Rahnella aquatilis JZ-GX1 Alleviate Salt Stress in Cinnamomum camphora by Regulating Oxidative Metabolism and Ion Homeostasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Test Strains and Plant Materials
2.2. Seedling Inoculation and Salt Stress Treatment
2.3. Measurement of Plant Growth Parameters
2.4. Root System Conformation Determination
2.5. Moisture Condition Detection
2.6. Determination of Electrolyte Leakage and Malondialdehyde Content in Leaves
2.7. Observations of the Ultrastructure of C. camphora Leaves
2.8. Evaluation of Enzyme Antioxidants and Compatible Solute Content
2.9. Determination of Nutrient Elements and Ions
2.10. Quantitative Real-Time Polymerase Chain Reaction Analysis
2.11. Statistical Analysis
3. Results
3.1. Growth Promotion of Cinnamomum Camphora by Rahnella Aquatilis JZ-GX1
3.2. Changes in Root Parameters of Cinnamomum Camphora Caused by Rahnella Aquatilis JZ-GX1
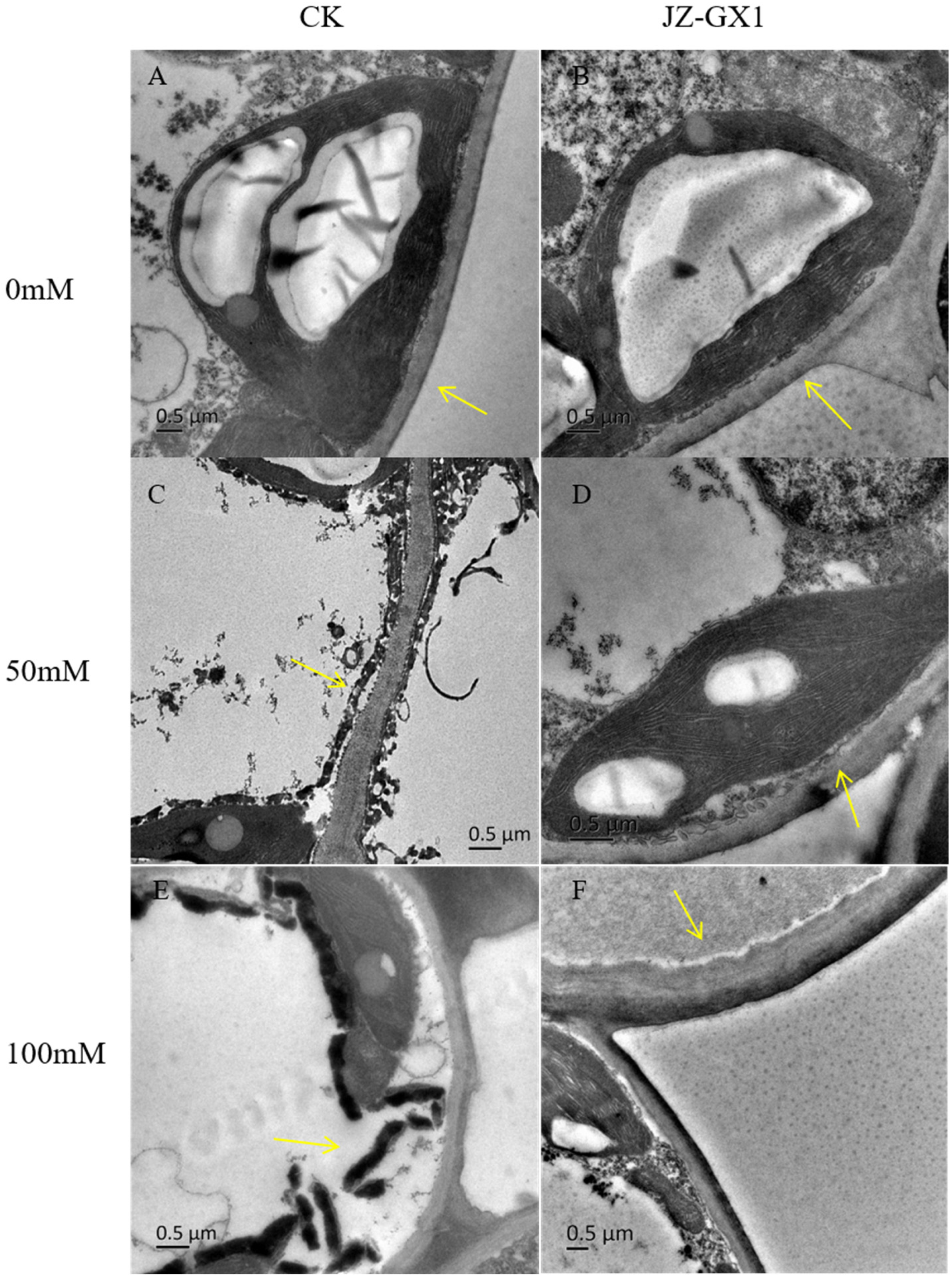
3.3. Rahnella Aquatilis JZ-GX1 Maintains Stability of the Cell Structure and Organelles of Cinnamomum Camphora Leaves
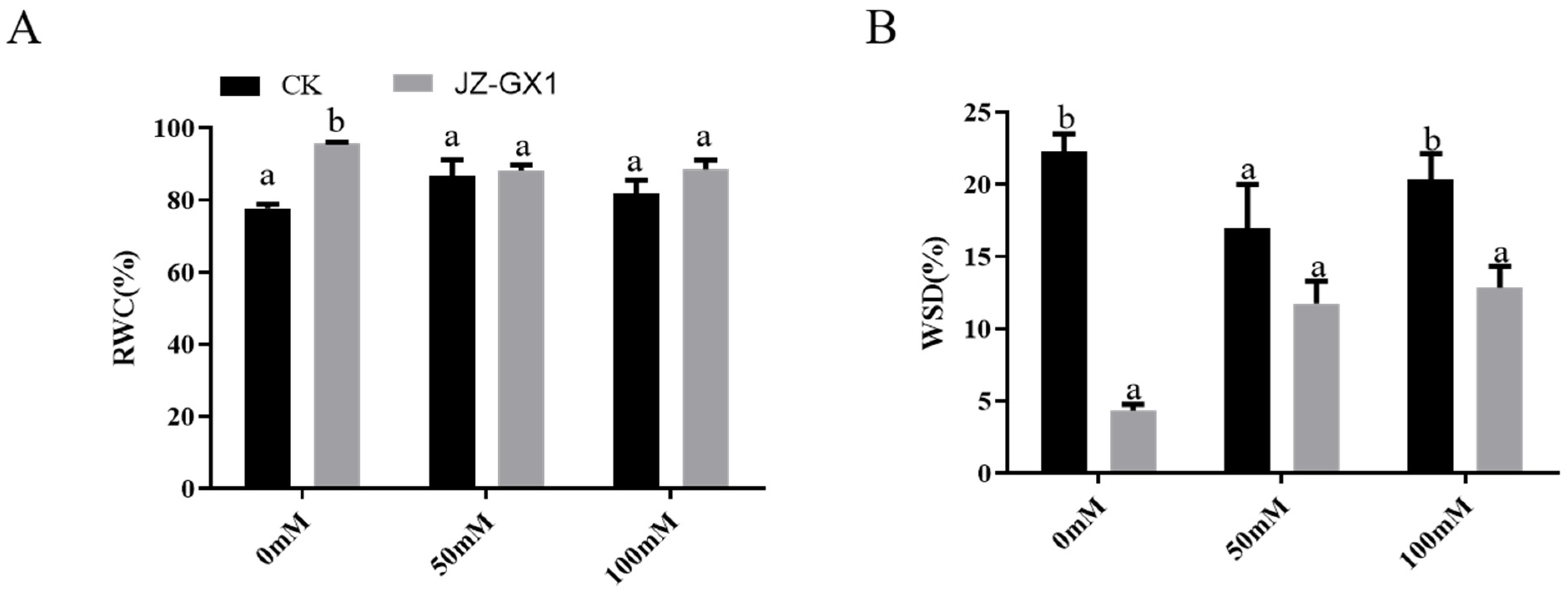
3.4. Improvement in the Water Status of Cinnamomum Camphora by Rahnella Aquatilis JZ-GX1
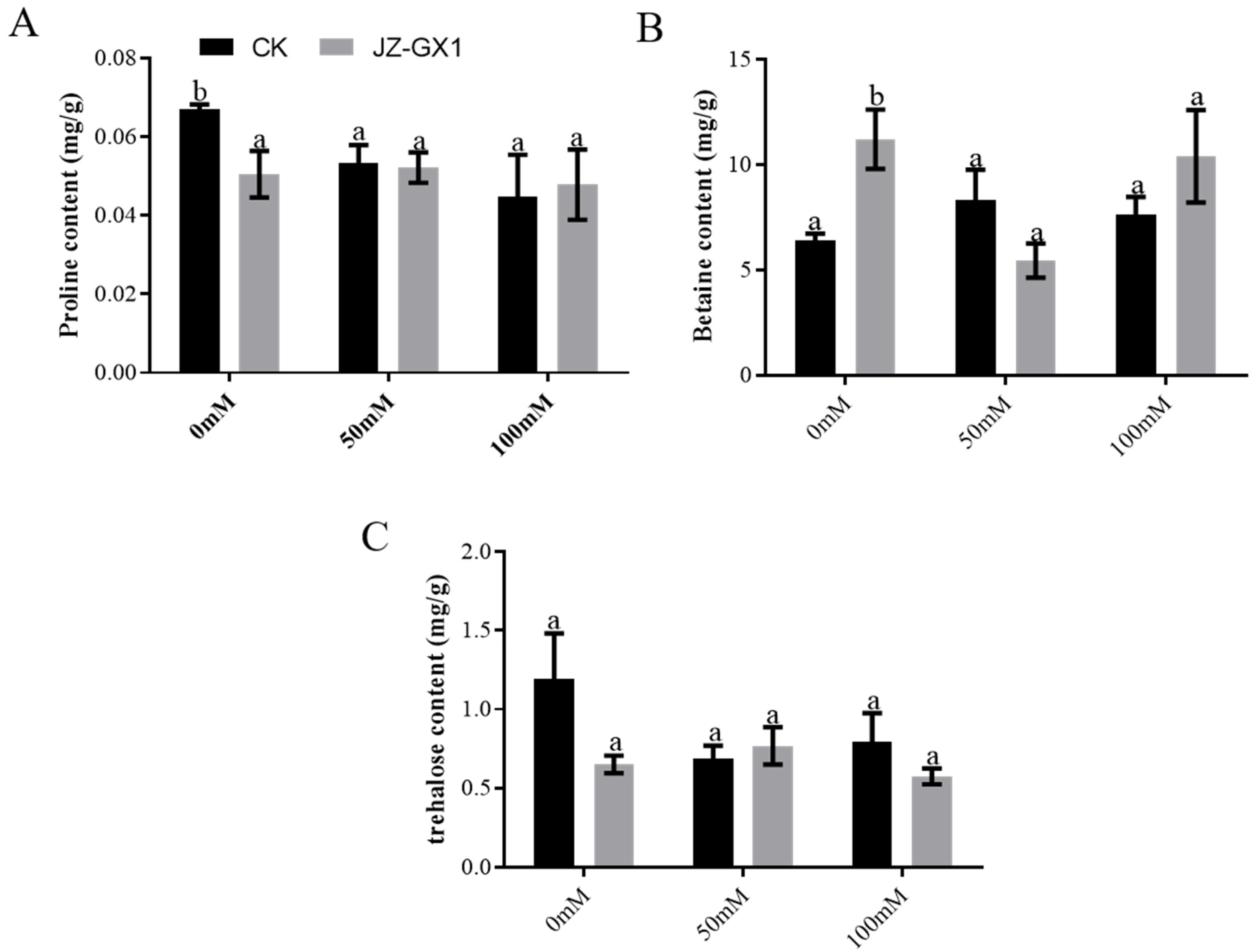
3.5. Enhancement of Membrane Integrity and Osmoregulatory Ability of C. camphora by R. Aquatilis JZ-GX1
3.6. Enhancement of the Antioxidant Capacity of C. camphora by R. aquatilis JZ-GX1
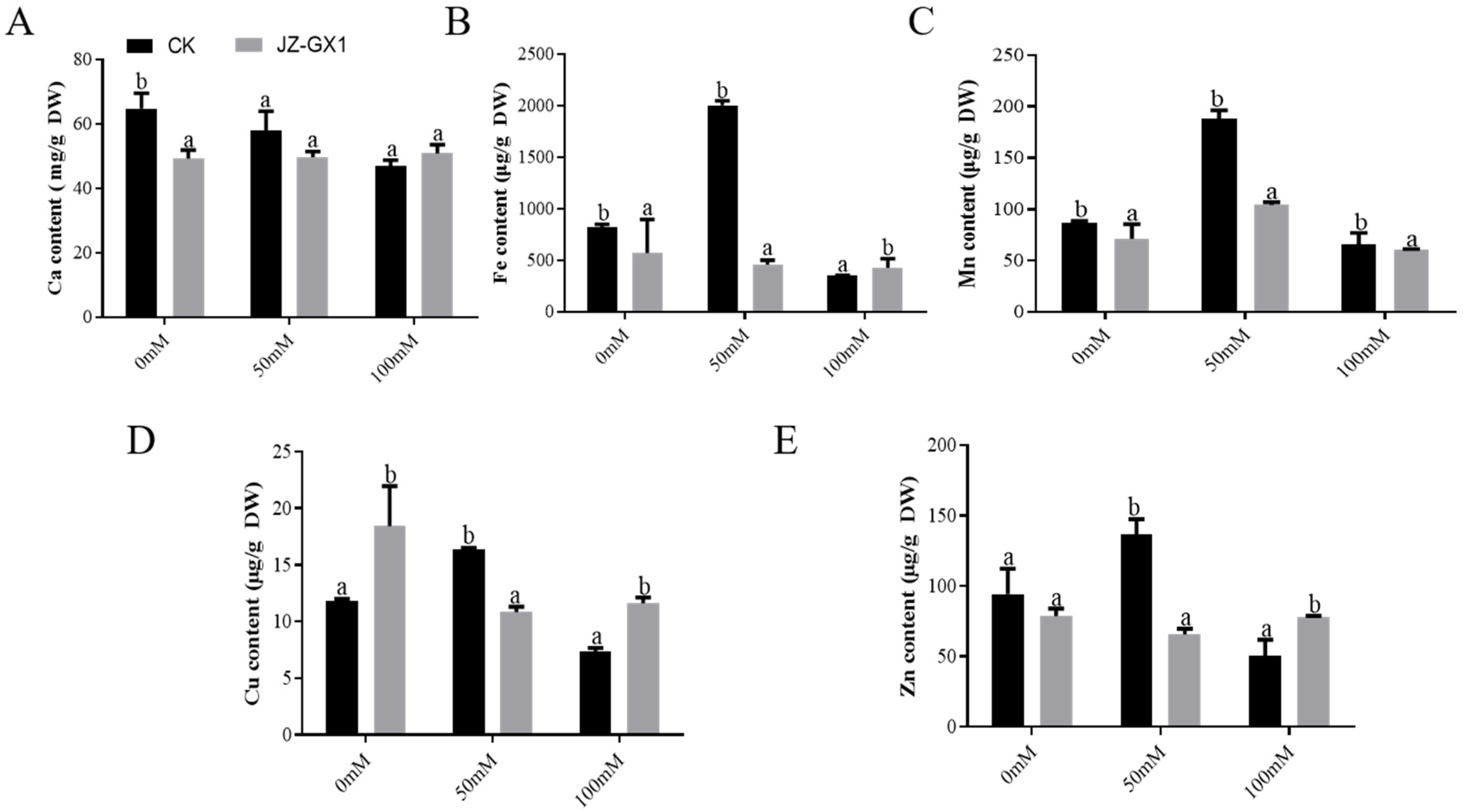
3.7. Nutrient Absorption and Ion Balance of Cinnamomum Camphora Promoted by Rahnella Aquatilis JZ-GX1
4. Discussion
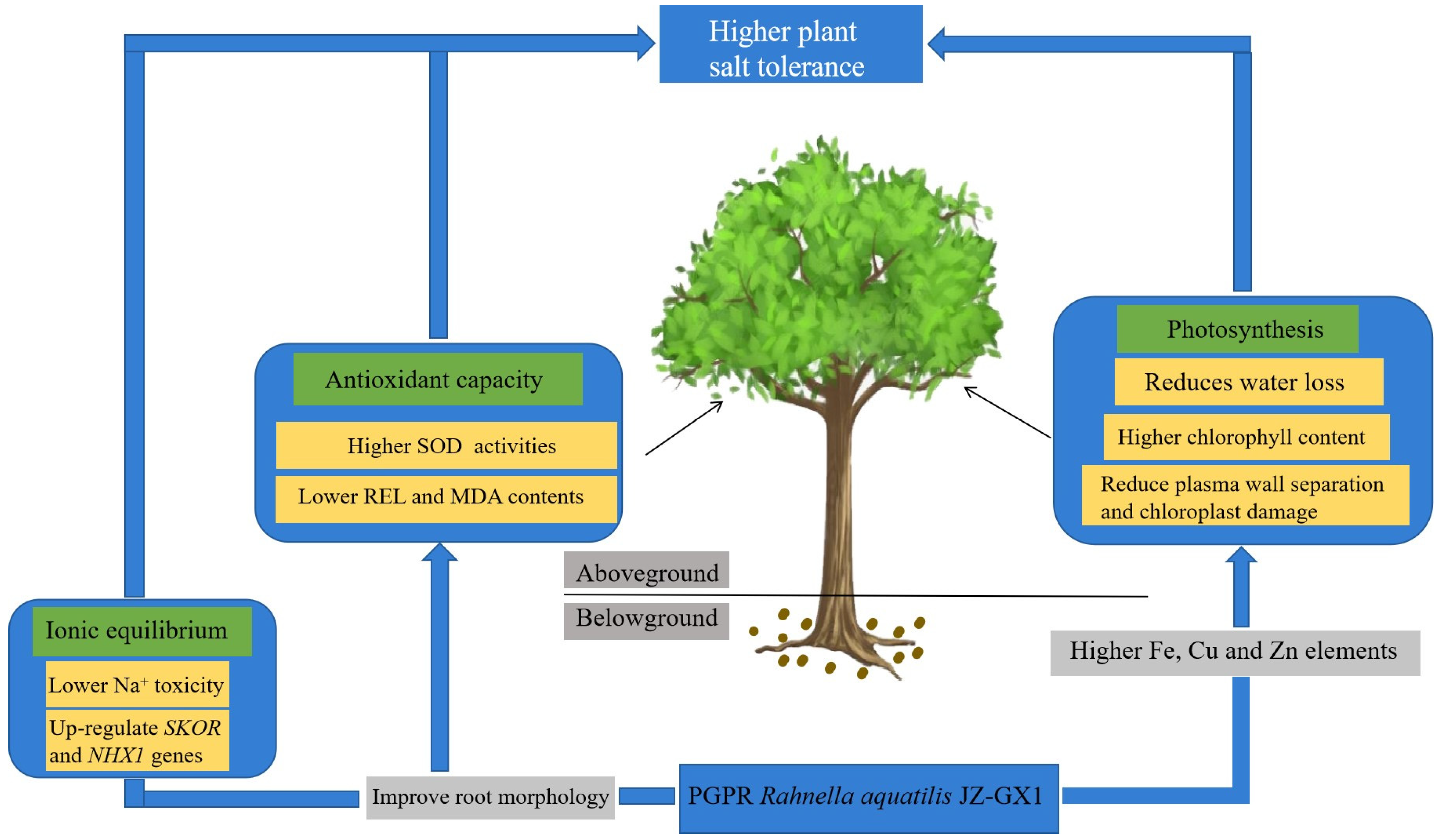
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; Al-Harrasi, A. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jia, L.; Zhang, B.; Ma, C. Progress in the screening and evaluation of salinity tolerance of coastal saline green plants. J. Southwest For. Univ. 2011, 31, 80–85. (In Chinese) [Google Scholar]
