The Role of Microorganisms in the Isolation of Nanocellulose from Plant Biomass
Abstract
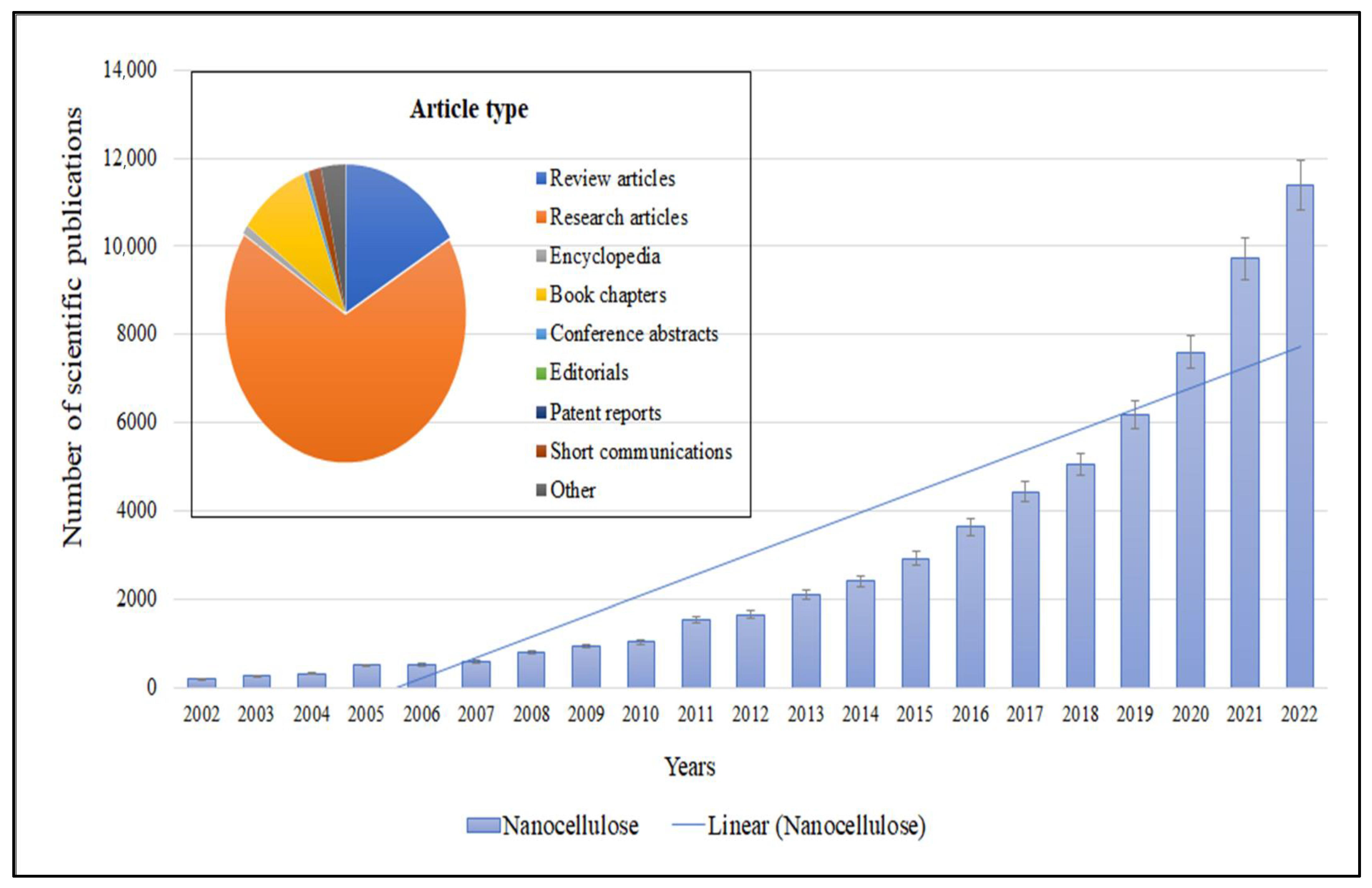
1. Introduction
2. Nanocellulose Functional Material
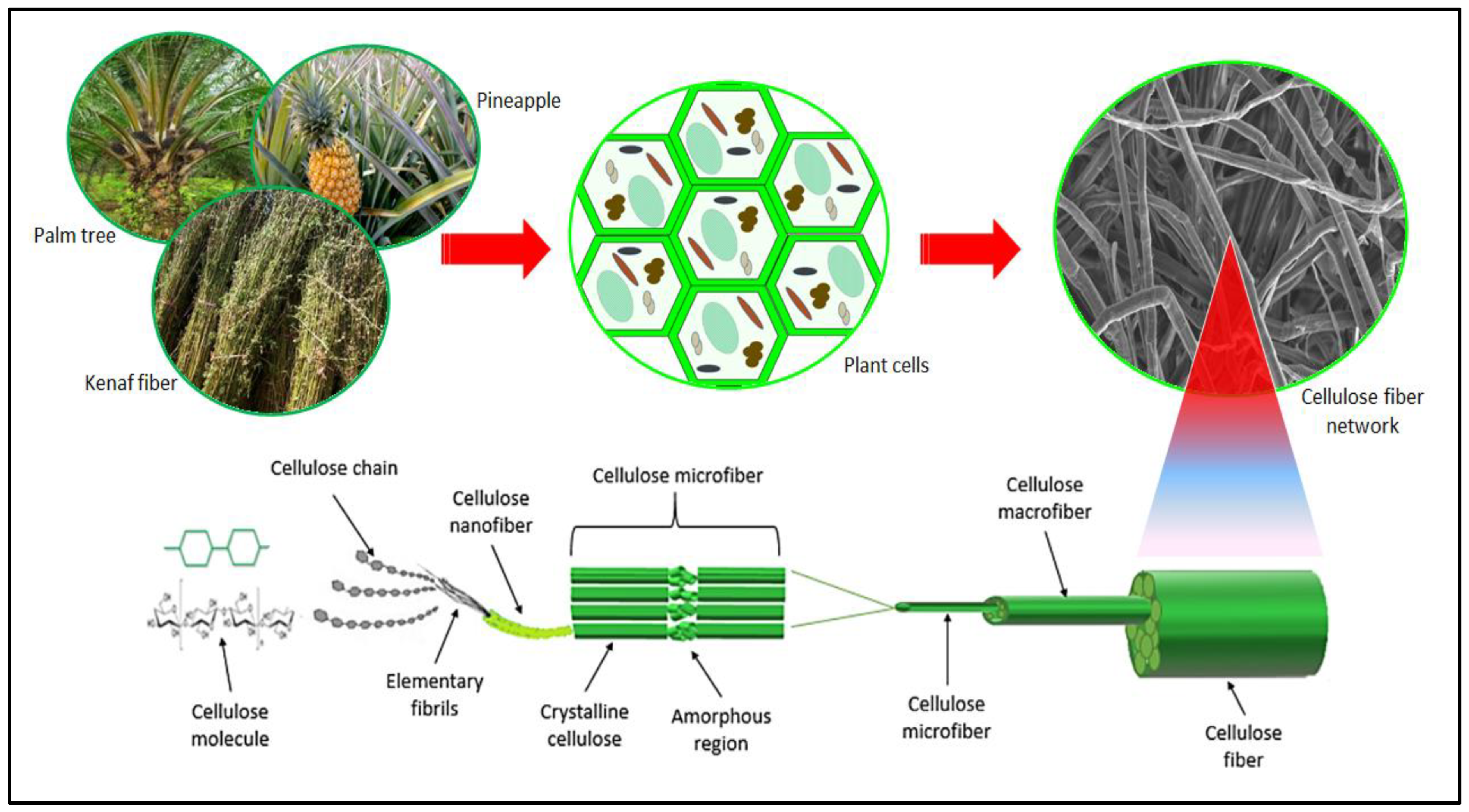
2.1. Structure and Properties of Plant-Based Nanocellulose
2.2. Structure and Properties of Microbial-Based Nanocellulose
2.3. Structure and Properties of Other Sources of Nanocellulose
2.4. Applications of Nanocellulose
3. The Role of Microorganisms in Nanocellulose Isolation
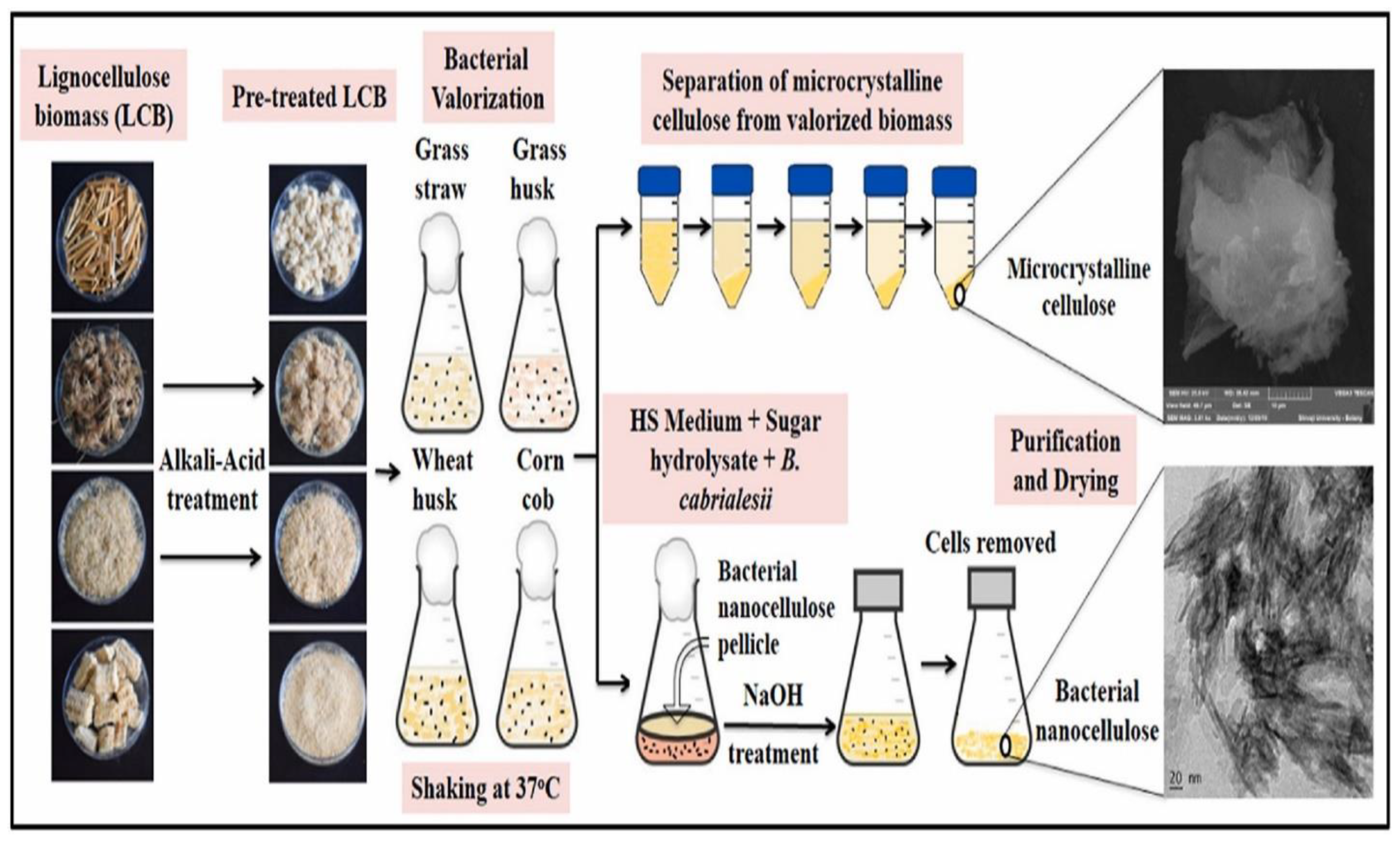
3.1. The Role of Bacteria
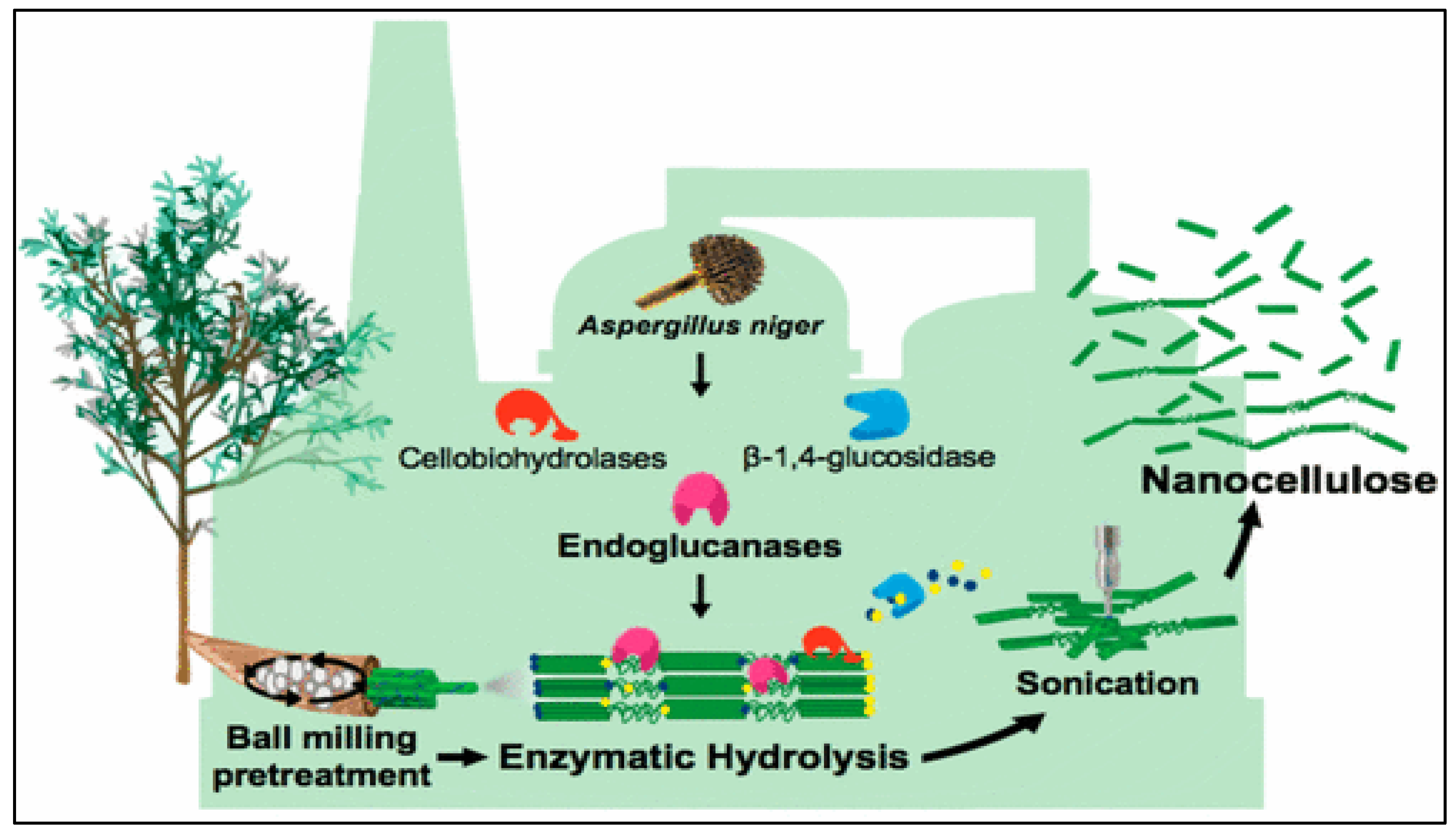
3.2. The Role of Fungi
4. Mechanism of Microbial Process in Nanocellulose Isolation
5. Advantages of Microbial Process Compared to Traditional Isolation Processes
5.1. Lower Energy Consumption
5.2. Higher Production Yields of Nanocellulose
5.3. Milder Reaction Conditions and Ease of Scale-Up
5.4. Controlled and Enhanced Properties of Nanocellulose
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osama, S.; Hussain, A.A.; Roushdy, M.M.; Shehabeldine, A.M.; Hasanin, M.S. Preliminary study for isolation, characterizations of the cellulolytic microorganisms: Green convert of microcrystalline cellulose to nanofibers. Egypt. J. Chem. 2022, 65, 1265–1273. [Google Scholar] [CrossRef]
- Abdul Khalil, H.; Adnan, A.; Yahya, E.B.; Olaiya, N.; Safrida, S.; Hossain, M.S.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.; Oyekanmi, A. A review on plant cellulose nanofibre-based aerogels for biomedical applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef]
- Magalhães, M.I.; Almeida, A.P. Nature-Inspired Cellulose-Based Active Materials: From 2D to 4D. Appl. Biosci. 2023, 2, 94–114. [Google Scholar] [CrossRef]
- Nasution, H.; Yahya, E.B.; Abdul Khalil, H.; Shaah, M.A.; Suriani, A.; Mohamed, A.; Alfatah, T.; Abdullah, C. Extraction and Isolation of Cellulose Nanofibers from Carpet Wastes Using Supercritical Carbon Dioxide Approach. Polymers 2022, 14, 326. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, F.; Tao, H.; Gao, W.; Guo, L.; Cui, B.; Yuan, C.; Liu, P.; Lu, L.; Wu, Z. Effects of different sources of cellulose on mechanical and barrier properties of thermoplastic sweet potato starch films. Ind. Crop. Prod. 2023, 194, 116358. [Google Scholar] [CrossRef]
- Kadier, A.; Ilyas, R.; Huzaifah, M.; Harihastuti, N.; Sapuan, S.; Harussani, M.; Azlin, M.; Yuliasni, R.; Ibrahim, R.; Atikah, M. Use of industrial wastes as sustainable nutrient sources for bacterial cellulose (BC) production: Mechanism, advances, and future perspectives. Polymers 2021, 13, 3365. [Google Scholar] [CrossRef] [PubMed]
- Nicu, R.; Ciolacu, F.; Ciolacu, D.E. Advanced functional materials based on nanocellulose for pharmaceutical/medical applications. Pharmaceutics 2021, 13, 1125. [Google Scholar] [CrossRef]
- Baltaci, M.O. Enhancement of cellulase production by co-culture of Streptomyces ambofaciens OZ2 and Cytobacillus oceanisediminis OZ5 isolated from rumen samples. Biocatal. Biotransformation 2022, 40, 144–152. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Kim, J.; Shim, J.; Chang, S.; Chung, W. Coconut mesocarp-based lignocellulosic waste as a substrate for cellulase production from high promising multienzyme-producing Bacillus amyloliquefaciens FW2 without pretreatments. Microorganisms 2022, 10, 327. [Google Scholar] [CrossRef]
- Zaki, M.; Abdul Khalil, H.P.S.; Sabaruddin, F.; Bairwan, R.; Oyekanmi, A.A.; Alfatah, T.; Danish, M.; Mistar, E.; Abdullah, C. Microbial treatment for nanocellulose extraction from marine algae and its applications as sustainable functional material. Bioresour. Technol. Rep. 2021, 16, 100811. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.H.; Kim, S.; Son, H.; Chun, Y.; Park, C.; Yoo, H.Y. Improved production of bacterial cellulose using Gluconacetobacter sp. LYP25, a strain developed in UVC mutagenesis with limited viability conditions. Int. J. Biol. Macromol. 2023, 232, 123230. [Google Scholar] [CrossRef]
- Adsul, M.G.; Dixit, P.; Saini, J.K.; Gupta, R.P.; Ramakumar, S.S.V.; Mathur, A.S. Morphologically favorable mutant of Trichoderma reesei for low viscosity cellulase production. Biotechnol. Bioeng. 2022, 119, 2167–2181. [Google Scholar] [CrossRef] [PubMed]
- Tsudome, M.; Tachioka, M.; Miyazaki, M.; Uchimura, K.; Tsuda, M.; Takaki, Y.; Deguchi, S. An ultrasensitive nanofiber-based assay for enzymatic hydrolysis and deep-sea microbial degradation of cellulose. Iscience 2022, 25, 104732. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.A.; Jummaat, F.; Yahya, E.B.; Olaiya, N.; Adnan, A.; Abdat, M.; NAM, N.; Halim, A.S.; Kumar, U.S.U.; Bairwan, R. A review on micro-to nanocellulose biopolymer scaffold forming for tissue engineering applications. Polymers 2020, 12, 2043. [Google Scholar] [CrossRef]
- Durand, H.; Smyth, M.; Bras, J. Nanocellulose: A new biopolymer for biomedical application. In Biopolymers for Biomedical and Biotechnological Applications; Wiley: Hoboken, NJ, USA, 2021; pp. 129–179. [Google Scholar]
