Quantification of Hydrolytic Sugars from Eucalyptus globulus Bio-Oil Aqueous Solution after Thermochemical Liquefaction
Abstract
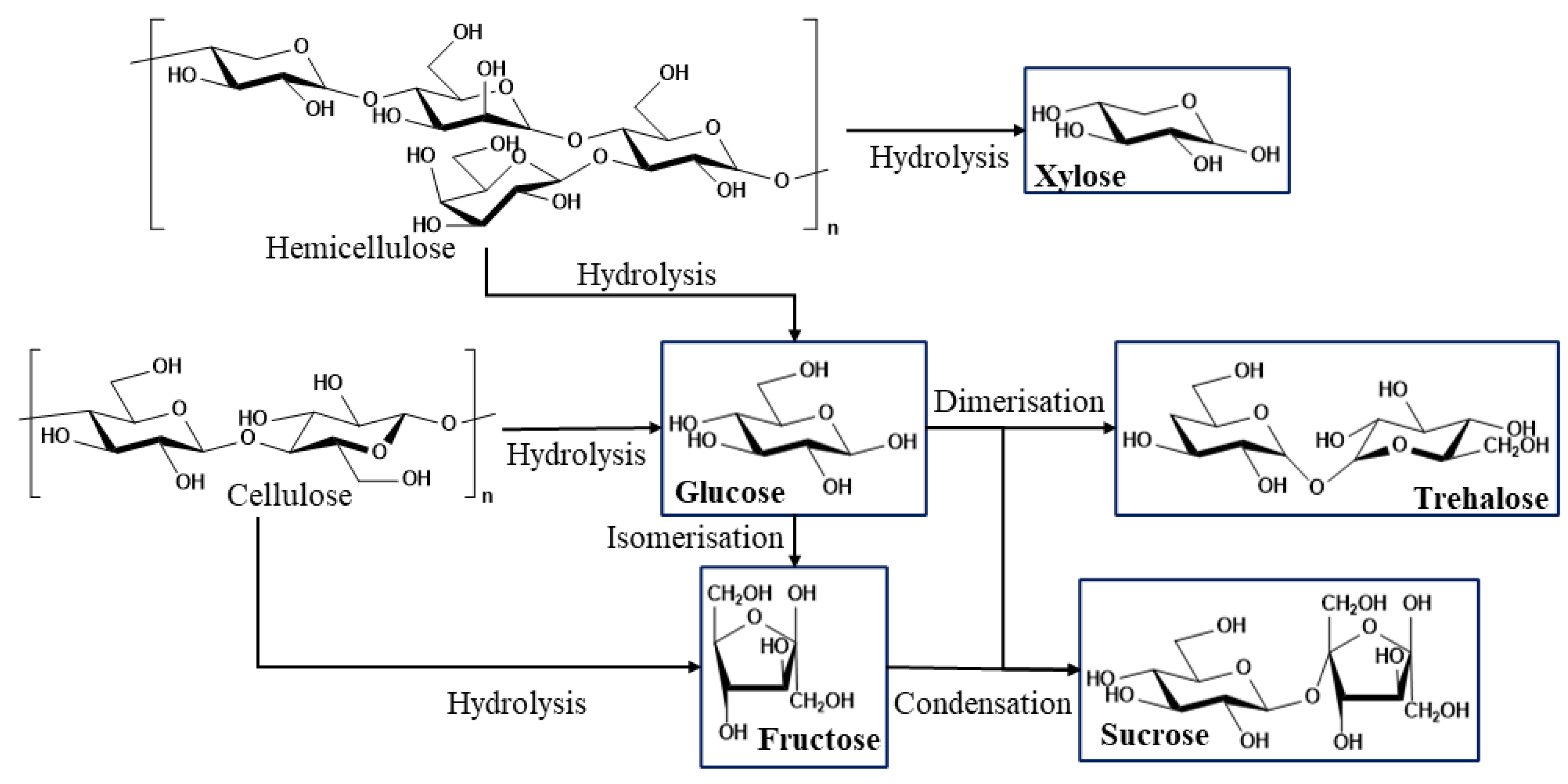
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Standards
2.2. Hydrolytic Sugars Sample Preparation
2.3. Elemental Analysis
2.4. HPLC Experiments
2.4.1. Preparation of Standard Solutions for Measuring Peak Detection Levels
2.4.2. Preparation of Samples of Aqueous Fractions
2.4.3. Optimisation of Chromatographic Separation
2.4.4. Column Temperature
2.4.5. HPLC Analysis
2.5. NMR
2.6. Fourier Transformed Infrared (FTIR-ATR)
3. Results
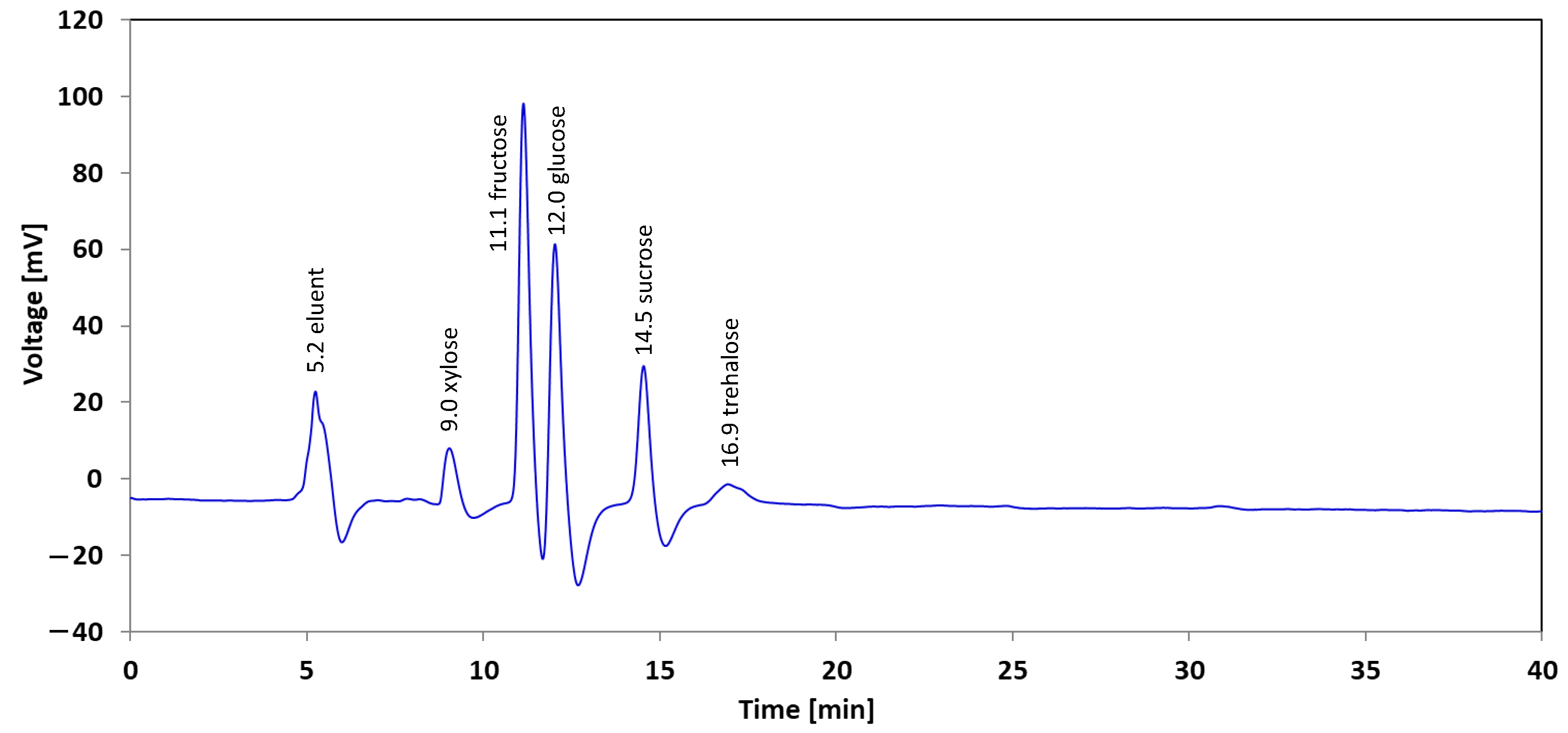
3.1. HPLC-RID Determination of Sugars in Aqueous Fractions of the Thermochemical Liquefaction of Eucalyptus globulus
3.1.1. Method Validation
3.1.2. Linearity
3.1.3. Detection Limit and Quantification Limit
3.1.4. Statistical Analysis
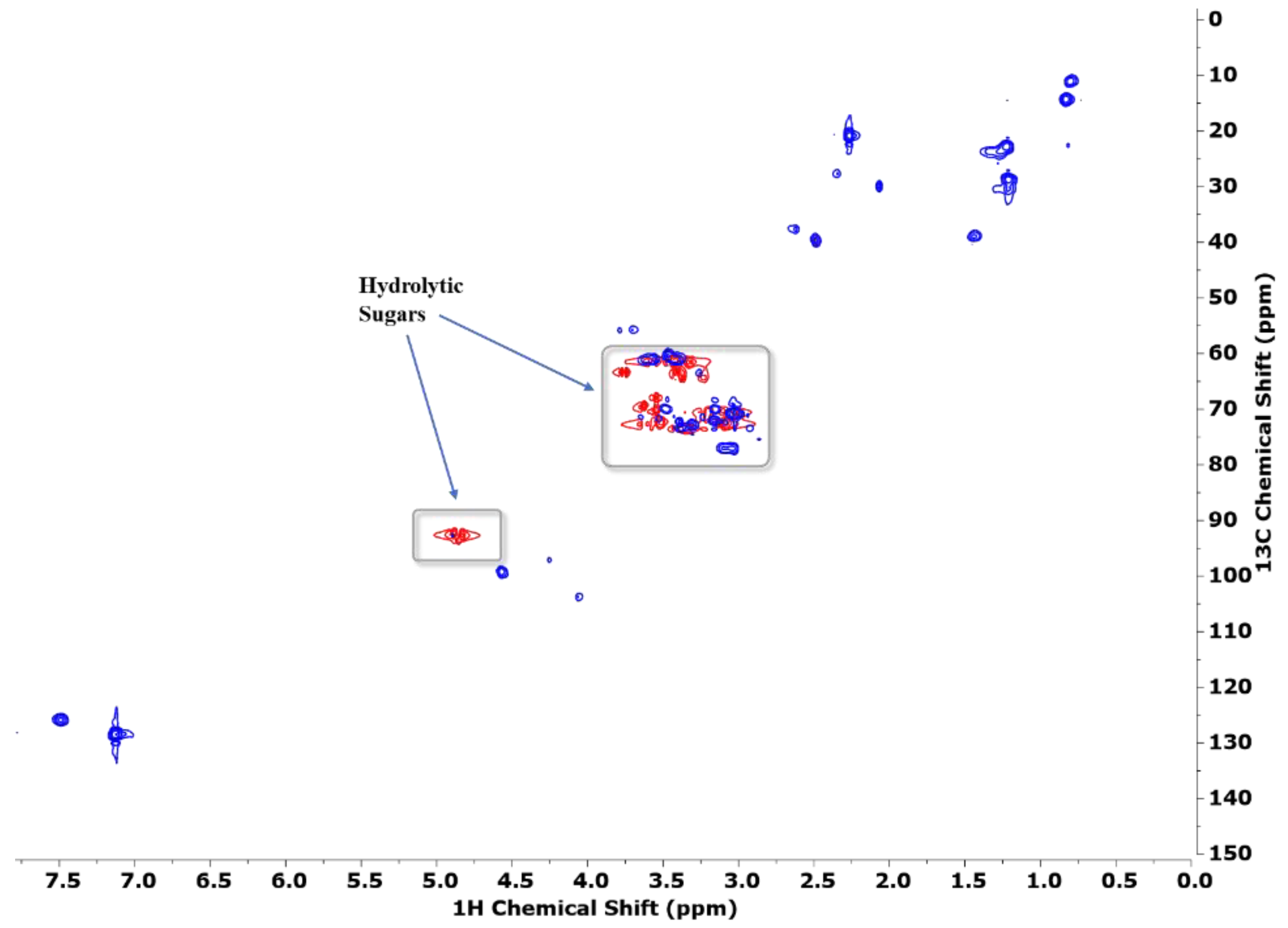
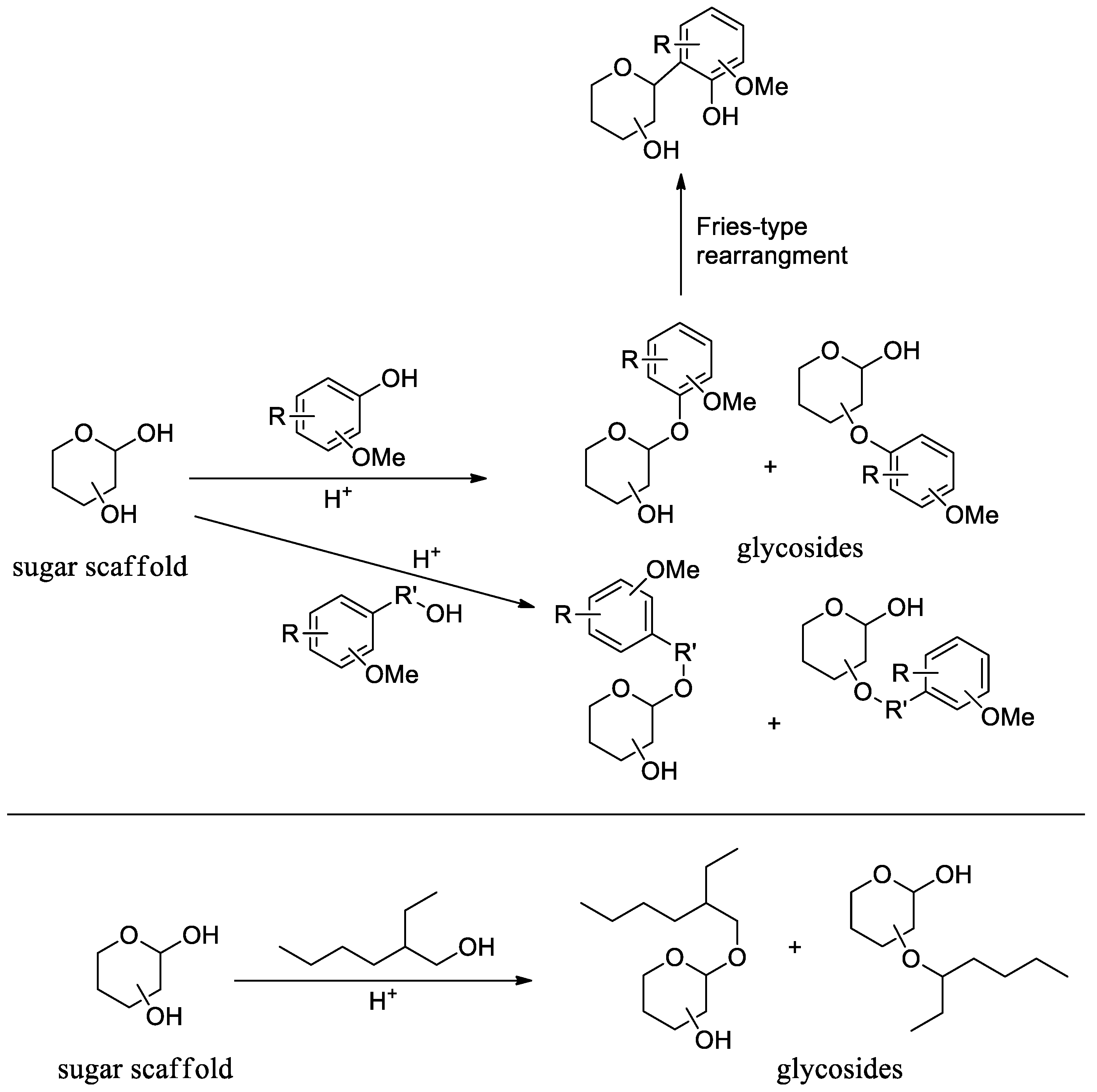
3.2. NMR Studies
3.3. FTIR Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mateus, M.M.; Guerreiro, D.; Ferreira, O.; Bordado, J.C.; Galhano dos Santos, R.; dos Santos, R. Heuristic Analysis of Eucalyptus Globulus Bark Depolymerization via Acid-Liquefaction. Cellulose 2017, 24, 659–668. [Google Scholar] [CrossRef]
- Huang, X.; Li, F.; Xie, J.; de Hoop, C.F.; Peng, X.; Qi, J.; Chen, Y.; Xiao, H. Preliminary Evaluation of Liquefaction Behavior of Eucalyptus Grandis Bark in Glycerol. J. For. Res. 2020, 31, 687–691. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Freire, C.S.R.; Domingues, M.R.M.; Silvestre, A.J.D.; Neto, C.P. Characterization of Phenolic Components in Polar Extracts of Eucalyptus Globulus Labill. Bark by High-Performance Liquid Chromatography-Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 9386–9393. [Google Scholar] [CrossRef]
