Identification of Eight Pterocarpus Species and Two Dalbergia Species Using Visible/Near-Infrared (Vis/NIR) Hyperspectral Imaging (HSI)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Equipment and Spectra Acquisition
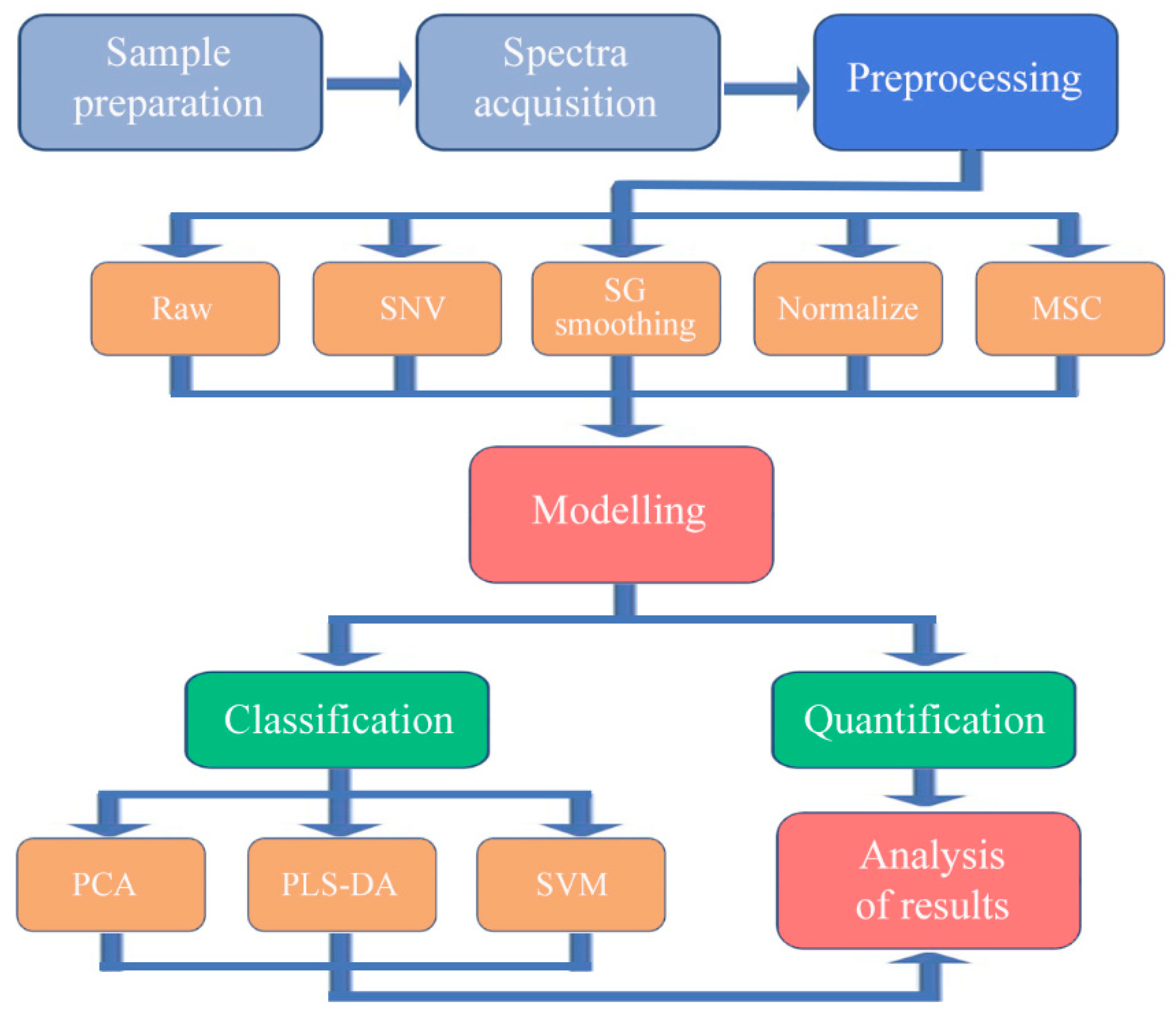
2.3. Model Development
3. Results
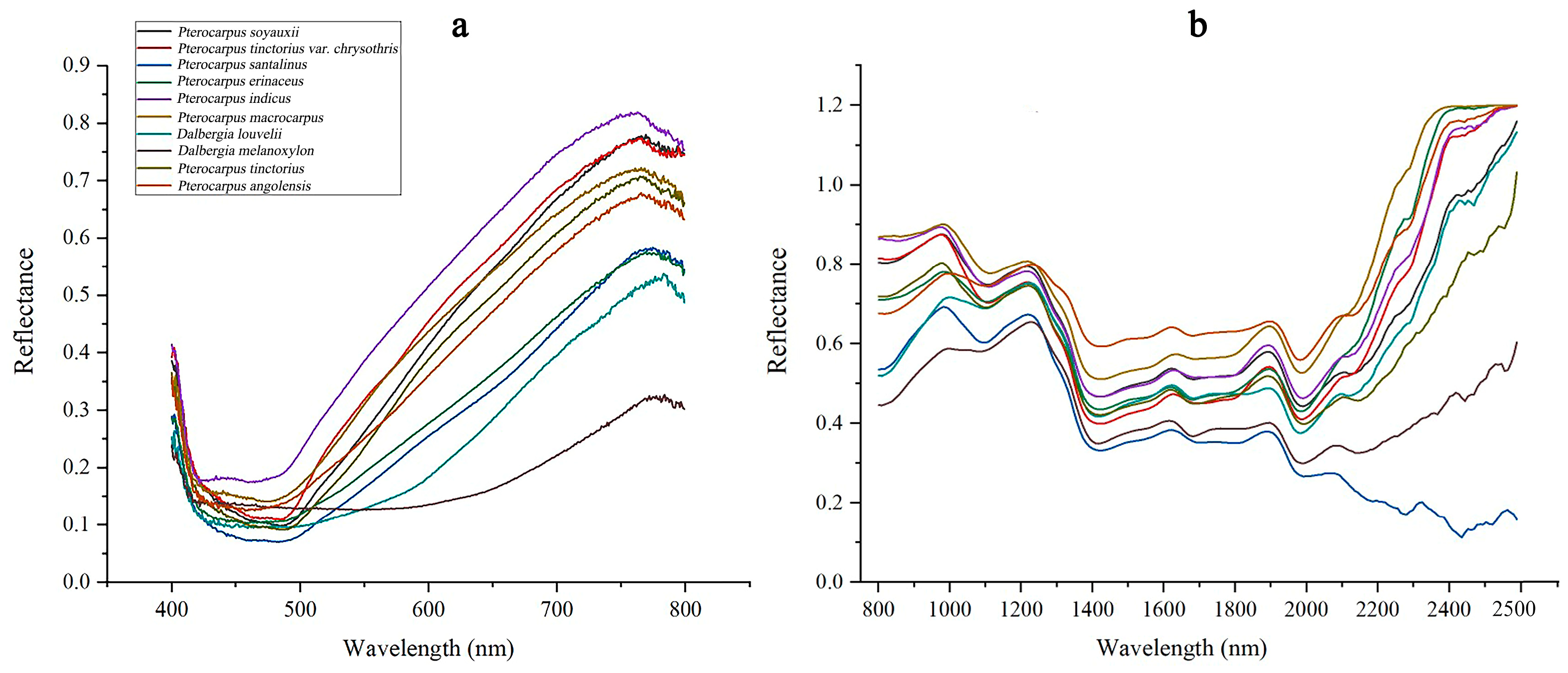
3.1. Spectroscopic Characterization
3.2. Principal Component Analysis
3.3. Results Using PLS-DA
3.4. Results Using SVM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karthikeyan, A.; Arunprasad, T. Growth Response of Pterocarpus santalinus Seedlings to Native Microbial Symbionts (Arbuscular mycorrhizal Fungi and Rhizobium aegyptiacum) under Nursery Conditions. J. For. Res. 2021, 32, 225–231. [Google Scholar] [CrossRef]
- Soundararajan, V. A Review on red sanders (Pterocarpus santalinus linn.)-Phyto-Chemistry and pharmacological importance. World J. Pharm. Pharm. Sci. 2016, 5, 667–689. [Google Scholar]
- Kukrety, S.; Dwivedi, P.; Jose, S.; Alavalapati, J.R.R. Stakeholders’ Perceptions on Developing Sustainable Red Sanders (Pterocarpus santalinus L.) Wood Trade in Andhra Pradesh, India. For. Policy Econ. 2013, 26, 43–53. [Google Scholar] [CrossRef]
- Prakash, E.; Sha Valli Khan, P.S.; Sreenivasa Rao, T.J.V.; Meru, E.S. Micropropagation of Red Sanders (Pterocarpus santalinus L.) Using Mature Nodal Explants. J. Res. 2006, 11, 329–335. [Google Scholar] [CrossRef]
- Arunkumar, A.N.; Joshi, G. Pterocarpus santalinus (Red Sanders) an Endemic, Endangered Tree of India: Current Status, Improvement and the Future. J. Trop. For. Environ. 2014, 4, 1–10. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Martin, S.; Perinbam, K.; Arockianathan, P.M.; Beatrice, V. Free Radical Scavenging Activity and HPTLC Finger Print of Pterocarpus santalinus L.—An in Vitro Study. Indian J. Sci. Technol. 2008, 1, 1–3. [Google Scholar] [CrossRef]
- Jiao, L.; Yu, M.; Wiedenhoeft, A.C.; He, T.; Li, J.; Liu, B.; Jiang, X.; Yin, Y. DNA Barcode Authentication and Library Development for the Wood of Six Commercial Pterocarpus Species: The Critical Role of Xylarium Specimens. Sci. Rep. 2018, 8, 1945. [Google Scholar] [CrossRef] [Green Version]
