A Simpler Fabrication for Thermal Energy Storage Wood
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of TESPs
2.3. Test Section
- M1—Mass of TESP when absolute dry (g)
- M0—Mass of OP when absolute dry (g)
3. Results and Discussion
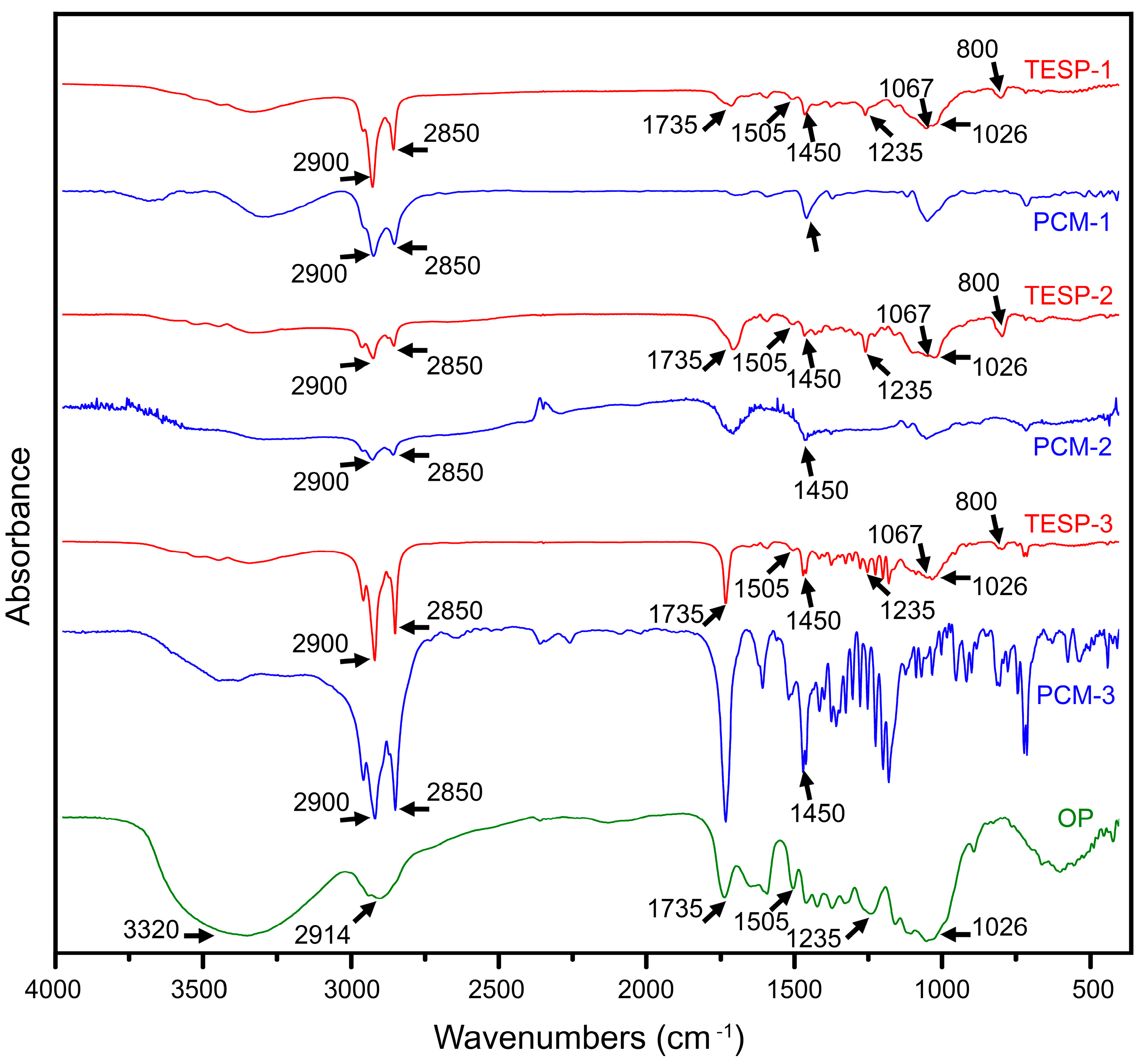
3.1. FT-IR Analysis
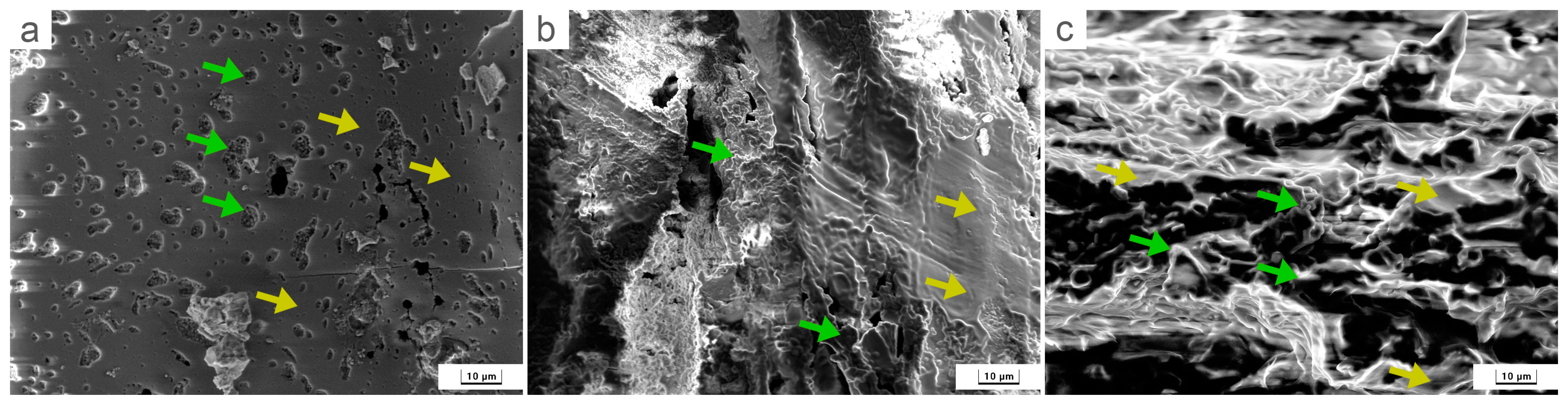
3.2. SEM and EDS Analysis
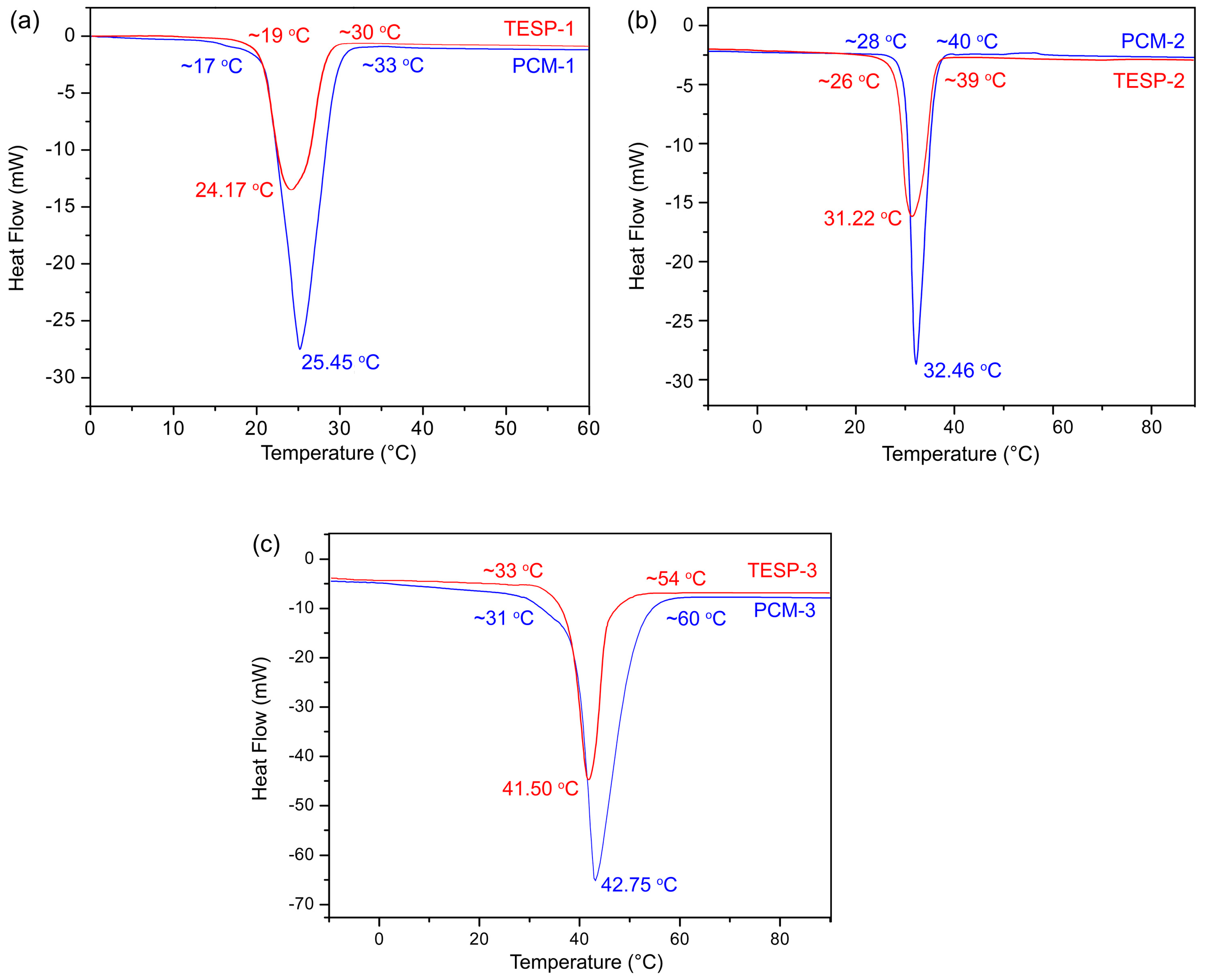
3.3. DSC Analysis
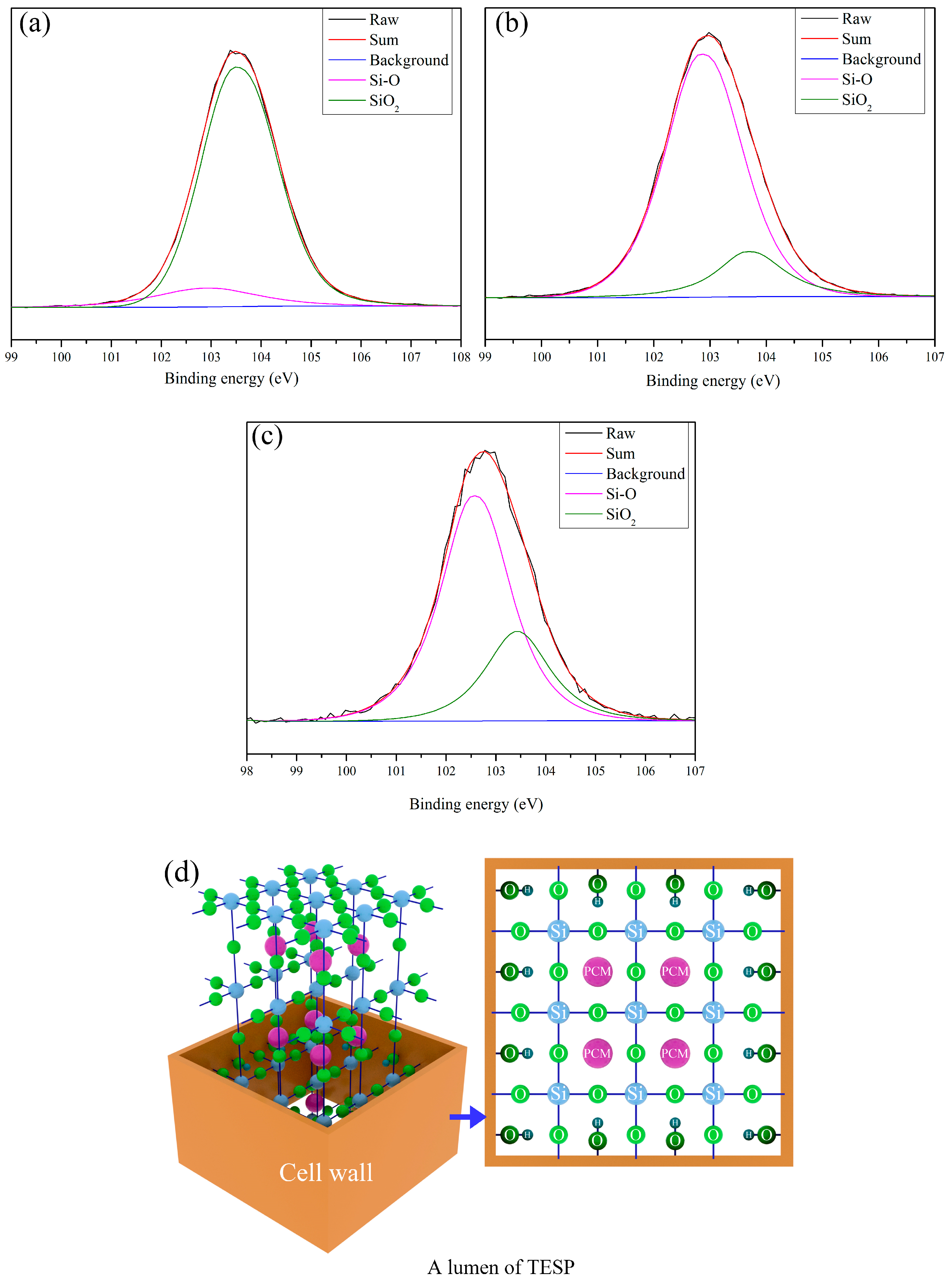
3.4. The Composite Mechanism of TESPs
3.5. Thermal Energy Storage and Heat Resistance
3.6. Mechanical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, X.; Jia, S.; Liu, J.; Wang, W.; Cao, H.; Guo, X.; Sun, W. Fabrication of thermal energy storage wood based on graphene aerogel encapsulated polyethylene glycol as phase change material. Mater. Res. Express 2020, 7, 095503. [Google Scholar] [CrossRef]
- Liu, J.; Jia, S.; Lin, X.; Cao, H.; Wang, W.; Guo, X.; Sun, W. Fabrication of thermal energy storage wood composite based on shape-stable phase change material. Mater. Res. Express 2021, 8, 055304. [Google Scholar] [CrossRef]
- Montanari, C.; Li, Y.; Chen, H.; Yan, M.; Berglund, L.A. Transparent wood for thermal energy storage and reversible optical transmittance. ACS Appl. Mater. Interfaces 2019, 11, 20465–20472. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cao, H.; Liu, J.; Jia, S.; Ma, L.; Guo, X.; Sun, W. A thermal energy storage composite by incorporating microencapsulated phase change material into wood. RSC Adv. 2020, 10, 8097. [Google Scholar] [CrossRef]
- Damien, M.; Pierre, B.; Véronic, L.; Philippe, L. Thermal characterization of bio-based phase changing materials in decorative wood-based panels for thermal energy storage. Green Energy Environ. 2019, 4, 56–65. [Google Scholar]
