Impacting Factors of Changes in Dynamic Viscosity and Interfacial Tension of Wood Xylem Sap
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Morphological Analysis
2.3. Sap Velocity Measurement
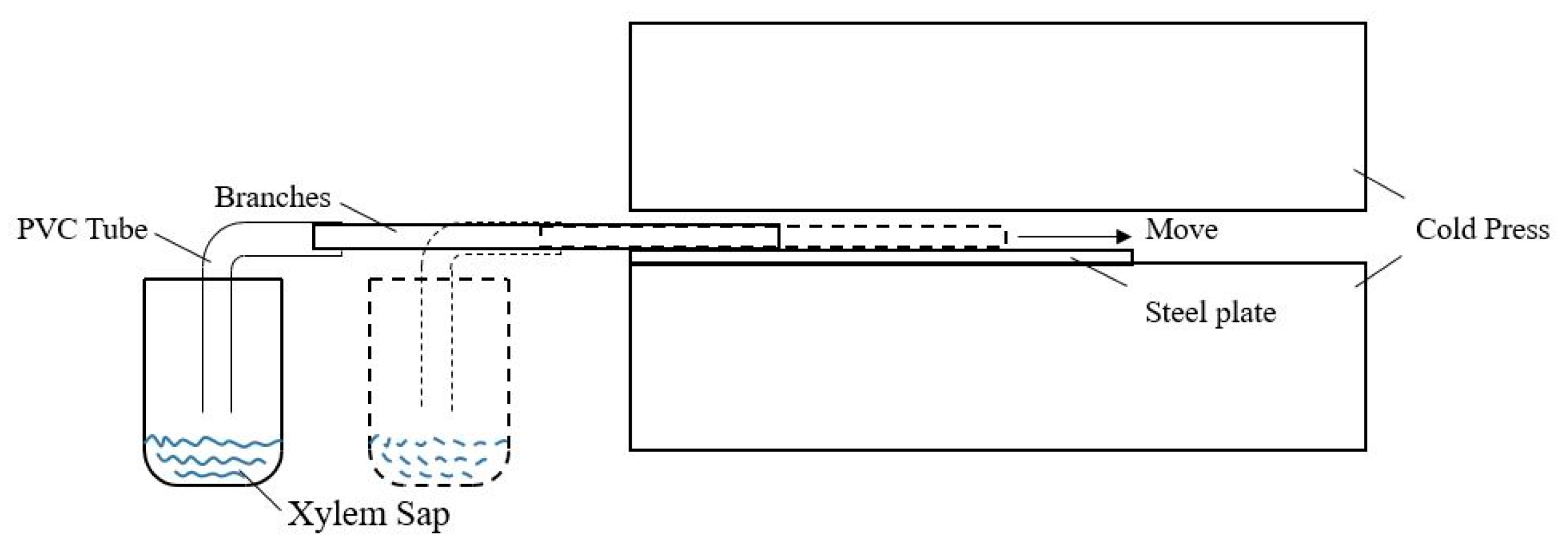
2.4. Xylem Sap Extraction
2.5. Contamination Control
2.6. Dynamic Viscosity Measurement
- ηt—the dynamic viscosity of the specimen at temperature t, mPa·s;
- C—viscometer constant, mm2/s2;
- τ—the average flow time of the specimen, s;
- ρt—the density of the specimen at temperature t, g/cm3.
