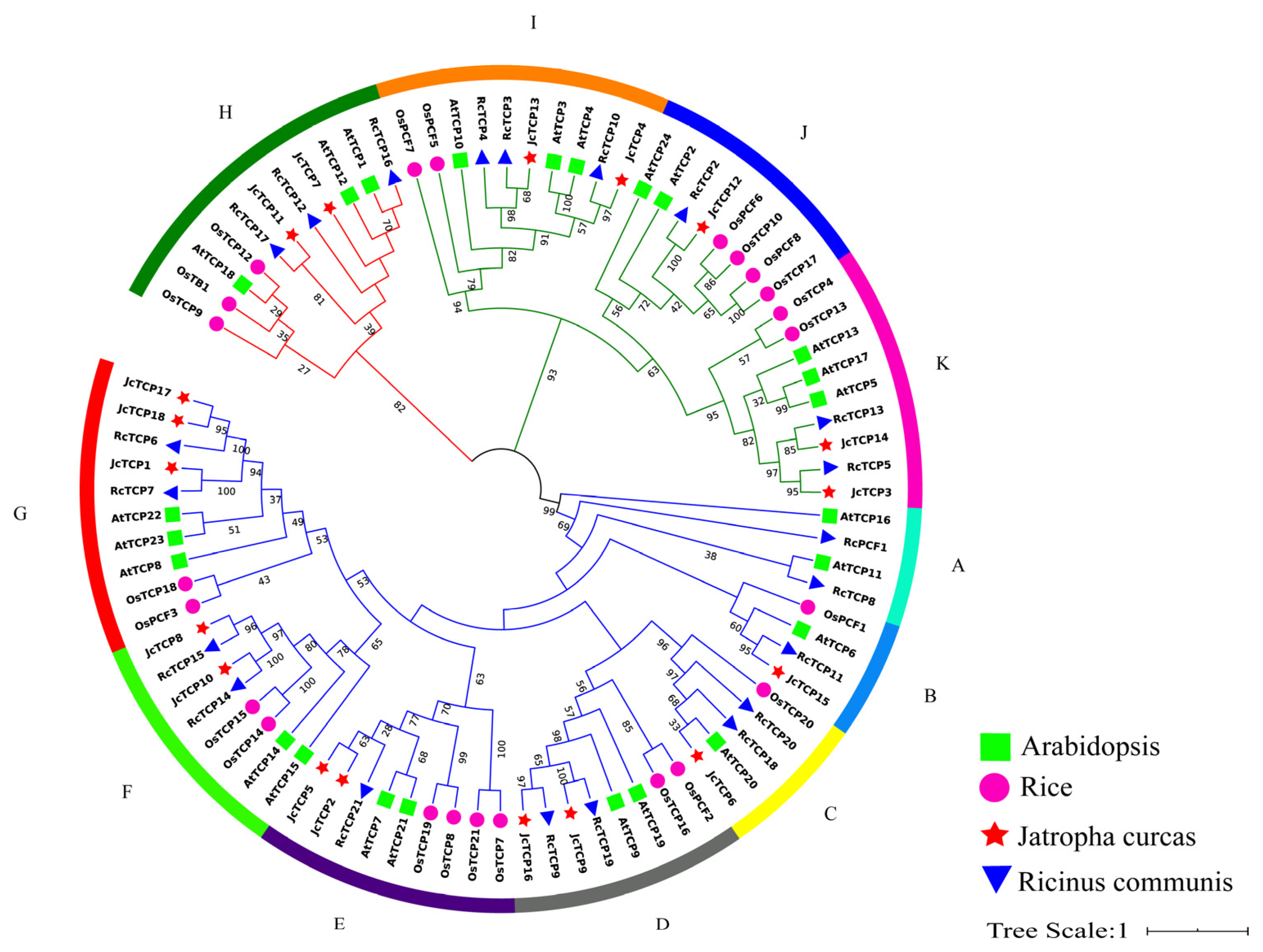
3.5. Analysis of Phylogenetic and Selection Pressures
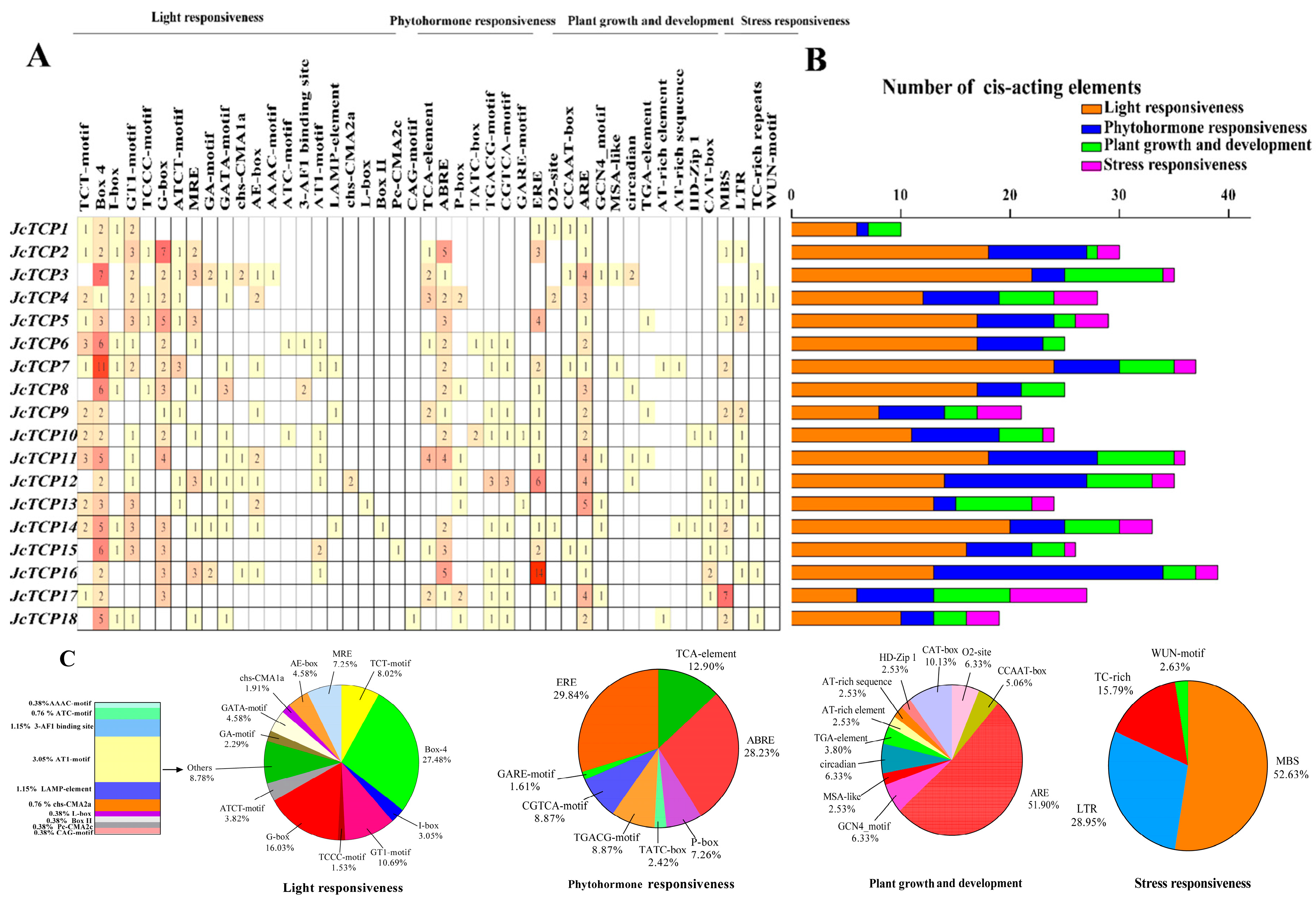
3.6. Promoter Component Analysis
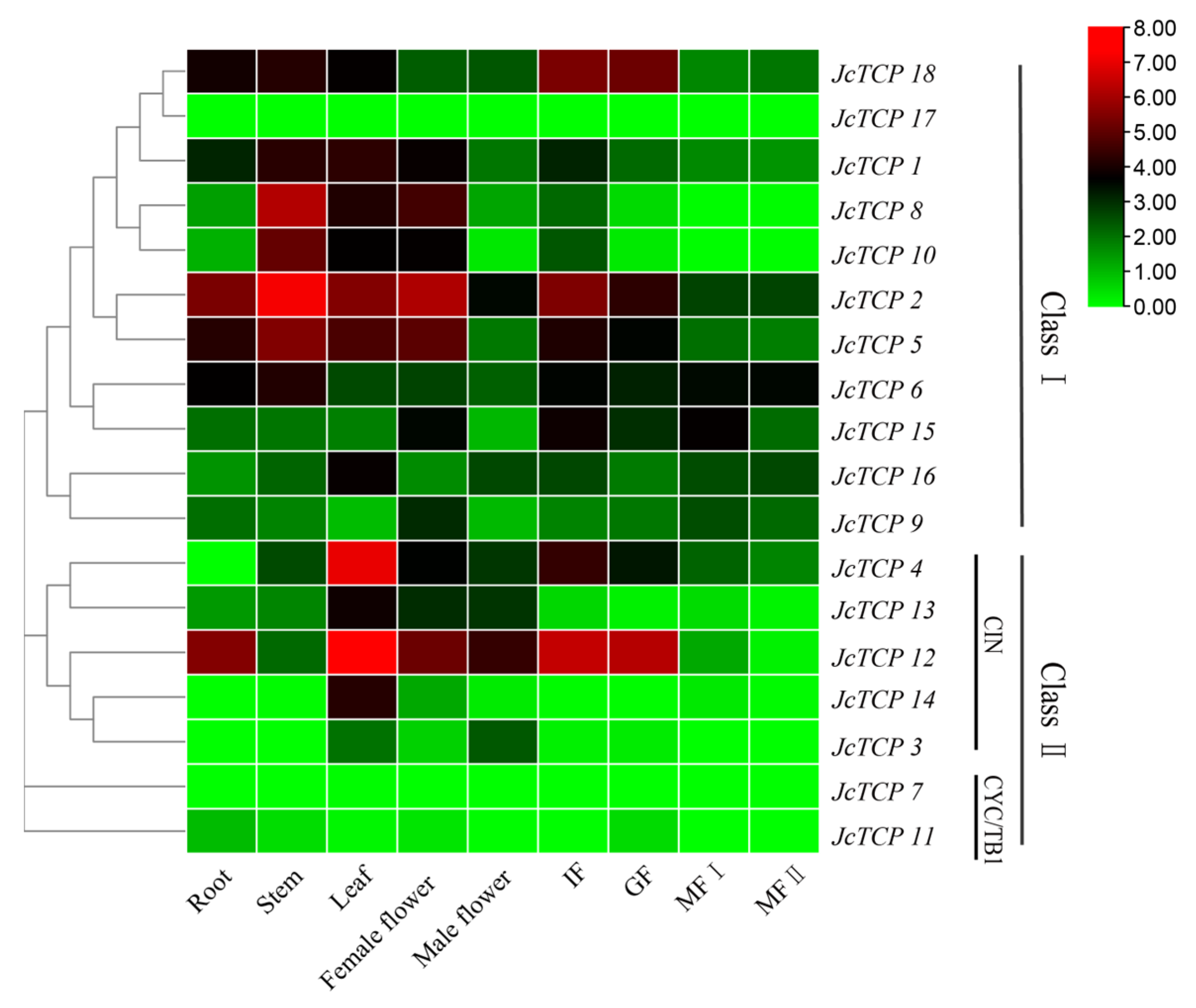
3.7. Expression Profiles of the Jc TCP Genes in Different Organs during Development
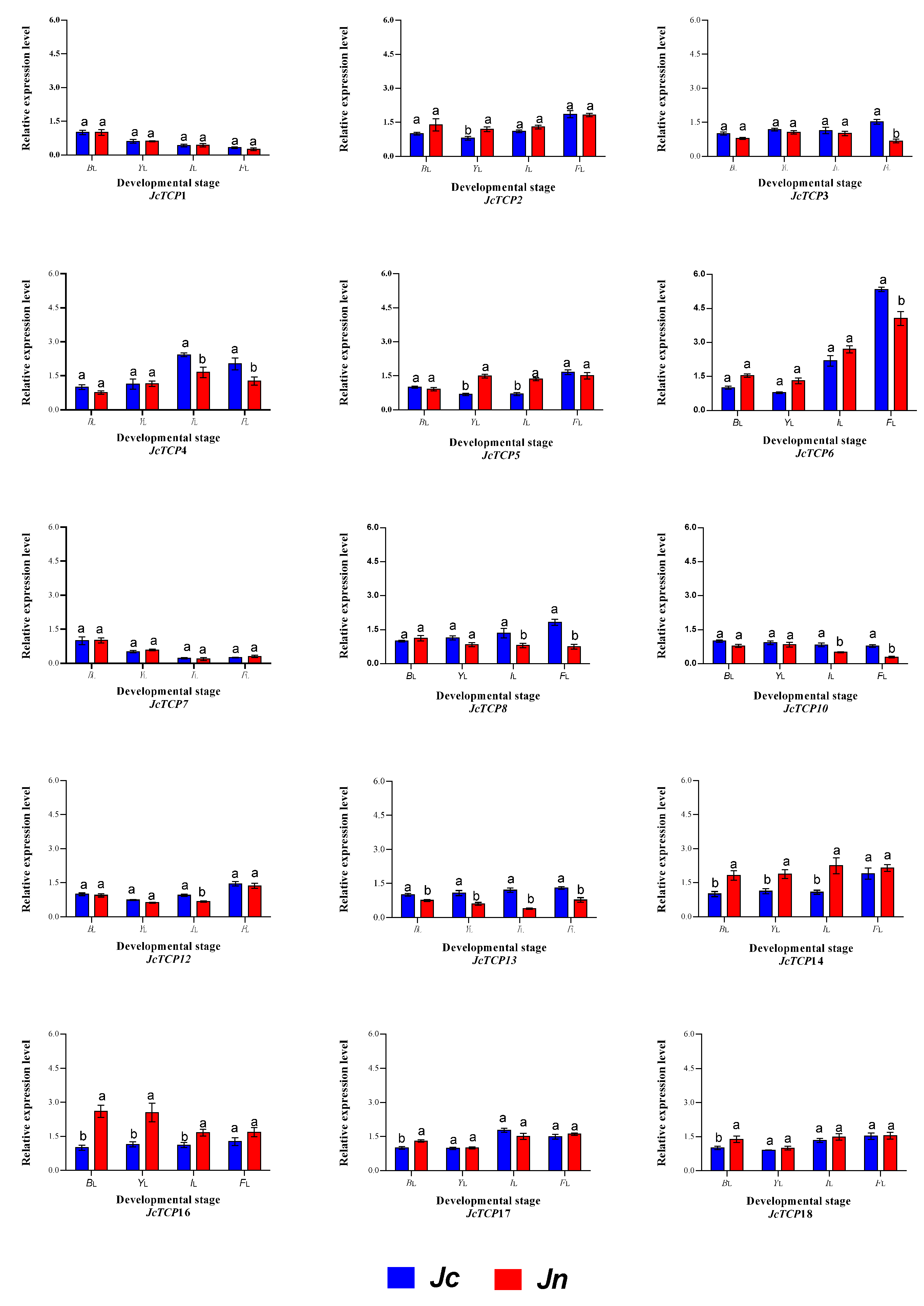
3.8. Expression Profiles of TCP Genes at Different Developmental Stages of Two Shapes of Leaves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| qRT-PCR | Quantitative real-time PCR |
| ARF | Auxin Response Factor |
| SCF | Skp1/Cullin/F-box |
| AA | Amino acid numbers |
| ORF | The length of the Open Reading Frame |
| MW | The protein molecular weight |
| PI | Isoelectric point |
| II | The instability index |
| GRAVY | Aliphatic index and grand average of hydropathicity |
| WGD | Whole-genome duplications |
| Ks | Synonymous substitution rate |
| Ka | Nonsynonymous substitution rate |
References
- Montes, J.M.; Melchinger, A.E. Domestication and Breeding of Jatropha Curcas L. Trends Plant Sci. 2016, 21, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Ezeldin Osman, M.; Sheshko, T.F.; Dipheko, T.D.; Abdallah, N.E.; Hassan, E.A.; Ishak, C.Y. Synthesis and Improvement of Jatropha Curcas L. Biodiesel Based on Eco-Friendly Materials. Int. J. Green Energy 2021, 18, 1396–1404. [Google Scholar] [CrossRef]
- Giadrossich, F.; Cohen, D.; Schwarz, M.; Seddaiu, G.; Contran, N.; Lubino, M.; Valdés-Rodríguez, O.A.; Niedda, M. Modeling Bio-Engineering Traits of Jatropha Curcas L. Ecol. Eng. 2016, 89, 40–48. [Google Scholar] [CrossRef]
- Ha, J.; Shim, S.; Lee, T.; Kang, Y.J.; Hwang, W.J.; Jeong, H.; Laosatit, K.; Lee, J.; Kim, S.K.; Satyawan, D.; et al. Genome Sequence of Jatropha Curcas L., a Non-Edible Biodiesel Plant, Provides a Resource to Improve Seed-Related Traits. Plant Biotechnol. J. 2019, 17, 517–530. [Google Scholar] [CrossRef]
- Yang, C.Y.; Lu, W.H.; Wu, X.H.; Xu, Z.F.; Long, Y.F. Analysis on Components of Fatty Acids from New Jatropha Cultivars Seed Oils and Their Potential of Preparing Biodiesel. Bot. Res. 2015, 4, 16–24. [Google Scholar] [CrossRef]
- Carins Murphy, M.R.; Jordan, G.J.; Brodribb, T.J. Differential Leaf Expansion Can Enable Hydraulic Acclimation to Sun and Shade. Plant Cell Environ. 2012, 35, 1407–1418. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C. Leaf Venation: Structure, Function, Development, Evolution, Ecology and Applications in the Past, Present and Future. New Phytol. 2013, 198, 983–1000. [Google Scholar] [CrossRef]
- Scoffoni, C.; Kunkle, J.; Pasquet-Kok, J.; Vuong, C.; Patel, A.J.; Montgomery, R.A.; Givnish, T.J.; Sack, L. Light-Induced Plasticity in Leaf Hydraulics, Venation, Anatomy, and Gas Exchange in Ecologically Diverse Hawaiian Lobeliads. New Phytol. 2015, 207, 43–58. [Google Scholar] [CrossRef]