- Fang, G.; Li, H.; Liu, X. Preparation and properties of lauric acid/silicon dioxide composites as form-stable phase change materials for thermal energy storage. Mater. Chem. Phys. 2010, 122, 533–536. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Q.; Li, L. Delignified wood/capric acid-palmitic acid mixture stable-form phase change material for thermal storage. Sol. Energy Mater. Sol. Cells 2019, 194, 215–221. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Yu, Q.; Cao, G.; Yang, R.; Ke, J.; Di, X.; Liu, F.; Zhang, W.; Wang, C. Composite phase change materials with good reversible thermochromic ability in delignified wood substrate for thermal energy storage. Appl. Energy 2018, 212, 455–464. [Google Scholar] [CrossRef]
- Yang, H.; Chao, W.; Wang, S.; Yu, Q.; Cao, G.; Yang, T.; Liu, F.; Di, X.; Li, J.; Wang, C.; et al. Self-luminous wood composite for both thermal and light energy storage. Energy Storage Mater. 2019, 18, 15–22. [Google Scholar] [CrossRef]
- Yang, H.; Chao, W.D.; Yang, Z.; Yang, T.; Yu, Q.; Liu, F.; Li, J.; Li, G.; Wang, C. Multifunctional wood based composite phase change materials for magnetic-thermal and solar-thermal energy conversion and storage. Energy Convers. Manag. 2019, 200, 112029. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Zhang, W.; Zhao, J.; Wang, B.; Fang, X.; Guo, H.; Liu, Y. A photo-to-thermal energy conversion and heat regulation wood supported by alkylated carbon black for thermal conductive filler. Sol. Energy Mater. Sol. Cells 2023, 251, 112165. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H.; Wang, Y.; Ma, C.; Luo, S.; Wu, Z.; Zhang, Z.; Li, W.; Liu, S. Fluorescent thermochromic wood-based composite phase change materials based on aggregation-induced emission carbon dots for visual solar-thermal energy conversion and storage. Chem. Eng. J. 2021, 424, 130426. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, N.; Pan, X.; Zhong, W.; Qiu, B.; Cai, Y.; Yuan, Y. Thermal properties of biomass-based form-stable phase change material for latent heat thermal energy storage. Int. J. Energy Res. 2021, 45, 20372–20383. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Barzin, R.; Farid, M.M. Composite of wood-plastic and micro-encapsulated phase change material (MEPCM) used for thermal energy storage. Appl. Therm. Eng. 2016, 112, 82–88. [Google Scholar] [CrossRef]
- Geng, X.; Wang, Y.; Han, N.; Zhang, X.; Li, W. Influences of PVA modification on performance of microencapsulated reversible thermochromic phase change materials for energy storage application. Sol. Energy Mater. Sol. Cells 2021, 222, 110938. [Google Scholar] [CrossRef]
- Jeong, S.; Jeon, J.; Seo, J.; Lee, J.; Kim, S. Performance evaluation of the microencapsulated PCM for wood-based flooring application. Energy Convers. Manag. 2012, 64, 516–521. [Google Scholar] [CrossRef]
- Lin, X.; Chen, X.; Weng, L.; Hu, D.; Qiu, C.; Liu, P.; Zhang, Y.; Fan, M.; Sun, W.; Guo, X. In-situ copper ion reduction and micro encapsulation of wood-based composite PCM with effective anisotropic thermal conductivity and energy storage. Sol. Energy Mater. Sol. Cells 2022, 242, 111762. [Google Scholar] [CrossRef]
- Mathis, D.; Blanchet, P.; Landry, V.; Lagiere, P. Impregnation of Wood with Microencapsulated Bio-Based Phase Change Materials for High Thermal Mass Engineered Wood Flooring. Appl. Sci. 2018, 8, 2696. [Google Scholar] [CrossRef]
