Effects of Drought and Flooding on Growth and Physiology of Cinnamomum camphora Seedlings
Abstract
:1. Introduction
2. Materials and Methods
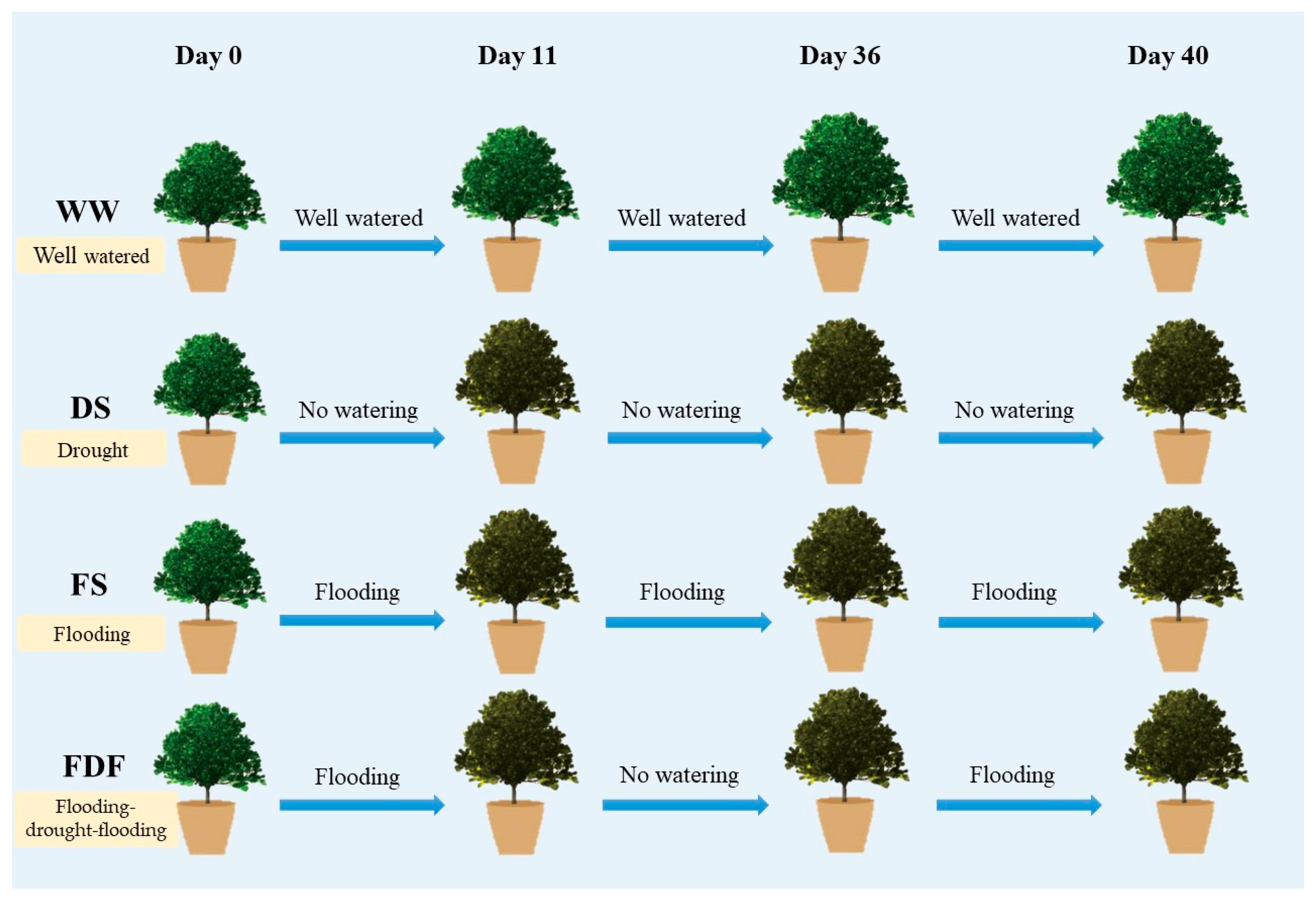
2.1. Plant Materials and Experimental Design
2.2. Biomass Measurements
2.3. Gas Exchange and Relative Chlorophyll Content (SPAD) Measurements
2.4. Water Potential Measurements
2.5. Biochemical Measurements
2.6. Statistical Analyses
3. Results
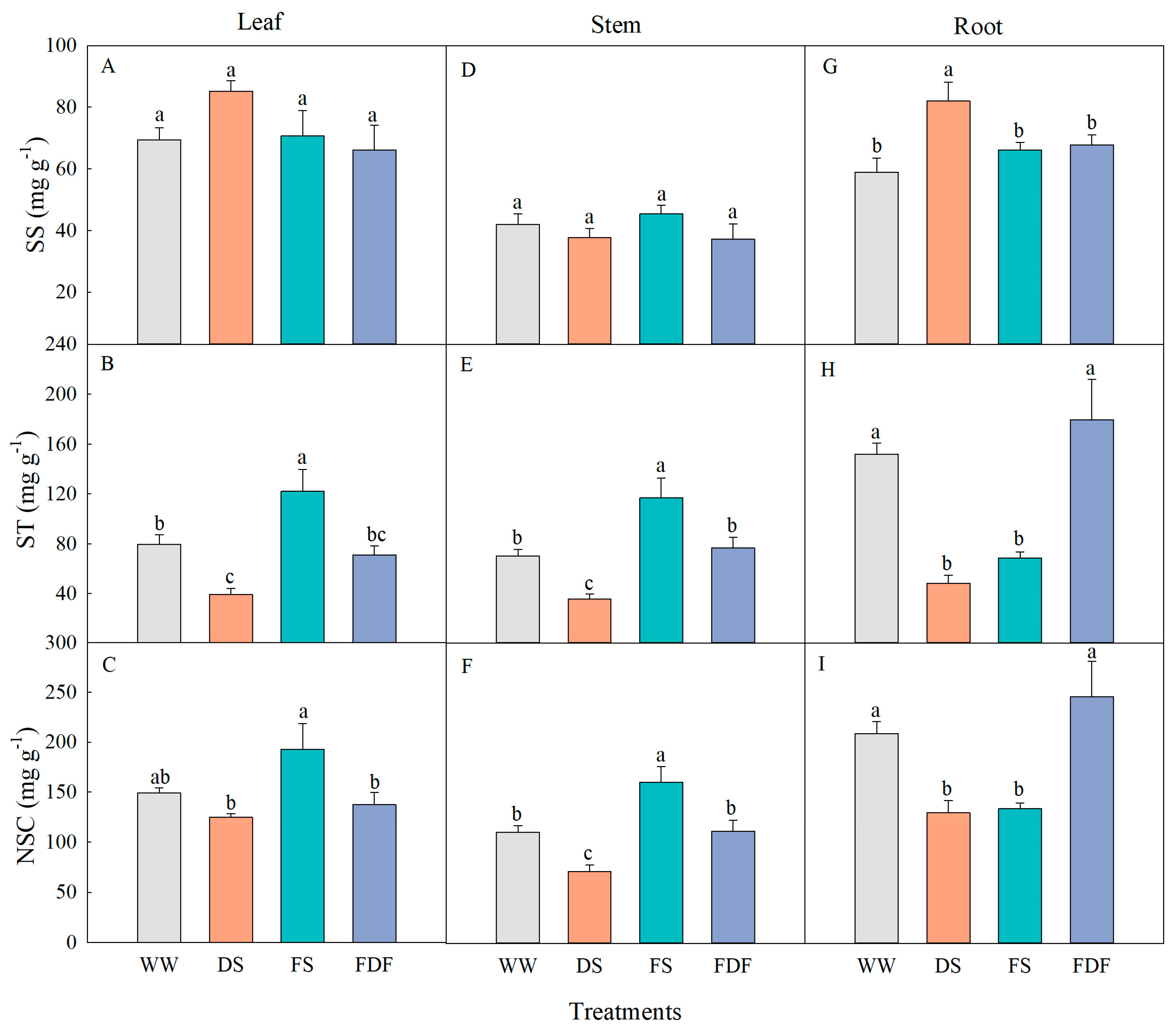
3.1. Morphological Responses
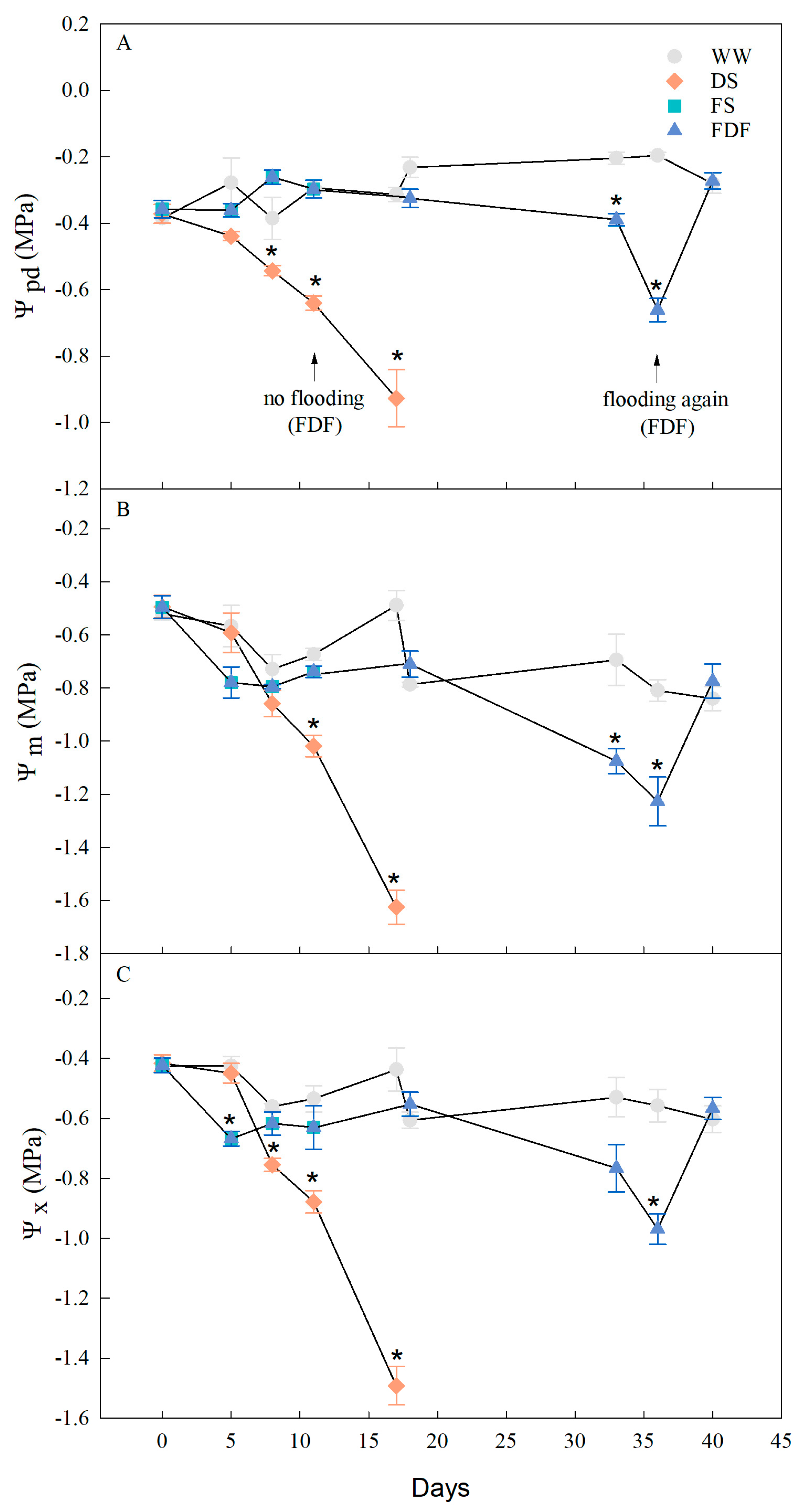
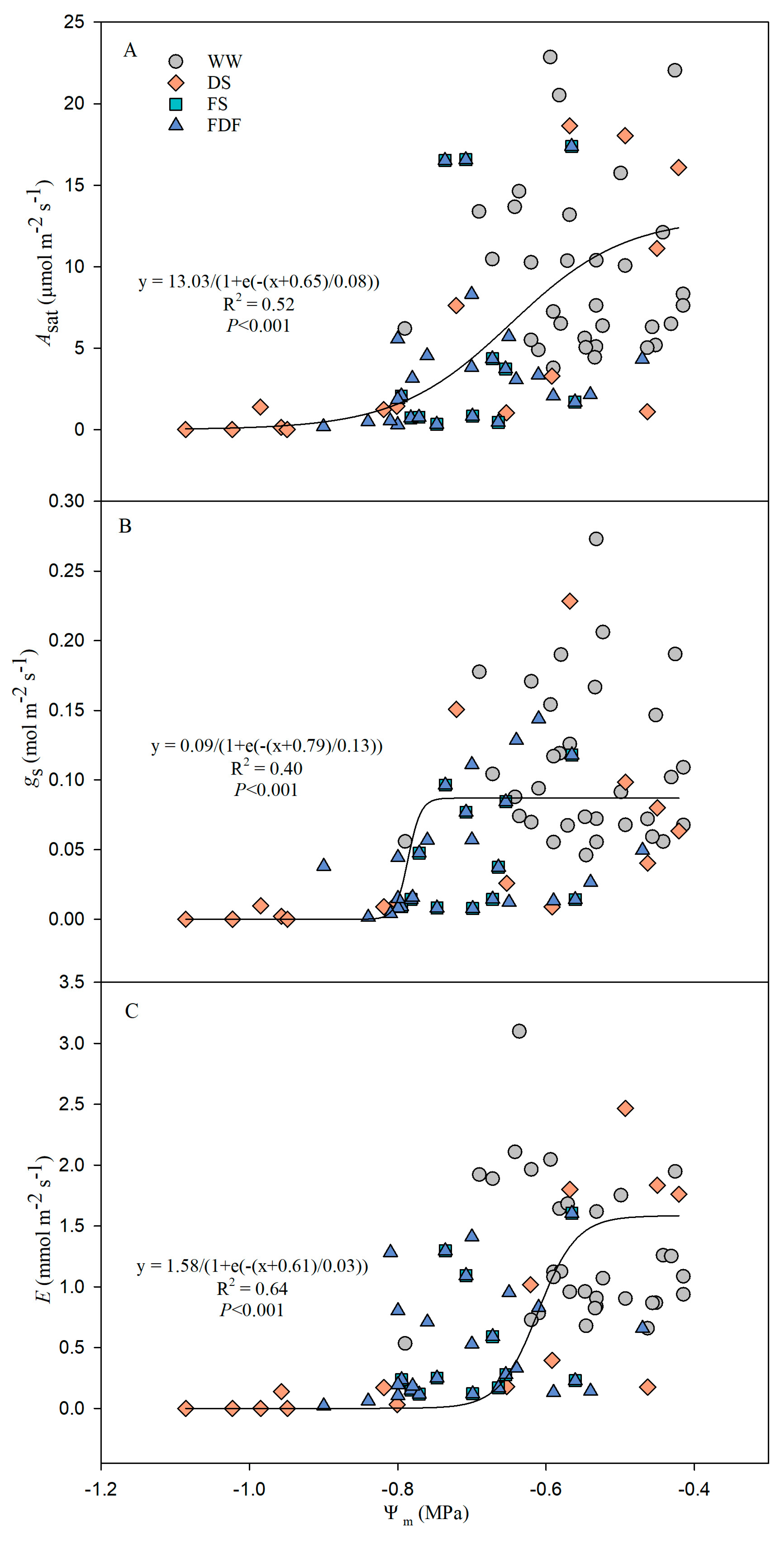
3.2. Water Potential
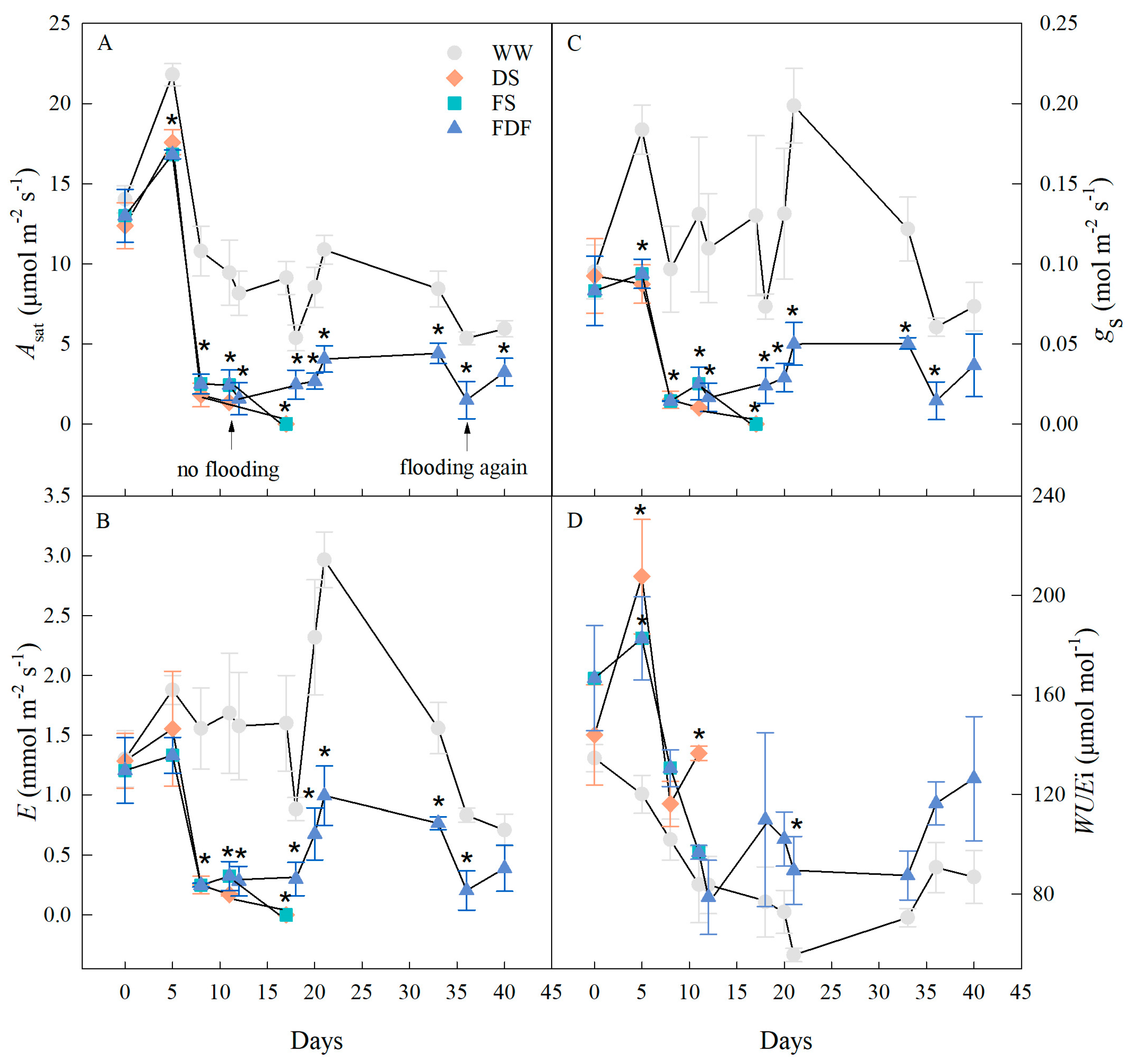
3.3. Gas Exchange
3.4. Non-Structural Carbohydrates
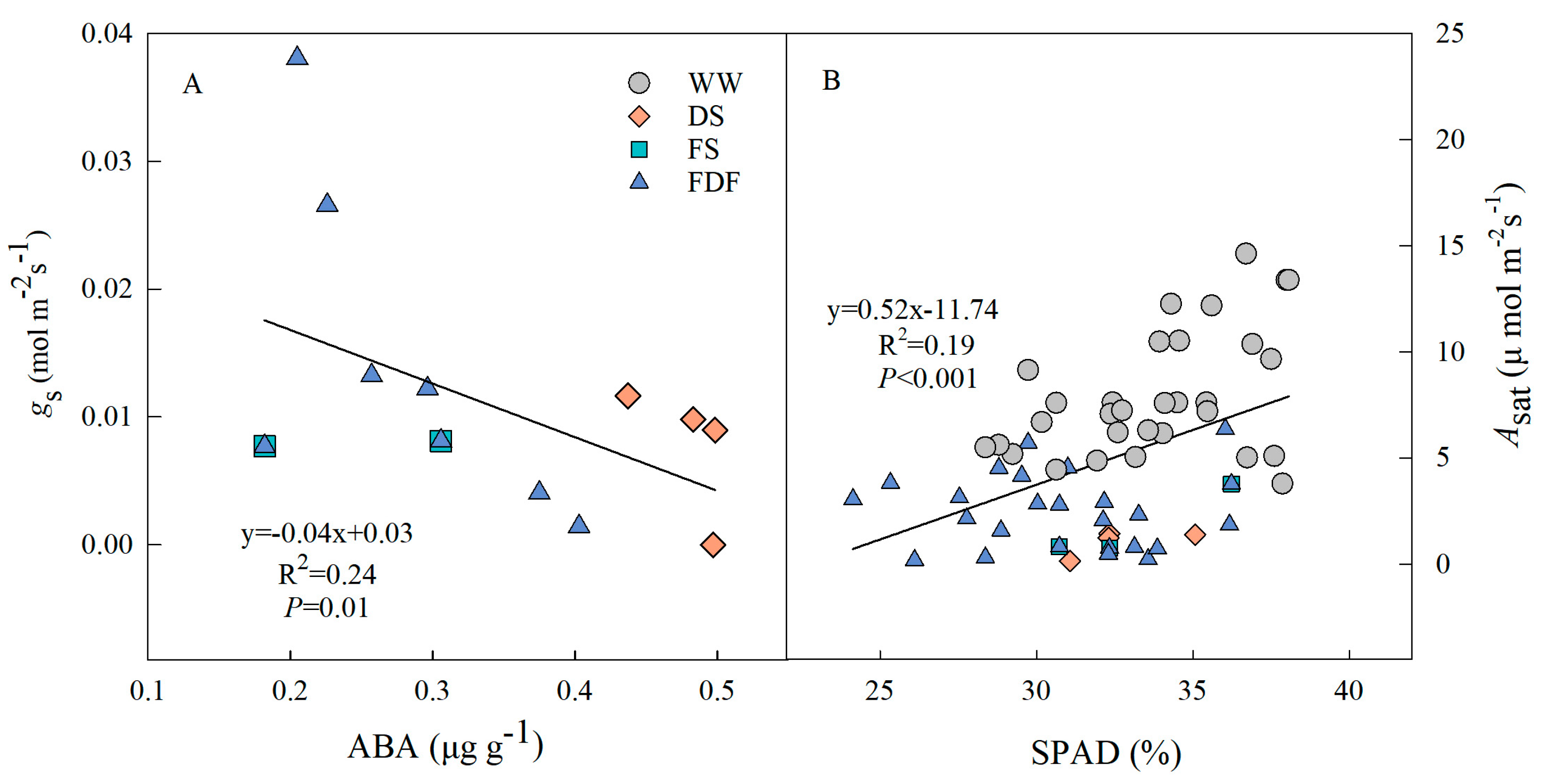
3.5. ABA and Proline
3.6. Element Stoichiometry and 13C Composition
4. Discussion
4.1. Plant Biomass and Allocation Responses
4.2. Gas Exchange and Water Potential Responses
4.3. NSC, Proline, and Nutrient Responses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change 2021 the Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- Kahraman, A.; Kendon, E.J.; Chan, S.C.; Fowler, H.J. Quasi-Stationary intense rainstorms spread across Europe under climate change. Geophys. Res. Lett. 2021, 48, e2020GL092361. [Google Scholar] [CrossRef]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.B.; Chen, M.X.; Wang, L.N.; Sasidharan, R.; Voesenek, L.A.; Xiao, S. Multi-stress resilience in plants recovering from submergence. Plant Biotechnol. J. 2022, 21, 466–481. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Chen, J.; Duan, B.; Wang, M.; Korpelainen, H.; Li, C. Intra-and inter-sexual competition of Populus cathayana under different watering regimes. Funct. Ecol. 2014, 28, 124–136. [Google Scholar] [CrossRef]
- Duan, H.L.; Li, Y.; Xu, Y.; Zhou, S.; Liu, J.; David, T.T.; Liu, J. Contrasting drought sensitivity and post-drought resilience among three co-occurring tree species in subtropical China. Agric. For. Meteorol. 2019, 272–273, 55–68. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Santos, D.L.; Moutinho-Pereira, J.M.; Goncalves, B.C.; Ferreira, H.F.; Correia, C.M. Immediate responses and adaptative strategies of three olive cultivars under contrasting water availability regimes: Changes on structure and chemical composition of foliage and oxidative damage. Plant Sci. 2006, 170, 596–605. [Google Scholar] [CrossRef]
- Boughalleb, F.; Hajlaoui, H. Physiological and anatomical changes induced by drought in two olive cultivars (cv Zalmati and Chemlali). Acta Physiol. Plant 2011, 33, 53–65. [Google Scholar] [CrossRef]
- Azeem, A.; Sun, J.; Javed, Q.; Jabran, K.; Du, D. The effect of submergence and eutrophication on the trait’s performance of Wedelia trilobata over its congener native Wedelia chinensis. Water 2020, 12, 934. [Google Scholar] [CrossRef] [Green Version]
- Ferner, E.; Rennenberg, H.; Kreuzwieser, J. Effect of flooding on C metabolism of flood-tolerant (Quercus robur) and non-tolerant (Fagus sylvatica) tree species. Tree Physiol. 2012, 32, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.; Ma, M.; Chen, J.; Wu, S. Plant morphological, physiological and anatomical adaption to flooding stress and the underlying molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 1088. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cheng, R.; Xiao, W.; Guo, Q.; Wang, N. Effect of off-season flooding on growth, photosynthesis, carbohydrate partitioning, and nutrient uptake in Distylium chinense. PLoS ONE 2014, 9, e107636. [Google Scholar] [CrossRef] [PubMed]
- Else, M.A.; Coupland, D.; Dutton, L.; Jackson, M.B. Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinus communis) despite diminished delivery of ABA from the roots to shoots in xylem sap. Physiol. Plant. 2001, 111, 46–54. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Rennenberg, H. Molecular and physiological responses of trees to waterlogging stress. Plant Cell Environ. 