Abstract
Fraxinus mandshurica is a widely used greening and ornamental tree species. However, its genetic transformation system has been hampered by problems such as low transformation efficiency, among others, which can hinder research related to molecular breeding and the analysis of functional genes. Thus, in this study, a novel genetic transformation method for efficient transformation of the embryonic callus of Fraxinus mandshurica was investigated. The method was optimized in terms of factors such as antibiotics, infection solution concentrations, co-culture time, and somatic embryo maturation. The results indicated that the optimal antibiotic concentration was 10 mg·L−1 of hygromycin (Hyg). At this point, the callus proliferation multiple was only 0.12. The highest transformation efficiency was found to be 93.93% when the absorbance of the infection solution concentration at OD600 was 0.4. Interestingly, transformation efficiency was found to be highest (77.9%) at 48 h of co-culture, with a GUS staining rate of 88.23%. The medium for somatic embryo maturation of transformed callus was half-strength MS medium (MS 1/2) containing 60 g·L−1 polyethylene glycol, 1 mg·L−1 abscisic acid, 400 mg·L−1 casein enzymatic hydrolysate (CH), 20 g·L−1 sucrose, 1 g·L−1 activated charcoal, and 5 g·L−1 gellan gum. The medium for somatic embryo germination was MS ½, containing 0.2 mg·L−1 of N-(Phenylmethyl)-9H-purin-6-amine(6-BA) and 5.0 mg·L−1 of gibberellin (GA). These results are of significance for the verification of the gene function and future genetic improvement of Fraxinus mandshurica.
1. Introduction
Fraxinus mandshurica is a cold-resistant deciduous tree belonging to the family Oleaceae. It can grow in relatively poor soil conditions, and it is valued for its timber, which is characterized by a hard, dense, and beautiful texture [1]. It is mainly distributed in northeastern and western China, South Korea, Japan, and eastern Russia [2]. F. mandshurica is one of the most valuable hardwood species in China and is often used for landscaping and afforestation [3,4,5]. F. mandshurica also has significant medicinal value, and its extracts are used to reduce pain and inflammation [6,7]. Due to its economic and ecological importance, there is significant interest in developing innovative breeding programs that can effectively combine biotechnology and traditional breeding [8].
Traditional methods for the breeding of forest trees, such as introduction and breeding and hybrid breeding [9,10,11], have the disadvantages of long breeding cycles and low efficiency. Somatic embryogenesis is widely used in plant callus culture. It can substantially shorten the breeding cycle and improve low breeding efficiency. Genetic transformation of plants is important in crop improvement and breeding and is widely used in the breeding of forest trees. However, efficient transformation and regeneration remain major problems for the breeding of many plants [12,13,14]. Therefore, there is an urgent need to develop an efficient sagrobacterium-mediated transformation scheme for F. mandshurica that can produce a large number of transgenic plants in a short time.
Agrobacterium-mediated transformation plays an important role in both functional gene analysis and molecular breeding. A number of important species and crops can be genetically transformed by agrobacterium-mediated methods [15,16,17]. At present, F. mandshurica seeds have been used to induce embryogenic callus and establish a complete somatic embryogenesis system [18]. Calli culture systems of F. mandshurica and the genetic transformation of adventitious buds have been established, and while the potential effects of ultrasonic and vacuum treatment on transformation have been investigated, the transformation efficiency was found to be relatively low at only 7.31% [1]. The main problem associated with the genetic transformation of F. mandshurica is that of low transformation efficiency and instability [1], which can effectively limit the industrial use of F. mandshurica. Thus, improvement of the conversion rate of F. mandshurica genetic transformation requires urgent investigation. In this study, genetic transformation was carried out using embryonic calli as the starting materials. The loose structure of the calli facilitates the successful entry of agrobacterium. Thus, more transgenic materials could be obtained by using agrobacterium to infect embryonic calli and thus improve the transformation rate, establishing an efficient agrobacterium-mediated genetic transformation system of F. mandshurica.
2. Materials and Methods
2.1. Plant Materials
The embryogenic callus of F. mandshurica was induced from immature zygotic embryos and obtained by subculture [18]. The embryonic callus induction medium consisted of 1/2 Murashige Skoog medium [8], supplemented with 5 mg·L−1 naphthylacetic acid (NAA; PhytoTech Labs, Lenexa, KS, USA), 2 mg·L−1 N-(Phenylmethyl)-9H-purin-6-amine(6-BA; PhytoTech Labs, USA), 400 mg·L−1 casein hydrolysate (CH; Biotopped Labs, Shanghai, China), 75 g·L−1 sucrose (PhytoTech Labs, USA), and 3 g·L−1 gellan gum (Gelrite, G1910, Sigma-Aldrich Co., St. Louis, MO, USA). The F. mandshurica cell line 2-1 induced by the above method was explanted, and the fresh embryogenic callus cultured for approximately 10 to 15 days was used as the transformation material.
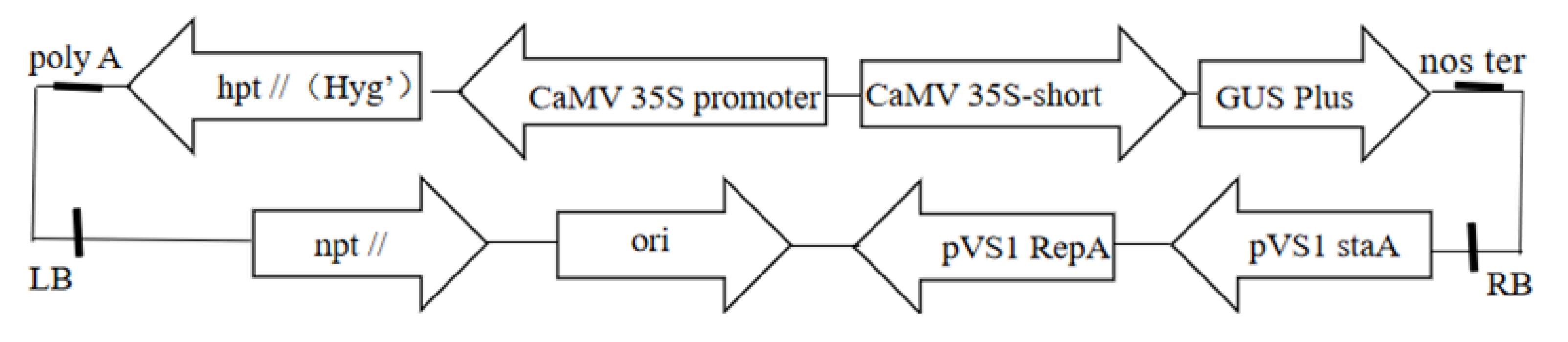
Escherichia coli DH5α used in this study was purchased from “TransGen Biotech”, agrobacterium strain GV3101 maintained in our laboratory contains plasmid pBI 121. The vector strain used in this study was VB191103-1905rcy. The plasmid contained the β-glucosidase gene (GUS), which was kindly provided by the State Key Laboratory of Forest Genetics and Breeding of Northeast Forestry University. The plasmid map is shown in Figure 1.
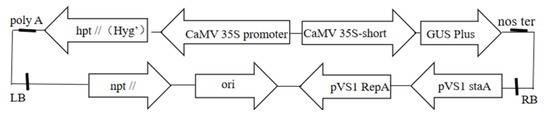
Figure 1.
Plasmid map.