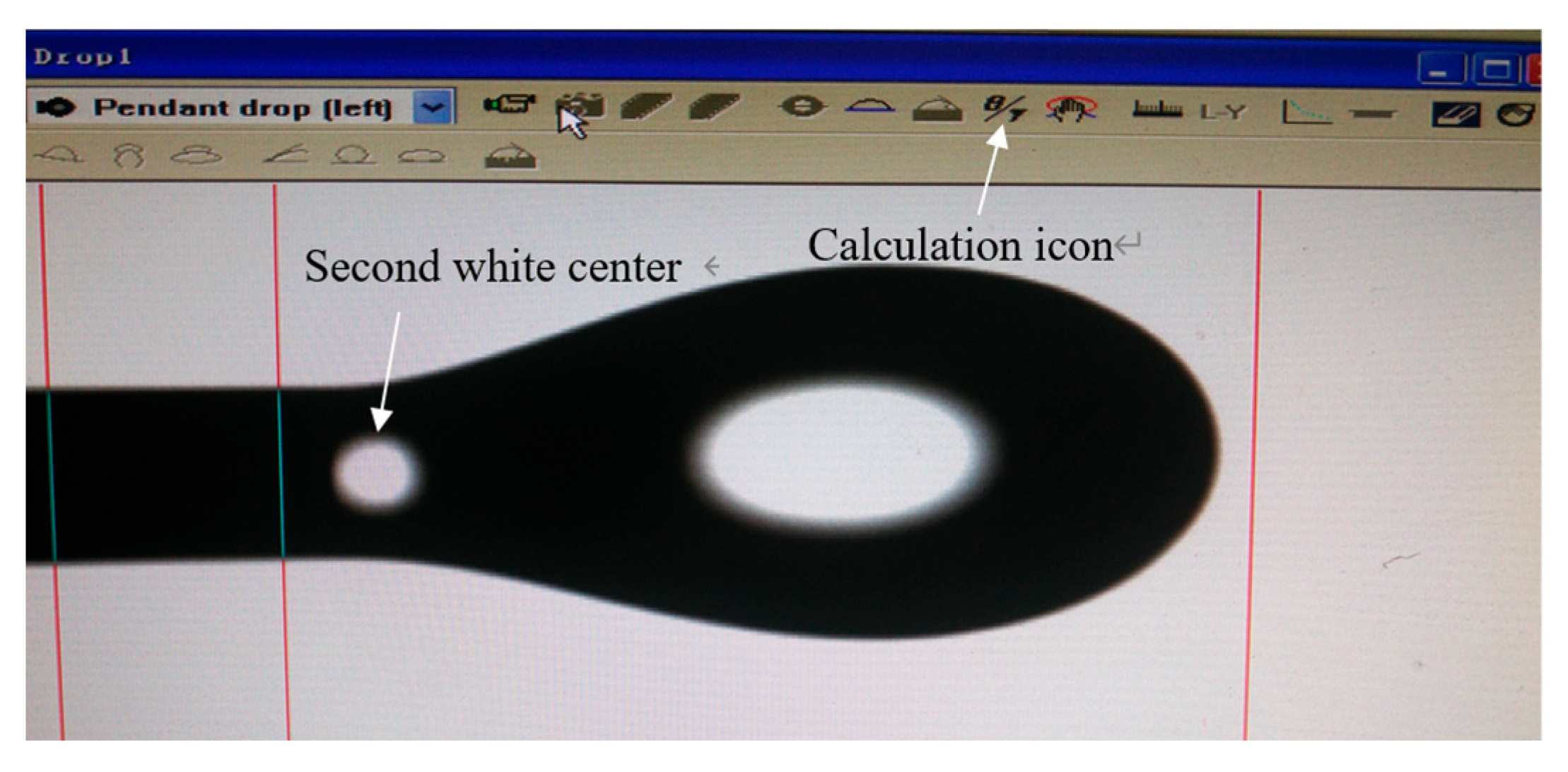
2.7. Interfacial Tension Measurement
2.8. Organic Component Analysis
2.9. Inorganic Cation Analysis