- Braga, J.W.B.; Pastore, T.C.M.; Coradin, V.T.R.; Camargos, J.A.A.; Silva, A.R. da The Use of near Infrared Spectroscopy to Identify Solid Wood Specimens of Swietenia Macrophylla0 (Cites Appendix II). IAWA J. 2011, 32, 285–296. [Google Scholar] [CrossRef]
- MacLachlan, I.R.; Gasson, P. PCA of Cites Listed Pterocarpus santalinus (Leguminosae) Wood. IAWA J. 2010, 31, 121–138. [Google Scholar] [CrossRef] [Green Version]
- Gasson, P. How Precise Can Wood Identification Be? Wood Anatomy’s Role in Support of the Legal Timber Trade, Especially Cites. IAWA J. 2011, 32, 137–154. [Google Scholar] [CrossRef]
- Ravindran, P.; Thompson, B.J.; Soares, R.K.; Wiedenhoeft, A.C. The XyloTron: Flexible, Open-Source, Image-Based Macroscopic Field Identification of Wood Products. Front. Plant Sci. 2020, 11, 1015. [Google Scholar] [CrossRef] [PubMed]
- Brunswick, P.; Cuthbertson, D.; Yan, J.; Chua, C.C.; Duchesne, I.; Isabel, N.; Evans, P.D.; Gasson, P.; Kite, G.; Bruno, J.; et al. A Practical Study of CITES Wood Species Identification by Untargeted DART/QTOF, GC/QTOF and LC/QTOF Together with Machine Learning Processes and Statistical Analysis. Environ. Adv. 2021, 5, 100089. [Google Scholar] [CrossRef]
- Sun, X.; Li, H.; Yi, Y.; Hua, H.; Guan, Y.; Chen, C. Rapid Detection and Quantification of Adulteration in Chinese Hawthorn Fruits Powder by Near-Infrared Spectroscopy Combined with Chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 250, 119346. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Z.; Xu, L.-L.; Tang, G.-Q.; Song, Q.-Q.; Feng, Q.-X. Rapid Detection of Surface Color of Shatian Pomelo Using Vis-NIR Spectrometry for the Identification of Maturity. Food Anal. Methods 2016, 9, 192–201. [Google Scholar] [CrossRef]
- da Silva, V.A.G.; Talhavini, M.; Peixoto, I.C.F.; Zacca, J.J.; Maldaner, A.O.; Braga, J.W.B. Non-Destructive Identification of Different Types and Brands of Blue Pen Inks in Cursive Handwriting by Visible Spectroscopy and PLS-DA for Forensic Analysis. Microchem. J. 2014, 116, 235–243. [Google Scholar] [CrossRef]
- Wu, D.; He, Y.; Nie, P.; Cao, F.; Bao, Y. Hybrid Variable Selection in Visible and Near-Infrared Spectral Analysis for Non-Invasive Quality Determination of Grape Juice. Anal. Chim. Acta 2010, 659, 229–237. [Google Scholar] [CrossRef]