- Fernandes, F.; Matos, S.; Gaspar, D.; Silva, L.; Paulo, I.; Vieira, S.; Pinto, P.C.R.; Bordado, J.; dos Santos, R.G. Boosting the Higher Heating Value of Eucalyptus Globulus via Thermochemical Liquefaction. Sustainability 2021, 13, 3717. [Google Scholar] [CrossRef]
- Heidari, A.; Khaki, E.; Younesi, H.; Lu, H.R. Evaluation of Fast and Slow Pyrolysis Methods for Bio-Oil and Activated Carbon Production from Eucalyptus Wastes Using a Life Cycle Assessment Approach. J. Clean. Prod. 2019, 241, 118394. [Google Scholar] [CrossRef]
- Vidal, M.; Bastos, D.; Silva, L.; Gaspar, D.; Paulo, I.; Matos, S.; Vieira, S.; Bordado, J.M.; Galhano dos Santos, R. Up-Cycling Tomato Pomace by Thermochemical Liquefaction—A Response Surface Methodology Assessment. Biomass Bioenergy 2022, 156, 106324. [Google Scholar] [CrossRef]
- Mateus, M.M.; Ventura, P.; Rego, A.; Mota, C.; Castanheira, I.; Moura Bordado, J.; Galhano, R.; Santos, D. Acid Liquefaction of Potato (Solanum tuberosum) and Sweet Potato (Ipomoea batatas) Cultivars Peels—Pre-Screening of Antioxidant Activity/Total Phenolic and Sugar Contents. Bioresources 2017, 12, 1463–1478. [Google Scholar] [CrossRef]
- Xu, J.; Xie, X.; Wang, J.; Jiang, J. Directional Liquefaction Coupling Fractionation of Lignocellulosic Biomass for Platform Chemicals. Green. Chem. 2016, 18, 3124–3138. [Google Scholar] [CrossRef]
- Raj, T.; Chandrasekhar, K.; Naresh Kumar, A.; Kim, S.H. Lignocellulosic Biomass as Renewable Feedstock for Biodegradable and Recyclable Plastics Production: A Sustainable Approach. Renew. Sustain. Energy Rev. 2022, 158, 112130. [Google Scholar] [CrossRef]