- Rizal, S.; Alfatah, T.; Abdul Khalil, H.; Yahya, E.B.; Abdullah, C.; Mistar, E.M.; Ikramullah, I.; Kurniawan, R.; Bairwan, R. Enhanced Functional Properties of Bioplastic Films Using Lignin Nanoparticles from Oil Palm-Processing Residue. Polymers 2022, 14, 5126. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.; Sapuan, S.; Norrrahim, M.N.F.; Yasim-Anuar, T.A.T.; Kadier, A.; Kalil, M.S.; Atikah, M.; Ibrahim, R.; Asrofi, M.; Abral, H. Nanocellulose/starch biopolymer nanocomposites: Processing, manufacturing, and applications. In Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 65–88. [Google Scholar]
- Xu, Y.; Xu, Y.; Chen, H.; Gao, M.; Yue, X.; Ni, Y. Redispersion of dried plant nanocellulose: A review. Carbohydr. Polym. 2022, 294, 119830. [Google Scholar] [CrossRef]
- de Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; de Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. A review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Mishra, R.; Sabu, A.; Tiwari, S. Materials chemistry and the futurist eco-friendly applications of nanocellulose: Status and prospect. J. Saudi Chem. Soc. 2018, 22, 949–978. [Google Scholar] [CrossRef]
- Moon, R.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Bertoncini, P.; Cathala, B.; Capron, I. Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions. Soft Matter 2013, 9, 952–959. [Google Scholar] [CrossRef]
- Bettaieb, F.; Khiari, R.; Dufresne, A.; Mhenni, M.F.; Belgacem, M.N. Mechanical and thermal properties of Posidonia oceanica cellulose nanocrystal reinforced polymer. Carbohydr. Polym. 2015, 123, 99–104. [Google Scholar] [CrossRef]
- Choi, S.M.; Shin, E.J. The nanofication and functionalization of bacterial cellulose and its applications. Nanomaterials 2020, 10, 406. [Google Scholar] [CrossRef]
- Islam, M.N.; Rahman, F. Production and modification of nanofibrillated cellulose composites and potential applications. In Green Composites for Automotive Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 115–141. [Google Scholar]
- Vieira, D. Obtenção e Caracterização de Nanocelulose a Partir de Fibras de Chorisia Speciosa St. Hil. Master’s Thesis, Universidade Estadual Paulista, São Paulo, Brazil, 2015. [Google Scholar]
- Das, R.; Lindström, T.; Sharma, P.R.; Chi, K.; Hsiao, B.S. Nanocellulose for sustainable water purification. Chem. Rev. 2022, 122, 8936–9031. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, A.; Dutta, S.D.; Ganguly, K.; Patil, T.V.; Patel, D.K.; Lim, K.-T. A review of properties of nanocellulose, its synthesis, and potential in biomedical applications. Appl. Sci. 2022, 12, 7090. [Google Scholar] [CrossRef]
- Madsen, B.; Gamstedt, E.K. Wood versus plant fibers: Similarities and differences in composite applications. Adv. Mater. Sci. Eng. 2013, 2013, 564346. [Google Scholar] [CrossRef]
- Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Emerging technologies for the production of nanocellulose from lignocellulosic biomass. Carbohydr. Polym. 2022, 285, 119258. [Google Scholar] [CrossRef]
- Louis, A.C.F.; Venkatachalam, S. Energy efficient process for valorization of corn cob as a source for nanocrystalline cellulose and hemicellulose production. Int. J. Biol. Macromol. 2020, 163, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Oun, A.A.; Rhim, J.-W. Isolation of oxidized nanocellulose from rice straw using the ammonium persulfate method. Cellulose 2018, 25, 2143–2149. [Google Scholar] [CrossRef]
- Rai, R.; Dhar, P. Biomedical engineering aspects of nanocellulose: A review. Nanotechnology 2022, 33, 362001. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Sherje, A.P. Cellulose nanocrystals: Fundamentals and biomedical applications. Carbohydr. Polym. 2022, 275, 118668. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.L.; Tiong, S.I.X.; Siva, S.P.; Ahamed, F.; Chan, C.-H.; Lee, C.L.; Chew, I.M.L.; Ho, Y.K. Morphological control of cellulose nanocrystals via sulfuric acid hydrolysis based on sustainability considerations: An overview of the governing factors and potential challenges. J. Environ. Chem. Eng. 2022, 10, 108145. [Google Scholar] [CrossRef]
- Zhao, X.; Bhagia, S.; Gomez-Maldonado, D.; Tang, X.; Wasti, S.; Lu, S.; Zhang, S.; Parit, M.; Rencheck, M.L.; Korey, M. Bioinspired design toward nanocellulose-based materials. Mater. Today 2023, 66, 409–430. [Google Scholar] [CrossRef]
- Surendran, G.; Sherje, A.P. Cellulose nanofibers and composites: An insight on basics and biomedical applications. J. Drug Deliv. Sci. Technol. 2022, 75, 103601. [Google Scholar] [CrossRef]
- Meftahi, A.; Momeni Heravi, M.E.; Barhoum, A.; Samyn, P.; Najarzadeh, H.; Alibakhshi, S. Cellulose Nanofibers: Synthesis, Unique Properties, and Emerging Applications. In Handbook of Nanocelluloses: Classification, Properties, Fabrication, and Emerging Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 233–262. [Google Scholar]
- Bourmaud, A.; Mayer-Laigle, C.; Baley, C.; Beaugrand, J. About the frontier between filling and reinforcement by fine flax particles in plant fibre composites. Ind. Crop. Prod. 2019, 141, 111774. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45. [Google Scholar] [CrossRef]