- Wang, J.-H.; Cai, Y.-F.; Li, S.-F.; Zhang, S.-B. Differences in Leaf Physiological and Morphological Traits between Camellia japonica and Camellia reticulata. Plant Divers. 2020, 42, 181–188. [Google Scholar] [CrossRef]
- Kalve, S.; De Vos, D.; Beemster, G.T.S. Leaf Development: A Cellular Perspective. Front. Plant Sci 2014, 5, 362. [Google Scholar] [CrossRef]
- Martín-Trillo, M.; Cubas, P. TCP Genes: A Family Snapshot Ten Years Later. Trends Plant Sci. 2010, 15, 31–39. [Google Scholar] [CrossRef]
- Doebley, J.; Stec, A.; Hubbard, L. The Evolution of Apical Dominance in Maize. Nature 1997, 386, 485–488. [Google Scholar] [CrossRef]
- Luo, D.; Carpenter, R.; Vincent, C.; Copsey, L.; Coen, E. Origin of Floral Asymmetry in Antirrhinum. Nature 1996, 383, 794–799. [Google Scholar] [CrossRef]
- Kosugi, S.; Ohashi, Y. PCF1 and PCF2 Specifically Bind to Cis Elements in the Rice Proliferating Cell Nuclear Antigen Gene. Plant Cell 1997, 9, 1607–1619. [Google Scholar] [CrossRef]
- Cubas, P.; Lauter, N.; Doebley, J.; Coen, E. The TCP Domain: A Motif Found in Proteins Regulating Plant Growth and Development. Plant J. 1999, 18, 215–222. [Google Scholar] [CrossRef]
- Yin, Z.; Li, Y.; Zhu, W.; Fu, X.; Han, X.; Wang, J.; Lin, H.; Ye, W. Identification, Characterization, and Expression Patterns of TCP Genes and MicroRNA319 in Cotton. Int. J. Mol. Sci. 2018, 19, 3655. [Google Scholar] [CrossRef]
- Wen, Y.; Raza, A.; Chu, W.; Zou, X.; Cheng, H.; Hu, Q.; Liu, J.; Wei, W. Comprehensive In Silico Characterization and Expression Profiling of TCP Gene Family in Rapeseed. Front. Genet. 2021, 12, 794297. [Google Scholar] [CrossRef]
- Liu, D.-K.; Zhang, C.; Zhao, X.; Ke, S.; Li, Y.; Zhang, D.; Zheng, Q.; Li, M.-H.; Lan, S.; Liu, Z.-J. Genome-Wide Analysis of the TCP Gene Family and Their Expression Pattern in Cymbidium goeringii. Front. Plant Sci. 2022, 13, 1068969. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Z.; Zhao, K.; Yang, W.; Cheng, T.; Wang, J.; Zhang, Q. Genome-Wide Identification, Characterization and Expression Analysis of the TCP Gene Family in Prunus mume. Front. Plant Sci. 2016, 7, 1301. [Google Scholar] [CrossRef]
- Martín-Trillo, M.; Grandío, E.G.; Serra, F.; Marcel, F.; Rodríguez-Buey, M.L.; Schmitz, G.; Theres, K.; Bendahmane, A.; Dopazo, H.; Cubas, P. Role of Tomato BRANCHED1-like Genes in the Control of Shoot Branching. Plant J. 2011, 67, 701–714. [Google Scholar] [CrossRef]