- Mateus, M.M.; Gaspar, D.; Matos, S.; Rego, A.; Motta, C.; Castanheira, I.; Bordado, J.M.; Galhano Dos Santos, R. Converting a Residue from an Edible Source (Ceratonia Siliqua L.) into a Bio-Oil. J. Environ. Chem. Eng. 2019, 7, 103004. [Google Scholar] [CrossRef]
- Rodrigues José; Puls Jürgen; Faix Oskar; Pereira Helena Determination of Monosaccharide Composition of Eucalyptus. Holzforschung 2001, 55, 265–269. [CrossRef]
- Salazar, M.M.; Grandis, A.; Pattathil, S.; Neto, J.L.; Camargo, E.L.O.; Alves, A.; Rodrigues, J.C.; Squina, F.; Cairo, J.P.F.; Buckeridge, M.S.; et al. Eucalyptus Cell Wall Architecture: Clues for Lignocellulosic Biomass Deconstruction. Bioenergy Res. 2016, 9, 969–979. [Google Scholar] [CrossRef]
- Kunaver, M.; Anžlovar, A.; Žagar, E. The Fast and Effective Isolation of Nanocellulose from Selected Cellulosic Feedstocks. Carbohydr. Polym. 2016, 148, 251–258. [Google Scholar] [CrossRef]
- Bose, S.K.; Barber, V.A.; Alves, E.F.; Kiemle, D.J.; Stipanovic, A.J.; Francis, R.C. An Improved Method for the Hydrolysis of Hardwood Carbohydrates to Monomers. Carbohydr. Polym. 2009, 78, 396–401. [Google Scholar] [CrossRef]
- Alves, E.F.; Bose, S.K.; Francis, R.C.; Colodette, J.L.; Iakovlev, M.; van Heiningen, A. Carbohydrate Composition of Eucalyptus, Bagasse and Bamboo by a Combination of Methods. Carbohydr. Polym. 2010, 82, 1097–1101. [Google Scholar] [CrossRef]
- Grembecka, M.; Lebiedzińska, A.; Szefer, P. Simultaneous Separation and Determination of Erythritol, Xylitol, Sorbitol, Mannitol, Maltitol, Fructose, Glucose, Sucrose and Maltose in Food Products by High Performance Liquid Chromatography Coupled to Charged Aerosol Detector. Microchem. J. 2014, 117, 77–82. [Google Scholar] [CrossRef]