- Shabala, S.; Bose, J.; Fuglsang, A.T.; Pottosin, I. On a quest for stress tolerance genes: Membrane transporters in sensing and adapting to hostile soils. J. Exp. Bot. 2015, 67, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y. Enhancement of Plant Drought Tolerance by Microbes; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Walia, H.; Wilson, C.; Condamine, P.; Liu, X.; Ismail, A.M.; Zeng, L.; Wanamaker, S.I.; Mandal, J.; Xu, J.; Cui, X.; et al. Comparative transcriptional profiling of two contrasting rice genotypes under salinity stress during the vegetative growth stage. Plant Physiol. 2005, 139, 822–835. [Google Scholar] [CrossRef]
- Liu, S.; Hao, H.; Lu, X.; Zhao, X.; Wang, Y.; Zhang, Y.; Xie, Z.; Wang, R. Transcriptome profiling of genes involved in induced systemic salt tolerance conferred by Bacillus amyloliquefaciens FZB42 in Arabidopsis thaliana. Sci. Rep. 2017, 7, 10795. [Google Scholar] [CrossRef]
- Babalola, O.O. Beneficial bacteria of agricultural importance. Biotechnol. Lett. 2010, 32, 1559–1570. [Google Scholar]
- Mahmoud, M.B.; Hidri, R.; Talbi-Zribi, O.; Taamalli, W.; Abdelly, C.; Djebalic, N. Auxin and proline producing rhizobacteria mitigate salt induced growth inhibition of barley plants by enhancing water and nutrient status. S. Afr. J. Bot. 2020, 128, 209–217. [Google Scholar] [CrossRef]
- Santoyo, G.; Ma, O.M.; Campos-Garcia, J.; Glick, B.R.; Zetter-Salmon, E. The production of acc deaminase and trehalose by the plant growth promoting bacterium pseudomonas sp. uw4 synergistically protect tomato plants against salt stress. Front. Microbiol. 2019, 10, 1392. [Google Scholar]
- Sukweenadhi, J.; Balusamy, S.R.; Kim, Y.-J.; Lee, C.H.; Kim, Y.-J.; Koh, S.C.; Yang, D.C. A growth-promoting bacteria, Paenibacillus yonginensis DCY84T enhanced salt stress tolerance by activating defense-related systems in Panax ginseng. Front. Plant Sci. 2018, 9, 813. [Google Scholar] [CrossRef]
