Impact of Downed Logs of Masson Pine (Pinus massoniana Lamb.) on Soil Microbial Community in a Climate Transitional Forest of Central China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
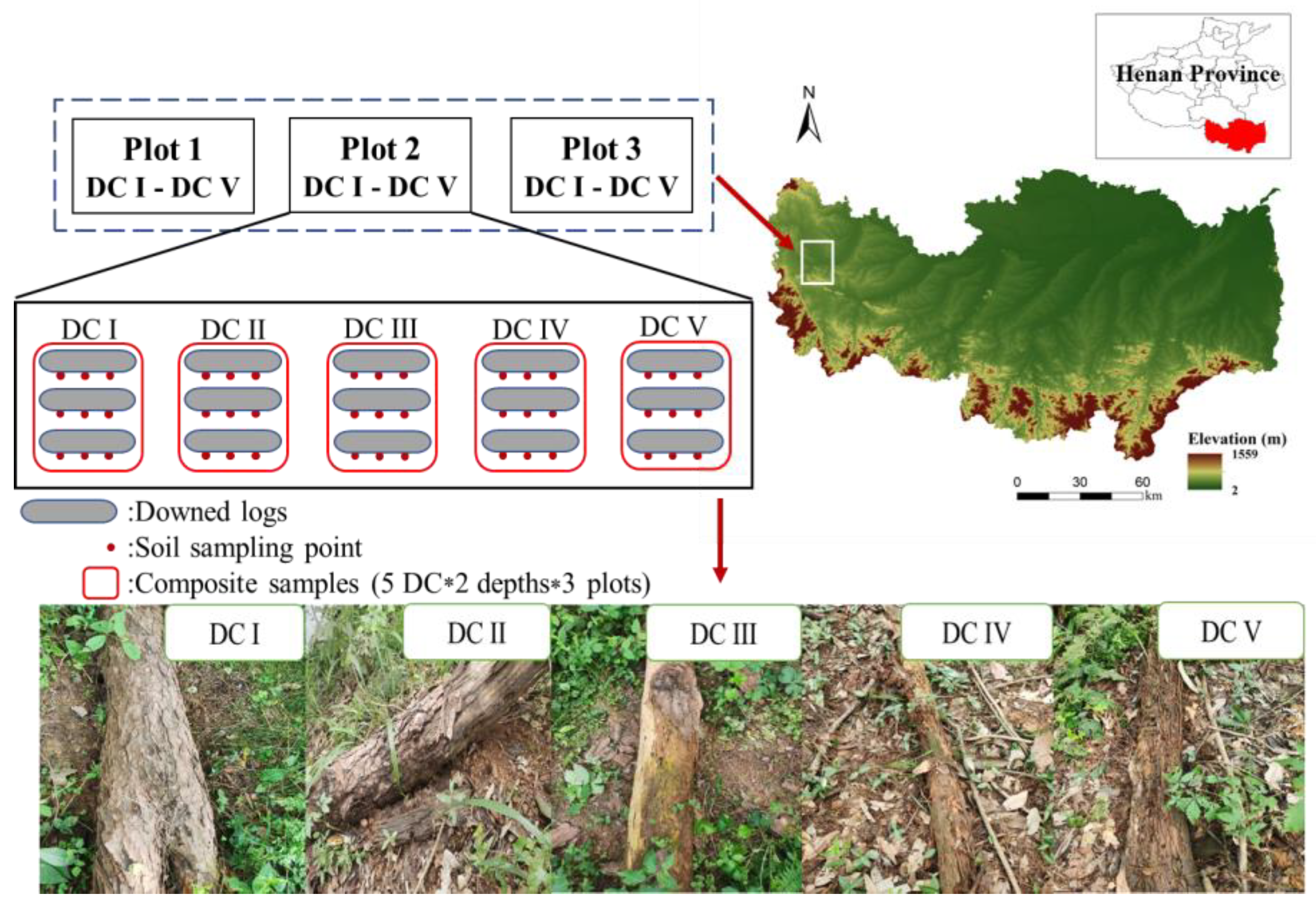
2.2. Experimental Design and Soil Collection
2.3. Laboratory Analysis
2.4. Statistical Analysis
3. Results
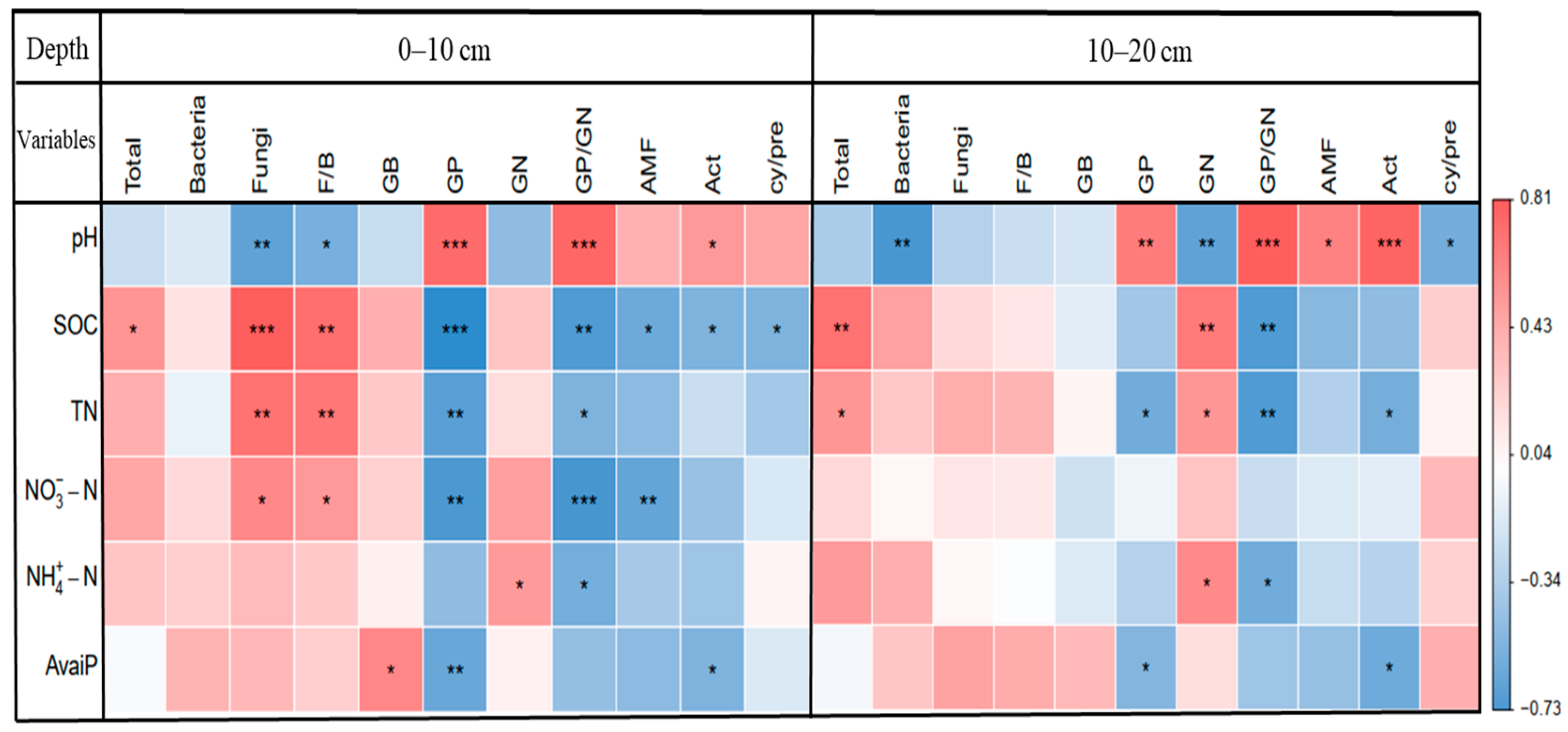
3.1. Soil Properties
3.2. Soil Microbial Biomass and Community Composition
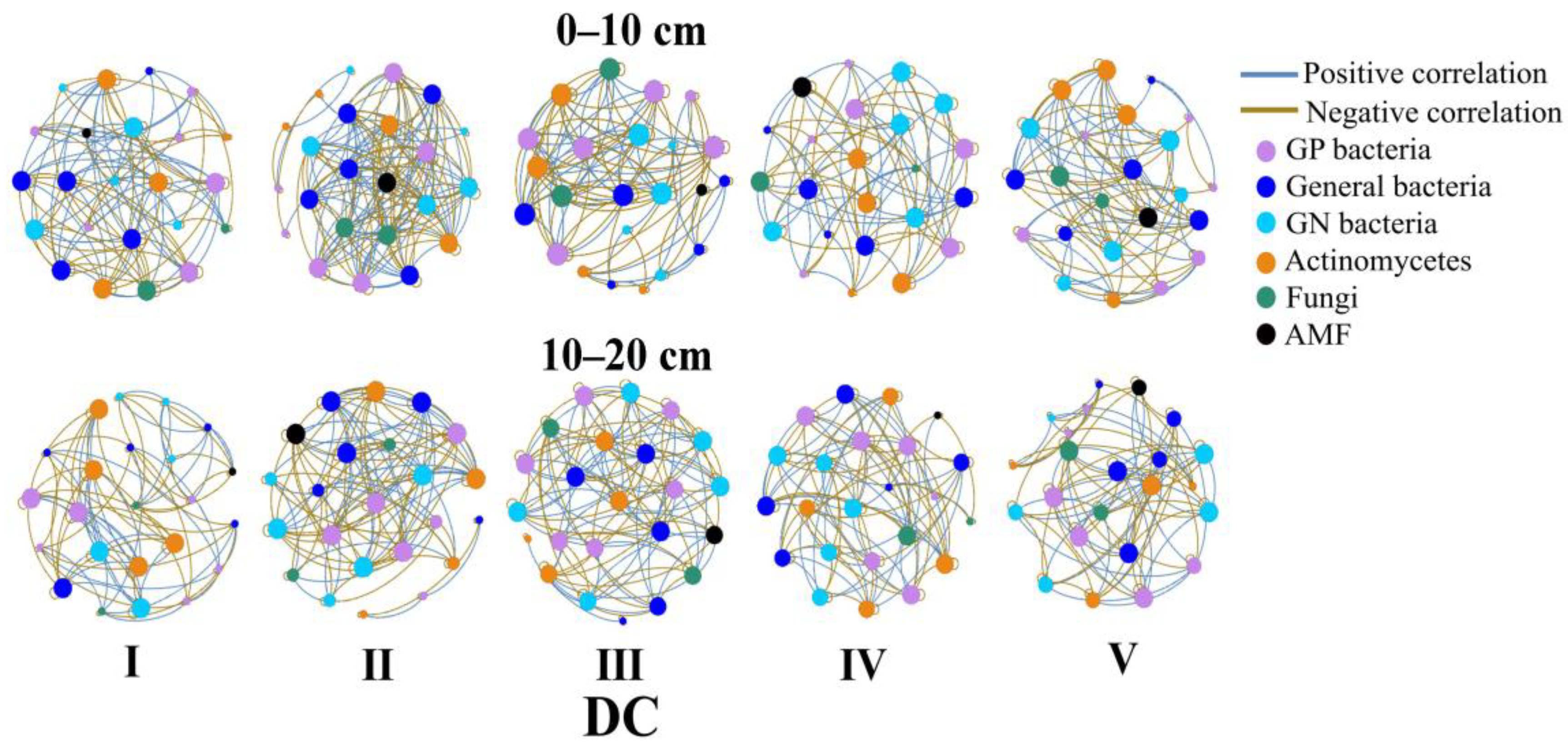
3.3. Soil Microbial Network Analysis
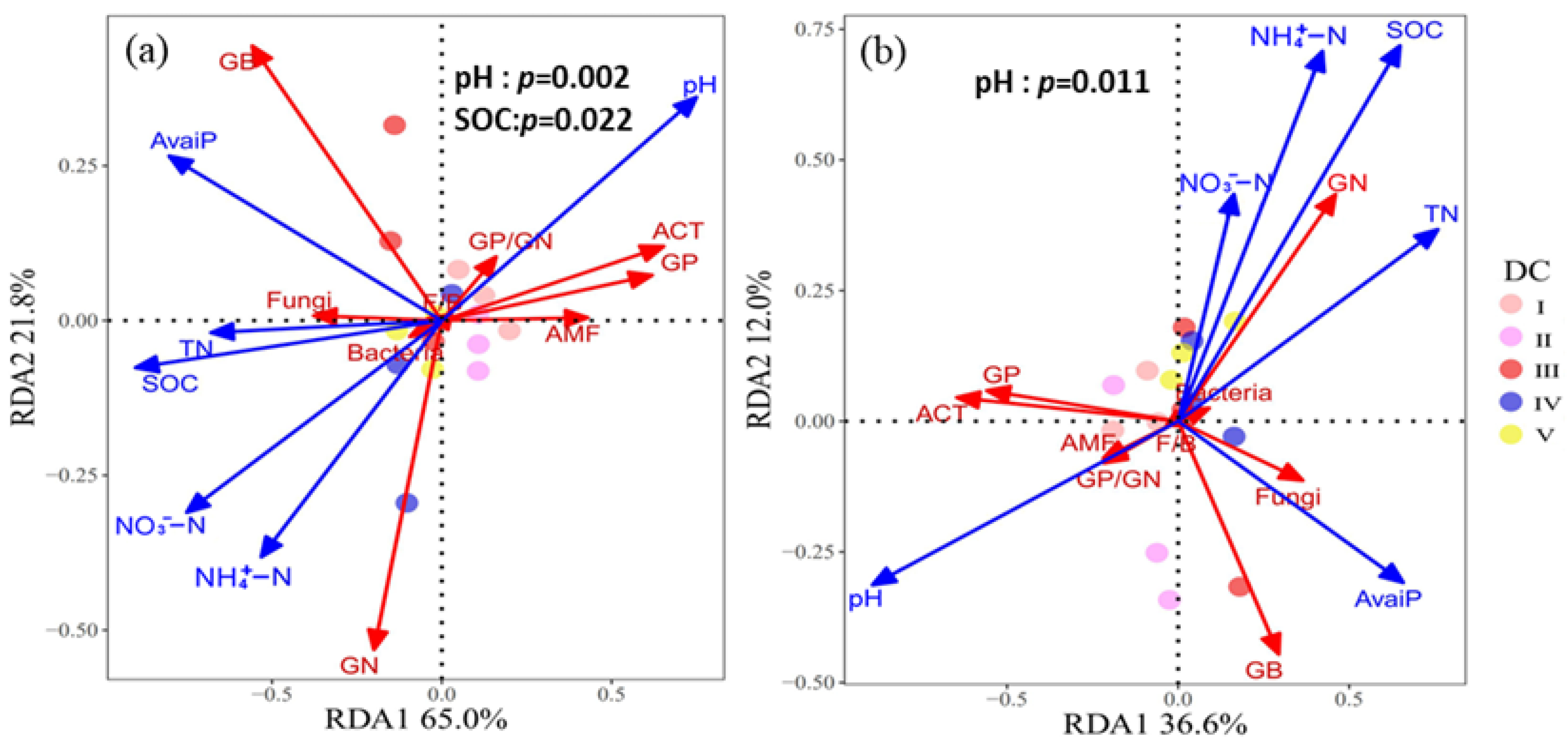
3.4. Factors Affecting the Soil Microbial Community
4. Discussion
4.1. Effects of DWD on Soil Microbial Biomass and Composition
4.2. Controlling Factors of Soil Microbial Community Response to DWD
4.3. Effects of DWD Decay Vary with Soil Depth
4.4. Implications of DWD for Soil Function
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Reichstein, M.; Bahn, M.; Ciais, P.; Frank, D.; Mahecha, M.D.; Seneviratne, S.I.; Zscheischler, J.; Beer, C.; Buchmann, N.; Frank, D.C.; et al. Climate extremes and the carbon cycle. Nature 2013, 500, 287–295. [Google Scholar] [CrossRef]
- Hu, Z.; Michaletz, S.T.; Johnson, D.J.; McDowell, N.G.; Huang, Z.; Zhou, X.; Xu, C. Traits drive global wood decomposition rates more than climate. Glob. Chang. Biol. 2018, 24, 5259–5269. [Google Scholar] [CrossRef]
- Jabin, M.; Mohr, D.; Kappes, H.; Topp, W. Influence of deadwood on density of soil macro-arthropods in a managed oakbeech forest. For. Ecol. Manag. 2004, 194, 61–69. [Google Scholar] [CrossRef]
- Creed, I.F.; Morrison, D.L.; Nicholas, N.S. Is coarse woody debris a net sink or source of nitrogen in the red spruce-Fraser fir forest of the southern Appalachians, USA? Can. J. For. Res. 2004, 34, 716–727. [Google Scholar] [CrossRef]
- Lassauce, A.; Paillet, Y.; Jactel, H.; Bouget, C. Deadwood as a surrogate for forest biodiversity: Meta-analysis of correlations between deadwood volume and species richness of saproxylic organisms. Ecol. Indic. 2011, 11, 1027–1039. [Google Scholar] [CrossRef]
- Perreault, L.; Forrester, J.A.; Mladenoff, D.J.; Lewandowski, T.E. Deadwood Reduces the Variation in Soil Microbial Communities Caused by Experimental Forest Gaps. Ecosystems 2021, 24, 1928–1943. [Google Scholar] [CrossRef]
- Magnússon, R.Í.; Tietema, A.; Cornelissen, J.H.C.; Hefting, M.M.; Kalbitz, K. Tamm Review: Sequestration of carbon from coarse woody debris in forest soils. For. Ecol. Manag. 2016, 377, 1–15. [Google Scholar] [CrossRef]
- Moghimian, N.; Jalali, S.G.; Kooch, Y.; Rey, A. Downed logs improve soil properties in old-growth temperate forests of Northern Iran. Pedosphere 2017, 30, 378–389. [Google Scholar] [CrossRef]