2.2. Subculture Proliferation of the Embryonic Callus of Fraxinus mandshurica
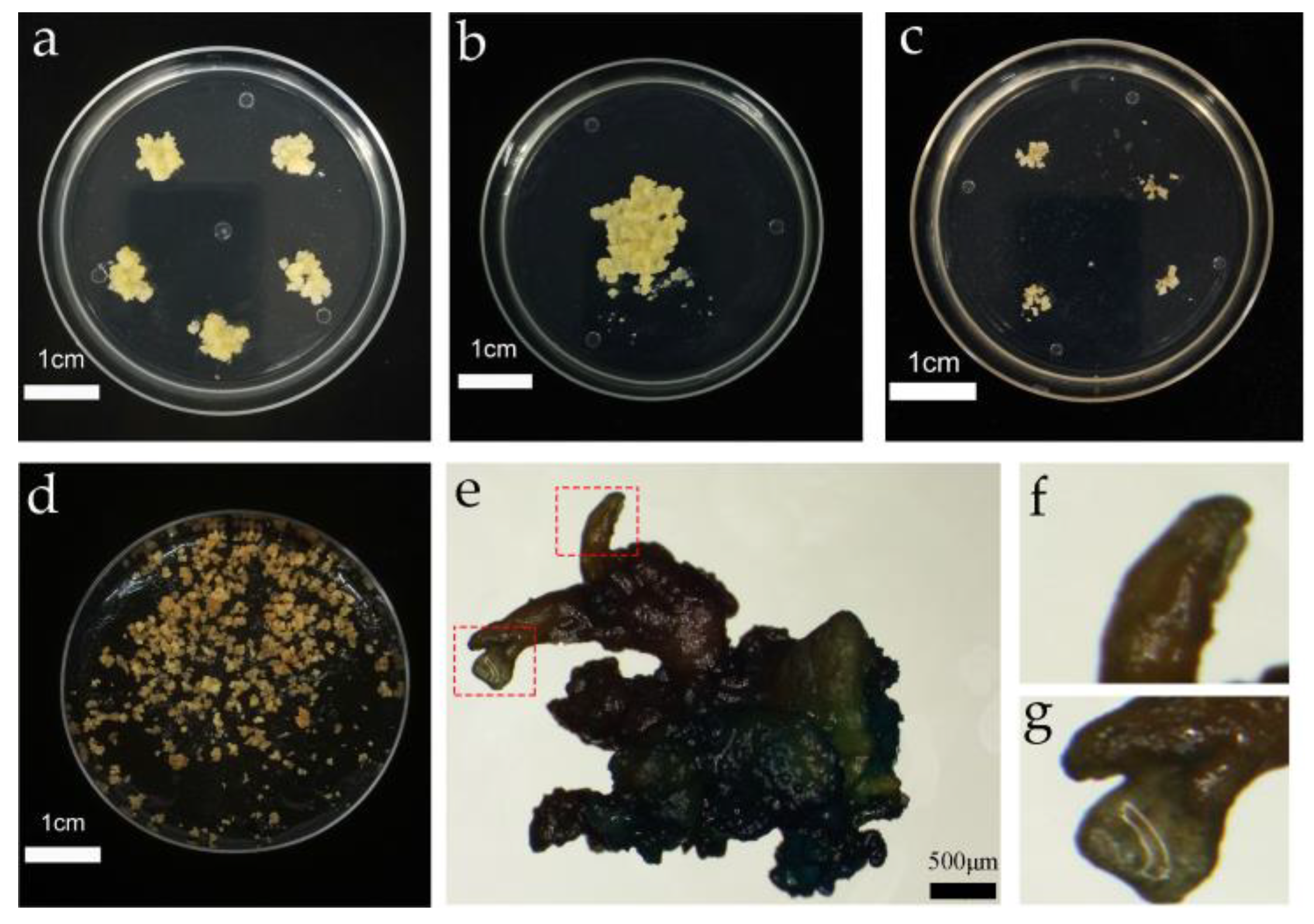
After 20 days of subculture and proliferation, the callus block was placed in a sterile environment. The fresh light yellow callus on the surface of the callus block was placed on woody plant medium (WPM; PhytoTech Labs, USA) (Supplementary File S1), supplemented with 20 g·L−1 sucrose, 3.5 g·L−1 gellan gum, 0.15 mg·L−1 2,4-dichlorophenoxyaceticacid (2,4-D; PhytoTech Labs, USA), and 0.1 mg·L−1 6-BA, pH = 5.8, and incubated in the dark at 25 ± 2 °C for 20 days. The proliferation is shown in Figure 2a.
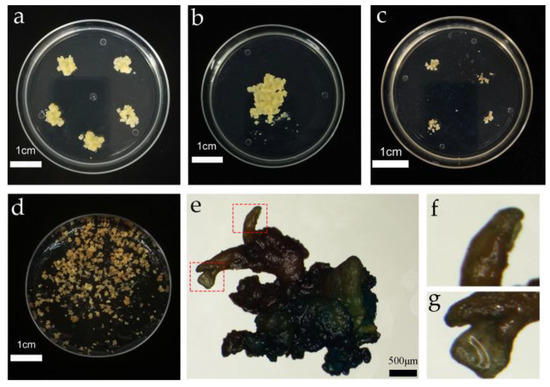
Figure 2.
Genetic transformation of the embryogenic callus and somatic embryo induction. (a) Proliferation of the cultured embryogenic callus; (b) co-culture of the embryogenic callus; (c) screening culture of the embryogenic callus; (d) somatic embryo induction of the embryogenic callus; (e) GUS detection of resistant somatic embryo mature material; (f) abnormal somatic embryos; and (g) normal somatic embryos.
2.3. Medium Preparation
The main components of the media used for embryonic callus subculture and genetic transformation of F. mandshurica are shown in Table 1. The medium for bacterial proliferation contained 5 g·L−1 yeast extract, 10 g·L−1 tryptone, and 10 g·L−1 NaCl, while all other media contained 20 g·L−1 sucrose and 400 mg·L−1 CH.

Table 1.
Main components of media.
2.4. Anti-Sensitivity Test of Embryogenic Callus
Embryogenic callus samples weighing 0.3 g were placed on induction medium containing Kanamycin (Kan) or Hygromycin (Hyg), with each petri dish containing three callus blocks. The concentration gradient of Kan was 0, 100, 200, 400, and 600 mg·L−1, and the concentration gradient of Hyg was 0, 5, 10, 20, and 30 mg·L−1. The cultures were then incubated in the dark at 25 ± 1 °C. After 15 days, the fresh weight of the embryonic callus of F. mandshurica was measured, with three replicates for each treatment.
2.5. Activation of Bacteria
The bacterial solution stored at −80 °C was thawed on ice, and a small amount was collected on a sterile inoculation loop. The bacterial solution was thereafter separated on a yeast extract-mannitol broth solid medium containing 50 mg·L−1 of Kan, Gen, and Rif and cultured in the dark at 28 °C for 1 to 2 days. Single colonies were incubated in 20 mL of liquid YEB medium containing 20 mg·L−1 Kan and 20 mg·L−1 Rif and cultured at 28 °C with shaking at 200 rpm in the dark for 12 to 16 h. Thereafter, 1 to 2 mL of culture was transferred into 50 mL of liquid YEB medium containing 50 mg·L−1 Kan and 50 mg·L−1 Rif and cultured for 4 to 8 h until the growth of agrobacterium reached the logarithmic phase.
2.6. Selection of Infectant Concentration and Co-Culture Cycle
2.6.1. Selection of Infectant Concentration
Cultures of agrobacterium in the logarithmic growth phase were centrifuged at 4 °C, 2000 rpm, for 5 min to collect the bacteria. After washing with a small amount of suspension, the bacteria were resuspended as an infection solution. The OD600 of the infection solutions was adjusted to 0.2, 0.4, 0.6, and 0.8, respectively. The embryogenic calli of 3 g of F. mandshurica were immersed in four different concentrations of infection solution for 20 min.