- Tewari, S.; Arora, N.K. Role of salicylic acid from Pseudomonas aeruginosa PF23 EPS+ in growth promotion of sunflower in saline soils infested with phytopathogen Macrophomina phaseolina. Environ. Sustain. 2018, 1, 49–59. [Google Scholar] [CrossRef]
- Hong, B.H.; Joe, M.M.; Selvakumar, G.; Kim, K.Y.; Choi, J.H.; Sa, T.M. Influence of salinity variations on exocellular polysaccharide production, biofilm formation and flocculation in halotolerant bacteria. J. Environ. Biol. 2017, 38, 657–664. [Google Scholar] [CrossRef]
- Ledger, T.; Rojas, S.; Timmermann, T.; Pinedo, I.; Poupin, M.J.; Garrido, T.; Richter, P.; Tamayo, J.; Donoso, R. Volatile-Mediated effects predominate in Paraburkholderia phytofirmans growth promotion and salt stress tolerance of Arabidopsis thaliana. Front. Microbiol. 2016, 7, 1838. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kulkarni, J.; Jha, B. Halotolerant rhizobacteria promote growth and enhance salinity tolerance in peanut. Front. Microbiol. 2016, 7, 1600. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Wu, G.; Njeri, K.V.; Shen, Q.; Zhang, N.; Zhang, R. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol. Plant. 2016, 158, 34–44. [Google Scholar] [PubMed]
- Bharti, N.; Barnawal, D.; Awasthi, A.; Yadav, A.; Kalra, A. Plant growth promoting rhizobacteria alleviate salinity induced negative effects on growth, oil content and physiological status in Mentha arvensis. Acta Physiol. Plant. 2014, 36, 45–60. [Google Scholar] [CrossRef]
- Qurashi, A.W.; Sabri, A.N. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz. J. Microbiol. 2012, 43, 1183–1191. [Google Scholar] [CrossRef]
- Li, G.E.; Wu, X.Q. Study on the phytase characteristics of high-efficiency phytic acid salt-degrading bacterial strain JZ-GX1. J. Cent. South For. Univ. 2014, 34, 5. (In Chinese) [Google Scholar]
- Li, P.S.; Kong, W.L.; Wu, X.Q. Salt tolerance mechanism of the rhizosphere bacterium JZ-GX1 and its effects on tomato seed germination and seedling growth. Front. Microbiol. 2021, 8, 33–35. [Google Scholar] [CrossRef]