3. Results
3.1. Comparisons of Xylem Sap and Contamination Controls
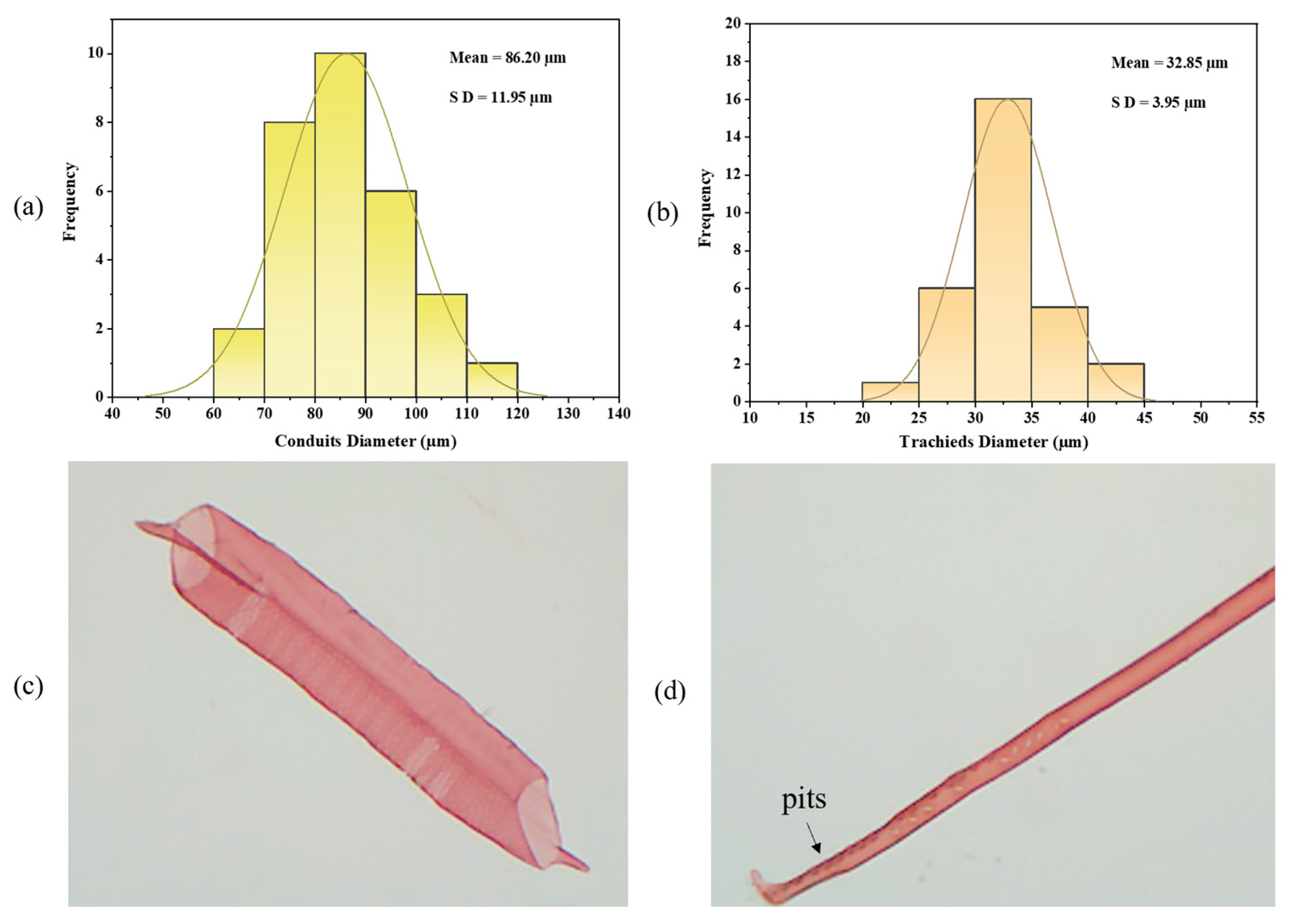
3.2. Morphological