- Faivre-Rampant, O.; Bryan, G.J.; Roberts, A.G.; Milbourne, D.; Viola, R.; Taylor, M.A. Regulated Expression of a Novel TCP Domain Transcription Factor Indicates an Involvement in the Control of Meristem Activation Processes in Solanum tuberosum. J. Exp. Bot. 2004, 55, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Aguilar Martinez, J.; Sinha, N. Analysis of the Role of Arabidopsis Class I TCP Genes AtTCP7, AtTCP8, AtTCP22, and AtTCP23 in Leaf Development. Front. Plant Sci. 2013, 4, 406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, Q.; Chen, F.; Wu, L.; Liu, B.; Li, F.; Zhang, J.; Bao, M.; Liu, G. Genome-Wide Identification, Characterization and Expression Analysis of TCP Transcription Factors in Petunia. Int. J. Mol. Sci. 2020, 21, 6594. [Google Scholar] [CrossRef] [PubMed]
- Danisman, S.; van der Wal, F.; Dhondt, S.; Waites, R.; de Folter, S.; Bimbo, A.; van Dijk, A.D.; Muino, J.M.; Cutri, L.; Dornelas, M.C.; et al. Arabidopsis Class I and Class II TCP Transcription Factors Regulate Jasmonic Acid Metabolism and Leaf Development Antagonistically. Plant Physiol. 2012, 159, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.A.; Sun, Y.; Blair, P.B.; Mukhtar, M.S. TCP Three-Way Handshake: Linking Developmental Processes with Plant Immunity. Trends Plant Sci. 2015, 20, 238–245. [Google Scholar] [CrossRef]
- Resentini, F.; Felipo-Benavent, A.; Colombo, L.; Blázquez, M.A.; Alabadi, D.; Masiero, S. TCP14 and TCP15 Mediate the Promotion of Seed Germination by Gibberellins in Arabidopsis thaliana. Mol. Plant 2021, 14, 1771. [Google Scholar] [CrossRef]
- Shang, X.; Han, Z.; Zhang, D.; Wang, Y.; Qin, H.; Zou, Z.; Zhou, L.; Zhu, X.; Fang, W.; Ma, Y. Genome-Wide Analysis of the TCP Gene Family and Their Expression Pattern Analysis in Tea Plant (Camellia sinensis). Front. Plant Sci. 2022, 13, 840350. [Google Scholar] [CrossRef]
- Zhan, W.; Cui, L.; Guo, G.; Zhang, Y. Genome-Wide Identification and Functional Analysis of the TCP Gene Family in Rye (Secale cereale L.). Gene 2023, 854, 147104. [Google Scholar] [CrossRef]
- Ding, S.; Cai, Z.; Du, H.; Wang, H. Genome-Wide Analysis of TCP Family Genes in Zea mays L. Identified a Role for ZmTCP42 in Drought Tolerance. Int. J. Mol. Sci. 2019, 20, 2762. [Google Scholar] [CrossRef]
- Hwarari, D.; Guan, Y.; Li, R.; Movahedi, A.; Chen, J.; Yang, L. Comprehensive Bioinformatics and Expression Analysis of TCP Transcription Factors in Liriodendron Chinense Reveals Putative Abiotic Stress Regulatory Roles. Forests 2022, 13, 1401. [Google Scholar] [CrossRef]
- Jiao, P.; Liu, T.; Zhao, C.; Fei, J.; Guan, S.; Ma, Y. ZmTCP14, a TCP Transcription Factor, Modulates Drought Stress Response in Zea mays L. Environ. Exp. Bot. 2023, 208, 105232. [Google Scholar] [CrossRef]
- Koyama, T.; Sato, F.; Ohme-Takagi, M. Roles of MiR319 and TCP Transcription Factors in Leaf Development. Plant Physiol. 2017, 175, 874–885. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, H.; Zhang, C.; Li, X.; Lyu, Y.; Qi, D.; Cui, Y.; Hu, L.; Wang, Z.; Liang, Z.; et al. TCP7 Functions Redundantly with Several Class I TCPs and Regulates Endoreplication in Arabidopsis. J. Integr. Plant Biol. 2019, 61, 1151–1170. [Google Scholar] [CrossRef]