- Xiao, Y.T.; Li, M.; Chen, F. Root Rot of Cinnamomum camphora (Linn) Presl Caused by Phytopythium vexans in China. Plants 2023, 12, 1072. [Google Scholar] [CrossRef]
- Zhang, L.H.; Wang, X.L.; Wang, M.Q.; Han, H.Z. Effects of soda saline-alkali stress on photosynthetic characteristics of Cinnamomum camphora seedlings. North. Hortic. 2012, 23, 91–93. (In Chinese) [Google Scholar]
- Mei, H.Y.; Wang, N.; Li, Z.Y.; Su, J.L. Effects of NaCl and Na2SO4 stress on physiological characteristics of Cinnamomum camphora seedlings. J. Northwest For. Univ. 2011, 26, 30–34. (In Chinese) [Google Scholar]
- Golombek, S.D.; Ali, I.Z. Effect of Drought and Nitrogen Availability on Osmotic Adjustment of Five Pearl Millet Cultivars in the Vegetative Growth Stage. J. Agron. Crop Sci. Z. Fur Acker-Und Pflanzenbau 2016, 55, 433–444. [Google Scholar]
- Li, H.S. Principles and Techniques of Plant Physiological and Biochemical Experiments; Beijing Higher Education Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Velázquez-Becerra, C.; Macías-Rodríguez, L.I.; López-Bucio, J.; Hernández, J.A.; Flores-Cortez, I.; Valencia-Cantero, E. A volatile organic compound analysis from arthrobacter agilis identifies dimethylhexadecylamine, an amino-containing lipid modulating bacterial growth and medicago sativa morphogenesis in vitro. Plant Soil 2011, 18, 329–340. [Google Scholar] [CrossRef]
- Pareek, A.; Singla, S.L.; Grover, A. Short-term salinity and high temperature stress-associated ultrastructural alterations in young leaf cells of Oryza sativa L. Ann. Bot. 1997, 80, 629–639. [Google Scholar] [CrossRef]
- Li, Y.; Yu, L.; Li, H.X.; Xu, L.; Jiao, J.G.; Hu, F. Screening, Identification and Characteristics of a Peanut Rhizosphere Growth-Promoting Bacteria. J. Ecol. Rural. Environ. 2012, 28, 416–421. (In Chinese) [Google Scholar]
- Zhang, J.J.; Zhang, L.W.; Li, Y.P.; Jiao, X.L.; Zhu, F.L.; Du, L. Selection of internal reference genes for real-time quantitative pcr of Cinnamomum camphora under different abiotic stresses. Biotechnol. Bull. 2016, 32, 7. (In Chinese) [Google Scholar]
