Composition of the Gas-Air Mixture in the Containment and Suppression of Forest Fires with Promising Extinguishing Agents
Abstract
1. Introduction
2. Materials and Properties
2.1. Forest Fuels
2.2. Firefighting Compositions
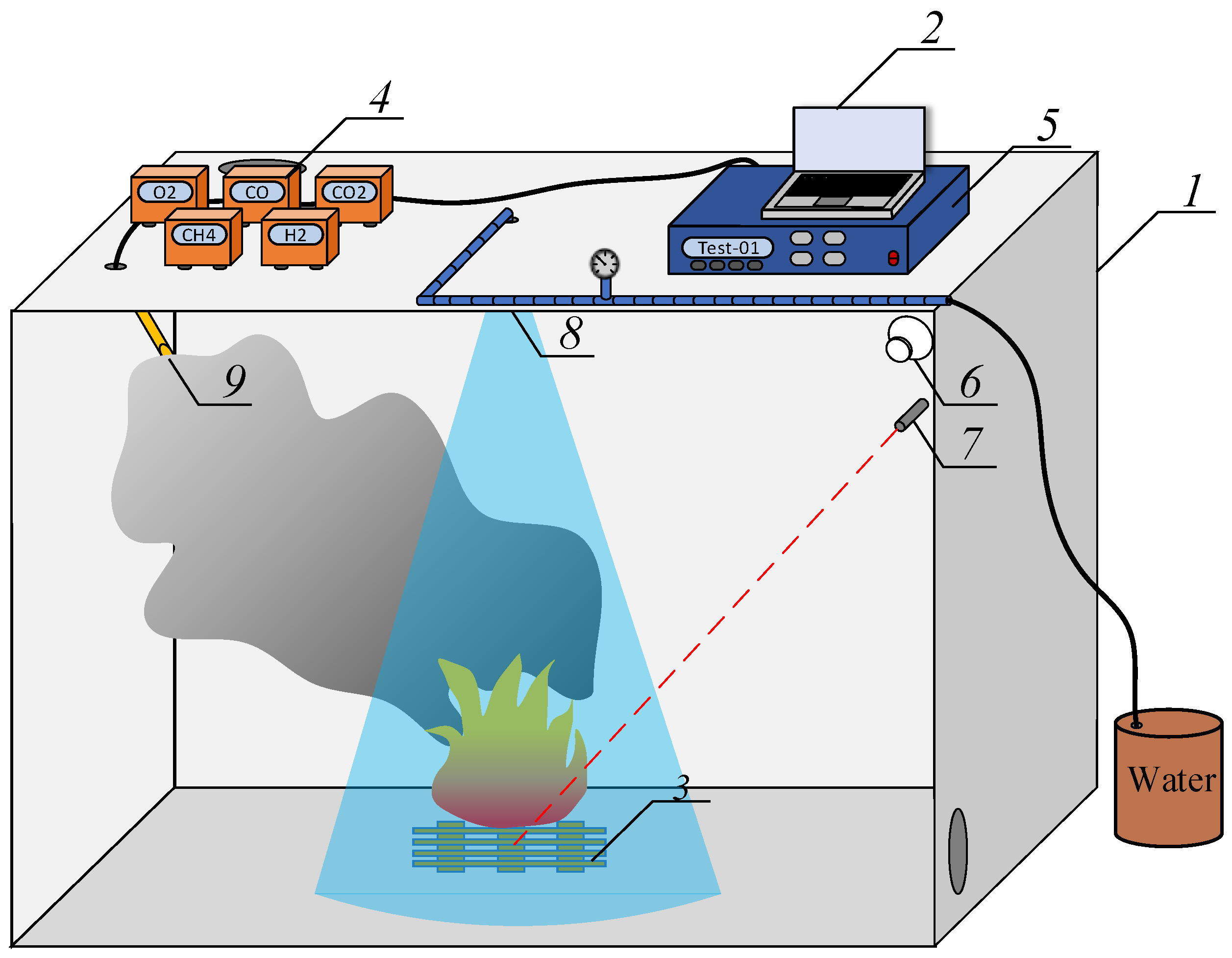
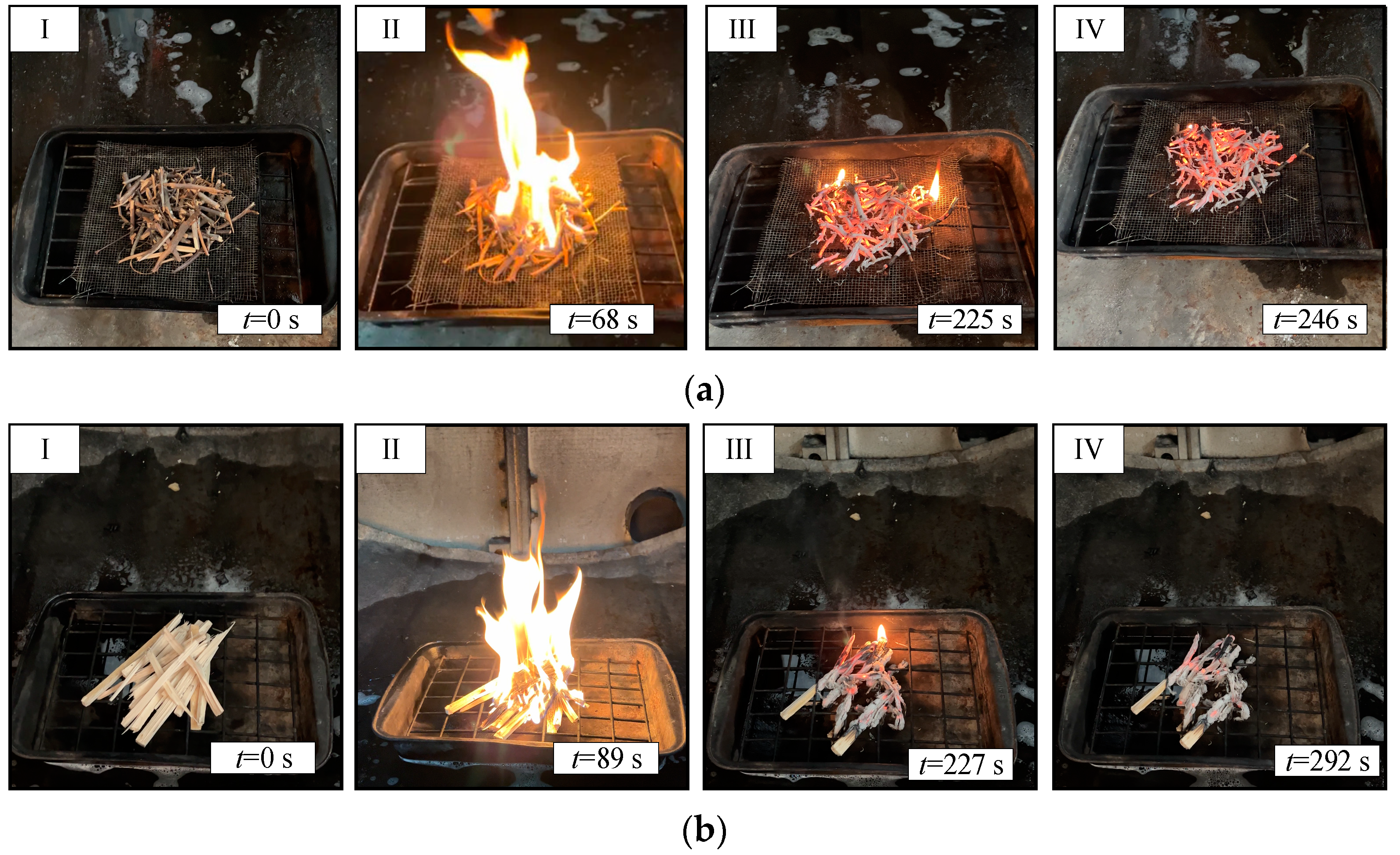
3. Experimental Technique
4. Results and Discussion
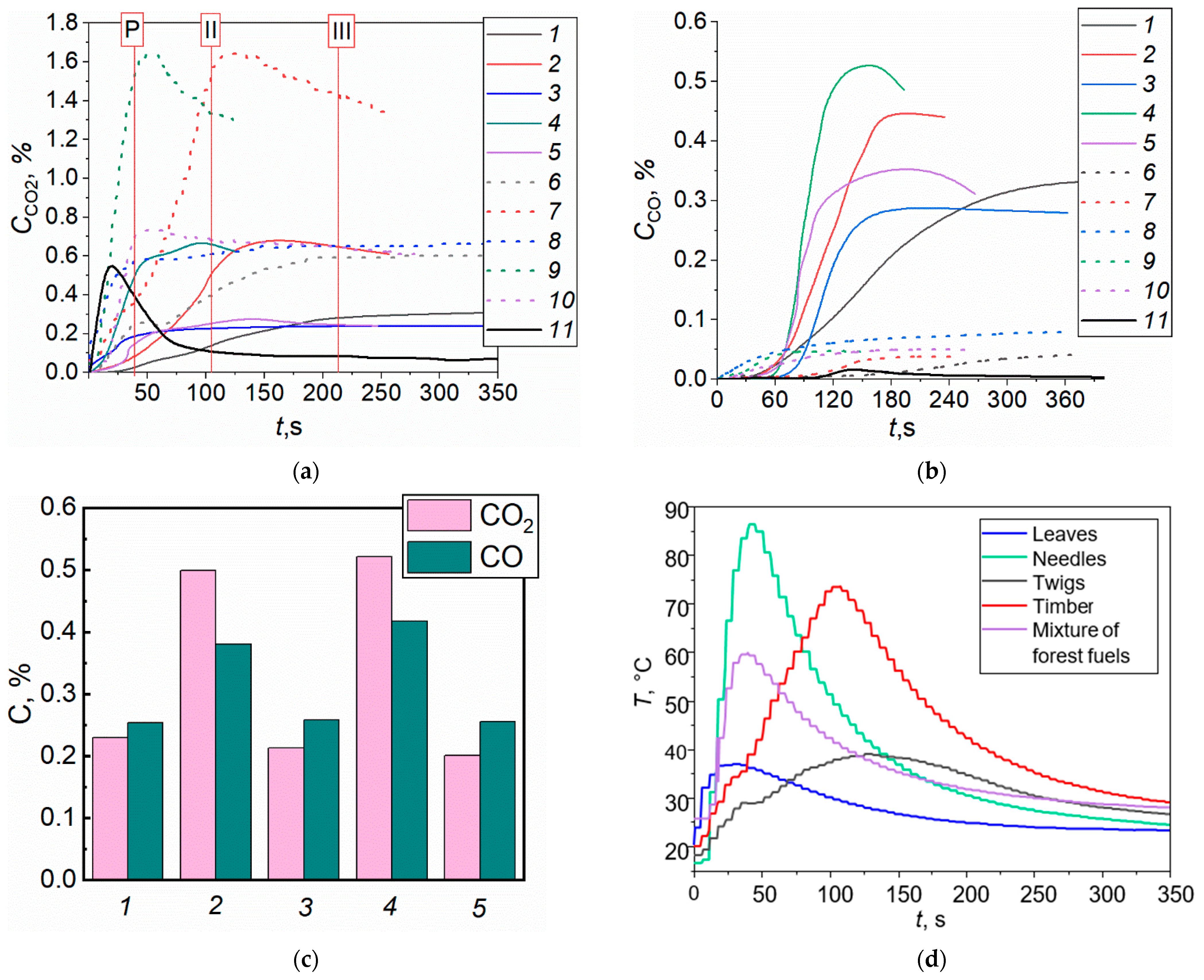
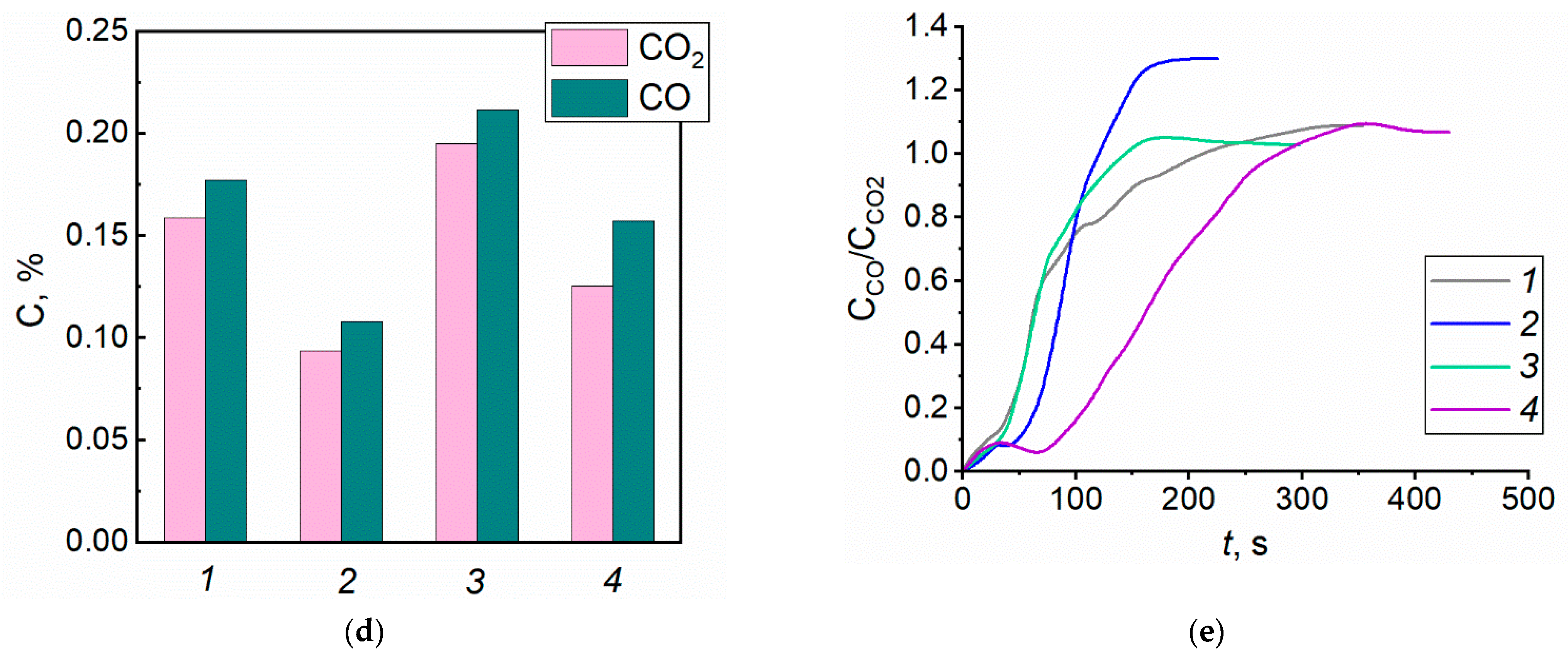
4.1. Gas Concentrations in the Free Burning of Forest Fuels
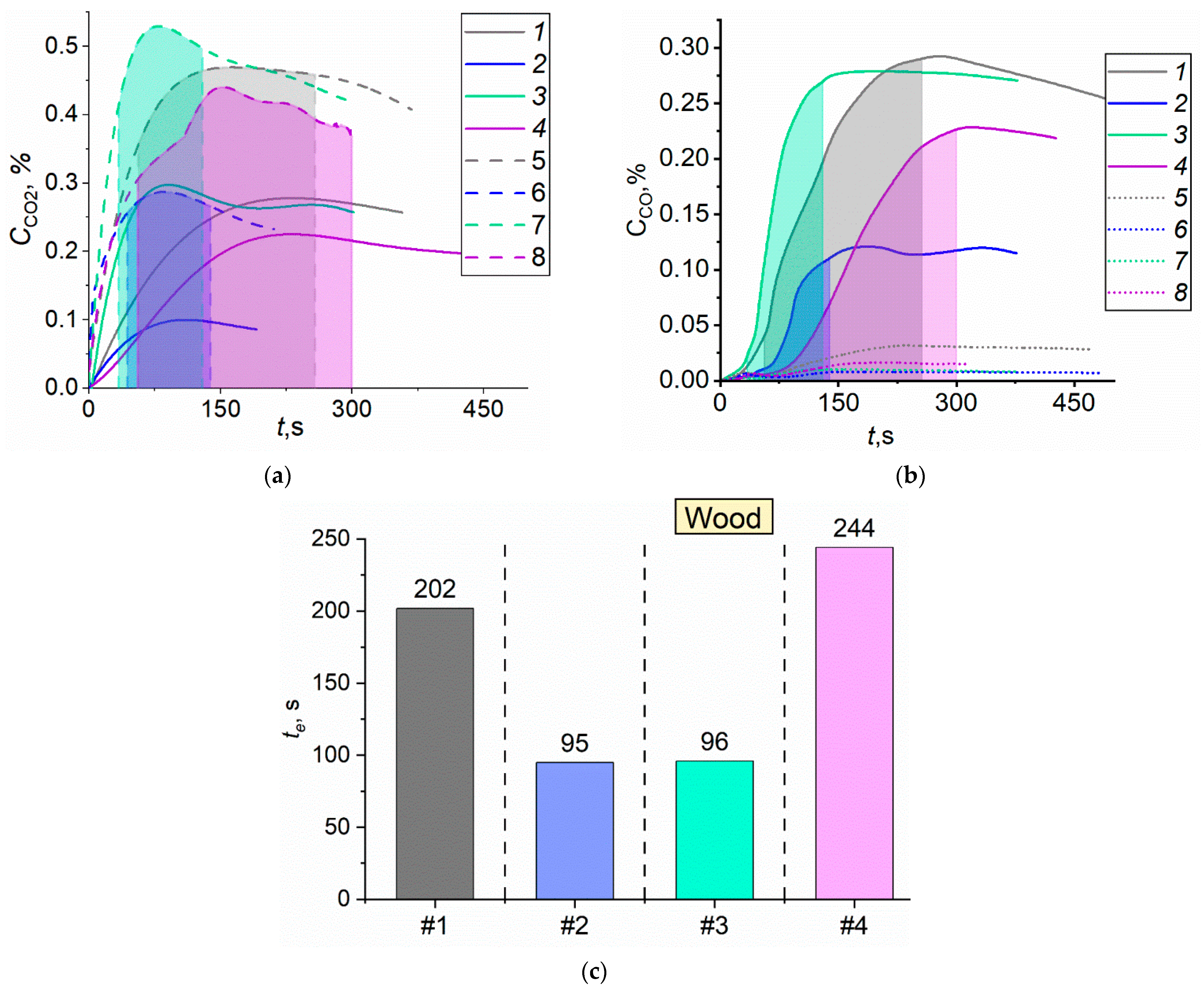
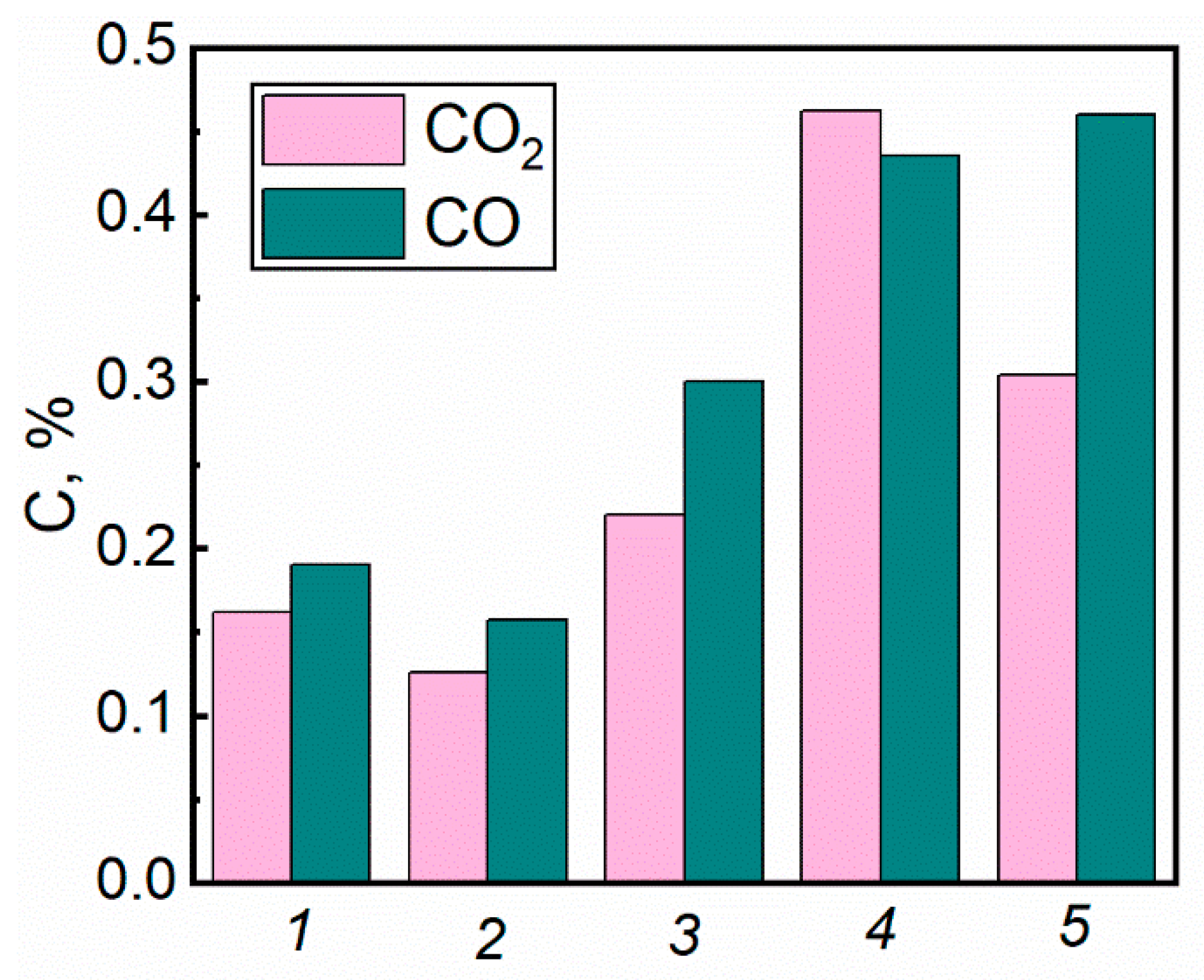
4.2. Gas Concentrations during the Suppression of Forest Fuel Fires Using Fire Extinguishing Compositions
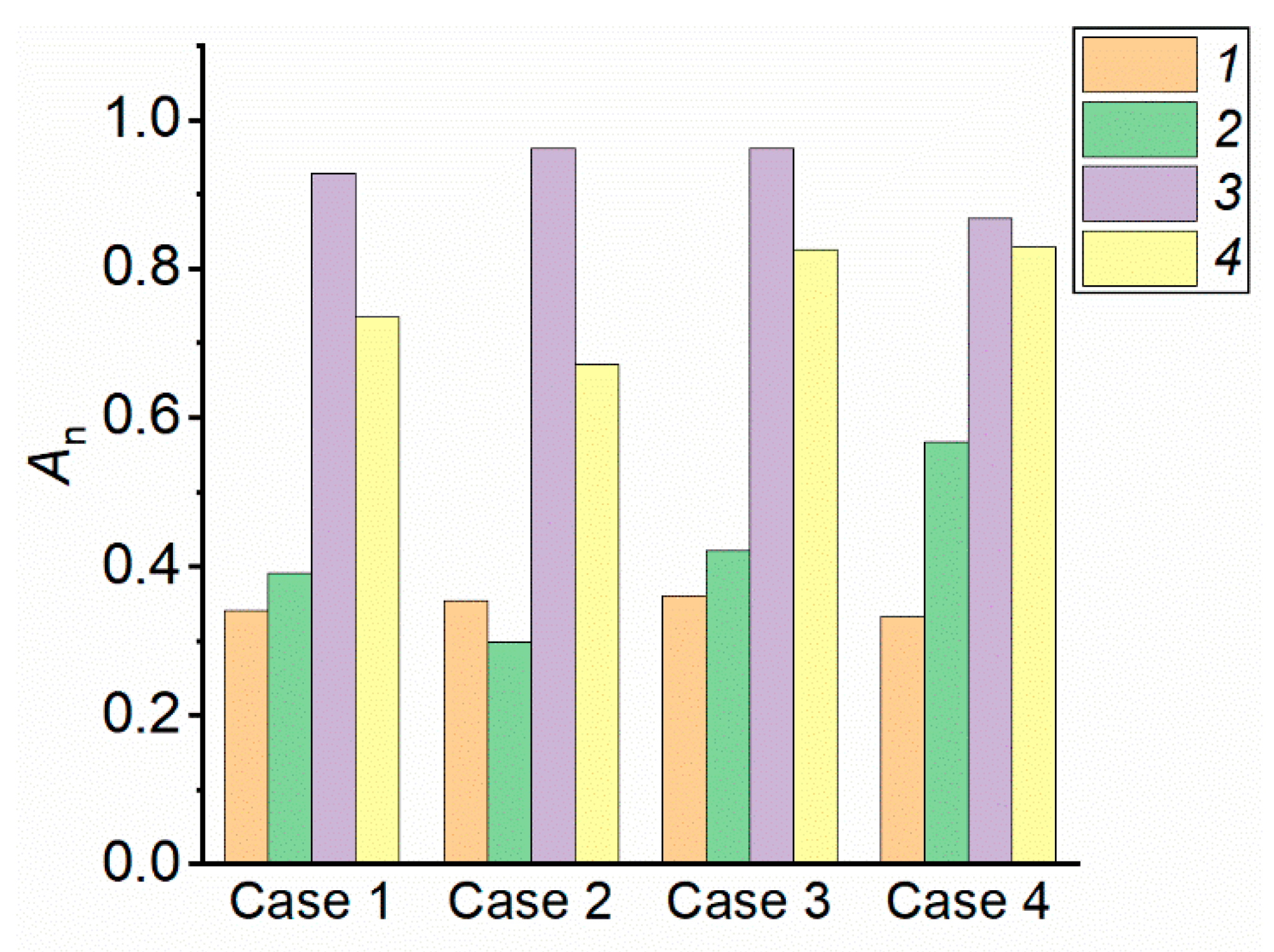
4.3. Relative Efficiency Indicators of Fire Extinguishing Agents
5. Conclusions
- (i)
- The conducted experiments demonstrated fundamental differences in the composition of forest fuel pyrolysis and combustion products with and without the application of promising fire extinguishing agents. Solutions, slurries, and emulsions were used. Utilizing two gas analysis systems provided important patterns of flue gas composition changes throughout the time during a burning forest fuel fire. The CO and CO2 concentrations for typical forest fuels (leaves, needles, twigs, a mixture of these, and timber) were found to differ by 35–52%.
- (ii)
- The comparison of continuous and cycling modes of applying fire extinguishing liquids to a forest fire revealed that the production of carbon monoxide and dioxide in cycling spraying is slightly higher (25–32%) than in continuous spraying. However, the amount of water required for extinguishment in cycling spraying is 1.4 times smaller. The firefighting liquid composition plays a decisive role. For instance, significant differences were identified between gas concentrations when spraying solutions, slurries, and emulsions. The emission factors of CO and CO2 when extinguishing timber fires with a bentonite slurry are 73% higher than when using water. The lowest emissions were recorded when a bischofite solution was used to extinguish fires involving timber. The yield of carbon monoxide and dioxide in this case was 63% lower than when water was used. The production of CO and CO2 can be reduced by 28% when using a foaming agent solution instead of water.