- Debebe, A.; Temesgen, S.; Redi-Abshiro, M.; Chandravanshi, B.S.; Ele, E. Improvement in Analytical Methods for Determination of Sugars in Fermented Alcoholic Beverages. J. Anal. Methods Chem. 2018, 2018, 4010298. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, G.; Winge, S.; Sandberg, H. Separation of Monosaccharides by Hydrophilic Interaction Chromatography with Evaporative Light Scattering Detection. J. Chromatogr. A 2005, 1092, 246–249. [Google Scholar] [CrossRef]
- Chávez-Servín, J.L.; Castellote, A.I.; López-Sabater, M.C. Analysis of Mono- and Disaccharides in Milk-Based Formulae by High-Performance Liquid Chromatography with Refractive Index Detection. J. Chromatogr. A 2004, 1043, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Azevedo, J.L.T. Estimating the Higher Heating Value of Biomass Fuels from Basic Analysis Data. Biomass Bioenergy 2005, 28, 499–507. [Google Scholar] [CrossRef]
- Gagic, T.; Perva-Uzunalic, A.; Knez, Ž.; Škerget, M. Hydrothermal Treatment of Sugars to Obtain High-Value Products. J. Serb. Chem. Soc. 2020, 85, 97–109. [Google Scholar] [CrossRef]
- Evtuguin, D.; Neto, C. Recent Advances in Eucalyptus Wood Chemistry: Structural Features Through the Prism of Technological Response. 2007. Available online: https://www.eucalyptus.com.br/icep03/20Evtuguin.text.pdf.pdf (accessed on 15 March 2021).
- Sweygers, N.; Alewaters, N.; Dewil, R.; Appels, L. Microwave Effects in the Dilute Acid Hydrolysis of Cellulose to 5-Hydroxymethylfurfural. Sci. Rep. 2018, 8, 7719. [Google Scholar] [CrossRef]
- Stick, R.V.; Williams, S.J. Disaccharides, Oligosaccharides and Polysaccharides. Carbohydr. Essent. Mol. Life 2009, 2, 321–341. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Zanuso, E.; Lara-Flores, A.A.; Aguilar, D.L.; Velazquez-Lucio, J.; Aguilar, C.N.; Rodriguez-Jasso, R.M. Kinetic Modeling, Operational Conditions, and Biorefinery Products from Hemicellulose: Depolymerization and Solubilization during Hydrothermal Processing. In Hydrothermal Processing in Biorefineries: Production of Bioethanol and High. Added-Value Compounds of Second. and Third Generation Biomass; Springer: Berlin/Heidelberg, Germany, 2017; pp. 141–160. [Google Scholar] [CrossRef]
- Wang, Y.L.; Chow, S.Y.; Lin, Y.T.; Hsieh, Y.C.; Lee, G.C.; Liaw, S.H. Structures of Trehalose Synthase from Deinococcus Radiodurans Reveal That a Closed Conformation Is Involved in Catalysis of the Intramolecular Isomerization. Acta Crystallogr. D Biol. Crystallogr. 2014, 70, 3144–3154. [Google Scholar] [CrossRef] [PubMed]
- Garrote, G.; Kabel, M.A.; Schols, H.A.; Falqué, E.; Domínguez, H.; Parajó, J.C. Effects of Eucalyptus Globulus Wood Autohydrolysis Conditions on the Reaction Products. J. Agric. Food Chem. 2007, 55, 9006–9013. [Google Scholar] [CrossRef]
- Gullón, P.; González-Muñoz, M.J.; van Gool, M.P.; Schols, H.A.; Hirsch, J.; Ebringerová, A.; Parajó, J.C. Structural Features and Properties of Soluble Products Derived from Eucalyptus Globulus Hemicelluloses. Food Chem. 2011, 127, 1798–1807. [Google Scholar] [CrossRef]
- Zaky, A.S.; Pensupa, N.; Andrade-Eiroa, Á.; Tucker, G.A.; Du, C. A New HPLC Method for Simultaneously Measuring Chloride, Sugars, Organic Acids and Alcohols in Food Samples. J. Food Compos. Anal. 2017, 56, 25–33. [Google Scholar] [CrossRef]
- Snyder, L.R.; Kirkland, J.J.; Dolan, J.W. Introduction to Modern Liquid Chromatography; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Chinnici, F.; Spinabelli, U.; Riponi, C.; Amati, A. Optimization of the Determination of Organic Acids and Sugars in Fruit Juices by Ion-Exclusion Liquid Chromatography. J. Food Compos. Anal. 2005, 18, 121–130. [Google Scholar] [CrossRef]