- Irizarry, I.; White, J.F. Application of bacteria from non-cultivated plants to promote growth, alter root architecture and alleviate salt stress of cotton. J. Appl. Microbiol. 2017, 122, 1110–1120. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, S.; Hu, W.; Xiao, L.; Tang, M. Arbuscular mycorrhizal fungus Rhizophagus irregularis increased potassium content and expression of genes encoding potassium channels in Lyciumbarbarum. Front. Plant Sci. 2017, 8, 440. [Google Scholar]
- Hajiboland, R.; Aliasgharzadeh, N.; Laiegh, S.F.; Poschenrieder, C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 2010, 331, 313–327. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Azcon, R.; Ruiz-Lozano, J.M. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Nat root-to-shoot distribution. Mycorrhiza 2016, 26, 673–684. [Google Scholar] [CrossRef]
- Aroca, R.; Ruiz-Lozano, J.M.; Zamarreño, Á.M.; Paz, J.A.; García-Mina, J.M.; Pozo, M.J.; López-Ráez, J.A. Arbuscular mycorhizal symbiosis influences strigolactone production under salinity and alleviatessalt stress in lettuce plants. J. Plant Physiol. 2013, 170, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Tang, M.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhiza on organic solutes in maize leaves under salt stress. Mycorrhiza 2011, 21, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.A.; Bañón, S.; Vicente, M.J.; Miralles, J.; Martínez-Sánchez, J.J. Root development in horticultural plants grown under abiotic stress conditions—A review. J. Hortic. Sci. Biotechnol. 2011, 86, 543–556. [Google Scholar] [CrossRef]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Amual Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Evelin, H.; Giri, B.; Kapoor, R. Contribution of Glomus intraradices inoculation to nutri ent acquisitionand mitigation of ionic imbalance in NaCl-stressed Trigonella foenum-graecumn. Mycorrhiza 2012, 22, 203–217. [Google Scholar] [CrossRef]