- (iii)
- When generalizing the experimental findings, wide ranges of efficiency factors were defined for different forest fuels and liquids applied. The efficiency factor varied in the range between 0.29 and 0.96. The created database of values can be used to choose the type and volume of extinguishing agent as well as the discharge technique, taking the requirements and priorities into account.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, G.; Kumar, A.; Saikia, P.; Roy, P.S.; Khan, M.L. Ecological Impacts of Forest Fire on Composition and Structure of Tropical Deciduous Forests of Central India. Phys. Chem. Earth Parts A/B/C 2022, 128, 103240. [Google Scholar] [CrossRef]
- Bohórquez, L.; Gómez, I.; Santa, F. Methodology for the Discrimination of Areas Affected by Forest Fires Using Satellite Images and Spatial Statistics. Procedia Environ. Sci. 2011, 7, 389–394. [Google Scholar] [CrossRef]
- Yao, Q.; Fang, K.; Wang, Z. Fire History and Its Forcing in Northeastern Asia Boreal Forests. Nat. Hazards Res. 2022, 2, 166–171. [Google Scholar] [CrossRef]
- Bondur, V.G.; Tsidilina, M.N.; Kladov, V.L.; Gordo, K.A. Irregular Variability of Spatiotemporal Distributions of Wildfires and Emissions of Harmful Trace Gases in Europe Based on Satellite Monitoring Data. Dokl. Earth Sci. 2019, 485, 461–464. [Google Scholar] [CrossRef]
- Chew, Y.J.; Ooi, S.Y.; Pang, Y.H.; Wong, K.-S. A Review of Forest Fire Combating Efforts, Challenges and Future Directions in Peninsular Malaysia, Sabah, and Sarawak. Forests 2022, 13, 1405. [Google Scholar] [CrossRef]
- Lippok, D.; Beck, S.G.; Renison, D.; Gallegos, S.C.; Saavedra, F.V.; Hensen, I.; Schleuning, M. Forest Recovery of Areas Deforested by Fire Increases with Elevation in the Tropical Andes. For. Ecol. Manag. 2013, 295, 69–76. [Google Scholar] [CrossRef]
- Rakowska, J.; Szczygieł, R.; Kwiatkowski, M.; Porycka, B.; Radwan, K.; Prochaska, K. Application Tests of New Wetting Compositions for Wildland Firefighting. Fire Technol. 2017, 53, 1379–1398. [Google Scholar] [CrossRef]