2.6.2. Selection of te Co-Culture Cycle
Fresh embryogenic callus cultures weighing approximately 0.3 g were selected, immersed in infection solution, and evenly dispersed in the infection solution. After 20 min of infection, the cultures were incubated with the co-culture medium in the dark for 16 h, 24 h, 48 h, or 72 h at 25 ± 1 °C. It should be noted that the excess infection solution on the surface of the callus was sucked out with sterile filter paper before co-culture. The transformation efficiency was then assessed after screening the transformed callus.
2.7. Sterilization and Screening Culture
The co-cultured calli were rinsed twice with sterile water and then rinsed twice with the suspension or sterile water containing 500 mg·L−1 Cefotaxime (Cef), for 2 to 3 min each time. After sterilization, the infected calli were dried with sterile filter paper to absorb the excess liquid on the surface and were subsequently cut into small pieces with a diameter of about 0.5 ± 0.2 cm and spread on the screening medium for 15 days to obtain the resistant calli; this process was repeated three times. After 45 days, the number of resistant calli was counted, and the screening data are shown in Figure 2c.
2.8. Histochemical GUS Detection and Molecular Identification
2.8.1. Histochemical GUS Detection
Resistant calli of the different transgenic lines were selected and placed in 1.5 mL Eppendorf tubes, after which 700 μL of GUS staining solution were added to cover the callus. The tube containing the callus was then evacuated for 10 min, which was repeated three times until no bubbles were apparent in the tube, allowing full exposure of the callus to the GUS staining solution. The callus was then placed in a 37 °C water bath for 7 days, and non-transformed calli were used as negative controls. After one week, the staining status was observed and assessed under a stereomicroscope. Blue calli were considered transgenic, while unstained calli were considered false positives.
2.8.2. Molecular Identification
Genomic DNA was extracted from the different transgenic lines using the hexadecyl trimethyl ammonium bromide (CTAB) method, and PCR was performed using primer pairs (forward primer F:5′-CAAAGCAAGTGGATTGATGTGAT-3′; reverse primer R:5′-AGAGAAAAGGGTCCTAACCAAGA-3′). The plasmid pBI 121 contained the CaMV35S activated beta-glucosidase (GUS) and neomycin phosphatase II gene (npt II) as selective marker genes. The expected product size was 270 bp. A 20 μL PCR detection system was used, including 10 μL of Green Taq MIX (Vazyme Biotech Co., Ltd, Nanjing, China), 1 μL of upstream primer F, 1 μL of downstream primer R, 2 μL of DNA, and ddH2O up to the 20 μL mark. The wild type of callus was used as the negative control, and the agrobacterium solution as the positive control. The PCR procedure included pre-denaturation at 94 °C for 2 min, 94 °C for 30 s, annealing at 55 °C for 30 s, 72 °C extension for 40 s, with a total of 35 cycles. Thereafter, PCR products were analyzed by 1.0% (w/v) agarose gel electrophoresis.
2.9. Genetic Transformation System Verification
In order to verify the stability and efficiency of the transformation system of the embryonic callus of F. mandshurica, the LobHLH34 gene cloned from Larix olgensis was heterologously transferred into the embryogenic callus of F. mandshurica [19]. PCR was performed using the primer pairs (LobHLH34-F:5′-CGGGATCCATGATGGGATCACCTCAGAGC-3′, LobHLH34-R:5′-GCGTCGACGGCAACAGGAGGCCTTAGC-3′). The plasmid pBI 121 contained the CaMV35S activated beta-glucosidase (GUS) and neomycin phosphatase II gene (npt II) as selective marker genes. The expected product size was 750 bp. The resistant embryonic callus was detected by GUS and PCR to verify whether the gene was successfully transferred.
2.10. Somatic Embryo Maturation
2.10.1. Pretreatment
We improved the drying method previously described by Liu (Yang Liu et al., 2021). Briefly, 3 g of calli from each transgenic line were added to 10 mL of liquid medium (MS 1/2 + 20 g·L−1 sucrose). After mixing well, the mixture was poured into sterile filter paper, and the liquid was repeatedly absorbed by the filter paper until the callus was in a dry, granular state.
2.10.2. Mature Processing
Based on the results of the preliminary experiments, 0.5 g of calli from the transgenic line K7 were transferred to maturation medium. The half-strength MS medium was supplemented with different concentrations (0, 40, 60, and 80 g·L−1) of PEG, 1 mg·L−1 ABA, 400 mg·L−1 casein enzymatic hydrolysate (CH), 20 g·L−1 sucrose, 1 g·L−1 activated carbon, and 5 g·L−1 gellan gum at pH 5.8. The same materials without drying treatment were cultured on the medium without PEG, which was used as a control (CK). All the transformed calli described above were cultured at 25 ± 2 °C in the dark for 30 to 60 days.
2.11. Statistical Analysis
SPSS software was used for all data analysis, with p-values < 0.05 considered statistically significant. The tables and figures were compiled using Origin 64 and Excel 2010. The calculation of the various parameters in this study was as follows:
Transformation efficiency of embryogenic callus (%) = number of resistant tissues after screening/total number of infected embryogenic calli × 100%
Proliferation multiple = callus proliferation/callus initial weight × 100%
GUS staining rate (%) = Number of blue calli blocks after GUS staining/Number of resistant calli stained × 100%
PCR positive rate (%) = number of calli amplified by PCR/number of resistant tissues detected by PCR × 100%
3. Results
3.1. Effect of Kan on Proliferation of Embryogenic Callus
The wild-type embryogenic calli were spread on WPM medium supplemented with different concentrations of Kan. It was observed that increasing Kan concentrations did not affect the proliferation of the calli significantly compared with the control group. The proliferation ratio of embryogenic callus without Kan was 3.14, which was 0.04%, 0.32%, 0.07%, and 0.23% higher than that of embryogenic callus treated with 100, 200, 400, and 600 mg·L−1 Kan, respectively (Table 2). According to the analysis of variance, there was no significant difference in the fresh weight of embryogenic calli treated with the different concentrations of Kan after 14 days of proliferation (p > 0.05), indicating that Cef had no significant inhibitory effects on the proliferation of the embryogenic callus.

Table 2.
Effect of Kan on the proliferation of the embryonic callus.
3.2. Effect of Hyg on the Proliferation of Embryogenic Calluess
The antibiotic Hyg significantly affected the proliferation of the embryonic callus of F. mandshurica. It was observed that with the increase in Hyg concentration, the proliferation ratio of the embryonic callus of F. mandshurica with 14 days of proliferation decreased first and then tended to increase gradually. The results showed that when the concentration of Hyg was 5 mg·L−1, the effect on the proliferation of embryogenic callus was significant, but the proliferation multiple was 1.92 lower than that of the control (Table 3). When the concentration of Hyg was 10, 20, or 30 mg·L−1, the proliferation rates were found to be 0.12%, 0.11%, and 0.09%, respectively.

Table 3.
Effect of Hyg on the proliferation of the embryonic callus.