- Ribeiro Vasconcelos De Sá, L.; de Oliveira Moutta, R.; Pinto Da Silva Bon, E.; Christe Cammarota, M.; Ferreira-Leitão, V.S. Fermentative Biohydrogen Production Using Hemicellulose Fractions: Analytical Validation for C5 and C6-Sugars, Acids and Inhibitors by HPLC. Int. J. Hydrogen Energy 2015, 40, 13888–13900. [Google Scholar] [CrossRef]
- Yu, Y.; Chua, Y.W.; Wu, H. Characterization of Pyrolytic Sugars in Bio-Oil Produced from Biomass Fast Pyrolysis. Energy Fuels 2016, 30, 4145–4149. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Jesus, A.R.; Caio, J.M.; Rauter, A.P. Fries-Type Reactions for the C-Glycosylation of Phenols. Curr. Org. Chem. 2010, 15, 128–148. [Google Scholar] [CrossRef]
- Santos, R.G.; Xavier, N.M.; Bordado, J.C.; Rauter, A.P. Efficient and First Regio- and Stereoselective Direct C-Glycosylation of a Flavanone Catalysed by Pr(OTf)3 under Conventional Heating or Ultrasound Irradiation. Eur. J. Org. Chem. 2013, 2013, 1441–1447. [Google Scholar] [CrossRef]
- Mateus, M.M.; Vieira, S.; Bastos, D.; Matos, S.; Gaspar, D.; Bordado, J.C.; Galhano, R.; Santos, D. GC-MS Analysis and Characterization of Bio-Oil from Sweet Potato Peel-A Putative Bio-Fuel. J. Mater. Sci. Technol. Res. 2019, 6, 110–119. [Google Scholar] [CrossRef]
- Laurson, P.; Raudsepp, P.; Kaldmäe, H.; Kikas, A.; Mäeorg, U. The Deconvolution of FTIR-ATR Spectra to Five Gaussians for Detection of Small Changes in Plant-Water Clusters. AIP Adv. 2020, 10, 085214. [Google Scholar] [CrossRef]
- Xiao, B.; Sun, X.F.; Sun, R. Chemical, Structural, and Thermal Characterizations of Alkali-Soluble Lignins and Hemicelluloses, and Cellulose from Maize Stems, Rye Straw, and Rice Straw. Polym. Degrad. Stab. 2001, 74, 307–319. [Google Scholar] [CrossRef]