- Rakowska, J.; Prochaska, K.; Twardochleb, B.; Rojewska, M.; Porycka, B.; Jaszkiewicz, A. Selection of Surfactants as Main Components of Ecological Wetting Agent for Effective Extinguishing of Forest and Peat-Bog Fires. Chem. Pap. 2014, 68, 823–833. [Google Scholar] [CrossRef]
- Guo, X.; Pan, X.; Zhang, L.; Hua, M.; Li, H.; Li, S.; Zhang, H. The Inhibitory Combustion Reaction Performance and Tail Gas Analysis of Composite Ultra-Fine Dry Powder Extinguishing Agent Containing Different Additives. J. Loss Prev. Process Ind. 2020, 65, 104153. [Google Scholar] [CrossRef]
- Andersson, B.; Blomqvist, P. Experimental Study of Thermal Breakdown Products from Halogenated Extinguishing Agents. Fire Saf. J. 2011, 46, 104–115. [Google Scholar] [CrossRef]
- Roberts, A.J.; Crowley, L.M.; Sadler, J.P.; Nguyen, T.T.T.; Gardner, A.M.; Hayward, S.A.L.; Metcalfe, D.B. Effects of Elevated Atmospheric CO2 Concentration on Insect Herbivory and Nutrient Fluxes in a Mature Temperate Forest. Forests 2022, 13, 998, Correction in Forests 2022, 13, 1216. [Google Scholar] [CrossRef]
- Ping, X.; Chang, Y.; Liu, M.; Hu, Y.; Huang, W.; Shi, S.; Jia, Y.; Li, D. Carbon Emission and Redistribution among Forest Carbon Pools, and Change in Soil Nutrient Content after Different Severities of Forest Fires in Northeast China. Forests 2022, 13, 110. [Google Scholar] [CrossRef]
- Bowman, D.M.J.S.; Balch, J.K.; Artaxo, P.; Bond, W.J.; Carlson, J.M.; Cochrane, M.A.; D’Antonio, C.M.; DeFries, R.S.; Doyle, J.C.; Harrison, S.P.; et al. Fire in the Earth System. Science 2009, 324, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Mallongi, A.; Indra, R.; Arief, M.K.M.; Fais Satrianegara, M. Environmental Pollution and Health Problems Due to Forest Fires with CO2 Parameters. Med.-Leg. Updat. 2020, 20, 888–892. [Google Scholar]
- Xu, D.; Huang, G.; Guo, L.; Chen, Y.; Ding, C.; Liu, C. Enhancement of Catalytic Combustion and Thermolysis for Treating Polyethylene Plastic Waste. Adv. Compos. Hybrid Mater. 2022, 5, 113–129. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Evangelopoulos, P.; Sieradzka, M.; Zajemska, M.; Magdziarz, A. Pyrolysis of Agricultural Waste Biomass towards Production of Gas Fuel and High-Quality Char: Experimental and Numerical Investigations. Fuel 2021, 296, 120611. [Google Scholar] [CrossRef]