- Guo, X.; Cao, J.; Peng, Y.; Liu, R. Incorporation of microencapsulated dodecanol into wood flour/high-density polyethylene composite as a phase change material for thermal energy storage. Mater. Des. 2016, 89, 1325–1334. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, X.; Lin, W. Research on the wood properties of betula platyphylla and franxinus madschurica in natural secondary forest. For. Eng. 2017, 33, 35–40. [Google Scholar]
- Vance, E.D.; Loehle, C.; Wigley, T.B.; Weatherford, P. Scientific basis for sustainable management of eucalyptus and populus as short-rotation woody crops in the U.S. Forests 2014, 5, 901–918. [Google Scholar] [CrossRef]
- Zou, W.; Li, M.; Wang, Z.; Sun, D.; Zhang, P. Poplar-based thermochromic composites that change colour at 38 °C to 46 °C. Sci. Rep. 2021, 11, 16865. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Wang, Z.; Li, M.; Sun, D. Thermochromic poplar that changes colour at 16–30 °C. Eur. J. Wood Wood Prod. 2022, 80, 741–748. [Google Scholar] [CrossRef]
- Zou, W.; Wang, Z.; Sun, Z.; Jiang, X.; Yu, M.; Song, L.; Sun, D. A thermochromic wood that can change colour at 24–40 °C and collect heat for heating flooring. Ind. Crops Prod. 2022, 186, 115293. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Zuo, Y.; Wu, Y.; Li, X. Construction of a network structure in Chinese fir wood by Na2SiF6 crosslinked Na2SiO3. J. Mater. Res. Technol. 2020, 9, 14190–14199. [Google Scholar] [CrossRef]
- Yu, Z.; Yao, Y.; Yao, J.; Zhang, L.; Chen, Z.; Gao, Y.; Luo, H. Transparent wood containing CsxWO3 nanoparticles for heat-shielding-window applications. J. Mater. Chem. A 2017, 5, 6019–6024. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Q.; Rojas, R.; Yan, M.; Lawoko, M.; Berglund, L.A. Lignin-retaining transparent wood. Chemsuschem 2017, 10, 3445–3451. [Google Scholar] [CrossRef]