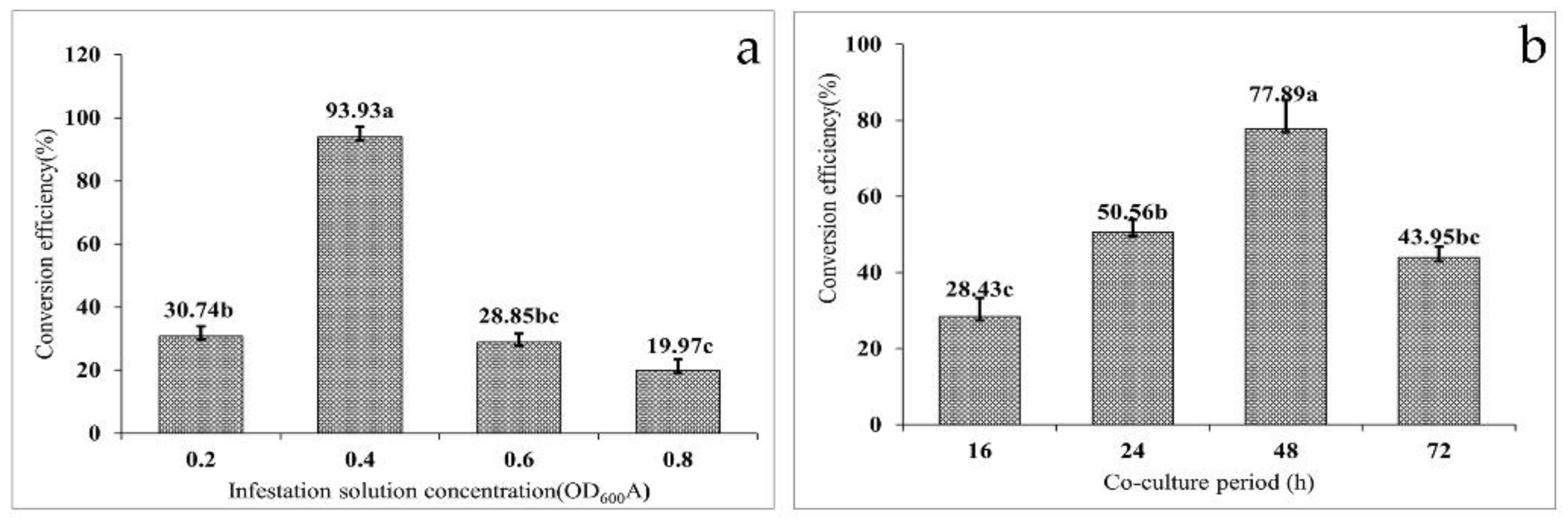
3.3. Effect of Infectant Concentration on Transformation Efficiency
To determine the potential effect of infection solution concentration on the transformation efficiency, four separate gradients of OD600 (0.2, 0.4, 0.6, and 0.8) were used. The agrobacterium-mediated transformation efficiency of the embryonic calli of F. mandshurica exhibited a trend of increasing first and then decreasing with increased concentrations of the infection solution (Figure 3a). The highest transformation efficiency (93.93%) was observed when the OD600 of the infection solution was 0.4. When the OD600 of the infection solution was 0.8, the conversion efficiency was the lowest at only 19.97%. However, the transformation efficiencies did not differ significantly when the OD600 of the infection solution was 0.2 and 0.8, seen as 30.74% and 28.85%, respectively. The results of the variance analysis showed that the concentration of infection solution had a significant effect on the transformation efficiency of the embryonic callus of F. mandshurica (p = 0.000).
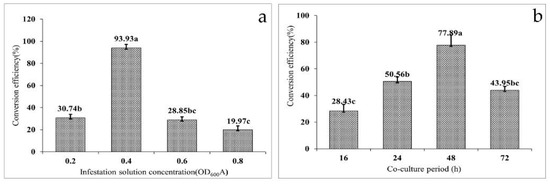
Figure 3.
Factors affecting the efficiency of the genetic transformation of Fraxinus mandshurica: (a) effect of infectant concentration on the transformation efficiency and (b) effect of the co-culture cycle on the transformation efficiency. (Data are expressed as the mean ± standard deviation. The different lowercase letters in the same column numbers indicate significant differences).
At the same time, when the OD600 of the infection solution was 0.6, high concentrations of the infection solution were able to promote high reproduction of agrobacterium. Interestingly, in the subsequent sterilization process, the use of a high Cef concentration for the multiple sterilizations was not able to inhibit the reproduction of agrobacterium, which could eventually lead to the death of the transformed embryogenic callus and thus significantly reduce the transformation efficiency.
3.4. Effect of the Co-Culture Cycle on Transformation Efficiency
Co-culture is the process of transferring T-DNA carried by agrobacterium into the nucleus of the receptor and then integrating with the receptor DNA; thus, the co-culture cycle has a significant influence on the transformation efficiency. It was found that with increased co-culture time, the conversion efficiency first increased and then decreased (Figure 3b). When the co-culture time was set at 16 h, the conversion efficiency was only 28.43%. When the co-culture time was 24 h, the conversion efficiency increased by 22.13%. However, the conversion efficiency reached a peak of 77.89% at 48 h, and on extension of the co-culture time to 72 h, the conversion efficiency decreased to 43.95%. The results indicated agrobacterium overgrowth when the co-culture time was more than 48 h, resulting in a callus wrapped in agrobacterium, thereby affecting the proliferation. Subsequently, 500–600 mg·L−1 Cef failed to control the growth of agrobacterium, which eventually led to the death of the callus and reduced transformation efficiency. When the infection time is too short, the agrobacterium infection is not complete, which will also lead to a decrease in transformation efficiency. The co-culture procedure and results are shown in Figure 2b.
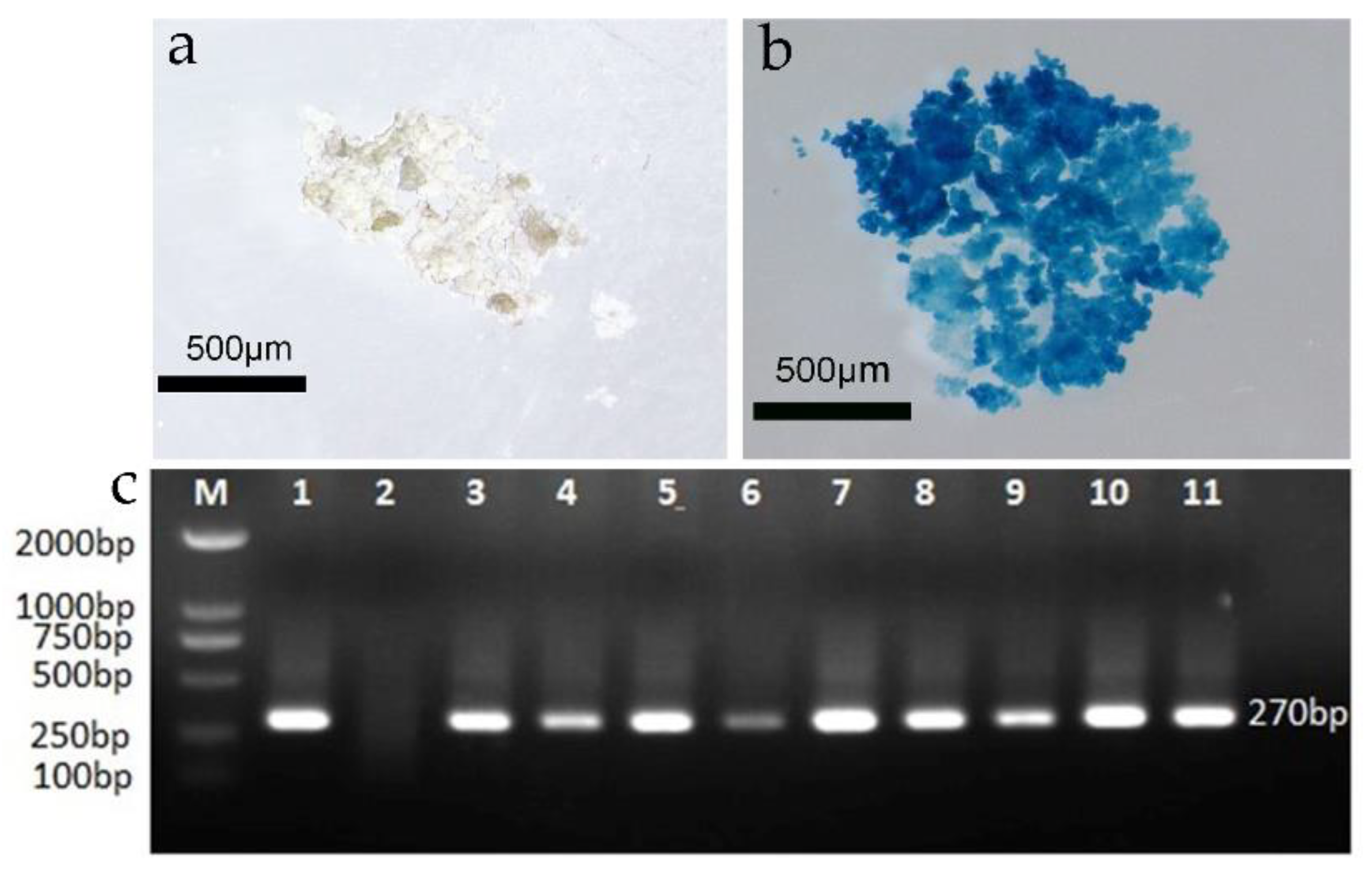
3.5. Histochemical GUS Detection
To construct a genetic transformation system for F. mandshurica and then successfully obtain the embryonic callus with a blank vector, the callus was initially infected by agrobacterium carrying the pBI 121 vector. The resistant calli after recovery culture were then replaced in a 1.5 mL Eppendorf tube and stained using the histochemical GUS method. The resistant callus was colored blue after staining and was thus a GUS-positive callus, indicating that the vector containing the GUS gene had been successfully transferred into the callus (Figure 4a,b). A total of 34 callus blocks were evaluated, 30 of which were blue; the GUS staining rate was thus 88.23%, and the false positive rate was 11.77%.