- He, K.; Zhong, M.; Li, Z.; Liu, J. Near-Infrared Spectroscopy for the Concurrent Quality Prediction and Status Monitoring of Gasoline Blending. Control Eng. Pract. 2020, 101, 104478. [Google Scholar] [CrossRef]
- Jingyan, L.I.; Xiaoli, C.H.U.; Pu, C.; Songbai, T. Application of Spectral Automatic Retrieval Algorithm on the Rapid Establishment of Gasoline Spectral Database. Acta Pet. Sin. (Pet. Process. Sect.) 2017, 33, 131. [Google Scholar]
- Lin, Z.D.; Wang, Y.B.; Wang, R.J.; Wang, L.S.; Lu, C.P.; Zhang, Z.Y.; Song, L.T.; Liu, Y. Improvements of the Vis-NIRS Model in the Prediction of Soil Organic Matter Content Using Spectral Pretreatments, Sample Selection, and Wavelength Optimization. J. Appl. Spectrosc. 2017, 84, 529–534. [Google Scholar] [CrossRef]
- Mishra, P.; Herrmann, I.; Angileri, M. Improved Prediction of Potassium and Nitrogen in Dried Bell Pepper Leaves with Visible and Near-Infrared Spectroscopy Utilising Wavelength Selection Techniques. Talanta 2021, 225, 121971. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, C.; Takeda, F. Nondestructive Detection and Quantification of Blueberry Bruising Using Near-Infrared (NIR) Hyperspectral Reflectance Imaging. Sci. Rep. 2016, 6, 35679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, I.A.; Conceição, D.G.; Viana, M.B.; de J. Silva, G.; Santos, L.S.; Ferrão, S.P.B. NIR and MIR Spectroscopy for Quick Detection of the Adulteration of Cocoa Content in Chocolates. Food Chem. 2021, 349, 129095. [Google Scholar] [CrossRef] [PubMed]
- Lakeh, M.A.; Karimvand, S.K.; Khoshayand, M.R.; Abdollahi, H. Analysis of Residual Moisture in a Freeze-Dried Sample Drug Using a Multivariate Fitting Regression Model. Microchem. J. 2020, 154, 104516. [Google Scholar] [CrossRef]
- Amaral, E.A.; dos Santos, L.M.; Hein, P.R.G.; Costa, E.V.S.; Rosado, S.C.S.; Trugilho, P.F. Evaluating Basic Density Calibrations Based on NIR Spectra Recorded on the Three Wood Faces and Subject to Different Mathematical Treatments. N. Z. J. For. Sci. 2021, 51. [Google Scholar] [CrossRef]
- Blaschek, M.; Roudier, P.; Poggio, M.; Hedley, C.B. Prediction of Soil Available Water-Holding Capacity from Visible near-Infrared Reflectance Spectra. Sci. Rep. 2019, 9, 12833. [Google Scholar] [CrossRef] [Green Version]