2014, 37, 2245–2259. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.F.; Yang, F. Sex-specific responses to winter flooding, spring waterlogging and post-flooding recovery in Populus deltoides. Sci. Rep. 2017, 7, 2534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, A. Responses to flooding of plant water relations and leaf gas exchange in tropical tolerant trees of a black-water wetland. Front. Plant Sci. 2013, 4, 106. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sun, H.; Sheng, J.; Jin, S.; Zhou, F.; Hu, Z.; Diao, Y. Transcriptome, physiological and biochemical analysis of Triarrhena sacchariflora in response to flooding stress. BMC Genet. 2019, 20, 88. [Google Scholar] [CrossRef]
- Loreti, E.; Valeri, M.C.; Novi, G.; Perata, P. Gene regulation and survival under hypoxia requires starch availability and metabolism. Plant Physiol. 2018, 176, 1286–1298. [Google Scholar] [CrossRef]
- Irfan, M.; Hayat, S.; Hayat, Q.; Afroz, S.; Ahmad, A. Physiological and biochemical changes in plants under waterlogging. Protoplasma 2010, 241, 3–17. [Google Scholar] [CrossRef]
- Zuniga-Feest, A.; Bustos-Salazar, A.; Alves, F.; Martinez, V.; Smith-Ramirez, C. Physiological and morphological responses to permanent and intermittent waterlogging in seedlings of four evergreen trees of temperate swamp forests. Tree Physiol. 2017, 37, 779–789. [Google Scholar] [CrossRef] [Green Version]