Characteristics of Xylem Elements in Different Tree Species
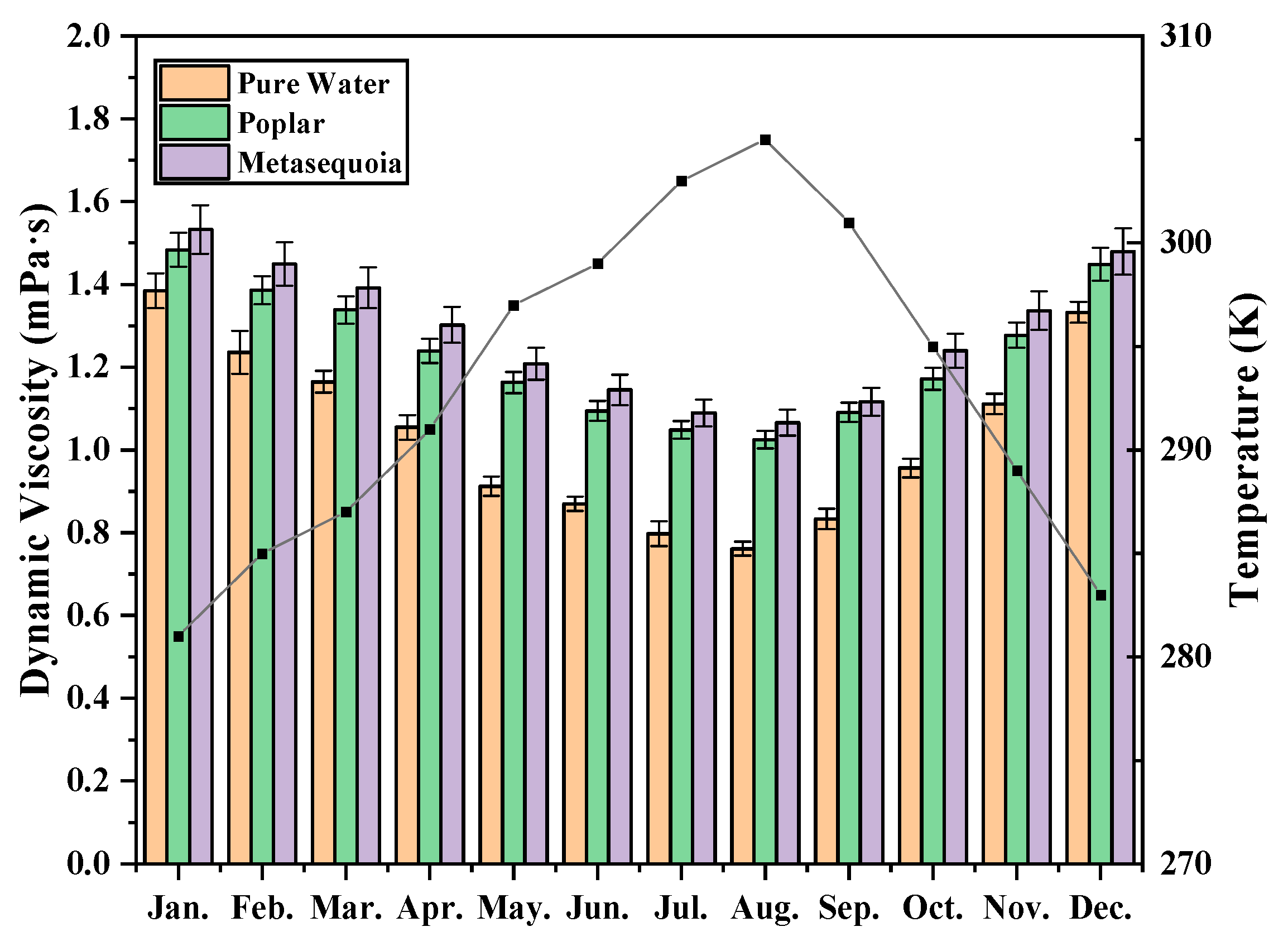
3.3. Monthly Variation of Dynamic Viscosity