- Hervé, C.; Dabos, P.; Bardet, C.; Jauneau, A.; Auriac, M.C.; Ramboer, A.; Lacout, F.; Tremousaygue, D. In Vivo Interference with AtTCP20 Function Induces Severe Plant Growth Alterations and Deregulates the Expression of Many Genes Important for Development. Plant Physiol. 2009, 149, 1462–1477. [Google Scholar] [CrossRef]
- Palatnik, J.F.; Allen, E.; Wu, X.; Schommer, C.; Schwab, R.; Carrington, J.C.; Weigel, D. Control of Leaf Morphogenesis by MicroRNAs. Nature 2003, 425, 257–263. [Google Scholar] [CrossRef]
- Efroni, I.; Blum, E.; Goldshmidt, A.; Eshed, Y. A Protracted and Dynamic Maturation Schedule Underlies Arabidopsis Leaf Development. Plant Cell 2008, 20, 2293–2306. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, L.; Wang, W.; Tian, P.; Wang, W.; Wang, K.; Gao, Z.; Liu, S.; Zhang, Y.; Irish, V.F.; et al. TCP5 Controls Leaf Margin Development by Regulating KNOX and BEL-like Transcription Factors in Arabidopsis. J. Exp. Bot. 2021, 72, 1809–1821. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, H.; Gao, Y.; Xiong, R.; Wu, M.; Zhang, K.; Xiang, Y. The TCP Transcription Factor PeTCP10 Modulates Salt Tolerance in Transgenic Arabidopsis. Plant Cell Rep. 2021, 40, 1971–1987. [Google Scholar] [CrossRef]
- Navaud, O.; Dabos, P.; Carnus, E.; Tremousaygue, D.; Hervé, C. TCP Transcription Factors Predate the Emergence of Land Plants. J. Mol. Evol. 2007, 65, 23–33. [Google Scholar] [CrossRef]
- Yao, X.; Ma, H.; Wang, J.; Zhang, D. Genome-Wide Comparative Analysis and Expression Pattern of TCP Gene Families in Arabidopsis Thaliana and Oryza Sativa. J. Integr. Plant Biol. 2007, 49, 885–897. [Google Scholar] [CrossRef]
- Parapunova, V.; Busscher, M.; Busscher-Lange, J.; Lammers, M.; Karlova, R.; Bovy, A.G.; Angenent, G.C.; de Maagd, R.A. Identification, Cloning and Characterization of the Tomato TCP Transcription Factor Family. BMC Plant Biol. 2014, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, S.; Ohashi, Y. DNA Binding and Dimerization Specificity and Potential Targets for the TCP Protein Family. Plant J. 2002, 30, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Ori, N.; Cohen, A.R.; Etzioni, A.; Brand, A.; Yanai, O.; Shleizer, S.; Menda, N.; Amsellem, Z.; Efroni, I.; Pekker, I.; et al. Regulation of LANCEOLATE by MiR319 Is Required for Compound-Leaf Development in Tomato. Nat. Genet. 