- Pace, J.-H.C.; de F. Latorraca, J.-V.; Hein, P.; da Silva, C.-E.S. Wood Species Identification from Atlantic Forest by near Infrared Spectroscopy. For. Syst. 2019, 28, 3. [Google Scholar]
- Xue, X.; Chen, Z.; Wu, H.; Gao, H. Identification of Guiboutia Species by NIR-HSI Spectroscopy. Sci. Rep. 2022, 12, 11507. [Google Scholar] [CrossRef]
- Ziyang, W.; Shikui, Y.I.N.; Ying, L.I.; Yaoxiang, L.I. Identification of common wood species in northeast China using Vis/NIR spectroscopy. J. Zhejiang AF Univ. 2019, 36, 162–169. [Google Scholar]
- Zhao, P.; Li, Z.-Y.; Wang, C.-K. Wood Species Recognition Based on Visible and Near-Infrared Spectral Analysis Using Fuzzy Reasoning and Decision-Level Fusion. J. Spectrosc. 2021, 2021, e6088435. [Google Scholar] [CrossRef]
- Sandak, J.; Sandak, A.; Zitek, A.; Hintestoisser, B.; Picchi, G. Development of Low-Cost Portable Spectrometers for Detection of Wood Defects. Sensors 2020, 20, 545. [Google Scholar] [CrossRef] [Green Version]
- Palacios-Morillo, A.; Jurado, J.M.; Alcázar, A.; Pablos, F. Differentiation of Spanish Paprika from Protected Designation of Origin Based on Color Measurements and Pattern Recognition. Food Control 2016, 62, 243–249. [Google Scholar] [CrossRef]
- Andrés, S.; Murray, I.; Navajas, E.A.; Fisher, A.V.; Lambe, N.R.; Bünger, L. Prediction of Sensory Characteristics of Lamb Meat Samples by near Infrared Reflectance Spectroscopy. Meat Sci. 2007, 76, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Emsley, A.M.; Herman, H.; Heywood, R.J. Spectroscopic Studies of the Ageing of Cellulosic Paper. Polymer 2001, 42, 2893–2900. [Google Scholar] [CrossRef]
- Núñez-Sánchez, N.; Martínez-Marín, A.L.; Polvillo, O.; Fernández-Cabanás, V.M.; Carrizosa, J.; Urrutia, B.; Serradilla, J.M. Near Infrared Spectroscopy (NIRS) for the Determination of the Milk Fat Fatty Acid Profile of Goats. Food Chem. 2016, 190, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Badaró, A.T.; Morimitsu, F.L.; Ferreira, A.R.; Clerici, M.T.P.S.; Fernandes Barbin, D. Identification of Fiber Added to Semolina by near Infrared (NIR) Spectral Techniques. Food Chem. 2019, 289, 195–203. [Google Scholar] [CrossRef]