- Perreault, L.; Forrester, J.A.; Wurzburger, N.; Mladenoff, D.J. Emergent properties of downed woody debris in canopy gaps: A response of the soil ecosystem to manipulation of forest structure. Soil Biol. Biochem. 2020, 151, 108053. [Google Scholar] [CrossRef]
- Perron, T.; Kouakou, A.; Simon, C.; Mareschal, L.; Frederic, G.; Soumahoro, M.; Kouassi, D.; Rakotondrazafy, N.; Rapidel, B.; Laclau, J.P.; et al. Logging residues promote rapid restoration of soil health after clear-cutting of rubber plantations at two sites with contrasting soils in Africa. Sci. Total Environ. 2022, 816, 151526. [Google Scholar] [CrossRef]
- Shao, P.; Liang, C.; Rubert-Nason, K.; Li, X.; Xie, H.; Bao, X. Secondary successional forests undergo tightly-coupled changes in soil microbial community structure and soil organic matter. Soil Biol. Biochem. 2019, 128, 56–65. [Google Scholar] [CrossRef]
- Gonzalez-Polo, M.; Fernández-Souto, A.; Austin, A.T. Coarse woody debris stimulates soil enzymatic activity and litter decomposition in an Old-Growth temperate forest of Patagonia, Argentina. Ecosystems 2013, 16, 1025–1038. [Google Scholar] [CrossRef]
- Błońska, E.; Kacprzyk, M.; Spólnik, A. Effect of deadwood of different tree species in various stages of decomposition on biochemical soil properties and carbon storage. Ecol. Res. 2017, 32, 193–203. [Google Scholar] [CrossRef]
- Kwak, J.H.; Chang, S.; Naeth, M.A.; Schaaf, W. Coarse woody debris increases microbial community functional diversity but not enzyme activities in reclaimed oil sands soils. PLoS ONE 2015, 10, e0143857. [Google Scholar] [CrossRef]
- Persoh, D.; Borken, W. Impact of woody debris of different tree species on the microbial activity and community of an underlying organic horizon. Soil Biol. Biochem. 2017, 115, 516–525. [Google Scholar] [CrossRef]
- Lagomarsino, A.; De Meo, I.; Agnelli, A.E.; Paletto, A.; Mazza, G.; Bianchetto, E.; Pastorelli, R. Decomposition of black pine (Pinus nigra J. F. Arnold) deadwood and its impact on forest soil components. Sci. Total Environ. 2021, 754, 142039. [Google Scholar] [CrossRef]
- Brant, J.B.; Sulzman, E.W.; Myrold, D.D. Microbial community utilization of added carbon substrates in response to long-term carbon input manipulation. Soil Biol. Biochem. 2006, 38, 2219–2232. [Google Scholar] [CrossRef]
- Kaiser, C.; Franklin, O.; Dieckmann, U.; Richter, A. Microbial community dynamics alleviate stoichiometric constraints during litter decay. Ecol. Lett. 2014, 17, 680–690. [Google Scholar] [CrossRef]