- Zhou, G.; Taylor, G.; Polle, A. FTIR-ATR-Based Prediction and Modelling of Lignin and Energy Contents Reveals Independent Intra-Specific Variation of These Traits in Bioenergy Poplars. Plant. Methods 2011, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, D.; Orišková, S.; Matos, S.; Machado, H.; Vieira, S.; Bastos, D.; Gaspar, D.; Paiva, R.; Bordado, J.C.; Rodrigues, A.; et al. Thermochemical Liquefaction as a Cleaner and Efficient Route for Valuing Pinewood Residues from Forest Fires. Molecules 2021, 26, 7156. [Google Scholar] [CrossRef]
- Doulati Ardejani, F.; Badii, K.; Limaee, N.Y.; Shafaei, S.Z.; Mirhabibi, A.R. Adsorption of Direct Red 80 Dye from Aqueous Solution onto Almond Shells: Effect of PH, Initial Concentration and Shell Type. J. Hazard. Mater. 2008, 151, 730–737. [Google Scholar] [CrossRef]
- Mohebby, B. Application of ATR Infrared Spectroscopy in Wood Acetylation. J. Agric. Sci. Technol. 2008, 10, 253–259. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of Irradiated Starches by Using FT-Raman and FTIR Spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef]
- Yona, A.M.C.; Budija, F.; Kričej, B.; Kutnar, A.; Pavlič, M.; Pori, P.; Tavzes, Č.; Petrič, M. Production of Biomaterials from Cork: Liquefaction in Polyhydric Alcohols at Moderate Temperatures. Ind. Crops Prod. 2014, 54, 296–301. [Google Scholar] [CrossRef]


| Elemental Composition (%) | |||
|---|---|---|---|
| Samples | C | H | O |
| Aqueous extract | 43.01 | 9.20 | 47.79 |
| Bio-oil [12] | 69.53 | 10.14 | 17.82 |
| Eucalyptus globulus [12] | 48.72 | 5.99 | 44.67 |
| Compound | HPLC Concentration (mg/mL) | Aqueous Extract Concentration (mg/mL) |
|---|---|---|
| Fructose | 9.57 | 105.27 |
| Glucose | 8.72 | 95.92 |
| Sucrose | 3.96 | 43.56 |
| Trehalose | 1.26 | 13.86 |
| Xylose | 2.65 | 29.15 |