- Guillermo, N.D.; Silvia, E.M.; María, E.R.; Virginia, M.C.L. Physiological responses to alternative flooding and drought stress episodes in two willow (Salix spp.) clones. Can. J. For. Res. 2016, 47, 174–182. [Google Scholar]
- CMA Climate Change Centre. Blue Book on Climate Change in China Science; CMA Climate Change Centre: Beijing, China, 2022. [Google Scholar]
- Li, X.H.; Zhang, Q.; Zhang, D.; Ye, X.C. Investigation of the drought-flood abrupt alternation of streamflow in Poyang Lake catchment during the last 50 years. Hydrol. Res. 2017, 48, 1402–1417. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Wei, X.; Huang, G.; Fan, H.; Zhou, S.; Wan, S. The decoupling between gas exchange and water potential of Cinnamomum camphora seedlings during drought recovery and its relation to ABA accumulation in leaves. J. Plant Ecol. 2020, 13, 683–692. [Google Scholar] [CrossRef]
- Choi, D.S.; Hwang, B.K. Proteomics and functional analyses of pepper abscisic acid–responsive 1 (ABR1), which is involved in cell death and defense signaling. Plant Cell 2011, 23, 823–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Lu, C.; Feng, X.; Guo, G. Effect of autumn foliar application of fertilizers on cold resistance of Red Globe grapes. J. Northwest AF Univ. 2014, 42, 126–132. [Google Scholar]