- Krähmer, A.; Engel, A.; Kadow, D.; Ali, N.; Umaharan, P.; Kroh, L.W.; Schulz, H. Fast and Neat—Determination of Biochemical Quality Parameters in Cocoa Using near Infrared Spectroscopy. Food Chem. 2015, 181, 152–159. [Google Scholar] [CrossRef]
- Lequeue, G.; Draye, X.; Baeten, V. Determination by near Infrared Microscopy of the Nitrogen and Carbon Content of Tomato (Solanum lycopersicum L.) Leaf Powder. Sci. Rep. 2016, 6, 33183. [Google Scholar] [CrossRef]
- Bonaccorsi, I.; Cacciola, F.; Utczas, M.; Inferrera, V.; Giuffrida, D.; Donato, P.; Dugo, P.; Mondello, L. Characterization of the Pigment Fraction in Sweet Bell Peppers (Capsicum annuum L.) Harvested at Green and Overripe Yellow and Red Stages by Offline Multidimensional Convergence Chromatography/Liquid Chromatography-Mass Spectrometry. J. Sep. Sci. 2016, 39, 3281–3291. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, B.; Ren, C.; Guo, M.; Wang, J.; Shi, K.; Wu, X.; Feng, Y. Rapid Identification Model Based on Decision Tree Algorithm Coupling with 1H NMR and Feature Analysis by UHPLC-QTOFMS Spectrometry for Sandalwood. J. Chromatogr. B 2020, 1161, 122449. [Google Scholar] [CrossRef]
- Berrueta, L.A.; Alonso-Salces, R.M.; Héberger, K. Supervised Pattern Recognition in Food Analysis. J. Chromatogr. A 2007, 1158, 196–214. [Google Scholar] [CrossRef]
- Lestander, T.A.; Lindeberg, J.; Eriksson, D.; Bergsten, U. Prediction of Pinus Sylvestris Clear-Wood Properties Using NIR Spectroscopy and Biorthogonal Partial Least Squares Regression. Can. J. For. Res. 2008, 38, 2052–2062. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Wang, Z.; Han, X.; Hou, H.; Zheng, R. Comparative Investigation of Partial Least Squares Discriminant Analysis and Support Vector Machines for Geological Cuttings Identification Using Laser-Induced Breakdown Spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2014, 102, 52–57. [Google Scholar] [CrossRef]
- Li, Y.; Via, B.K.; Young, T.; Li, Y. Visible-Near Infrared Spectroscopy and Chemometric Methods for Wood Density Prediction and Origin/Species Identification. Forests 2019, 10, 1078. [Google Scholar] [CrossRef] [Green Version]
- Estimation of Pinus Radiata D. Don Tracheid Morphological Characteristics by near Infrared Spectroscopy. Available online: https://www.degruyter.com/document/doi/10.1515/HF.2004.009/html (accessed on 3 May 2023).