- Hurtado, P.L.; Rouilly, A.; Vandenbossche, V.; Raynaud, C. A review on the properties of cellulose fibre insulation. Build. Environ. 2016, 96, 170–177. [Google Scholar] [CrossRef]
- Schiros, T.N.; Antrobus, R.; Farías, D.; Chiu, Y.-T.; Joseph, C.T.; Esdaille, S.; Sanchirico, G.K.; Miquelon, G.; An, D.; Russell, S.T. Microbial nanocellulose biotextiles for a circular materials economy. Environ. Sci. Adv. 2022, 1, 276–284. [Google Scholar] [CrossRef]
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Shi, Z.; Gauthier, M.; Yang, G. Bacterial cellulose: Molecular regulation of biosynthesis, supramolecular assembly, and tailored structural and functional properties. Prog. Mater. Sci. 2022, 129, 100972. [Google Scholar] [CrossRef]
- Jozala, A.F.; de Lencastre-Novaes, L.C.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa-Jr, A.; Grotto, D.; Gerenutti, M.; Chaud, M.V. Bacterial nanocellulose production and application: A 10-year overview. Appl. Microbiol. Biotechnol. 2016, 100, 2063–2072. [Google Scholar] [CrossRef]
- Stanisławska, A. Bacterial nanocellulose as a microbiological derived nanomaterial. Adv. Mater. Sci. 2016, 16, 45–57. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Thomas, D.; Madhavan, A.; Sindhu, R.; Binod, P.; Varjani, S.; Awasthi, M.K.; Pandey, A. Bacterial nanocellulose: Engineering, production, and applications. Bioengineered 2021, 12, 11463. [Google Scholar]
- Skočaj, M. Bacterial nanocellulose in papermaking. Cellulose 2019, 26, 6477–6488. [Google Scholar] [CrossRef]
- Cottet, C.; Ramirez-Tapias, Y.A.; Delgado, J.F.; de la Osa, O.; Salvay, A.G.; Peltzer, M.A. Biobased materials from microbial biomass and its derivatives. Materials 2020, 13, 1263. [Google Scholar] [CrossRef] [PubMed]
- Gregory, D.A.; Tripathi, L.; Fricker, A.T.; Asare, E.; Orlando, I.; Raghavendran, V.; Roy, I. Bacterial cellulose: A smart biomaterial with diverse applications. Mater. Sci. Eng. R Rep. 2021, 145, 100623. [Google Scholar] [CrossRef]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef]
- Moniri, M.; Boroumand Moghaddam, A.; Azizi, S.; Abdul Rahim, R.; Bin Ariff, A.; Zuhainis Saad, W.; Navaderi, M.; Mohamad, R. Production and status of bacterial cellulose in biomedical engineering. Nanomaterials 2017, 7, 257. [Google Scholar] [CrossRef]
- Pacheco, G.; de Mello, C.V.; Chiari-Andréo, B.G.; Isaac, V.L.B.; Ribeiro, S.J.L.; Pecoraro, É.; Trovatti, E. Bacterial cellulose skin masks—Properties and sensory tests. J. Cosmet. Dermatol. 2018, 17, 840–847. [Google Scholar] [CrossRef]
- Lee, K.Y.; Buldum, G.; Mantalaris, A.; Bismarck, A. More than meets the eye in bacterial cellulose: Biosynthesis, bioprocessing, and applications in advanced fiber composites. Macromol. Biosci. 2014, 14, 10–32. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of bacterial cellulose in food. Food Hydrocoll. 2014, 35, 539–545. [Google Scholar] [CrossRef]
- Bar-Shai, N.; Sharabani-Yosef, O.; Zollmann, M.; Lesman, A.; Golberg, A. Seaweed cellulose scaffolds derived from green macroalgae for tissue engineering. Sci. Rep. 2021, 11, 11843. [Google Scholar] [CrossRef]
- Oyekanmi, A.A.; Saharudin, N.; Hazwan, C.M.; HPS, A.K.; Olaiya, N.G.; Abdullah, C.K.; Alfatah, T.; Gopakumar, D.A.; Pasquini, D. Improved hydrophobicity of macroalgae biopolymer film incorporated with kenaf derived CNF using silane coupling agent. Molecules 2021, 26, 2254. [Google Scholar] [CrossRef]
- Zanchetta, E.; Damergi, E.; Patel, B.; Borgmeyer, T.; Pick, H.; Pulgarin, A.; Ludwig, C. Algal cellulose, production and potential use in plastics: Challenges and opportunities. Algal Res. 2021, 56, 102288. [Google Scholar] [CrossRef]
- Jeong, T.S.; Choi, C.H.; Lee, J.Y.; Oh, K.K. Behaviors of glucose decomposition during acid-catalyzed hydrothermal hydrolysis of pretreated Gelidium amansii. Bioresour. Technol. 2012, 116, 435–440. [Google Scholar] [CrossRef]
- Albuquerque, J.C.S.; Araújo, M.L.H.; Rocha, M.V.P.; de Souza, B.W.S.; de Castro, G.M.C.; Cordeiro, E.M.S.; de Sousa Silva, J.; Benevides, N.M.B. Acid hydrolysis conditions for the production of fine chemicals from Gracilaria birdiae alga biomass. Algal Res. 2021, 53, 102139. [Google Scholar] [CrossRef]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.; Duarte, A.C. Chemical composition of red, brown and green macroalgae from Buarcos bay in Central West Coast of Portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef]
- Fraly Erbabley, N.Y.G.; Junianto, J. Chemical characteristics and phytochemicals of the brown alga Sargassum filipendulla from kelanit waters of southeast Maluku. Egypt. J. Aquat. Biol. Fish. 2020, 24, 535–547. [Google Scholar] [CrossRef]
- Ennab, R.M.; Aljabali, A.A.; Charbe, N.B.; Barhoum, A.; Alqudah, A.; Tambuwala, M.M. Nanocelluloses in Wound Healing Applications. In Handbook of Nanocelluloses: Classification, Properties, Fabrication, and Emerging Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–28. [Google Scholar]