- Duan, H.L.; Huang, G.; Zhou, S.; Tissue, D.T. Dry mass production, allocation patterns and water use efficiency of two conifers with different water use strategies under elevated [CO2], warming and drought conditions. Eur. J. For. Res. 2018, 5, 605–618. [Google Scholar] [CrossRef]
- Kasurinen, A.; Koikkalainen, K.; Anttonen, M.J.; Possen, B.; Oksanen, E.; Rousi, M.; Holopainen, T. Root morphology, mycorrhizal roots and extramatrical mycelium growth in silver birch (Betula pendula Roth) genotypes exposed to experimental warming and soil moisture manipulations. Plant Soil 2016, 407, 341–353. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C. Individual and interactive responses of woody plants’ biomass and leaf traits to drought and shade. Glob. Ecol. Biogeogr. 2022, 32, 35–48. [Google Scholar] [CrossRef]
- Eziz, A.; Yan, Z.; Tian, D.; Han, W.; Tang, Z.; Fang, J. Drought effect on plant biomass allocation: A meta-analysis. Ecol. Evol. 2017, 7, 11002–11010. [Google Scholar] [CrossRef]
- Yulianti, Y.; Sudrajat, D.J. Morphological responses, sensitivity and tolerance indices of four tropical trees species to drought and waterlogging. 2016, 17, 110–115. Biodivers. J. Biol. Divers.