- Hoque, M.E.; Rashid, F.; Aziz, M. Gasification and Power Generation Characteristics of Rice Husk, Sawdust, and Coconut Shell Using a Fixed-Bed Downdraft Gasifier. Sustainability 2021, 13, 2027. [Google Scholar] [CrossRef]
- Hoque, M.; Rashid, F.; Prodhan, M.; Arman, A. Analysis of Energy Consumption and Efficiency to Reduce Power Losses in Industrial Equipment. In Proceedings of the International Conference on Mechanical Engineering, Dhaka, Bangladesh, 23–24 December 2017; pp. 18–20. [Google Scholar]
- Dhall, A.; Dhasade, A.; Nalwade, A.; VK, M.R.; Kulkarni, V. A Survey on Systematic Approaches in Managing Forest Fires. Appl. Geogr. 2020, 121, 102266. [Google Scholar] [CrossRef]
- Kim, H.-S.; Noulèkoun, F.; Noh, N.-J.; Son, Y.-W. Future Projection of CO2 Absorption and N2O Emissions of the South Korean Forests under Climate Change Scenarios: Toward Net-Zero CO2 Emissions by 2050 and Beyond. Forests 2022, 13, 1076. [Google Scholar] [CrossRef]
- Mamkin, V.; Varlagin, A.; Yaseneva, I.; Kurbatova, J. Response of Spruce Forest Ecosystem CO2 Fluxes to Inter-Annual Climate Anomalies in the Southern Taiga. Forests 2022, 13, 1019. [Google Scholar] [CrossRef]
- Jia, J.; Huang, R.; Wang, Y. Study on the Combustion Characteristics of Mountain Forest Vegetation. Forests 2022, 13, 1443. [Google Scholar] [CrossRef]
- Nestola, E.; Sgrigna, G.; Pallozzi, E.; Caccavale, L.; Guidolotti, G.; Calfapietra, C. Experimental Characterization of Particulate and Gaseous Emissions from Biomass Burning of Six Mediterranean Species and Litter. Forests 2022, 13, 322. [Google Scholar] [CrossRef]
- Barboni, T.; Leonelli, L.; Santoni, P.-A.; Tihay-Felicelli, V. Study of the Burning of Pteridium Aquilinum L. and Risk for the Personnel Involved: Thermal Properties and Chemical Risk. Fire Saf. J. 2019, 110, 102904. [Google Scholar] [CrossRef]