- Pandey, K.K. A study of chemical structure of soft and hardwood and wood polymers by FT-IR spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- Pandey, K.K.; Pitman, A.J. FT-IR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Rana, R.; Langenfeld-Heyser, R.; Finkeldey, R.; Polle, A. FT-IR spectroscopy, chemical and histochemical characterisation of wood and lignin of five tropical timber wood species of the family of dipterocarpaceae. Wood Sci. Technol. 2010, 2, 225–242. [Google Scholar] [CrossRef]
- Zhang, W.; Johnson, A.M.; Barone, J.R.; Renneckar, S. Reducing the heterogeneity of xylan through processing. Carbohydr. Polym. 2016, 150, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Su, X.J.; Hou, G.L. Effects of silicon content on microstructure and photocatalytic activity of TiO2/SiO2 composite aerogels. J. Inorg. Mater. 2010, 25, 911–915. [Google Scholar] [CrossRef]
- Fu, Y.; Ding, X.; Meng, C.; Dou, T.; Yang, H. Synthesis and pore structure of porous SiO2/TiO2 composite aerogel. Mater. Sci. Technol. 2015, 23, 1–7. [Google Scholar]
- Zhang, X.; Liu, D.; Wang, D.; Liu, F.; Bai, X. Effect of ammonia on the superamphiphobic coating prepared by sol-gel method. Mater. Prot. 2019, 52, 96–102. [Google Scholar]
- Chen, Z.; Qi, L.; Wang, Z. Preparation and flame retardant application of new hybrid silica sol composite. New Chem. Mater. 2019, 47, 230–234+242. [Google Scholar]

| C (wt%) | O (wt%) | Si (wt%) | |
|---|---|---|---|
| TESP-1 (Longitudinal-cutting section) | 75.02 | 24.20 | 0.78 |
| TESP-1 (Radial-cutting section) | 63.01 | 27.13 | 9.86 |
| TESP-2 (Longitudinal-cutting section) | 75.90 | 22.18 | 1.92 |
| TESP-2 (Radial-cutting section) | 67.80 | 26.59 | 5.61 |
| TESP-3 (Longitudinal-cutting section) | 85.96 | 13.22 | 0.83 |