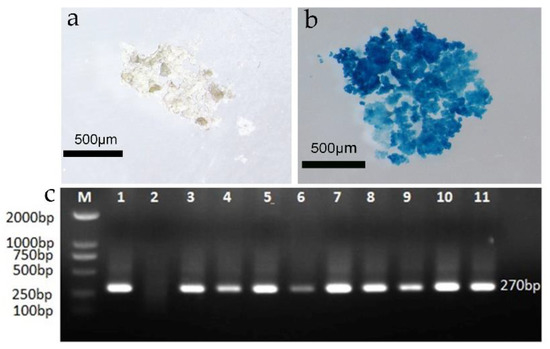
Figure 4.
GUS and PCR valuation of the transformed callus; (a) GUS staining of the wild-type callus; (b) GUS staining of the post-transformation callus; (c) PCR valuation of the resistant callus (M: DNA maker; 1: positive control; 2: negative control; 3–11: resistant embryonic callus).
3.6. Molecular Detection
A PCR analysis was performed using the total DNA of the putative callus as a template to detect the possible presence of these genes in the putatively transformed embryogenic calli. The results indicated that the amplification products with fragment lengths of about 270 bp were detected in the Hyg resistant callus but were not found in untransformed embryogenic calli. Total DNA was extracted from 9 of 30 calli randomly selected for the PCR evaluation, and 9 transformants were found to contain the target band (Figure 4c).
3.7. Verification of the Genetic Transformation System
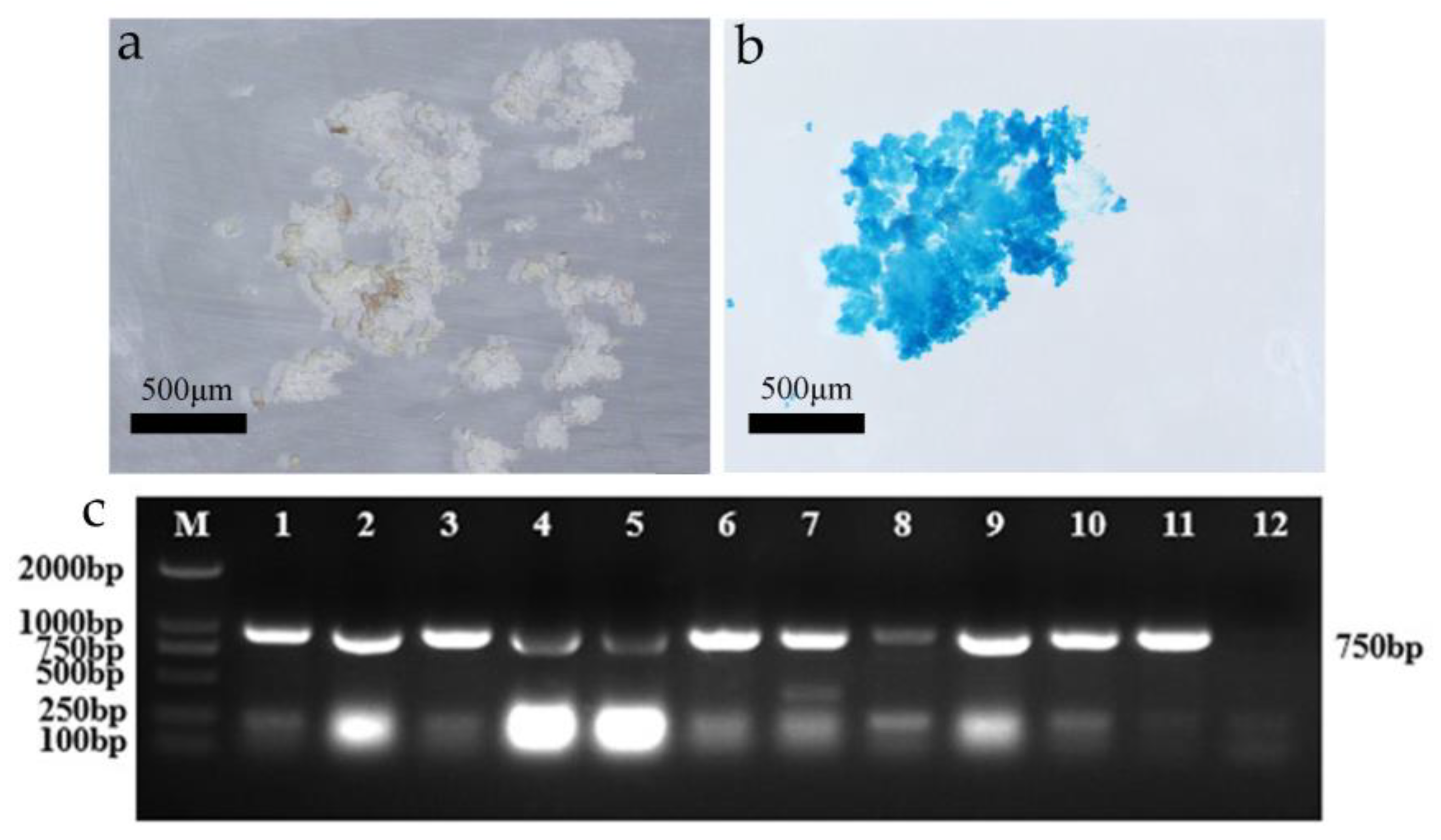
The LobHLH34 gene was transferred into the embryogenic callus of F. mandshurica, and 30 putative transformed calli were obtained. The GUS detection results showed that 26 of the 30 putatively transformed calli were blue (Figure 3a,b), and the conversion rate was 86.67%. Ten of the 26 resistant calli were randomly selected for total DNA extraction and PCR detection. Amplified product bands with fragment lengths of about 750 bp were detected in 10 transformants (Figure 5), indicating that the positive rate of transformed calli obtained by embryogenic callus infection was very high. The system was thus considered stable and efficient.
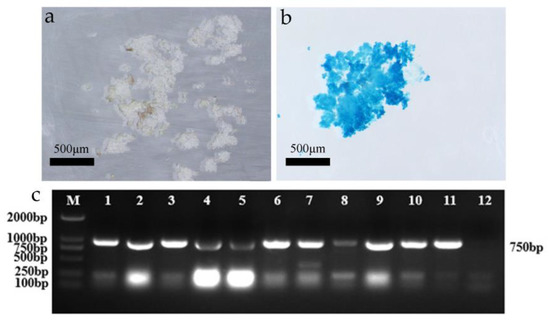
Figure 5.