- Zhang, M.; Haga, A.; Sekiguchi, H.; Hirano, S. Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int. J. Biol. Macromol. 2000, 27, 99–105. [Google Scholar] [CrossRef]
- Calvo, V.; González-Domínguez, J.M.; Benito, A.M.; Maser, W.K. Synthesis and Processing of Nanomaterials Mediated by Living Organisms. Angew. Chem. 2022, 134, e202113286. [Google Scholar] [CrossRef]
- Noremylia, M.; Hassan, M.Z.; Ismail, Z. Recent advancement in isolation, processing, characterization and applications of emerging nanocellulose: A review. Int. J. Biol. Macromol. 2022, 206, 954–976. [Google Scholar] [CrossRef]
- Fang, B.; Wan, Y.-Z.; Tang, T.-T.; Gao, C.; Dai, K.-R. Proliferation and osteoblastic differentiation of human bone marrow stromal cells on hydroxyapatite/bacterial cellulose nanocomposite scaffolds. Tissue Eng. Part A 2009, 15, 1091–1098. [Google Scholar] [CrossRef]
- Yahya, E.B.; Amirul, A.; HPS, A.K.; Olaiya, N.G.; Iqbal, M.O.; Jummaat, F.; AK, A.S.; Adnan, A. Insights into the role of biopolymer aerogel scaffolds in tissue engineering and regenerative medicine. Polymers 2021, 13, 1612. [Google Scholar] [CrossRef]
- Eskilson, O.; Zattarin, E.; Berglund, L.; Oksman, K.; Hanna, K.; Rakar, J.; Sivlér, P.; Skog, M.; Rinklake, I.; Shamasha, R. Nanocellulose composite wound dressings for real-time pH wound monitoring. Mater. Today Bio 2023, 19, 100574. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, N.; Sun, Y.; Shao, J.; Liu, Q.; Zhuang, X.; Twebaze, C.B. Nanocellulose aerogels from banana pseudo-stem as a wound dressing. Ind. Crop. Prod. 2023, 194, 116383. [Google Scholar] [CrossRef]
- Puppala, N.V.; Doddipatla, P.; Mohannath, G. Use of nanocellulose in the intracellular delivery of biological and non-biological drugs: A review. Cellulose 2023, 30, 1335–1354. [Google Scholar] [CrossRef]
- Bellmann, T.; Thamm, J.; Beekmann, U.; Kralisch, D.; Fischer, D. In situ Formation of Polymer Microparticles in Bacterial Nanocellulose Using Alternative and Sustainable Solvents to Incorporate Lipophilic Drugs. Pharmaceutics 2023, 15, 559. [Google Scholar] [CrossRef]
- de Assis, S.C.; Morgado, D.L.; Scheidt, D.T.; de Souza, S.S.; Cavallari, M.R.; Ando Junior, O.H.; Carrilho, E. Review of Bacterial Nanocellulose-Based Electrochemical Biosensors: Functionalization, Challenges, and Future Perspectives. Biosensors 2023, 13, 142. [Google Scholar] [CrossRef]
- Eldhose, M.; Roy, R.; George, C.; Joseph, A. Sensing and Biosensing Applications of Nanocellulose. In Handbook of Biopolymers; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–26. [Google Scholar]
- Vilarinho, F.; Sanches Silva, A.; Vaz, M.F.; Farinha, J.P. Nanocellulose in green food packaging. Crit. Rev. Food Sci. Nutr. 2018, 58, 1526–1537. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kitaoka, T. Ultraselective gas separation by nanoporous metal− organic frameworks embedded in gas-barrier nanocellulose films. Adv. Mater. 2016, 28, 1765–1769. [Google Scholar] [CrossRef]
- Aulin, C.; Karabulut, E.; Tran, A.; Wågberg, L.; Lindström, T. Transparent nanocellulosic multilayer thin films on polylactic acid with tunable gas barrier properties. ACS Appl. Mater. Interfaces 2013, 5, 7352–7359. [Google Scholar] [CrossRef]
- Parvathy, S.; Hema, S.; Sajith, M.; Sulthan, R.; Sreelekshmi, C.; Sambhudevan, S.; Shankar, B. A Review on Barrier Properties of Nanocellulose and Polylactic acid Composites. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2022; p. 012017. [Google Scholar]
- Rizal, S.; Olaiya, F.G.; Saharudin, N.; Abdullah, C.; NG, O.; Mohamad Haafiz, M.; Yahya, E.B.; Sabaruddin, F.; Ikramullah; Abdul Khalil, H.P.S. Isolation of textile waste cellulose nanofibrillated fibre reinforced in polylactic acid-chitin biodegradable composite for green packaging application. Polymers 2021, 13, 325. [Google Scholar] [CrossRef]
- Ginja, G.A.; da Costa, J.P.d.C.; Gounella, R.H.; Izquierdo, J.E.E.; Carmo, J.P.; Fonseca, F.J.; Cavallari, M.R.; Junior, O.H.A.; de Souza, S.S. A Humidity Sensor Based on Bacterial Nanocellulose Membrane (BNC). IEEE Sens. J. 2023, 23, 3485–3492. [Google Scholar] [CrossRef]
- Suman; Kardam, A.; Gera, M.; Jain, V. A novel reusable nanocomposite for complete removal of dyes, heavy metals and microbial load from water based on nanocellulose and silver nano-embedded pebbles. Environ. Technol. 2015, 36, 706–714. [Google Scholar] [CrossRef]
- Tasrin, S.; Mohamed Madhar Fazil, S.; Senthilmurugan, S.; Selvaraju, N. Facile preparation of nanocellulose embedded polypyrrole for dye removal: Unary and binary process optimization and seed toxicity. Int. J. Environ. Sci. Technol. 2021, 18, 365–378. [Google Scholar] [CrossRef]
- Maqbool, Q.; Barucca, G.; Sabbatini, S.; Parlapiano, M.; Ruello, M.L.; Tittarelli, F. Transformation of industrial and organic waste into titanium doped activated carbon–cellulose nanocomposite for rapid removal of organic pollutants. J. Hazard. Mater. 2022, 423, 126958. [Google Scholar] [CrossRef]
- Yin, Z.; Cheng, Y.; Deng, Y.; Li, Z.; Liu, K.; Li, M.; Chen, X.; Xue, M.; Ou, J.; Lei, S. Functional and versatile colorful superhydrophobic nanocellulose-based membrane with high durability, high-efficiency oil/water separation and oil spill cleanup. Surf. Coat. Technol. 2022, 445, 128714. [Google Scholar] [CrossRef]