- Zheng, H.; Yang, T.; Bao, Y.; He, P.; Yang, K.; Mei, X.; Banerjee, S. Network analysis and subsequent culturing reveal keystone taxa involved in microbial litter decomposition dynamics. Soil Biol. Biochem. 2021, 157, 108230. [Google Scholar] [CrossRef]
- Yang, Y.; Dou, Y.; Wang, B.; Xue, Z.; Wang, Y.; An, S.; Chang, S.X. Deciphering factors driving soil microbial life-history strategies in restored grasslands. iMeta 2023, 2, e66. [Google Scholar] [CrossRef]
- Andrews, J.H.; Harris, R.F. R-selection and K-selection and microbial ecology. Adv. Microb. Ecol. 1986, 9, 99–147. [Google Scholar]
- Spears, J.D.H.; Lajtha, K. The Imprint of Coarse Woody Debris on Soil Chemistry in the Western Oregon Cascades. Biogeochemistry 2004, 71, 163–175. [Google Scholar] [CrossRef]
- Khan, K.; Hussain, A.; Jamil, M.A.; Duan, W.; Chen, L.; Khan, A. Alteration in Forest Soil Biogeochemistry through Coarse Wood Debris in Northeast China. Forests 2022, 13, 1861. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Wu, Y.; Gutknecht, J.; Nadrowski, K.; Geissler, C.; Kuehn, P.; Scholten, T.; Both, S.; Erfmeier, A.; Boehnke, M.; Bruelheide, H.; et al. Relationships Between Soil Microorganisms, Plant Communities, and Soil Characteristics in Chinese Subtropical Forests. Ecosystems 2012, 15, 624–636. [Google Scholar] [CrossRef]
- Rousk, J.; Baath, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, T.; Wang, S.; Wang, Z. Soil pH and C/N ratio determines spatial variations in soil microbial communities and enzymatic activities of the agricultural ecosystems in Northeast China: Jilin Province case. Appl. Soil Ecol. 2020, 155, 103629. [Google Scholar] [CrossRef]
- Oberle, B.; Dunham, K.; Milo, A.M.; Walton, M.; Young, D.F.; Zanne, A.E. Progressive, idiosyncratic changes in wood hardness during decay: Implications for dead wood inventory and cycling. For. Ecol. Manag. 2014, 323, 1–9. [Google Scholar] [CrossRef]
- Wambsganss, J.; Stutz, K.P.; Lang, F. European beech deadwood can increase soil organic carbon sequestration in forest topsoils. For. Ecol. Manag. 2017, 405, 200–209. [Google Scholar] [CrossRef]
- Yang, Y.; Li, T.; Pokharel, P.; Liu, L.; Qiao, J.; Wang, Y.; An, S.; Chang, S.X. Global effects on soil respiration and its temperature sensitivity depend on nitrogen addition rate. Soil Biol. Biochem. 2022, 174, 108814. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Reed, S.C.; Keller, A.B.; Nemergut, D.R.; O’Neill, S.P.; Ostertag, R.; Vitousek, P.M. Litter quality versus soil microbial community controls over decomposition: A quantitative analysis. Oecologia 2014, 174, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Makipaa, R.; Rajala, T.; Schigel, D.; Rinne, K.T.; Pennanen, T.; Abrego, N.; Ovaskainen, O. Interactions between soil- and dead wood-inhabiting fungal communities during the decay of Norway spruce logs. ISME J. 2017, 11, 1964–1974. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Yang, Z.; Li, G.; Liu, S. Wild boar grubbing causes organic carbon loss from both top-and sub-soil in an oak forest in central China. For. Ecol. Manag. 2020, 464, 118059. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, H.; Li, J.; Wang, H.; Liu, S.; Liu, X. Reduced precipitation neutralizes the positive impact of soil warming on soil microbial community in a temperate oak forest. Sci. Total Environ. 2022, 806, 150957. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Song, J.; Li, S.; Li, Z.; Hao, Y.; Di, M.; Wan, S. Understanding the effects of fire and nitrogen addition on soil respiration of a field study by combining observations with a meta-analysis. Agric. For. Meteorol. 2020, 292, 108106. [Google Scholar] [CrossRef]
- Song, Y.; Yan, G.; Zhang, G. Light Competition Contributes to the Death of Masson Pines of Coniferous-Broadleaf Mixed Forests in Subtropical China. Forests 2022, 13, 85. [Google Scholar] [CrossRef]
- Hou, S.; Hattenschwiler, S.; Yang, J.; Sistla, S.; Wei, H.; Zhang, Z.; Hu, Y.; Wang, R.; Cui, S.; Lü, X.; et al. Increasing rates of long-term nitrogen deposition consistently increased litter decomposition in a semi-arid grassland. New Phytol. 2021, 229, 296–307. [Google Scholar] [CrossRef]
- Guo, L.; Deng, M.; Yang, S.; Liu, W.; Wang, X.; Wang, J.; Liu, L. The coordination between leaf and fine root litter decomposition and the difference in their controlling factors. Glob. Ecol. Biogeogr. 2021, 30, 2286–2296. [Google Scholar] [CrossRef]
- Bragazza, L.; Robroek BJ, M.; Jassey VE, J.; Arif, M.S.; Marchesini, R.; Guglielmin, M.; Cannone, N. Soil microbial community structure and enzymatic activity along a plant cover gradient in Victoria Land (continental Antarctica). Geoderma 2019, 353, 144–151. [Google Scholar] [CrossRef]
- Chang, E.H.; Chen, C.P.; Tian, G.; Chiu, C.Y. Replacement of natural hardwood forest with planted bamboo and cedar in a humid subtropical mountain affects soil microbial community. Appl. Soil Ecol. 2018, 124, 146–154. [Google Scholar] [CrossRef]
- Rouvinen, S.; Kuuluvainen, T.; Karjalainen, L. Coarse woody debris in old Pinus sylvestris dominated forests along a geographic and human impact gradient in boreal Fennoscandia. Can. J. For. Res. 2002, 32, 2184–2200. [Google Scholar] [CrossRef]
- Zelles, L.; Bai, Q.Y.; Beck, T.; Beese, F. Signature fatty acids in phospholipids and lipopolysaccharides as indicators of microbial biomass and community structure in agricultural soils. Soil Biol. Biochem. 1992, 24, 317–323. [Google Scholar] [CrossRef]