- Ibrahim, W.; Ahmed, I.M.; Chen, X.H.; Cao, F.B.; Zhu, S.J.; Wu, F.B. Genotypic diferences in photosynthetic performance, antioxidlant capacity, ultrastructure and nutrients in response to combined stress of salinity and Cd in cotton. Biometals 2015, 28, 1063–1078. [Google Scholar] [CrossRef]
- Gill, S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Pei, B.; Zhang, G.C.; Zhang, S.Y.; Wu, Q.; Xu, Z.Q.; Xu, P. Effects of soil drought stress on photosynthesis and antioxidant enzyme activities in sea buckthorn leaves. J. Ecol. 2013, 33, 1386–1396. (In Chinese) [Google Scholar]
- Talaat, N.B.; Shawky, B.T. Protective effects of arbuscular mycorrhizal fungi on wheat (Triicum aestivum L.) plants exposed to salinity. Environ. Exp. Bot. 2014, 98, 20–31. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, J.S.; Saxena, A.K.; Singh, D.P. Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol. 2012, 14, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Nabti, E.; Sahnoune, M.; Ghoul, M.; Fischer, D.; Hofmann, A.; Rothballer, M.; Schmid, M.; Hartmann, A. Restoration of growth of gurum wheat (Triticum durum var. waha) under saline conditions due to inoculation with the rhizosphere bacterium Azospirillun brasilense NH and extracts of the marinealga Ulva lactuca. Plant Growth Regul. 2010, 29, 6–22. [Google Scholar] [CrossRef]
- Wu, T.Y.; Wang, Y.H.; Wu, F.; Wu, X.Q. Dual inoculation with rhizosphere-promoting bacterium Bacillus cereus and beneficial fungus Peniophora cinerea improves salt stress tolerance and productivity in willow. Microbiol. Res. 2023, 268, 127280. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.Q.; Ji, R.L. Effects of exogenous salicylic acid on SOD activity in Cinnamomum camphora leaves under low temperature stress. Anhui Agric. Bull. 2014, 20, 19–20. (In Chinese) [Google Scholar]
- Sawada, H.; Shim, I.S.; Usui, K.; Kobayashi, K.; Fujihara, S. Adaptive mechanism of Echinochloa crusgalli Beauv. var. formosensis Ohwi under salt stress: Effect of salicylic acid on salt sensitivity. Plant Sci. 2008, 174, 583–589. [Google Scholar] [CrossRef]