- Parad, G.A.; Zarafshar, M.; Striker, G.G.; Sattarian, A. Some physiological and morphological responses of Pyrus boissieriana to flooding. Trees 2013, 27, 1387–1393. [Google Scholar] [CrossRef]
- Striker, G.G. Time is on our side: The importance of considering a recovery period when assessing flooding tolerance in plants. Ecol. Res. 2012, 27, 983–987. [Google Scholar] [CrossRef]
- Yan, W.; Zhong, Y.; Shangguan, Z. Elevated temperature exacerbates the effects of drought on the carbon and hydraulic characteristics of Robinia pseudoacacia seedlings. Agric. For. Meteorol. 2020, 280, 107794. [Google Scholar] [CrossRef]
- Creek, D.; Lamarque, L.J.; Torres-Ruiz, J.M.; Parise, C.; Burlett, R.; Tissue, D.T.; Delzon, S. Xylem embolism in leaves does not occur with open stomata: Evidence from direct observations using the optical visualization technique. J. Exp. Bot. 2020, 71, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Cochard, H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol. 2009, 149, 575–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juzoń, K.; Czyczyło-Mysza, I.; Ostrowska, A.; Marcińska, I.; Skrzypek, E. Chlorophyll fluorescence for prediction of yellow lupin (Lupinus luteus L.) and pea (Pisum sativum L.) susceptibility to drought. Photosynthetica 2019, 57, 950–959. [Google Scholar] [CrossRef] [Green Version]
- Estill, K.; Delaney, R.H.; Smith, W.K.; Ditterline, R.L. Water relations and productivity of alfalfa leaf chlorophyll variants. Crop Sci. 1991, 31, 1229–1233. [Google Scholar] [CrossRef]
- Yang, Y.J.; Bi, M.H.; Nie, Z.F.; Jiang, H.; Liu, X.D.; Fang, X.W.; Brodribb, T.J. Evolution of stomatal closure to optimize water-use efficiency in response to dehydration in ferns and seed plants. New Phytol. 2021, 230, 2001–2010. [Google Scholar] [CrossRef]
- Rengifo, E.; Tezara, W.; Herrera, A. Water relations, chlorophyll a fluorescence, and contents of saccharides in tree species of a tropical forest in response to flood. Photosynthetica 2005, 43, 203–210. [Google Scholar] [CrossRef]
- Martorell, S.; Diaz-Espejo, A.; Medrano, H.; Ball, M.C.; Choat, B. Rapid hydraulic recovery in Eucalyptus pauciflora after drought: Linkages between stem hydraulics and leaf gas exchange. Plant Cell Environ. 2014, 37, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Trumbore, S. Understanding the roles of non-structural carbohydrates in forest trees from what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Liu, H.; Qi, Y.; Liu, F.; Zhu, X. Patterns in nonstructural carbohydrate contents at the tree organ level in response to drought duration. Glob. Chang. Biol. 2020, 26, 3627–3638. [Google Scholar] [CrossRef]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.S.; Gear, L.; Hultine, K.R.; Koch, G.W.; Ogle, K. Non-structural carbohydrate dynamics associated with antecedent stem water potential and air temperature in a dominant desert shrub. Plant Cell Environ. 2020, 43, 1467–1483. [Google Scholar] [CrossRef]
- Sapes, G.; Demaree, P.; Lekberg, Y.; Sala, A. Plant carbohydrate depletion impairs water relations and spreads via ectomycorrhizal networks. New Phytol. 2021, 229, 3172–3183. [Google Scholar] [CrossRef]
- Blumstein, M.; Gersony, J.; Martinez-Vilalta, J.; Sala, A. Global variation in nonstructural carbohydrate stores in response to climate. Glob. Chang. Biol. 2022, 29, 1854–1869. [Google Scholar] [CrossRef]
- Galvez, D.A.; Landhausser, S.M.; Tyree, M.T. Root carbon reserve dynamics in aspen seedlings: Does simulated drought induce reserve limitation? Tree Physiol. 2011, 31, 250–257. [Google Scholar] [CrossRef]