- Hu, M.; Wang, J.; Lu, L.; Shao, P.; Zhou, Z.; Wang, D.; Han, S.; Osborne, B.; Chen, J. Post-fire soil extracellular enzyme activities in subtropical-warm temperate climate transitional forests. Land Degrad. Dev. 2023, 34, 1973–1983. [Google Scholar] [CrossRef]
- Pollierer, M.M.; Ferlian, O.; Scheu, S. Temporal dynamics and variation with forest type of phospholipid fatty acids in litter and soil of temperate forests across regions. Soil Biol. Biochem. 2015, 91, 248–257. [Google Scholar] [CrossRef]
- Strimmer, K. Fdrtool a versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics 2008, 24, 1461–1462. [Google Scholar] [CrossRef]
- Minnich, C.; Persoh, D.; Poll, C.; Borken, W. Changes in Chemical and Microbial Soil Parameters Following 8 Years of Deadwood Decay: An Experiment with Logs of 13 Tree Species in 30 Forests. Ecosystems 2021, 24, 955–967. [Google Scholar] [CrossRef]
- Wan, X.; Xiao, L.; Vadeboncoeur, M.A.; Johnson, C.E.; Huang, Z. Response of mineral soil carbon storage to harvest residue retention depends on soil texture: A meta-analysis. For. Ecol. Manag. 2018, 408, 9–15. [Google Scholar] [CrossRef]
- Bantle, A.; Borken, W.; Ellerbrock, R.H.; Schulze, E.D.; Weisser, W.W.; Matzner, E. Quantity and quality of dissolved organic carbon released from coarse woody debris of different tree species in the early phase of decomposition. For. Ecol. Manag. 2014, 329, 287–294. [Google Scholar] [CrossRef]
- Kappes, H.; Topp, W.; Zach, P.; Kulfan, J. Coarse woody debris, soil properties and snails (Mollusca: Gastropoda) in European primeval forests of different environmental conditions. Eur. J. Soil. Biol. 2006, 42, 139–146. [Google Scholar] [CrossRef]
- Piaszczyk, W.; Lasota, J.; Blonska, E. Effect of Organic Matter Released from Deadwood at Different Decomposition Stages on Physical Properties of Forest Soil. Forests 2020, 11, 24. [Google Scholar] [CrossRef]
- Fanin, N.; Kardol, P.; Farrell, M.; Nilsson, M.C.; Gundale, M.J.; Wardle, D.A. The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biol. Biochem. 2019, 128, 111–114. [Google Scholar] [CrossRef]
- Zalamea, M.; González, G.; Ping, C.L.; Michaelson, G. Soil organic matter dynamics under decaying wood in a subtropical wet forest: Effect of tree species and decay stage. Plant Soil 2007, 296, 173–185. [Google Scholar] [CrossRef]
- Hernandez, D.J.; David, A.S.; Menges, E.S.; Searcy, C.A.; Afkhami, M.E. Environmental stress destabilizes microbial networks. ISME J. 2021, 15, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhang, Q.; Zhu, H.; Reich, P.B.; Banerjee, S.; van der Heijden, M.G.A.; Sadowsky, M.J.; Ishii, S.; Jia, X.; Shao, M.; et al. Erosion reduces soil microbial diversity, network complexity and multifunctionality. ISME J. 2021, 15, 2474–2489. [Google Scholar] [CrossRef] [PubMed]