| Compound | Standard Curve a (Linearity from 1 to 25 mg/mL) | R2 | LOD b (mg/mL) | LOQ c (mg/mL) |
|---|---|---|---|---|
| Fructose | y = 25.331x − 55.865 | 0.9972 | 3.22 | 9.76 |
| Glucose | y = 24.877x − 78.006 | 0.9954 | 4.17 | 12.63 |
| Sucrose | y = 248.64x − 8.555 | 0.9861 | 7.22 | 21.89 |
| Trehalose | y = 206.17x − 16.704 | 0.9927 | 5.24 | 15.88 |
| Xylose | y = 191.38x − 106.23 | 0.9894 | 6.33 | 19.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.; Orišková, S.; Gonçalves, D.; Paulo, I.; Condeço, J.; Monteiro, M.; Xavier, N.M.; Rauter, A.P.; Bordado, J.M.; Galhano dos Santos, R. Quantification of Hydrolytic Sugars from Eucalyptus globulus Bio-Oil Aqueous Solution after Thermochemical Liquefaction. Forests 2023, 14, 799. https://doi.org/10.3390/f14040799
Silva L, Orišková S, Gonçalves D, Paulo I, Condeço J, Monteiro M, Xavier NM, Rauter AP, Bordado JM, Galhano dos Santos R. Quantification of Hydrolytic Sugars from Eucalyptus globulus Bio-Oil Aqueous Solution after Thermochemical Liquefaction. Forests. 2023; 14(4):799. https://doi.org/10.3390/f14040799
Chicago/Turabian StyleSilva, Luciana, Sofia Orišková, Diogo Gonçalves, Ivo Paulo, José Condeço, Miguel Monteiro, Nuno M. Xavier, Amélia P. Rauter, João M. Bordado, and Rui Galhano dos Santos. 2023. "Quantification of Hydrolytic Sugars from Eucalyptus globulus Bio-Oil Aqueous Solution after Thermochemical Liquefaction" Forests 14, no. 4: 799. https://doi.org/10.3390/f14040799
APA StyleSilva, L., Orišková, S., Gonçalves, D., Paulo, I., Condeço, J., Monteiro, M., Xavier, N. M., Rauter, A. P., Bordado, J. M., & Galhano dos Santos, R. (2023). Quantification of Hydrolytic Sugars from Eucalyptus globulus Bio-Oil Aqueous Solution after Thermochemical Liquefaction. Forests, 14(4), 799. https://doi.org/10.3390/f14040799