- Xiong, Z.; Lin, J.; Li, X.; Bian, F.; Wang, J. Hierarchically structured nanocellulose-implanted air filters for high-efficiency particulate matter removal. ACS Appl. Mater. Interfaces 2021, 13, 12408–12416. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, Z.; Guo, L.; Hu, S.; Wang, H.; Meng, L.; Tang, M.; Qi, H. Acetylated tunicate nanocellulose-based high-efficient air filter media with antibacterial property. J. Membr. Sci. 2023, 669, 121307. [Google Scholar] [CrossRef]
- Dias, O.A.T.; Konar, S.; Leão, A.L.; Yang, W.; Tjong, J.; Sain, M. Current state of applications of nanocellulose in flexible energy and electronic devices. Front. Chem. 2020, 8, 420. [Google Scholar] [CrossRef]
- Fan, W.; Wang, J.; Zhang, Z.; Li, Z. Synergistic enhancement of UV-resistance and electrical conductivity of waterborne polyurethane composite with combination of functionalized 2D graphene oxide and 1D nanocellulose. Prog. Org. Coat. 2021, 151, 106017. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, Q.; Liu, J.; Sun, J.; Zhu, Q.; Chen, H. Processing nanocellulose to bulk materials: A review. Cellulose 2019, 26, 7585–7617. [Google Scholar] [CrossRef]
- Ilyas, R.; Asyraf, M.; Aisyah, H.; Sapuan, S.; Norrrahim, M.; Ibrahim, R.; Atikah, M.; Atiqah, A.; Zainudin, E.; Ishak, M. Introduction to nanocellulose production from biological waste. In Industrial Applications of Nanocellulose and Its Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–37. [Google Scholar]
- Squinca, P.; Bilatto, S.; Badino, A.C.; Farinas, C.S. The use of enzymes to isolate cellulose nanomaterials: A systematic map review. Carbohydr. Polym. Technol. Appl. 2022, 3, 100212. [Google Scholar] [CrossRef]
- Oktiarni, D.; Nofyan, E.; Kasmiarti, G. Isolation and identification cellulolytic bacteria from termite gut obtained from Indralaya peatland area. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; p. 012024. [Google Scholar]
- Singh, R.S.; Singh, T.; Pandey, A. Microbial enzymes—An overview. In Advances in Enzyme Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–40. [Google Scholar]
- Straub, C.T.; Bing, R.G.; Otten, J.K.; Keller, L.M.; Zeldes, B.M.; Adams, M.W.; Kelly, R.M. Metabolically engineered Caldicellulosiruptor bescii as a platform for producing acetone and hydrogen from lignocellulose. Biotechnol. Bioeng. 2020, 117, 3799–3808. [Google Scholar] [CrossRef]
- Straub, C.T.; Bing, R.G.; Wang, J.P.; Chiang, V.L.; Adams, M.W.; Kelly, R.M. Use of the lignocellulose-degrading bacterium Caldicellulosiruptor bescii to assess recalcitrance and conversion of wild-type and transgenic poplar. Biotechnol. Biofuels 2020, 13, 43. [Google Scholar] [CrossRef]
- Zhu, X.; Xin, S.; Ding, H.; Yang, Y.; Chen, Y.; Li, X.; Shi, H.; Tan, Z.; Zhou, J.; Liu, P. Functional characterization of a noncatalytic protein, Athe_0181, from Caldicellulosiruptor bescii in promoting lignocellulose hydrolysis. BioResources 2022, 17, 3067–3081. [Google Scholar] [CrossRef]
- Ghosh, T.; Ngo, T.-D.; Kumar, A.; Ayranci, C.; Tang, T. Cleaning carbohydrate impurities from lignin using Pseudomonas fluorescens. Green Chem. 2019, 21, 1648–1659. [Google Scholar] [CrossRef]
- Mortabit, D.; Zyani, M.; Haggoud, A.; Housaini Iraqui, M.; Houari, A.; Fikri Benbrahim, K.; Koraichi Ibnsouda, S.; Koraichi, S. Carboxymethyl cellulase production by moroccan bacillus isolates. Moroc. J. Biol. 2010, 1, 43–49. [Google Scholar]
- Adorian, T.J.; Jamali, H.; Farsani, H.G.; Darvishi, P.; Hasanpour, S.; Bagheri, T.; Roozbehfar, R. Effects of probiotic bacteria Bacillus on growth performance, digestive enzyme activity, and hematological parameters of Asian sea bass, Lates calcarifer (Bloch). Probiotics Antimicrob. Proteins 2019, 11, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Kuebutornye, F.K.; Abarike, E.D.; Lu, Y. A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol. 2019, 87, 820–828. [Google Scholar] [CrossRef]
- Sadalage, P.S.; Pawar, K.D. Production of microcrystalline cellulose and bacterial nanocellulose through biological valorization of lignocellulosic biomass wastes. J. Clean. Prod. 2021, 327, 129462. [Google Scholar] [CrossRef]
- Mariño, M.; Lopes da Silva, L.; Durán, N.; Tasic, L. Enhanced materials from nature: Nanocellulose from citrus waste. Molecules 2015, 20, 5908–5923. [Google Scholar] [CrossRef] [PubMed]
- Yarbrough, J.M.; Zhang, R.; Mittal, A.; Vander Wall, T.; Bomble, Y.J.; Decker, S.R.; Himmel, M.E.; Ciesielski, P.N. Multifunctional cellulolytic enzymes outperform processive fungal cellulases for coproduction of nanocellulose and biofuels. ACS Nano 2017, 11, 3101–3109. [Google Scholar] [CrossRef] [PubMed]
- Septiani, D.; Suryadi, H.; Mun'im, A.; Mangunwardoyo, W. Production of cellulase from Aspergillus niger and Trichoderma reesei mixed culture in carboxymethylcellulose medium as sole carbon. Biodiversitas J. Biol. Divers. 2019, 20. [Google Scholar] [CrossRef]