- Bååth, E.; Nilsson, L.O.; Goransson, H.; Wallander, H. Can the extent of degradation of soil fungal mycelium during soil incubation be used to estimate ectomycorrhizal biomass in soil? Soil Biol. Biochem. 2004, 36, 2105–2109. [Google Scholar] [CrossRef]
- Bååth, E.; Anderson, T.H. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol. Biochem. 2003, 35, 955–963. [Google Scholar] [CrossRef]
- Krashevska, V.; Klarner, B.; Widyastuti, R.; Maraun, M.; Scheu, S. Impact of tropical lowland rainforest conversion into rubber and oil palm plantations on soil microbial communities. Biol. Fertil. Soils 2015, 51, 697–705. [Google Scholar] [CrossRef]
- Ge, R. Soil microbcs at dinghushan natural reserve and their adaptability to acidity. Chin. J. Ecol. 1993, 12, 11–18. [Google Scholar]
- Ouzounidou, G.; Skiada, V.; Papadopoulou, K.K.; Stamatis, N.; Kavvadias, V.; Eleftheriadis, E.; Gaitis, F. Effects of soil pH and arbuscular mycorrhiza (AM) inoculation on growth and chemical composition of chia (Salvia hispanica L.) leaves. Braz. J. Bot. 2015, 38, 487–495. [Google Scholar] [CrossRef]
- Ingwersen, J.; Poll, C.; Streck, T.; Kandeler, E. Micro-scale modelling of carbon turnover driven by microbial succession at a biogeochemical interface. Soil Biol. Biochem. 2008, 40, 864–878. [Google Scholar] [CrossRef]
- Blagodatskaya, E.V.; Anderson, T.H. Interactive effects of pH and substrate quality on the fungal-to-bacterial ratio and qCO2 of microbial communities in forest soils. Soil Biol. Biochem. 1998, 30, 1269–1274. [Google Scholar] [CrossRef]
- Siciliano, S.D.; Palmer, A.S.; Winsley, T.; Lamb, E.; Bissett, A.; Brown, M.V.; van Dorst, J.; Ji, M.; Ferrari, B.C.; Grogan, P.; et al. Soil fertility is associated with fungal and bacterial richness, whereas pH is associated with community composition in polar soil microbial communities. Soil Biol. Biochem. 2014, 78, 10–20. [Google Scholar] [CrossRef]
- Wang, C.; Lu, X.; Mori, T.; Mao, Q.; Zhou, K.; Zhou, G.; Nie, Y.; Mo, J. Responses of soil microbial community to continuous experimental nitrogen additions for 13 years in a nitrogen-rich tropical forest. Soil Biol. Biochem. 2018, 121, 103–112. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. Organic amendments: Microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef]
- Li, S.; Hu, M.; Shi, J.; Tian, X. Improving long-term crop productivity and soil quality through integrated straw-return and tillage strategies. Agron. J. 2022, 114, 1500–1511. [Google Scholar] [CrossRef]