- Islam, M.A.; Macdonald, S. Ecophysiological adaptations of black spruce (Picea mariana) and tamarack (Larix laricina) seedlings to flooding. Trees 2004, 18, 35–42. [Google Scholar] [CrossRef]
- Ahmed, C.B.; Rouina, B.B.; Sensoy, S.; Boukhris, M.; Abdallah, F.B. Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. Environ. Exp. Bot. 2009, 67, 345–352. [Google Scholar] [CrossRef]
- Ditmarová, L.; Kurjak, D.; Palmroth, S.; Kmet, J.; Střelcová, K. Physiological responses of Norway spruce (Picea abies) seedlings to drought stress. Tree Physiol. 2010, 30, 205–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, E.C.; Nogueira, R.J.M.C.; Da Silva, M.A.; de Albuquerque, M.B. Drought stress and plant nutrition. Plant Stress 2011, 5, 32–41. [Google Scholar]
- Gessler, A.; Schaub, M.; McDowell, N.G. The role of nutrients in drought-induced tree mortality and recovery. New Phytol. 2017, 214, 513–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Alcántara, B.; Jover, S.; Quinones, A.; Forner-Giner, M.Á.; Rodríguez-Gamir, J.; Legaz, F.; Iglesias, D.J. Flooding affects uptake and distribution of carbon and nitrogen in citrus seedlings. J. Plant Physiol. 2012, 169, 1150–1157. [Google Scholar] [CrossRef]
- Wang, A.F.; Roitto, M.; Lehto, T.; Sutinen, S.; Heinonen, J.; Zhang, G.; Repo, T. Photosynthesis, nutrient accumulation and growth of two Betula species exposed to waterlogging in late dormancy and in the early growing season. Tree Physiol. 2017, 37, 767–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey-Serres, J.; Voesenek, L. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Parameter | Treatments | |||
|---|---|---|---|---|
| WW | DS | FS | FDF | |
| Total biomass (g) | 40.83 ± 0.60 a | 23.58 ± 0.57 b | 30.30 ± 1.75 b | 23.88 ± 4.05 b |
| Root-to-shoot ratio | 0.55 ± 0.02 b | 0.68 ± 0.06 a | 0.41 ± 0.04 c | 0.63 ± 0.02 ab |
| Root mass ratio | 0.35 ± 0.01 a | 0.40 ± 0.02 a | 0.29 ± 0.02 b | 0.39 ± 0.01 a |
| Stem mass ratio | 0.39 ± 0.01 ab | 0.42 ± 0.03 ab | 0.36 ± 0.02 b | 0.46 ± 0.03 a |
| Leaf mass ratio | 0.26 ± 0.01 b | 0.18 ± 0.02 c | 0.35 ± 0.03 a | 0.15 ± 0.03 c |
| Parameter | Treatments | |||
|---|---|---|---|---|
| WW | DS | FS | FDF | |
| C concentration (mg/g) | 280.06 ± 40.99 a | 285.81 ± 2.96 a | 272.23 ± 8.58 a | 282.52 ± 7.16 a |
| N concentration (mg/g) | 9.68 ± 0.30 a | 10.73 ± 0.71 a | 9.02 ± 1.09 a | 8.99 ± 0.66 a |
| P concentration (mg/g) | 1.99 ± 0.12 a | 1.99 ± 0.03 a | 2.01 ± 0.05 a | 2.17 ± 0.14 a |
| C:N (%) | 28.75 ± 3.34 a | 26.85 ± 1.61 a | 31.21 ± 4.30 a | 31.68 ± 1.76 a |
| C:P (%) | 141.60 ± 21.44 a | 143.41 ± 0.77 a | 135.75 ± 6.55 a | 131.41 ± 9.37 a |
| N:P (%) | 4.89 ± 0.33 a | 5.38 ± 0.37 a | 4.48 ± 0.46 a | 4.16 ± 0.26 a |
| 13C (‰) | −34.27 ± 0.40 b | −32.98 ± 0.40 a | −33.68 ± 0.33 ab | −33.52 ± 0.25 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, D.; Duan, H. Effects of Drought and Flooding on Growth and Physiology of Cinnamomum camphora Seedlings. Forests 2023, 14, 1343. https://doi.org/10.3390/f14071343
Zhao Y, Wang D, Duan H. Effects of Drought and Flooding on Growth and Physiology of Cinnamomum camphora Seedlings. Forests. 2023; 14(7):1343. https://doi.org/10.3390/f14071343
Chicago/Turabian StyleZhao, Yongju, Defu Wang, and Honglang Duan. 2023. "Effects of Drought and Flooding on Growth and Physiology of Cinnamomum camphora Seedlings" Forests 14, no. 7: 1343. https://doi.org/10.3390/f14071343
APA StyleZhao, Y., Wang, D., & Duan, H. (2023). Effects of Drought and Flooding on Growth and Physiology of Cinnamomum camphora Seedlings. Forests, 14(7), 1343. https://doi.org/10.3390/f14071343