- Barboni, T.; Leonelli, L.; Santoni, P.-A.; Tihay-Felicelli, V. Aerosols and Carbonaceous and Nitrogenous Compounds Emitted during the Combustion of Dead Shrubs According to Twigs’ Diameter and Combustion Phases. Fire Saf. J. 2020, 113, 102988. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, S.; Zhu, Z.; Wang, G.; Tigabu, M.; Guo, Y.; Zheng, W.; Guo, F. Emissions of Gaseous Pollutants Released by Forest Fire in Relation to Litter Fuel Moisture Content. Atmos. Environ. 2022, 284, 119215. [Google Scholar] [CrossRef]
- Pallozzi, E.; Lusini, I.; Cherubini, L.; Hajiaghayeva, R.A.; Ciccioli, P.; Calfapietra, C. Differences between a Deciduous and a Conifer Tree Species in Gaseous and Particulate Emissions from Biomass Burning. Environ. Pollut. 2018, 234, 457–467. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Liu, X.; Ma, Y.; Zhou, Q.; Sa, R.; Zhang, Q. Emissions Released by Forest Fuel in the Daxing’an Mountains, China. Forests 2022, 13, 1220. [Google Scholar] [CrossRef]
- Li, Y.Z.; Ingason, H. Influence of Fire Suppression on Combustion Products in Tunnel Fires. Fire Saf. J. 2018, 97, 96–110. [Google Scholar] [CrossRef]
- Kropotova, S.S.; Kuznetsov, G.V.; Strizhak, P.A. Identifying Products of Pyrolysis and Combustion of Materials at Incipient Stages of Fires. Fire Saf. J. 2022, 132, 103643. [Google Scholar] [CrossRef]
- Antonov, D.V.; Volkov, R.S.; Voitkov, I.S.; Zhdanova, A.O.; Kuznetsov, G. V Influence of Special Additives in a Water Aerosol on the Suppression of a Forest Fire with It. J. Eng. Phys. Thermophys. 2018, 91, 1250–1259. [Google Scholar] [CrossRef]
- Atroshenko, Y.K.; Kuznetsov, G.V.; Strizhak, P.A.; Volkov, R.S. Protective Lines for Suppressing the Combustion Front of Forest Fuels: Experimental Research. Process Saf. Environ. Prot. 2019, 131, 73–88. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Islamova, A.G.; Orlova, E.G.; Strizhak, P.A.; Feoktistov, D. V Physicochemical Features of the Effect of Special Water-Based Fire Retardants on Forest Materials. Fire Saf. J. 2021, 123, 103371. [Google Scholar] [CrossRef]
- Zhdanova, A.; Islamova, A.; Kurapov, R.; Volkov, R. Evaporation of Promising Fire Extinguishing Agent Droplets. Forests 2023, 14, 301. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Kropotova, S.S.; Voytkov, I.S.; Strizhak, P.A. Influence of the Component Composition of Extinguishing Fluids on the Droplet Distribution in an Aerosol Cloud. Powder Technol. 2022, 395, 838–849. [Google Scholar] [CrossRef]