- Wang, C.-K.; Zhao, P.; Li, Z.-Y.; Li, X.-H. Comparison of VIS/NIR Spectral Curves plus RGB Images with Hyperspectral Images for the Identification of Pterocarpus Species. Holzforschung 2022, 76, 579–591. [Google Scholar] [CrossRef]
- Bächle, H.; Zimmer, B.; Wegener, G. Classification of Thermally Modified Wood by FT-NIR Spectroscopy and SIMCA. Wood Sci. Technol. 2012, 46, 1181–1192. [Google Scholar] [CrossRef]
- Bächle, H.; Zimmer, B.; Windeisen, E.; Wegener, G. Evaluation of Thermally Modified Beech and Spruce Wood and Their Properties by FT-NIR Spectroscopy. Wood Sci. Technol. 2010, 44, 421–433. [Google Scholar] [CrossRef]
- Karthick, M.; Parthiban, K.T. Chemical Characterization of Pterocarpus santalinus Wood Using GC-MS. J. Pharm. Phytochem. 2019, 8, 380–382. [Google Scholar]



| Scientific Classification | Calibration Set | Validation Set |
|---|---|---|
| Pterocarpus soyauxii | 60 | 20 |
| Pterocarpus tinctorius var. chrysothris | 60 | 20 |
| Pterocarpus santalinus | 60 | 20 |
| Pterocarpus erinaceus | 60 | 20 |
| Pterocarpus indicus | 60 | 20 |
| Pterocarpus macrocarpus | 60 | 20 |
| Dalbergia louvelii | 60 | 20 |
| Dalbergia melanoxylon | 60 | 20 |
| Pterocarpus tinctorius | 60 | 20 |
| Pterocarpus angolensis | 60 | 20 |
| 400~800 nm | 800~2500 nm | 400~2500 nm | |||||
|---|---|---|---|---|---|---|---|
| Calibration Set (%) | Validation Set (%) | Calibration Set (%) | Validation Set (%) | Calibration Set (%) | Validation Set (%) | ||
| Preprocessing | Raw | 88 | 84.5 | 96.7 | 96.5 | 90.3 | 94 |
| SNV | 79.2 | 76 | 92.8 | 92 | 86 | 88 | |
| SG Smoothing | 88.8 | 85.5 | 96.8 | 96.5 | 90.3 | 94 | |
| Normalization | 88.8 | 85 | 96.5 | 96 | 90.3 | 94 | |
| MSC | 88.8 | 85.5 | 92.7 | 92 | 85.8 | 88.5 | |
| 400~800 nm | 800~2500 nm | 400~2500 nm | |||||
|---|---|---|---|---|---|---|---|
| Calibration Set (%) | Validation Set (%) | Calibration Set (%) | Validation Set (%) | Calibration Set (%) | Validation Set (%) | ||
| Preprocessing | Raw | 96.3 | 94.5 | 99.7 | 99.5 | 99.8 | 99.5 |
| SNV | 93.2 | 92.5 | 97.3 | 99.5 | 95.8 | 98 | |
| SG Smoothing | 96.5 | 95 | 99.7 | 99.5 | 99.8 | 99.5 | |
| Normalization | 96.5 | 95 | 99.7 | 100 | 99.8 | 100 | |
| MSC | 92.2 | 91.5 | 97.3 | 99.5 | 96.7 | 98.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, X.; Chen, Z.; Wu, H.; Gao, H.; Nie, J.; Li, X. Identification of Eight Pterocarpus Species and Two Dalbergia Species Using Visible/Near-Infrared (Vis/NIR) Hyperspectral Imaging (HSI). Forests 2023, 14, 1259. https://doi.org/10.3390/f14061259
Xue X, Chen Z, Wu H, Gao H, Nie J, Li X. Identification of Eight Pterocarpus Species and Two Dalbergia Species Using Visible/Near-Infrared (Vis/NIR) Hyperspectral Imaging (HSI). Forests. 2023; 14(6):1259. https://doi.org/10.3390/f14061259
Chicago/Turabian StyleXue, Xiaoming, Zhenan Chen, Haoqi Wu, Handong Gao, Jiajie Nie, and Xinyang Li. 2023. "Identification of Eight Pterocarpus Species and Two Dalbergia Species Using Visible/Near-Infrared (Vis/NIR) Hyperspectral Imaging (HSI)" Forests 14, no. 6: 1259. https://doi.org/10.3390/f14061259