- Wu, D.; Qu, F.; Li, D.; Zhao, Y.; Li, X.; Niu, S.; Zhao, M.; Qi, H.; Wei, Z.; Song, C. Effect of Fenton pretreatment and bacterial inoculation on cellulose-degrading genes and fungal communities during rice straw composting. Sci. Total Environ. 2022, 806, 151376. [Google Scholar] [CrossRef]
- Fritz, C.; Jeuck, B.; Salas, C.; Gonzalez, R.; Jameel, H.; Rojas, O.J. Nanocellulose and proteins: Exploiting their interactions for production, immobilization, and synthesis of biocompatible materials. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Springer: Berlin/Heidelberg, Germany, 2016; pp. 207–224. [Google Scholar]
- Janardhnan, S.; Sain, M.M. Isolation of cellulose microfibrils–an enzymatic approach. Bioresources 2006, 1, 176–188. [Google Scholar] [CrossRef]
- López-Rubio, A.; Lagaron, J.; Ankerfors, M.; Lindström, T.; Nordqvist, D.; Mattozzi, A.; Hedenqvist, M.S. Enhanced film forming and film properties of amylopectin using micro-fibrillated cellulose. Carbohydr. Polym. 2007, 68, 718–727. [Google Scholar] [CrossRef]
- Svagan, A.J.; Azizi Samir, M.A.; Berglund, L.A. Biomimetic polysaccharide nanocomposites of high cellulose content and high toughness. Biomacromolecules 2007, 8, 2556–2563. [Google Scholar] [CrossRef]
- Squinca, P.; Bilatto, S.; Badino, A.C.; Farinas, C.S. Nanocellulose production in future biorefineries: An integrated approach using tailor-made enzymes. ACS Sustain. Chem. Eng. 2020, 8, 2277–2286. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; Wang, L.; Song, X.; Qin, C.; Wang, S. Tuning of size and properties of cellulose nanofibers isolated from sugarcane bagasse by endoglucanase-assisted mechanical grinding. Ind. Crop. Prod. 2020, 146, 112201. [Google Scholar] [CrossRef]
- Colonia, B.S.O.; Woiciechowski, A.L.; Malanski, R.; Letti, L.A.J.; Soccol, C.R. Pulp improvement of oil palm empty fruit bunches associated to solid-state biopulping and biobleaching with xylanase and lignin peroxidase cocktail produced by Aspergillus sp. LPB-5. Bioresour. Technol. 2019, 285, 121361. [Google Scholar] [CrossRef] [PubMed]
- Anand, G.; Leibman-Markus, M.; Elkabetz, D.; Bar, M. Method for the production and purification of plant immuno-active xylanase from Trichoderma. Int. J. Mol. Sci. 2021, 22, 4214. [Google Scholar] [CrossRef] [PubMed]
- Legodi, L.M.; La Grange, D.C.; van Rensburg, E.L.J. Production of the Cellulase Enzyme System by Locally Isolated Trichoderma and Aspergillus Species Cultivated on Banana Pseudostem during Solid-State Fermentation. Fermentation 2023, 9, 412. [Google Scholar] [CrossRef]
- Butt, K.Y.; Saleemi, K.B.; Gilani, S.R.; Ghori, M.I. Kinetics of cellobiohydrolase from a phytopathogenic fungus Trichoderma harzianum. Pak. J. Life Soc. Sci. 2018, 16, 72–76. [Google Scholar]
- Hasanin, M.S.; Mostafa, A.M.; Mwafy, E.A.; Darwesh, O.M. Eco-friendly cellulose nano fibers via first reported Egyptian Humicola fuscoatra Egyptia X4: Isolation and characterization. Environ. Nanotechnol. Monit. Manag. 2018, 10, 409–418. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, J.; Durán, N.; Tasic, L. Nanocellulose and bioethanol production from orange waste using isolated microorganisms. J. Braz. Chem. Soc. 2013, 24, 1537–1543. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Deng, X.-Y.; Shen, W.-H.; Jia, M.-Y. Preparation and characterization of the spherical nanosized cellulose by the enzymatic hydrolysis of pulp fibers. Carbohydr. Polym. 2018, 181, 879–884. [Google Scholar] [CrossRef]
- Teixeira, R.S.S.; da Silva, A.S.A.; Jang, J.-H.; Kim, H.-W.; Ishikawa, K.; Endo, T.; Lee, S.-H.; Bon, E.P. Combining biomass wet disk milling and endoglucanase/β-glucosidase hydrolysis for the production of cellulose nanocrystals. Carbohydr. Polym. 2015, 128, 75–81. [Google Scholar] [CrossRef]
- Black, K.; Walker, G. Yeast Fermentation for Production of Neutral Distilled Spirits. Appl. Sci. 2023, 13, 4927. [Google Scholar] [CrossRef]
- Chung, S.Y.; Seki, H.; Fujisawa, Y.; Shimoda, Y.; Hiraga, S.; Nomura, Y.; Saito, K.; Ishimoto, M.; Muranaka, T. A cellulose synthase-derived enzyme catalyses 3-O-glucuronosylation in saponin biosynthesis. Nat. Commun. 2020, 11, 5664. [Google Scholar] [CrossRef]
- Giese, E.C.; Cadete, R.M.; Pierozzi, M.; Philippini, R.R.; Martiniano, S.E.; Pagnocca, F.C.; Rosa, C.A.; Da Silva, S.S. Cellulases production by new yeast isolates from Brazilian biodiversity. Curr. Opin. Biotechnol. 2011, 22, S147–S148. [Google Scholar] [CrossRef]
- Jimenez, M.; Gonzalez, A.; Martinez, M.; Martinez, A.; Dale, B. Screening of yeasts isolated from decayed wood for lignocellulose-degrading enzyme activities. Mycol. Res. 1991, 95, 1299–1302. [Google Scholar] [CrossRef]
- Božič, M.; Liu, P.; Mathew, A.P.; Kokol, V. Enzymatic phosphorylation of cellulose nanofibers to new highly-ions adsorbing, flame-retardant and hydroxyapatite-growth induced natural nanoparticles. Cellulose 2014, 21, 2713–2726. [Google Scholar] [CrossRef]