| TESP-3 (Radial-cutting section) | 81.77 | 15.32 | 2.91 |
| Time (min) | OP (°C) | TESP-1 (°C) | TESP-2 (°C) | TESP-3 (°C) |
|---|---|---|---|---|
| 0 | ~48 | ~49 | ~49 | ~49 |
| 5 | ~32 | ~36 | ~37 | ~39 |
| 10 | ~26 | ~29 | ~31 | ~37 |
| 15 | ~24 | ~27 | ~29 | ~34 |
| 20 | ~22 | ~24 | ~27 | ~32 |
| 25 | ~19 | ~22 | ~24 | ~26 |
| 30 | ~18 | ~21 | ~23 | ~22 |
| 35 | ~17 | ~20 | ~21 | ~20 |
| 40 | ~17 | ~18 | ~19 | ~17 |
| 45 | ~17 | ~17 | ~18 | ~17 |
| 50 | ~17 | ~17 | ~17 | ~17 |
| OP | TESP-1 | TESP-2 | TESP-3 | |
|---|---|---|---|---|
| Average weight (g) | 4.38 ± 0.06 | 5.87 ± 0.05 | 5.91 ± 0.05 | 6.23 ± 0.06 |
| Type of Mechanical Test | OP | TESP-1 | TESP-2 | TESP-3 |
|---|---|---|---|---|
| Longitudinal compressive strength (MPa) | 32.14 ± 2.11 | 32.33 ± 2.30 | 33.19 ± 1.97 | 36.93 ± 2.02 |
| Radial bending strength (MPa) | 66.98 ± 3.12 | 101.04 ± 3.31 | 113.97 ± 4.02 | 114.07 ± 3.61 |
| Radial bending elastic modulus (GPa) | 7.51 ± 0.25 | 11.05 ± 0.18 | 12.57 ± 0.14 | 13.11 ± 0.18 |
| Tangential section hardness (kN) | 2.34 ± 0.04 | 3.91 ± 0.03 | 4.02 ± 0.02 | 4.23 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, W.; Li, C.; Sun, D.; Zou, N. A Simpler Fabrication for Thermal Energy Storage Wood. Forests 2023, 14, 1190. https://doi.org/10.3390/f14061190
Zou W, Li C, Sun D, Zou N. A Simpler Fabrication for Thermal Energy Storage Wood. Forests. 2023; 14(6):1190. https://doi.org/10.3390/f14061190
Chicago/Turabian StyleZou, Weihua, Cong Li, Delin Sun, and Naike Zou. 2023. "A Simpler Fabrication for Thermal Energy Storage Wood" Forests 14, no. 6: 1190. https://doi.org/10.3390/f14061190
APA StyleZou, W., Li, C., Sun, D., & Zou, N. (2023). A Simpler Fabrication for Thermal Energy Storage Wood. Forests, 14(6), 1190. https://doi.org/10.3390/f14061190