- Baltruschat, H.; Fodor, J.; Harrach, B.D.; Niemczyk, E.; Barna, B.; Gullner, G.; Janeczko, A.; Kogel, K.H.; Schafer, P.; Schwarczinger, I.; et al. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol. 2018, 180, 501–510. [Google Scholar] [CrossRef]
- Evelin, H.; Giri, B.; Kapoor, R. Ultrastructural evidence for AMF mediated salt stress mitigation in Trigonella foenum-graecum. Mycorrhiza 2013, 23, 71–86. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, D.P. Effect of salt tolerant plant growth promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol. 2015, 17, 288–293. [Google Scholar] [CrossRef]
- Rahman, M.; Mostofa, M.G.; Islam, M.; Keya, S.S.; Das, A.K.; Miah, M.G.; Kawser, A.Q.M.R.; Ahsan, S.M.; Hashem, A.; Tabassum, B.; et al. Acetic acid: A cost-effective agent for mitigation of seawater-induced salt toxicity in mung bean. Sci. Rep. 2019, 9, 15186. [Google Scholar] [CrossRef]
- Yuan, H.J.; Ma, Q.; Wu, G.Q.; Wang, P.; Hu, J.; Wang, S.M. Zxnhx controls Na+ and K+ homeostasis at the whole-plant level in zygophyllum xanthoxylum through feedback regulation of the expression of genes involved in their transport. Ann. Bot. 2015, 115, 495–507. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, M.; Sun, Y.; Dowd, S.E.; Shi, H.; Pare, P.W. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol. Plant-Microbe Interact. 2008, 21, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Aziz, M.; Qiao, Y.; Han, Q.Q.; Li, J.; Wang, Y.Q.; Shen, X.; Wang, S.M.; Pare, P.W. Soil microbe Bacillus subtilis (GB03) induces biomass accumulation and salt tolerance with lower sodium accumulation in wheat. Crop Pasture Sci. 2014, 65, 423–427. [Google Scholar] [CrossRef]
- Han, Q.Q.; Lü, X.P.; Bai, J.P.; Qiao, Y.; Paré, P.W.; Wang, S.M.; Zhang, J.L.; Wu, Y.N.; Pang, X.P.; Xu, W.B.; et al. Beneficial soil bacterium Bacillus subtilis (GB03) augments salt tolerance of white clover. Front. Plant Sci. 2014, 5, 525. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef] [PubMed]