- Ghodszad, L.; Reyhanitabar, A.; Maghsoodi, M.R.; Lajayer, B.A.; Chang, S.X. Biochar affects the fate of phosphorus in soil and water: A critical review. Chemosphere 2021, 283, 131176. [Google Scholar] [CrossRef]
- Huang, J.; Hu, B.; Qi, K.; Chen, W.; Pang, X.; Bao, W.; Tian, G. Effects of phosphorus addition on soil microbial biomass and community composition in a subalpine spruce plantation. Eur. J. Soil Biol. 2016, 72, 35–41. [Google Scholar] [CrossRef]
- Feng, J.; Li, Z.; Hao, Y.; Wang, J.; Ru, J.; Song, J.; Wan, S. Litter removal exerts greater effects on soil microbial community than understory removal in a subtropical-warm temperate climate transitional forest. For. Ecol. Manag. 2022, 505, 119867. [Google Scholar] [CrossRef]
- van Diepen, L.T.A.; Lilleskov, E.A.; Pregitzer, K.S.; Miller, R.M. Simulated nitrogen deposition causes a decline of intra- and extraradical abundance of arbuscular mycorrhizal fungi and changes in microbial community structure in northern hardwood forests. Ecosystems 2010, 13, 683–695. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.; Zhu, D.; Wang, W.; Liu, H.; Li, J.; Shi, N.; Ma, L.; Fu, S. Fine root biomass and morphology in a temperate forest are influenced more by the nitrogen treatment approach than the rate. Ecol. Indic. 2021, 130, 108031. [Google Scholar] [CrossRef]
- Spears, J.D.H.; Holub, S.M.; Harmon, M.E.; Lajtha, K. The influence of decomposing logs on soil biology and nutrient cycling in an old-growth mixed coniferous forest in Oregon, USA. Can. J. For. Res. 2003, 33, 2193–2201. [Google Scholar] [CrossRef]
- Castle, S.C.; Sullivan, B.W.; Knelman, J.; Hood, E.; Nemergut, D.R.; Schmidt, S.K.; Cleveland, C.C. Nutrient limitation of soil microbial activity during the earliest stages of ecosystems development. Oecologia 2017, 185, 513–524. [Google Scholar] [CrossRef]
- Piaszczyk, W.; Lasota, J.; Gaura, G.; Blonska, E. Effect of Deadwood Decomposition on the Restoration of Soil Cover in Landslide Areas of the Karpaty Mountains, Poland. Forests 2021, 12, 237. [Google Scholar] [CrossRef]
- von Lützow, M.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions? A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Swift, M.J. The estimation of mycelial biomass by determination of the hexosamine content of wood tissue decayed by fungi. Soil Biol. Biochem. 1973, 5, 321–332. [Google Scholar] [CrossRef]
- Dove, N.C.; Keeton, W.S. Structural Complexity Enhancement increases fungal species richness in northern hardwood forests. Fungal Ecol. 2015, 13, 181–192. [Google Scholar] [CrossRef]


| Depth (cm) | DC | pH | SOC (g kg−1) | TN (g kg−1) | NO3−-N (mg kg−1) | NH4+-N (mg kg−1) | Avai P (mg kg−1) |