- Dorokhov, V.V.; Kuznetsov, G.V.; Nyashina, G.S.; Strizhak, P.A. Composition of a Gas and Ash Mixture Formed during the Pyrolysis and Combustion of Coal-Water Slurries Containing Petrochemicals. Environ. Pollut. 2021, 285, 117390. [Google Scholar] [CrossRef]
- Dombrovsky, L.A.; Levashov, V.Y.; Kryukov, A.P.; Dembele, S.; Wen, J.X. A Comparative Analysis of Shielding of Thermal Radiation of Fires Using Mist Curtains Containing Droplets of Pure Water or Sea Water. Int. J. Therm. Sci. 2020, 152, 106299. [Google Scholar] [CrossRef]
- Gupta, M.; Rajora, R.; Sahai, S.; Shankar, R.; Ray, A.; Kale, S.R. Experimental Evaluation of Fire Suppression Characteristics of Twin Fluid Water Mist System. Fire Saf. J. 2012, 54, 130–142. [Google Scholar] [CrossRef]
- Fateh, T.; Richard, F.; Batiot, B.; Rogaume, T.; Luche, J.; Zaida, J. Characterization of the Burning Behavior and Gaseous Emissions of Pine Needles in a Cone Calorimeter–FTIR Apparatus. Fire Saf. J. 2016, 82, 91–100. [Google Scholar] [CrossRef]
- Vicente, A.; Alves, C.; Calvo, A.I.; Fernandes, A.P.; Nunes, T.; Monteiro, C.; Almeida, S.M.; Pio, C. Emission Factors and Detailed Chemical Composition of Smoke Particles from the 2010 Wildfire Season. Atmos. Environ. 2013, 71, 295–303. [Google Scholar] [CrossRef]
- Glushkov, D.O.; Nyashina, G.S.; Anand, R.; Strizhak, P.A. Composition of Gas Produced from the Direct Combustion and Pyrolysis of Biomass. Process Saf. Environ. Prot. 2021, 156, 43–56. [Google Scholar] [CrossRef]



| Forest Fuel | Component | Layer Density, kg/m3 | Particle Density, kg/m3 | Porosity (Fraction of Pores) |
|---|---|---|---|---|
| Pine | Twigs with needles | 0.05–0.12 | 320–500 | 0.583 |
| Pine needles | needles | 30 | 650 | 0.794 |
| Leaves | leaves | 8–12 | 300 | 0.787 |
| Timber (pine) | Timber | 448 | 448 | 0.7 |
| Name of Composition | Properties of Additives and Fire Extinguishing Agents Prepared | Fire Suppression Method |
|---|---|---|
| Water | Density: 998.2 kg/m3 Surface tension: 72.7 × 10−3 N/m Dynamic viscosity: 1.004 × 10−3 Pa·s Thermal diffusivity: 14.3 × 10−8 m2/s Heat capacity: 4.183 kJ/(kg·K) Thermal conductivity: 0.599 W/(m·K) Average ability to remain on forest fuel: 60.5% | Cooling |