- Atatoprak, T.; Amorim, M.M.; Ribeiro, T.; Pintado, M.; Madureira, A.R. Grape stalk valorization for fermentation purposes. Food Chem. Mol. Sci. 2022, 4, 100067. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; He, Z.; Zheng, L.; Pande, H.; Ni, Y. Enzymatic treatment processes for the production of cellulose nanomaterials: A review. Carbohydr. Polym. 2022, 299, 120199. [Google Scholar] [CrossRef]
- Beltramino, F.; Roncero, M.B.; Vidal, T.; Valls, C. A novel enzymatic approach to nanocrystalline cellulose preparation. Carbohydr. Polym. 2018, 189, 39–47. [Google Scholar] [CrossRef]
- Banvillet, G.; Depres, G.; Belgacem, N.; Bras, J. Alkaline treatment combined with enzymatic hydrolysis for efficient cellulose nanofibrils production. Carbohydr. Polym. 2021, 255, 117383. [Google Scholar] [CrossRef]
- Tibolla, H.; Pelissari, F.M.; Rodrigues, M.I.; Menegalli, F.C. Cellulose nanofibers produced from banana peel by enzymatic treatment: Study of process conditions. Ind. Crop. Prod. 2017, 95, 664–674. [Google Scholar] [CrossRef]
- Zielińska, D.; Szentner, K.; Waśkiewicz, A.; Borysiak, S. Production of nanocellulose by enzymatic treatment for application in polymer composites. Materials 2021, 14, 2124. [Google Scholar] [CrossRef]
- Li, H.; Legere, S.; He, Z.; Zhang, H.; Li, J.; Yang, B.; Zhang, S.; Zhang, L.; Zheng, L.; Ni, Y. Methods to increase the reactivity of dissolving pulp in the viscose rayon production process: A review. Cellulose 2018, 25, 3733–3753. [Google Scholar] [CrossRef]
- Duan, C.; Verma, S.K.; Li, J.; Ma, X.; Ni, Y. Combination of mechanical, alkaline and enzymatic treatments to upgrade paper-grade pulp to dissolving pulp with high reactivity. Bioresour. Technol. 2016, 200, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Chen, L.; Huang, L.; Tian, C.; Zheng, L.; Ni, Y. A process for enhancing the accessibility and reactivity of hardwood kraft-based dissolving pulp for viscose rayon production by cellulase treatment. Bioresour. Technol. 2014, 154, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Houfani, A.A.; Anders, N.; Spiess, A.C.; Baldrian, P.; Benallaoua, S. Insights from enzymatic degradation of cellulose and hemicellulose to fermentable sugars–a review. Biomass Bioenergy 2020, 134, 105481. [Google Scholar] [CrossRef]
- de Aguiar, J.; Bondancia, T.J.; Claro, P.I.C.; Mattoso, L.H.C.; Farinas, C.S.; Marconcini, J.M. Enzymatic deconstruction of sugarcane bagasse and straw to obtain cellulose nanomaterials. ACS Sustain. Chem. Eng. 2020, 8, 2287–2299. [Google Scholar] [CrossRef]
- Afrin, S.; Karim, Z. Isolation and surface modification of nanocellulose: Necessity of enzymes over chemicals. ChemBioEng Rev. 2017, 4, 289–303. [Google Scholar] [CrossRef]
- Pires, J.R.; Souza, V.G.; Fernando, A.L. Valorization of energy crops as a source for nanocellulose production–current knowledge and future prospects. Ind. Crop. Prod. 2019, 140, 111642. [Google Scholar] [CrossRef]
- Ai, Y.; Zhang, L.; Cui, M.; Huang, R.; Qi, W.; He, Z.; Klemeš, J.J.; Su, R. Toward cleaner production of nanocellulose: A review and evaluation. Green Chem. 2022, 24, 6406–6434. [Google Scholar] [CrossRef]
- Eriksen, Ø.; Syverud, K.; Gregersen, Ø. The use of microfibrillated cellulose produced from kraft pulp as strength enhancer in TMP paper. Nord. Pulp Pap. Res. J. 2008, 23, 299–304. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef]
- Anderson, S.R.; Esposito, D.; Gillette, W.; Zhu, J.; Baxa, U.; Mcneil, S.E. Enzymatic preparation of nanocrystalline and microcrystalline cellulose. Tappi J. 2014, 13, 35–42. [Google Scholar] [CrossRef]
- Spence, K.L.; Venditti, R.A.; Rojas, O.J.; Habibi, Y.; Pawlak, J.J. A comparative study of energy consumption and physical properties of microfibrillated cellulose produced by different processing methods. Cellulose 2011, 18, 1097–1111. [Google Scholar] [CrossRef]
- Mohlin, U.; Pettersson, B. Improved papermaking by cellulase treatment before refining. ICBPPI 2001, 21, 291–299. [Google Scholar]
- Bajpai, P.; Mishra, S.P.; Mishra, O.P.; Kumar, S.; Bajpai, A. Use of enzymes for reduction in refining energy-laboratory studies. Tappi J. 2006, 5, 25. [Google Scholar]
- Lecourt, M.; Meyer, V.; Sigoillot, J.-C.; Petit-Conil, M. Energy reduction of refining by cellulases. Holzforschung 2010, 64, 441–446. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, Y.; Jiang, F. Sustainable isolation of nanocellulose from cellulose and lignocellulosic feedstocks: Recent progress and perspectives. Carbohydr. Polym. 2021, 267, 118188. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, Y.; Qin, C.; Yang, S.; Song, X.; Wang, S.; Li, K. Enzyme-assisted mechanical grinding for cellulose nanofibers from bagasse: Energy consumption and nanofiber characteristics. Cellulose 2018, 25, 7065–7078. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. Predicting the environmental impact of a future nanocellulose production at industrial scale: Application of the life cycle assessment scale-up framework. J. Clean. Prod. 2018, 174, 283–295. [Google Scholar] [CrossRef]
- Li, Q.; McGinnis, S.; Wong, A.; Renneckar, S. Nanocellulose life cycle assessment. ACS Sustain. Chem. Eng. 2013, 1, 919–928. [Google Scholar] [CrossRef]