2007, 39, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam Protein Families Database: Towards a More Sustainable Future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The Proteomics Server for in-Depth Protein Knowledge and Analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X Version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A Multiple Sequence Alignment Editor and Analysis Workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Wu, Z.-J.; Wang, W.-L.; Zhuang, J. Developmental Processes and Responses to Hormonal Stimuli in Tea Plant (Camellia sinensis) Leaves Are Controlled by GRF and GIF Gene Families. Funct. Integr. Genom. 2017, 17, 503–512. [Google Scholar] [CrossRef]
- Ling, J.; Jiang, W.; Zhang, Y.; Yu, H.; Mao, Z.; Gu, X.; Huang, S.; Xie, B. Genome-Wide Analysis of WRKY Gene Family in Cucumis Sativus. BMC Genom. 2011, 12, 471. [Google Scholar] [CrossRef]
- Chai, W.; Jiang, P.; Huang, G.; Jiang, H.; Li, X. Identification and Expression Profiling Analysis of TCP Family Genes Involved in Growth and Development in Maize. Physiol. Mol. Biol. Plants 2017, 23, 779–791. [Google Scholar] [CrossRef]
- Li, C.; Potuschak, T.; Colon-Carmona, A.; Gutierrez, R.; Doerner, P. Arabidopsis TCP20 Links Regulation of Growth and Cell Division Control Pathways. Proc. Natl. Acad. Sci. USA 2005, 102, 12978–12983. [Google Scholar] [CrossRef]
- Nicolas, M.; Cubas, P. TCP Factors: New Kids on the Signaling Block. Curr. Opin. Plant Biol. 2016, 33, 33–41. [Google Scholar] [CrossRef]
- Manassero, N.G.U.; Viola, I.L.; Welchen, E.; Gonzalez, D.H. TCP Transcription Factors: Architectures of Plant Form. Biomol. Concepts 2013, 4, 111–127. [Google Scholar] [CrossRef]
- Bresso, E.G.; Chorostecki, U.; Rodriguez, R.E.; Palatnik, J.F.; Schommer, C. Spatial Control of Gene Expression by MiR319-Regulated TCP Transcription Factors in Leaf Development. Plant Physiol. 2018, 176, 1694–1708. [Google Scholar] [CrossRef]
- Mao, Y.; Wu, F.; Yu, X.; Bai, J.; Zhong, W.; He, Y. MicroRNA319a-Targeted Brassica Rapa Ssp. Pekinensis TCP Genes Modulate Head Shape in Chinese Cabbage by Differential Cell Division Arrest in Leaf Regions. Plant Physiol. 2014, 164, 710–720. [Google Scholar] [CrossRef]
- Lin, J.; Zhu, M.; Cai, M.; Zhang, W.; Fatima, M.; Jia, H.; Li, F.; Ming, R. Identification and Expression Analysis of TCP Genes in Saccharum spontaneum L. Trop. Plant Biol. 2019, 12, 206–218. [Google Scholar] [CrossRef]
- Liu, M.-M.; Wang, M.-M.; Yang, J.; Wen, J.; Guo, P.-C.; Wu, Y.-W.; Ke, Y.-Z.; Li, P.-F.; Li, J.-N.; Du, H. Evolutionary and Comparative Expression Analyses of TCP Transcription Factor Gene Family in Land Plants. Int. J. Mol. Sci. 2019, 20, 3591. [Google Scholar] [CrossRef]
- Sánchez Moreano, J.P.; Xu, X.; Aucapiña Criollo, C.B.; Chen, X.; Lin, Y.; Munir, N.; Lai, Z. Genome-Wide Identification and Comprehensive Analyses of TCP Gene Family in Banana (Musa L.). Trop. Plant Biol. 2021, 14, 180–202. [Google Scholar] [CrossRef]