| Foaming agent solution (AFFF) 5 vol% | Properties of the 5% solution: Density: 1002–1187 kg/m3. Dynamic viscosity: 0.12 Pa·s Surface tension: 17.3 × 10−3 N/m Thermal diffusivity: 12.9 × 10−6 m2/s Heat capacity: 3.995 kJ/(kg·K) Thermal conductivity: 0.595 W/(m·K) Foam stability: 250 s. Average ability to remain on forest fuel: 74.5% | Cooling, smothering |
| Bentonite slurry 5 vol% | Fire protection efficiency group I melting point >1250 °C Properties of the 5% solution: Density: 1100 kg/m3 Surface tension: 68.5 × 10−3 N/m Dynamic viscosity: 24.3 × 10−3 Pa·s Thermal diffusivity: 5.44 × 10−4 m2/s Heat capacity: 3.83 kJ/(kg·K) Thermal conductivity: 0.704 W/(m·K) | Smothering |
| Bischofite solution 10 vol% | Fire protection efficiency group I Properties of the 10% solution: Thermal conductivity: 0.58 W/(m·K) Heat capacity: 3.603 kJ/(kg·K) Density: 1081.49 kg/m3 Dynamic viscosity: 1.015 × 10−3 Pa·s Surface tension: 75.8 × 10−3 N/m | Smothering |
| Component of Gas-Air Mixture | Types of Sensors | Measurement Range | Accuracy | Reaction Time |
|---|---|---|---|---|
| O2 | electrochemical | 0%–25% | ±0.2 vol% (absolute) | ≤15 s |
| H2 | polarographic | 0%–5% | ±0.2 vol% (absolute) | ≤35 s |
| CO2 | optical | 0%–30% | ±2% (basic percentage error) | ≤25 s |
| CH4 | optical | 0%–30% | ±5% (relative) | ≤25 s |
| CO | electrochemical | 0%–30% | ±5% (relative) | ≤35 s |
| H2S | electrochemical | 0–500 ppm | ±5% (relative) | ≤45 s |
| SO2 | electrochemical | 0–1000 ppm | ±5% (relative) | ≤45 s |
| Type of Detector | Measurement Range | Maximum Permissible Relative Error, δ % |
|---|---|---|
| Carbon dioxide (CO2) | 0.01–5 vol% | ±15 |
| Carbon monoxide (CO) | 0.1–300 mg/m3 | ±10 |
| Methane (CH4) | 0.01–2.5 vol% | ±10 |
| Oxygen (O2) | 0.1–30 vol% | ±5 |
| Hydrogen (H2) | 0.01–4 vol% | ±10 |
| Material | CO, g/g | CO2, g/g |
|---|---|---|
| Twigs | 0.714 | 0.676 |
| Timber | 0.375 | 0.491 |
| Needles | 0.822 | 0.636 |
| Leaves | 0.208 | 0.181 |
| Mixture | 0.127 | 0.177 |
| Water Discharge Technique | CO Yield, g/g | CO2 Yield, g/g | te, s | Discharge Density, l/(m2·s) |
|---|---|---|---|---|
| Cycling water discharge | 0.205 | 0.221 | 180 | 7.96 |