GUS and PCR detection of transgenic LobHLH34-resistant callus. (a) Control callus staining; (b) transgenic LobHLH34 callus staining; and (c) PCR detection of resistant callus (M: maker; 1–10: transgenic embryonic callus; 11: positive control; 12: negative control).
3.8. Somatic Embryo Maturation of Transformed Callus
The effects of PEG concentration on the maturation of somatic embryos are shown in Figure 2d and Table 4. It was found that calli cultured on non-PEG and non-dried medium (CK) for 50 days had high water contents and obvious agglomeration, with obvious proliferation, although no somatic embryos were present. After the drying treatment on the medium without PEG, there was no significant difference compared with the control group, and no somatic embryos were observed. However, on the medium containing 40 g·L−1 PEG, the calli became significantly dryer, larger, and granular, and a small part of the calli were dark brown. The somatic embryos appeared opaque and white, globular, or irregularly oval, and the number of somatic embryos was 10 pcs·g−1. In addition, on the medium containing 60 g·L−1 PEG, the calli were dry and granular, and half of the calli were dark brown in color. The somatic embryos were white and spherical or seen as long strips, and multiple somatic embryos were also apparent where they grew together. The number of somatic embryos was 22 pcs·g−1. On medium containing 80 g·L−1 PEG, the calli were dry, powdery, and uniformly distributed. Most of the somatic embryos were white, spherical, and uniformly distributed in the callus. The number of somatic embryos was 18 pcs·g−1, but during the study, it was observed that the number of deformed somatic embryos of F. mandshurica was relatively large and the maturation rate of somatic embryos was low, thus reducing the incidence of abnormal somatic embryos.

Table 4.
Effect of PEG concentration on somatic embryogenesis.
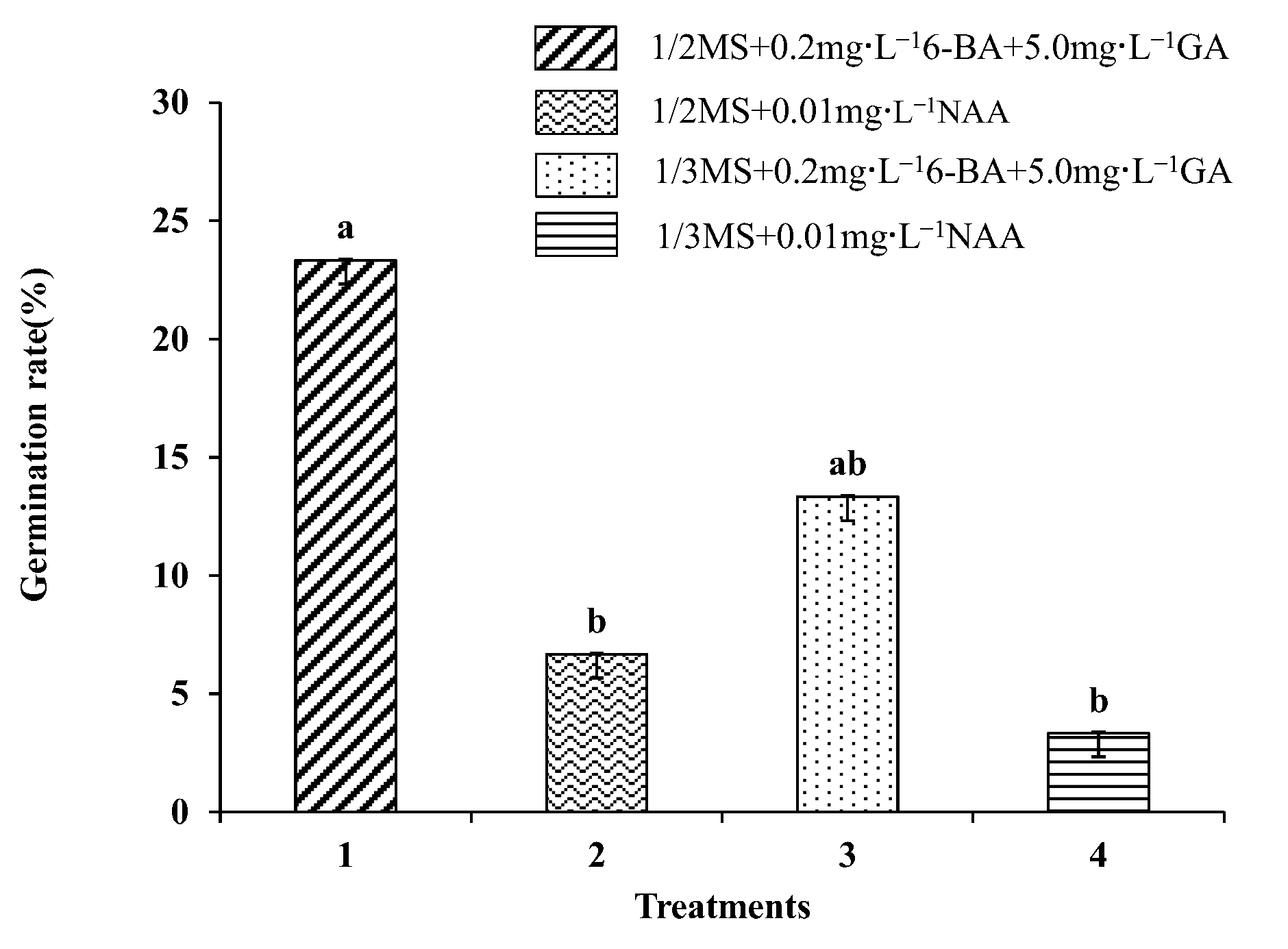
3.9. Somatic Embryo Germination
The somatic embryos were transferred to four germination media for germination, and the germination rate was then determined (Figure 6). The germination rate of somatic embryos was found to be highest (23.33%) on medium I. However, in medium II, the germination rate of somatic embryos was significantly decreased to 6.67%, indicating that a high concentration of GA and a low concentration of cytokinin were more suitable for somatic embryo germination. When the I and II basic media were replaced by 1/3 Murashige Skoog medium (MS 1/3) (Supplementary File S1), the germination rate of somatic embryos decreased significantly, indicating that half-strength MS medium was more suitable as the basic medium for somatic embryo germination of the transformation materials. Subsequently, GUS staining was performed on the somatic embryo germination transformation materials, and it was observed that the callus was stained blue (Figure 2e–g).
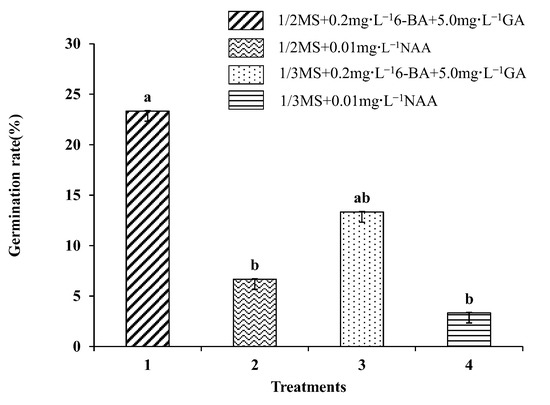
Figure 6.
Effects of different media on embryo germination of Fraxinus mandshurica cells. Note: Data are expressed as the mean ± standard deviation. The different lowercase letters in the same column numbers indicate significant differences.
4. Discussion
The main methods used for genetic transformation include polyethylene glycol-mediated transformation (PMT), restriction enzyme-mediated integration (REMI), electroporation, and agrobacterium-mediated transformation (ATMT) [19].