|---|---|---|---|---|---|---|---|
| 0–10 | Ⅰ | 4.69 ± 0.04a | 29.37 ± 1.62c | 2.20 ± 0.06b | 14.47 ± 0.93b | 15.84 ± 0.52ab | 12.57 ± 0.10b |
| Ⅱ | 4.33 ± 0.04b | 37.43 ± 1.27b | 2.53 ± 0.09ab | 15.26 ± 1.14ab | 13.73 ± 1.54b | 13.86 ± 0.63b | |
| Ⅲ | 4.25 ± 0.00b | 51.03 ± 0.26a | 3.27 ± 0.20ab | 19.07 ± 1.80ab | 17.46 ± 0.50ab | 17.10 ± 1.17a | |
| Ⅳ | 4.10 ± 0.01c | 56.81 ± 2.86a | 3.67 ± 0.41a | 21.26 ± 1.54a | 20.31 ± 2.10a | 14.75 ± 0.21ab | |
| Ⅴ | 4.23 ± 0.01bc | 54.74 ± 0.99a | 3.47 ± 0.47ab | 21.22 ± 1.24a | 17.14 ± 0.62ab | 14.53 ± 0.22ab | |
| 10–20 | Ⅰ | 4.75 ± 0.03a | 14.63 ± 0.67b | 1.23 ± 0.03a | 8.52 ± 0.63a | 8.57 ± 0.44b | 11.88 ± 0.12b |
| Ⅱ | 4.67 ± 0.02a | 14.73 ± 0.87b | 1.23 ± 0.03a | 8.09 ± 0.85a | 9.02 ± 0.89b | 13.23 ± 0.14ab | |
| Ⅲ | 4.50 ± 0.03b | 19.50 ± 0.81ab | 1.83 ± 0.23a | 8.96 ± 1.37a | 9.08 ± 0.56b | 15.33 ± 0.79a | |
| Ⅳ | 4.39 ± 0.01b | 28.60 ± 1.72a | 2.13 ± 0.39a | 9.49 ± 1.55a | 12.45 ± 0.40a | 13.98 ± 0.43ab | |
| Ⅴ | 4.49 ± 0.04b | 26.57 ± 4.20a | 1.70 ± 0.25a | 10.78 ± 1.49a | 10.74 ± 0.99ab | 13.33 ± 0.77ab |
| 0–10 cm | 10–20 cm | |||||
|---|---|---|---|---|---|---|
| Microbial PLFAs | Independent Variables | Partial R2 | p | Independent Variables | Partial R2 | p |
| Bacteria | NO | pH | 0.528 | 0.002 | ||
| Fungi | SOC | 0.716 | <0.001 | NO | ||
| F/B | SOC | 0.575 | 0.001 | NO | ||
| GB | Avai P | 0.399 | 0.012 | NO | ||
| GP | SOC | 0.703 | <0.001 | pH | 0.444 | 0.007 |
| GN | NH4+-N | 0.278 | 0.043 | SOC | 0.446 | 0.007 |
| GP/GN | pH | 0.625 | <0.001 | pH | 0.661 | <0.001 |
| AMF | NO3−-N | 0.435 | 0.008 | pH | 0.398 | 0.012 |
| Act | SOC | 0.321 | 0.028 | pH | 0.599 | <0.001 |
| cy/pre | SOC | 0.325 | 0.027 | pH | 0.345 | 0.021 |
| NH4+-N | 0.215 | 0.036 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Hu, M.; Wang, J.; Xu, X.; Gui, H.; Yan, X.; Miao, Y.; Wang, W.; Han, S. Impact of Downed Logs of Masson Pine (Pinus massoniana Lamb.) on Soil Microbial Community in a Climate Transitional Forest of Central China. Forests 2023, 14, 955. https://doi.org/10.3390/f14050955
Lu L, Hu M, Wang J, Xu X, Gui H, Yan X, Miao Y, Wang W, Han S. Impact of Downed Logs of Masson Pine (Pinus massoniana Lamb.) on Soil Microbial Community in a Climate Transitional Forest of Central China. Forests. 2023; 14(5):955. https://doi.org/10.3390/f14050955
Chicago/Turabian StyleLu, Longlong, Mengjun Hu, Jiali Wang, Xinchuang Xu, Haoran Gui, Xinyu Yan, Yuan Miao, Wenjie Wang, and Shijie Han. 2023. "Impact of Downed Logs of Masson Pine (Pinus massoniana Lamb.) on Soil Microbial Community in a Climate Transitional Forest of Central China" Forests 14, no. 5: 955. https://doi.org/10.3390/f14050955