| Continuous spraying | 0.153 | 0.150 | 250 | 11.06 |
| Material | Water | Bentonite Slurry | Bischofite Solution | Foaming Agent Solution | ||||
|---|---|---|---|---|---|---|---|---|
| CO, g/g | CO2, g/g | CO, g/g | CO2, g/g | CO, g/g | CO2, g/g | CO, g/g | CO2, g/g | |
| Twigs | 0.568 | 0.456 | ||||||
| Timber | 0.153 | 0.150 | 0.582 | 0.551 | 0.057 | 0.045 | 0.109 | 0.105 |
| Needles | 0.169 | 0.258 | ||||||
| Leaves | 0.181 | 0.149 | ||||||
| Mixture | 0.129 | 0.106 | ||||||
| Criteria | Water | Bentonite Slurry | Bischofite Solution | Foaming Agent Solution |
|---|---|---|---|---|
| Extinguishing time, s | 244 | 202 | 95 | 96 |
| CO yield, g/g | 0.153 | 0.582 | 0.057 | 0.109 |
| CO2 yield, g/g | 0.15 | 0.551 | 0.045 | 0.105 |
| Discharge density, l/(m2·s) | 10.8 | 3.6 | 4.6 | 3.3 |
| Criteria | Water | Bentonite Slurry | Bischofite Solution | Foaming Agent Solution |
|---|---|---|---|---|
| Extinguishing time, s | 0.39 | 0.47 | 1 | 0.99 |
| CO yield, g/g | 0.37 | 0.098 | 1 | 0.52 |
| CO2 yield, g/g | 0.3 | 0.082 | 1 | 0.43 |
| Discharge density, l/(m2·s) | 0.31 | 0.92 | 0.72 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kropotova, S.; Dorokhov, V.; Sviridenko, A.; Strizhak, P. Composition of the Gas-Air Mixture in the Containment and Suppression of Forest Fires with Promising Extinguishing Agents. Forests 2023, 14, 786. https://doi.org/10.3390/f14040786
Kropotova S, Dorokhov V, Sviridenko A, Strizhak P. Composition of the Gas-Air Mixture in the Containment and Suppression of Forest Fires with Promising Extinguishing Agents. Forests. 2023; 14(4):786. https://doi.org/10.3390/f14040786
Chicago/Turabian StyleKropotova, Svetlana, Vadim Dorokhov, Aleksandr Sviridenko, and Pavel Strizhak. 2023. "Composition of the Gas-Air Mixture in the Containment and Suppression of Forest Fires with Promising Extinguishing Agents" Forests 14, no. 4: 786. https://doi.org/10.3390/f14040786
APA StyleKropotova, S., Dorokhov, V., Sviridenko, A., & Strizhak, P. (2023). Composition of the Gas-Air Mixture in the Containment and Suppression of Forest Fires with Promising Extinguishing Agents. Forests, 14(4), 786. https://doi.org/10.3390/f14040786