Agrobacterium-mediated genetic transformation has emerged as the most commonly used method in plant genetic transformation due to its high transformation efficiency, low cost, and clear transfer of DNA fragments [20]. When using the agrobacterium-mediated method for infection, different tissue parts of the plant can be infected. The infection material used for the genetic transformation of Chinese cabbage was the common callus [21]. The infection materials employed for the genetic transformation of Paulownia elongata were leaves and petioles [22], whereas the infection material used for the genetic transformation of L. olgensis was the embryogenic callus [23]. We found that the structure of the embryonic callus of F. mandshurica was significantly loose, and this structure was easier for agrobacterium penetration compared with ordinary calli. However, in the study reported by Yang [18], hypocotyls were selected as explants for the regeneration and transformation of F. mandshurica, resulting in a transformation rate of 7.31%. In this study, the embryogenic callus of F. mandshurica was used for the genetic transformation, with a positive transformation rate of 88.23%.
There are several factors that can potentially affect the efficiency of genetic transformation, and transformation efficiency can be improved by optimizing these factors. Hence, comprehensive optimization of these factors can effectively improve the efficiency of genetic transformation. Antibiotics [24] play important roles in genetic transformation as they reduce the false positive rate of the transformation materials and thus improve the transformation efficiency. The study of the antibiotic sensitivity of receptor materials constitutes the basis for the selection of suitable antibiotics. Commonly used marker genes used in selection for genetic transformation are neomycin phosphotransferase, hygromycin phosphotransferase, and glufosinate acetate transferase [23]. Interestingly, in a prior study of antibiotic sensitivity, Kan and Hyg were used for screening, and the results found that the embryogenic callus was not sensitive to Kan. When 0, 100, 200, 400, and 600 mg·L−1 Kan were added, the proliferation multiple of the embryogenic callus exhibited no significant differences (p < 0.05), but embryogenic callus was very sensitive to Hyg. In addition, with incubation with Hyg, the proliferation multiple of the embryogenic callus was markedly reduced, and 5 mg·L−1 of Hyg could significantly attenuate the proliferation multiple of the embryogenic callus. The addition of 10, 20, or 30 mg·L−1 Hyg almost completely inhibited the callus growth. It is worth mentioning that in the three-screening culture process, the mortality rate of the resistant callus was substantially higher in the first screening stage, but as the screening number increased, the mortality rate gradually decreased, and there was almost no callus death during the third screening stage.
A suitable concentration of the infection solution is also an important factor for facilitating successful transformation [20,25]. It was found that as the concentration of the infection solution increased, the transformation efficiency first increased and then decreased. When the concentration of infection solution was too low, the concentration of A. tumefaciens attached to the explants was found to be extremely low, thus resulting in incomplete infection and low transformation efficiency. When the concentration of the infection solution was too high, it could lead to excessive reproduction of agrobacterium tumefaciens, inhibiting the growth of explants, and the transformation efficiency was also markedly reduced. Co-culture is one of the most important steps in plant genetic transformation and is the key to T-DNA incorporation into the plant genomic DNA [26]. Interestingly, several earlier studies found that the duration of co-culture can also affect the transformation efficiency, and longer co-culture periods could also cause overgrowth of agrobacterium and be harmful to plant cells [24,27], which was consistent with our experimental results.
Various factors affecting somatic embryogenesis have been identified. These include exogenous hormones [28], AgNO3 [29], light conditions [30], and abscisic acid (ABA) [31], amongst others. Several studies have shown that incorporation of PEG in media or PEG treatment can effectively facilitate somatic embryogenesis [32,33]. In this study, it was found that the number of somatic embryos was highest when 60 g·L−1 PEG was added, but the frequency of malformed embryos was also higher, and the germination rate of somatic embryos was related to the state of the initial somatic embryos. In previous studies, the hypocotyls, or zygotic embryos, of F. mandshurica were used as explants for genetic transformation. This method can result in a direct yield of the transformed buds, but there are many chimeras, and the transformation is unstable. We used loose embryogenic callus as the starting material, which has the advantage of high transformation efficiency and can lead to stable transformation. However, during the maturation of somatic embryos, it was found that the presence of somatic embryos was relatively low and the incidence of abnormal embryos was high. Thus, future experiments are needed to solve the problem associated with the prevalence of abnormal embryos.
5. Conclusions
In this study, we established and optimized a novel agrobacterium tumefaciens-mediated genetic transformation system for embryonic calli of F. mandshurica. We determined the various factors affecting the transformation efficiency, including the selection of antibiotics, the concentration of the infection solution, the duration of co-culture, and the effect of media and media components on the transformation efficiency during somatic embryo maturation. The results of this study help promote genetic improvement in breeding processes and lay the foundation for subsequent related transgenic research. In this report, the genetic transformation efficiency of F. mandshurica was found to be significantly improved, but the incidence of abnormal embryos was relatively high, and additional detailed investigations are required into the maturation process of somatic embryos.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14050957/s1, File S1: Basic medium components.
Author Contributions
S.L., L.Y. and H.S. conceived and designed the experiments; Y.A. and H.D. performed the experiments, analyzed the data, prepared the figures and tables, and contributed equally to this work; W.Z. reviewed drafts of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science and Technology Major Project, grant number 2022YFD22003020204, and the National Natural Science Foundation of China, grant number 31400535.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no competing interests.
References
- Qi, F.; Tang, M.; Wang, W.; Liu, L.; Cao, Y.; Jing, T.; Zhan, Y. In vitro adventitious shoot regeneration system for Agrobacterium-mediated genetic transformation of Fraxinus mandshurica Rupr. Trees 2022, 36, 1387–1399. [Google Scholar] [CrossRef]
- Kong, D.M.; Preece, J.E.; Shen, H.L. Somatic embryogenesis in immature cotyledons of Manchurian ash (Fraxinus mandshurica Rupr.). Plant Cell Tiss. Organ Cult. 2012, 108, 485–492. [Google Scholar] [CrossRef]
- He, L.; Xu, Y.; Zeng, F.; Tian, H.; Xiao, Y.; Liu, H.; Yu, L.; Zhan, Y. Establishment of a micropropagation supporting technology for the Fraxinus mandshurica × Fraxinus sogdiana. Vitr. Cell Dev. Biol.-Plant 2021, 57, 307–318. [Google Scholar] [CrossRef]