- Julkowska, M.M.; Testerink, C. Tuning plant signaling and growth to survive salt. Trends Plant Sci. 2015, 20, 586–594. [Google Scholar] [CrossRef]





| Gene Name | Gene Function | Primers |
|---|---|---|
| EF1α | Endogenous control, Reference gene | TCCAAGGCACGGTATGAT CCTGAAGAGGGAGACGAA |
| SKOR | Potassium channel | AAGCAGGCTTTTGCGACTTG TGTTCAAGATCGTTCGCCCA |
| NHX1 | Antiporter isoform | CCATCTCGCCTTCGTATGCT CAAACATTGGGCGCATGACA |
| NaCl (mM) | Treatment | Shoot Height (cm) | Shoot Height Growth Rate (%) | SPAD | SPAD Growth Rate (%) |
|---|---|---|---|---|---|
| 0 | CK JZ-GX1 | 7.12 ± 0.71 a 9.64 ± 0.37 b | 35.10 44.53 | 28.5 ± 3.05 a 37.88 ± 1.80 b | −17.65 14.89 |
| 50 | CK JZ-GX1 | 5.90 ± 0.72 a 9.12 ± 1.31 b | 6.31 51.24 | 26.52 ± 2.12 a 33.54 ± 4.07 b | −22.27 7.16 |
| 100 | CK JZ-GX1 | 5.70 ± 0.17 a 7.68 ± 0.33 b | 3.26 24.27 | 26.50 ± 4.85 a 37.12 ± 4.30 b | −24.35 10.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.-S.; Kong, W.-L.; Wu, X.-Q. Rahnella aquatilis JZ-GX1 Alleviate Salt Stress in Cinnamomum camphora by Regulating Oxidative Metabolism and Ion Homeostasis. Forests 2023, 14, 1110. https://doi.org/10.3390/f14061110
Li P-S, Kong W-L, Wu X-Q. Rahnella aquatilis JZ-GX1 Alleviate Salt Stress in Cinnamomum camphora by Regulating Oxidative Metabolism and Ion Homeostasis. Forests. 2023; 14(6):1110. https://doi.org/10.3390/f14061110
Chicago/Turabian StyleLi, Pu-Sheng, Wei-Liang Kong, and Xiao-Qin Wu. 2023. "Rahnella aquatilis JZ-GX1 Alleviate Salt Stress in Cinnamomum camphora by Regulating Oxidative Metabolism and Ion Homeostasis" Forests 14, no. 6: 1110. https://doi.org/10.3390/f14061110
APA StyleLi, P.-S., Kong, W.-L., & Wu, X.-Q. (2023). Rahnella aquatilis JZ-GX1 Alleviate Salt Stress in Cinnamomum camphora by Regulating Oxidative Metabolism and Ion Homeostasis. Forests, 14(6), 1110. https://doi.org/10.3390/f14061110