- Danisman, S.; van Dijk, A.D.J.; Bimbo, A.; van der Wal, F.; Hennig, L.; de Folter, S.; Angenent, G.C.; Immink, R.G.H. Analysis of Functional Redundancies within the Arabidopsis TCP Transcription Factor Family. J. Exp. Bot. 2013, 64, 5673–5685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, C.; Wu, P.; Chen, Y.; Li, M.; Jiang, H.; Wu, G. Genome-wide identification and expression analysis of TCP transcription factors in castor bean (Ricinus communis). PeerJ 2019, 7, e7776. [Google Scholar] [CrossRef]
- Wang, J.-L.; Wang, H.-W.; Cao, Y.-N.; Kan, S.-L.; Liu, Y.-Y. Comprehensive Evolutionary Analysis of the TCP Gene Family: Further Insights for Its Origin, Expansion, and Diversification. Front. Plant Sci. 2022, 13, 994567. [Google Scholar] [CrossRef]
- Ferrero, L.V.; Viola, I.L.; Ariel, F.D.; Gonzalez, D.H. Class I TCP Transcription Factors Target the Gibberellin Biosynthesis Gene GA20ox1 and the Growth-Promoting Genes HBI1 and PRE6 during Thermomorphogenic Growth in Arabidopsis. Plant Cell Physiol. 2019, 60, 1633–1645. [Google Scholar] [CrossRef]
- Lucero, L.E.; Uberti-Manassero, N.G.; Arce, A.L.; Colombatti, F.; Alemano, S.G.; Gonzalez, D.H. TCP15 Modulates Cytokinin and Auxin Responses during Gynoecium Development in Arabidopsis. Plant J. 2015, 84, 267–282. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, D.; An, J.; Yin, H.; Fang, S.; Chu, J.; Zhao, Y.; Li, J. TCP Transcription Factors Regulate Shade Avoidance via Directly Mediating the Expression of Both PHYTOCHROME INTERACTING FACTORs and Auxin Biosynthetic Genes. Plant Physiol. 2018, 176, 1850–1861. [Google Scholar] [CrossRef]
- Koyama, T.; Mitsuda, N.; Seki, M.; Shinozaki, K.; Ohme-Takagi, M. TCP Transcription Factors Regulate the Activities of ASYMMETRIC LEAVES1 and MiR164, as Well as the Auxin Response, during Differentiation of Leaves in Arabidopsis. Plant Cell 2010, 22, 3574–3588. [Google Scholar] [CrossRef]
- Ma, X.; Ma, J.; Fan, D.; Li, C.; Jiang, Y.; Luo, K. Genome-Wide Identification of TCP Family Transcription Factors from Populus Euphratica and Their Involvement in Leaf Shape Regulation. Sci. Rep. 2016, 6, 32795. [Google Scholar] [CrossRef]
- Luo, C.; Wang, S.; Ning, K.; Chen, Z.; Wang, Y.; Yang, J.; Wang, Q. LsAP2 Regulates Leaf Morphology by Inhibiting CIN-like TCP Transcription Factors and Repressing LsKAN2 in Lettuce. Hortic. Res. 2021, 8, 184. [Google Scholar] [CrossRef]

| Gene Name | Accession Number | Protein | MW(KD) | pI | GRAVY | Aliphatic Index | Loc |
|---|---|---|---|---|---|---|---|
| Jc TCP 1 | Jatcu.01g001829 | 177 | 18.59 | 5.76 | −0.399 | 47.09 | chr01,16313129-16313662 − |
| Jc TCP 2 | Jatcu.01g002405 | 239 | 24.4 | 9.79 | −3.222 | 49.9 | chr01,19888124-19888951 − |