- Gong, X.-W.; Hao, G.-Y. The synergistic effect of hydraulic and thermal impairments accounts for the severe crown damage in Fraxinus mandshurica seedlings following the combined drought-heatwave stress. Sci. Total Environ. 2023, 856, 159017. [Google Scholar] [CrossRef]
- He, L.; Zhang, J.; Guo, D.; Tian, H.; Cao, Y.; Ji, X.; Zhan, Y. Establishment of the technology of cambial meristematic cells (CMCs) culture from shoots and high expression of FmPHV (PHAVOLUTA) functions in identification and differentiation of CMCs and promoting the shoot regeneration by hypocotyl in Fraxinus mandshurica. Plant Physiol. Biochem. 2021, 160, 352–364. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, G.; Liu, F.; Gong, X. Immunosuppressive effect of extracts from leaves of Fraxinus Mandshurica Rupr. Bioengineered 2017, 8, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhao, H.; Ma, Z.; Zhang, S.; Li, M.; Zheng, Z.; Ren, X.; Ho, C.T.; Bai, N. Anti-Obesity and Gut Microbiota Modulation Effect of Secoiridoid-Enriched Extract from Fraxinus mandshurica Seeds on High-Fat Diet-Fed Mice. Molecules 2020, 25, 4001. [Google Scholar] [CrossRef]
- Yang, L.; Bian, L.; Shen, H.L.; Li, Y.-H. Somatic embryogenesis and plantlet regeneration from mature zygotic embryos of Manchurian ash (Fraxinus mandshurica Rupr.). Plant Cell Tiss. Organ Cult. 2013, 115, 115–125. [Google Scholar] [CrossRef]
- Ascough, G.D.; Novák, O.; Pěnčík, A.; Rolčík, J.; Strnad, M.; Erwin, J.E.; Van Staden, J. Hormonal and cell division analyses in Watsonia lepida seedlings. J. Plant Physiol. 2009, 166, 1497–1507. [Google Scholar] [CrossRef]
- de Oliveira, G.L.; Niederauer, G.F.; de Oliveira, F.A.; Rodrigues, C.S.; Hernandes, J.L.; de Souza, A.P.; Moura, M.F. Genetic diversity, population structure and parentage analysis of Brazilian grapevine hybrids after half a century of genetic breeding. Sci. Hortic. 2023, 311, 111825. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, P.; He, G.; Cao, Y.; Liu, Y.; Xu, L.; Ming, J. ITS sequence analysis used for parent selection in Lilium lancifolium Thunb. cross-breeding. J. Appl. Res. Med. Aromat. Plants 2022, 26, 100362. [Google Scholar] [CrossRef]
- Ramkumar, T.R.; Lenka, S.K.; Arya, S.S.; Bansal, K.C. A Short History and Perspectives on Plant Genetic Transformation. Methods Mol. Biol. 2020, 2124, 39–68. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Liu, Y.; Yuan, J.; Ye, L.; Zhang, Y.; Diao, S.; Han, W.; Suo, Y.; Li, H.; Hu, R.; et al. Establishment of an efficient genetic transformation system: A case study of RNAi-induced silencing of the transcription factor MeGI in Diospyros oleifera Cheng seedlings. Sci. Hortic. 2023, 308, 111560. [Google Scholar] [CrossRef]
- Mao, W.; Song, H.; Li, Y.; Wang, Y.; Lin, H.; Yao, C.; Zhou, W.; Yang, B.; Chen, X.; Li, P. Efficient plant regeneration and genetic transformation system of the precious fast-growing tree Toona ciliate. Ind. Crop. Prod. 2021, 172, 114015. [Google Scholar] [CrossRef]
- Kumlehn, J.; Serazetdinova, L.; Hensel, G.; Becker, D.; Loerz, H. Genetic transformation of barley (Hordeum vulgare L.) via infection of androgenetic pollen cultures with Agrobacterium tumefaciens. Plant Biotechnol. J. 2006, 4, 251–261. [Google Scholar] [CrossRef]
- Wang, G.; Liu, M.; Liu, G.; Bao, Z.; Ma, F. Establishment and optimization of Agrobacterium-mediated transformation in blueberry (Vaccinium species). Sci. Hortic. 2022, 304, 111258. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, S.; Liu, Y.; Chen, Y.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; Xu, L. Efficient Agrobacterium-mediated genetic transformation using cotyledons, hypocotyls and roots of ‘Duli’ (Pyrus betulifolia Bunge). Sci. Hortic. 2022, 296, 110906. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, C.; Wang, H.; Ma, X.; Shen, H. Indirect somatic embryogenesis and regeneration of Fraxinus mandshurica plants via callus tissue. J. For. Res. 2021, 32, 1613–1625. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, H.; Dong, S.; Wang, N.; Song, Y.; Zhang, H.; Li, S. Cloning and expression analysis of transcription factor LobHLH34 in Larix olgensis. Plant Res. 2022, 42, 112–120. [Google Scholar]
- Xu, J.-W. Genetic Transformation System. In The Lingzhi Mushroom Genome; Liu, C., Ed.; Compendium of Plant Genomes; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Zhao, Y.; Zong, P.; Zhan, Z.; Piao, Z. Establishment of A Simple and Efficient Agrobacterium-mediated Genetic Transformation System to Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Hortic. Plant J. 2021, 7, 117–128. [Google Scholar] [CrossRef]
- Bajaj, R.; Irvin, L.; Vaidya, B.N.; Dhekney, S.A.; Joshee, N. Optimizing plant regeneration and genetic transformation of Paulownia elongate. Biocatal. Agric. Biotechnol. 2021, 33, 101970. [Google Scholar] [CrossRef]
- Song, Y.; Bai, X.; Dong, S.; Yang, Y.; Dong, H.; Wang, N.; Zhang, H.; Li, S. Stable and Efficient Agrobacterium-Mediated Genetic Transformation of Larch Using Embryogenic Callus. Front. Plant Sci. 2020, 11, 584492. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, X.; Zhang, L.; Liu, H.; Zhu, C.; Ma, Z. Agrobacterium tumefaciens mediated genetic transformation of Tripterygium wilfordii and its application to enhance the accumulation of triptolide. Ind. Crop. Prod. 2022, 187, 115506. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Q.; Lin, S.; Zhang, Z.; Wang, Z.; Wang, Q.; Yan, X.; Bendahmane, M.; Bao, M.; Fu, X. Regeneration and Agrobacterium-mediated genetic transformation in Dianthus chinensis. Sci. Hortic. 2021, 287, 110279. [Google Scholar] [CrossRef]
- Han, J.-L.; Wang, H.; Ye, H.-C.; Liu, Y.; Li, Z.-Q.; Zhang, Y.; Zhang, Y.-S.; Yan, F.; Li, G.-F. High efficiency of genetic transformation and regeneration of Artemisia annua L. via Agrobacterium tumefaciens-mediated procedure. Plant Sci. 2005, 168, 73–80. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Z.; Shen, Y.; Li, F.; Yu, X.; Qu, S. In vitro regeneration and Agrobacterium tumefaciens-mediated genetic transformation of D. lotus (Diospyros lotus L.). Sci. Hortic. 2018, 236, 229–237. [Google Scholar] [CrossRef]
- de Almeida, N.V.; Rivas, E.B.; Cardoso, J.C. Somatic embryogenesis from flower tepals of Hippeastrum aiming regeneration of virus-free plants. Plant Sci. 2022, 317, 111191. [Google Scholar] [CrossRef]
- Manokari, M.; Priyadharshini, S.; Cokulraj, M.; Dey, A.; Faisal, M.; Alatar, A.A.; Alok, A.; Shekhawat, M.S. Exogenous implications of silver nitrate on direct and indirect somatic embryogenesis and germination of cold stored synseeds of Vanilla planifolia Jacks. ex Andrews. South Afr. J. Bot. 2022, 150, 129–138. [Google Scholar] [CrossRef]
- Sunandar, A.; Dorly; Supena, E.D.J. Induction of Somatic Embryogenesis in Sengon (Falcataria moluccana) With Thidiazuron and Light Treatments. HAYATI J. Biosci. 2017, 24, 105–108. [Google Scholar] [CrossRef]
- Domínguez, C.; Martínez, Ó.; Nieto, Ó.; Ferradás, Y.; González, M.V.; Rey, M. Involvement of polyamines in the maturation of grapevine (Vitis vinifera L. ‘Mencía’) somatic embryos over a semipermeable membrane. Sci. Hortic. 2023, 308, 111537. [Google Scholar] [CrossRef]
- Gnanaraj, M.; Sneka, C.; Sisubalan, N.; Baburajan, R.; Manikandan, R.; Muneeswaran, T. Polyethylene glycol induced somatic embryogenesis and plant regeneration from cotyledons of Vigna radiata (L.) Wilczek. South Afr. J. Bot. 2022, 150, 721–730. [Google Scholar] [CrossRef]
- Bhusare, B.P.; John, C.K.; Bhatt, V.P.; Nikam, T.C. Nikam. Induction of somatic embryogenesis in leaf and root explants of Digitalis lanata Ehrh.: Direct and indirect method. South Afr. J. Bot. 2020, 130, 356–365. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).