- Yassin, M.A.; Gad, A.A.M.; Ghanem, A.F.; Rehim, M.H.A. Green synthesis of cellulose nanofibers using immobilized cellulase. Carbohydr. Polym. 2019, 205, 255–260. [Google Scholar] [CrossRef]
- Ashkan, Z.; Hemmati, R.; Homaei, A.; Dinari, A.; Jamlidoost, M.; Tashakor, A. Immobilization of enzymes on nanoinorganic support materials: An update. Int. J. Biol. Macromol. 2021, 168, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Q.; Pang, G.-X.; Shen, W.-H.; Tong, X.; Jia, M.-Y. Preparation and characterization of the ribbon-like cellulose nanocrystals by the cellulase enzymolysis of cotton pulp fibers. Carbohydr. Polym. 2019, 207, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Yan, D.; Zhang, C.; Nie, S. Enzyme-assisted mechanical production of cellulose nanofibrils: Thermal stability. Cellulose 2018, 25, 5049–5061. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Pohlmann, B.C.; Calado, V.; Bojorge, N.; Pereira Jr, N. Production of nanocellulose by enzymatic hydrolysis: Trends and challenges. Eng. Life Sci. 2019, 19, 279–291. [Google Scholar] [CrossRef]
- Samyn, P.; Meftahi, A.; Geravand, S.A.; Heravi, M.E.M.; Najarzadeh, H.; Sabery, M.S.K.; Barhoum, A. Opportunities for bacterial nanocellulose in biomedical applications: Review on biosynthesis, modification and challenges. Int. J. Biol. Macromol. 2023, 231, 123316. [Google Scholar] [CrossRef]
- da Gama, F.M.P.; Dourado, F. Bacterial NanoCellulose: What future? BioImpacts BI 2018, 8, 1. [Google Scholar]
- Padmanaban, S.; Balaji, N.; Muthukumaran, C.; Tamilarasan, K. Statistical optimization of process parameters for exopolysaccharide production by Aureobasidium pullulans using sweet potato based medium. 3 Biotech 2015, 5, 1067–1073. [Google Scholar] [CrossRef]
- Cakar, F.; Özer, I.; Aytekin, A.Ö.; Şahin, F. Improvement production of bacterial cellulose by semi-continuous process in molasses medium. Carbohydr. Polym. 2014, 106, 7–13. [Google Scholar] [CrossRef]
- Kamal, I. Novel Approaches in Production Pathway of Microbial, Microcrystalline and Nano-Cellulose. 2022. Available online: https://www.researchgate.net/publication/271829336_Novel_approaches_In_Production_Pathway_Of_Microbial_Microcrystalline_and_Nano-Cellulose (accessed on 25 May 2022).
- Zhang, H.; Chen, C.; Yang, J.; Sun, B.; Lin, J.; Sun, D. Effect of Culture Conditions on Cellulose Production by a Komagataeibacter xylinus Strain. Macromol. Biosci. 2022, 22, 2100476. [Google Scholar] [CrossRef] [PubMed]
- Tapias, Y.A.R.; Di Monte, M.V.; Peltzer, M.A.; Salvay, A.G. Kombucha fermentation in yerba mate: Cellulose production, films formulation and its characterisation. Carbohydr. Polym. Technol. Appl. 2023, 5, 100310. [Google Scholar] [CrossRef]
- Blanco Parte, F.G.; Santoso, S.P.; Chou, C.-C.; Verma, V.; Wang, H.-T.; Ismadji, S.; Cheng, K.-C. Current progress on the production, modification, and applications of bacterial cellulose. Crit. Rev. Biotechnol. 2020, 40, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Mazhar, S.; Abidi, S.H.; Syed, Q.; Abbas, N.; Saleem, Y.; Nadeem, A.A.; Maryam, M.; Essa, R.; Ashfaq, S. Biobutanol production from sustainable biomass process of anaerobic ABE fermentation for industrial applications. Arch. Microbiol. 2022, 204, 672. [Google Scholar] [CrossRef]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of bacterial cellulose from industrial wastes: A review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Gorgieva, S.; Trček, J. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [PubMed]
| Characteristic/s | CNF | CNC | BNC | Ref |
|---|---|---|---|---|
| Degree of polymerization | ≥500 | 500–15,000 | 300–10,000 | [20,21] |
| Crystallinity (%) | 50–65 | 72–80 | 84–89 | [22] |
| Length (µm) | 0.5–2 µm | 0.05–0.5 µm | >1 µm | [20,23] |
| Width (nm) | 10–50 | 3–15 | 12–22 | [24,25] |
| Diameter (nm) | 5–50 | 2–20 | 10–50 | [26,27] |
| Young Modulus (GPa) | 50–100 | 140–160 | 15–130 | [28] |
| Purity | Low | Low | High | [29,30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahya, E.B.; Elarbash, S.S.; Bairwan, R.D.; Mohamed, M.M.I.; Khan, N.B.; Harlina, P.W.; Abdul Khalil, H.P.S. The Role of Microorganisms in the Isolation of Nanocellulose from Plant Biomass. Forests 2023, 14, 1457. https://doi.org/10.3390/f14071457
Yahya EB, Elarbash SS, Bairwan RD, Mohamed MMI, Khan NB, Harlina PW, Abdul Khalil HPS. The Role of Microorganisms in the Isolation of Nanocellulose from Plant Biomass. Forests. 2023; 14(7):1457. https://doi.org/10.3390/f14071457
Chicago/Turabian StyleYahya, Esam Bashir, Suhail Salem Elarbash, Rahul Dev Bairwan, Montaha Mohamed Ibrahim Mohamed, Niaz Bahadur Khan, Putri Widyanti Harlina, and H. P. S. Abdul Khalil. 2023. "The Role of Microorganisms in the Isolation of Nanocellulose from Plant Biomass" Forests 14, no. 7: 1457. https://doi.org/10.3390/f14071457
APA StyleYahya, E. B., Elarbash, S. S., Bairwan, R. D., Mohamed, M. M. I., Khan, N. B., Harlina, P. W., & Abdul Khalil, H. P. S. (2023). The Role of Microorganisms in the Isolation of Nanocellulose from Plant Biomass. Forests, 14(7), 1457. https://doi.org/10.3390/f14071457