| Jc TCP 3 | Jatcu.01g002698 | 344 | 38.57 | 8.69 | −0.828 | 42.68 | chr01,22051531-22053082 + |
| Jc TCP 4 | Jatcu.03g001473 | 370 | 40.18 | 6.31 | −0.601 | 51.99 | chr03,9416180-9417377 − |
| Jc TCP 5 | Jatcu.04g002308 | 95 | 9.86 | 10.94 | −0.409 | 48.04 | chr04,21737538-21737987 + |
| Jc TCP 6 | Jatcu.05g001498 | 313 | 33.54 | 9.01 | −0.776 | 57.76 | chr05,22093625-22094566 + |
| Jc TCP 7 | Jatcu.06g000882 | 418 | 46.91 | 9.27 | −0.786 | 36.23 | chr06,10403642-10405001 − |
| Jc TCP 8 | Jatcu.06g000960 | 355 | 38.8 | 8.93 | −0.52 | 68.6 | chr06,10815277-10816344 + |
| Jc TCP 9 | Jatcu.06g001627 | 412 | 43.77 | 8.49 | −0.248 | 51.26 | chr06,14359430-14361071 − |
| Jc TCP 10 | Jatcu.09g001368 | 356 | 38.56 | 8.95 | −0.449 | 63.36 | chr09,7223018-7224088 − |
| Jc TCP 11 | Jatcu.09g001633 | 238 | 26.97 | 7.72 | −0.841 | 59.93 | chr09,8690821-8692087 − |
| Jc TCP 12 | Jatcu.10g000691 | 526 | 57.1 | 9.53 | −0.847 | 48.5 | chr10,3612342-3616079 − |
| Jc TCP 13 | Jatcu.U000152 | 339 | 37.38 | 5.79 | −0.639 | 34.25 | scaffold35,825831-826850 + |
| Jc TCP 14 | Jatcu.U000413 | 261 | 28.98 | 9.51 | −0.723 | 52.35 | scaffold35:2332822-2333607 + |
| Jc TCP 15 | Jatcu.U001198 | 219 | 23.44 | 8.54 | −0.497 | 57.3 | scaffold41,1007626-1008285 − |
| Jc TCP 16 | Jatcu.U002150 | 333 | 35.63 | 9.20 | −0.383 | 55.08 | scaffold75,678700-679701 − |
| Jc TCP 17 | Jatcu.U003021 | 219 | 23.61 | 9.12 | 0.068 | 31.72 | scaffold134,14512-15280 + |
| Jc TCP 18 | Jatcu.U003022 | 428 | 46.11 | 6.81 | −0.669 | 50.55 | scaffold134,43667-44953 + |
| Gene Pairs | Ka | Ks | Ka/Ks | Gene Pairs | Ka | Ks | Ka/Ks |
|---|---|---|---|---|---|---|---|
| Jc TCP 13/Rc TCP 10 | 0.4130 | 3.3841 | 0.1220 | Jc TCP 15/Rc TCP 20 | 0.7093 | NaN | NaN |
| Jc TCP 13/Rc TCP 3 | 0.1394 | 0.7124 | 0.1956 | Jc TCP 15/Rc TCP 8 | 0.6850 | 2.0846 | 0.3286 |
| Jc TCP 13/Rc TCP 4 | 0.2189 | 0.7156 | 0.3059 | Jc TCP 6/RcPCF1 | 0.9058 | NaN | NaN |
| Jc TCP 4/Rc TCP 10 | 0.1621 | 0.7071 | 0.2293 | Jc TCP 6/Rc TCP 11 | 0.6338 | NaN | NaN |
| Jc TCP 4/Rc TCP 3 | 0.4605 | 2.1172 | 0.2175 | Jc TCP 6/Rc TCP 18 | 0.0522 | 0.4826 | 0.1082 |
| Jc TCP 4/Rc TCP 4 | 0.5321 | NaN | NaN | Jc TCP 6/Rc TCP 20 | 0.2489 | 1.7971 | 0.1385 |
| Jc TCP 10/Rc TCP 14 | 0.1420 | 0.9604 | 0.1478 | Jc TCP 6/Rc TCP 8 | 0.7873 | NaN | NaN |
| Jc TCP 10/Rc TCP 15 | 0.3016 | 2.5247 | 0.1195 | At TCP 5/Jc TCP 3 | 0.4985 | 3.4374 | 0.1450 |
| Jc TCP 8/Rc TCP 14 | 0.3556 | 2.3572 | 0.1509 | At TCP 17/Jc TCP 3 | 0.5780 | NaN | NaN |
| Jc TCP 8/Rc TCP 15 | 0.0749 | 1.0617 | 0.0706 | At TCP 4/Jc TCP 4 | 0.3458 | 2.9209 | 0.1184 |
| Jc TCP 14/Rc TCP 13 | 0.1728 | 0.6955 | 0.2484 | At TCP 3/Jc TCP 4 | 0.3466 | NaN | NaN |
| Jc TCP 14/Rc TCP 5 | 0.4281 | 1.0216 | 0.4190 | At TCP 11/Jc TCP 6 | 0.4484 | 4.3034 | 0.1042 |
| Jc TCP 2/Rc TCP 21 | 0.1439 | 2.0347 | 0.0707 | At TCP 20/Jc TCP 6 | 0.2611 | 2.0023 | 0.1304 |
| Jc TCP 5/Rc TCP 21 | 0.3036 | NaN | NaN | At TCP 12/Jc TCP 7 | 0.5767 | NaN | NaN |
| Jc TCP 12/Rc TCP 2 | 0.0618 | 0.5854 | 0.1056 | At TCP 15/Jc TCP 8 | 0.4612 | NaN | NaN |
| Jc TCP 1/Rc TCP 6 | 0.4281 | 4.0149 | 0.1066 | At TCP 15/Jc TCP 8 | 0.4612 | NaN | NaN |
| Jc TCP 1/Rc TCP 7 | 0.2230 | 1.1044 | 0.2019 | At TCP 19/Jc TCP 9 | 0.3622 | 2.2903 | 0.1581 |
| Jc TCP 17/Rc TCP 6 | 0.2806 | 0.9262 | 0.3029 | At TCP 14/Jc TCP 10 | 0.5362 | NaN | NaN |
| Jc TCP 17/Rc TCP 7 | 0.4946 | 1.6012 | 0.3089 | At TCP 15/Jc TCP 10 | 0.4090 | NaN | NaN |
| Jc TCP 18/Rc TCP 6 | 0.1634 | 0.8614 | 0.1898 | At TCP 18/Jc TCP 11 | 0.7134 | NaN | NaN |
| Jc TCP 18/Rc TCP 7 | 0.4529 | 1.4916 | 0.3036 | At TCP 24/Jc TCP 12 | 0.4573 | NaN | NaN |
| Jc TCP 11/Rc TCP 12 | 0.5601 | 1.5207 | 0.3683 | At TCP 2/Jc TCP 12 | 0.4196 | 1.7702 | 0.2371 |
| Jc TCP 11/Rc TCP 16 | 0.5988 | 1.7005 | 0.3521 | At TCP 10/Jc TCP 13 | 0.4913 | NaN | NaN |
| Jc TCP 11/Rc TCP 17 | 0.2948 | 1.0868 | 0.2712 | At TCP 13/Jc TCP 14 | 0.5941 | NaN | NaN |
| Jc TCP 7/Rc TCP 12 | 0.2545 | 1.3599 | 0.1872 | At TCP 6/Jc TCP 15 | 0.4941 | 3.0264 | 0.1632 |
| Jc TCP 7/Rc TCP 16 | 0.7803 | 2.0336 | 0.3837 | Jc TCP 3/Jc TCP 14 | 0.4562 | 1.2004 | 0.3801 |
| Jc TCP 7/Rc TCP 17 | 0.6804 | 3.6230 | 0.1878 | Jc TCP 4/Jc TCP 13 | 0.5218 | 2.6840 | 0.1944 |
| Jc TCP 15/RcPCF1 | 0.7057 | NaN | NaN | Jc TCP 7/Jc TCP 11 | 0.5186 | 1.9782 | 0.2622 |
| Jc TCP 15/Rc TCP 11 | 0.2099 | 1.1577 | 0.1813 | Jc TCP 8/Jc TCP 10 | 0.3031 | 2.2030 | 0.1376 |
| Jc TCP 15/Rc TCP 18 | 0.6691 | 1.9555 | 0.3422 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, R.; Peng, Y.; Zhao, Y.; Wang, X. Genome-Wide Identification and Expression Analysis of the TCP Genes in Jatropha curcas L. Reveals Its Roles in Involvement of Leaf Shape. Forests 2023, 14, 780. https://doi.org/10.3390/f14040780
Zou R, Peng Y, Zhao Y, Wang X. Genome-Wide Identification and Expression Analysis of the TCP Genes in Jatropha curcas L. Reveals Its Roles in Involvement of Leaf Shape. Forests. 2023; 14(4):780. https://doi.org/10.3390/f14040780
Chicago/Turabian StyleZou, Rong, Yang Peng, Yang Zhao, and Xiurong Wang. 2023. "Genome-Wide Identification and Expression Analysis of the TCP Genes in Jatropha curcas L. Reveals Its Roles in Involvement of Leaf Shape" Forests 14, no. 4: 780. https://doi.org/10.3390/f14040780
APA StyleZou, R., Peng, Y., Zhao, Y., & Wang, X. (2023). Genome-Wide Identification and Expression Analysis of the TCP Genes in Jatropha curcas L. Reveals Its Roles in Involvement of Leaf Shape. Forests, 14(4), 780. https://doi.org/10.3390/f14040780