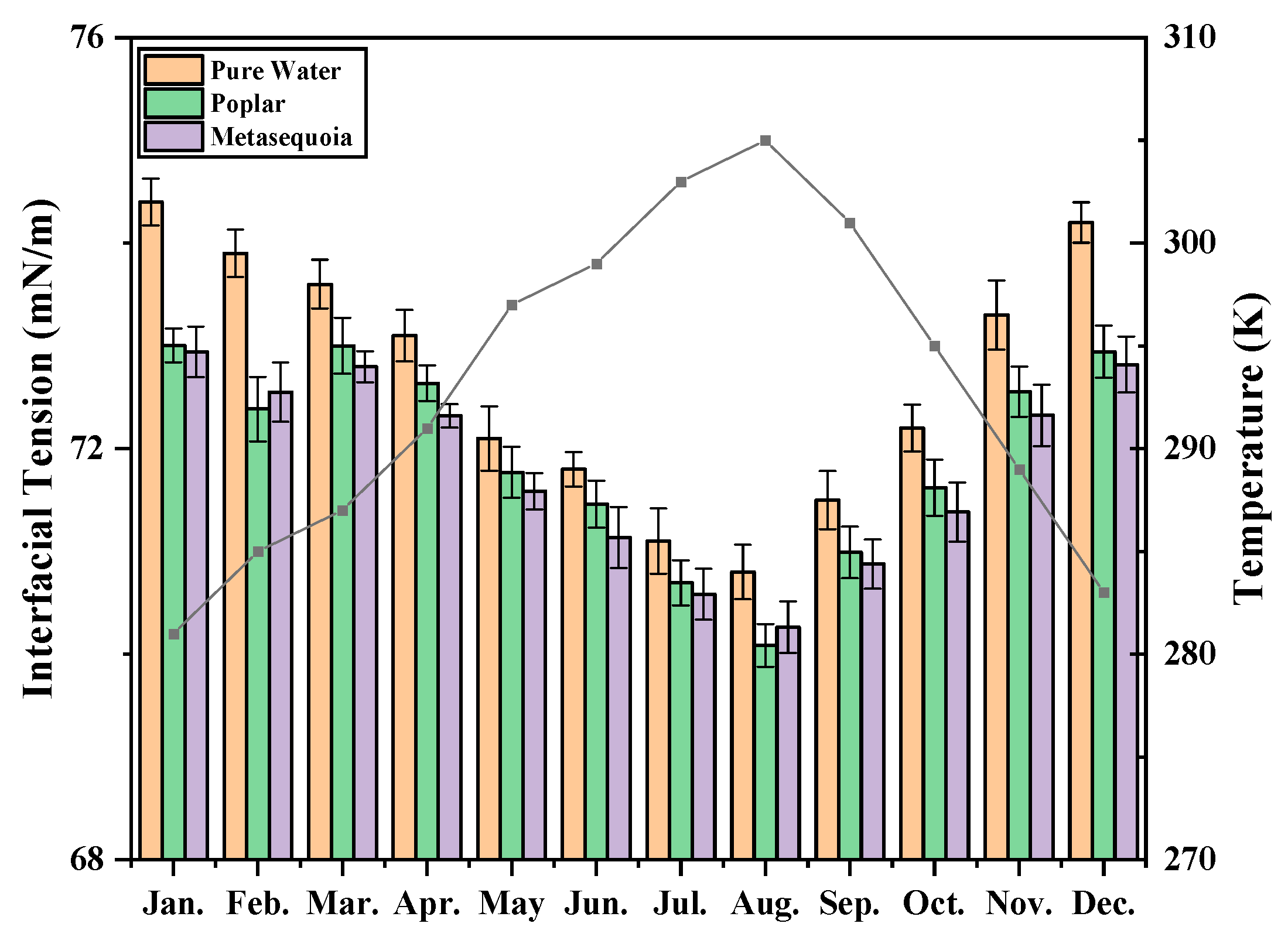
3.4. Monthly Variation of Interfacial Tension
3.5. Monthly Variation of Daily Maximum Sap Velocity
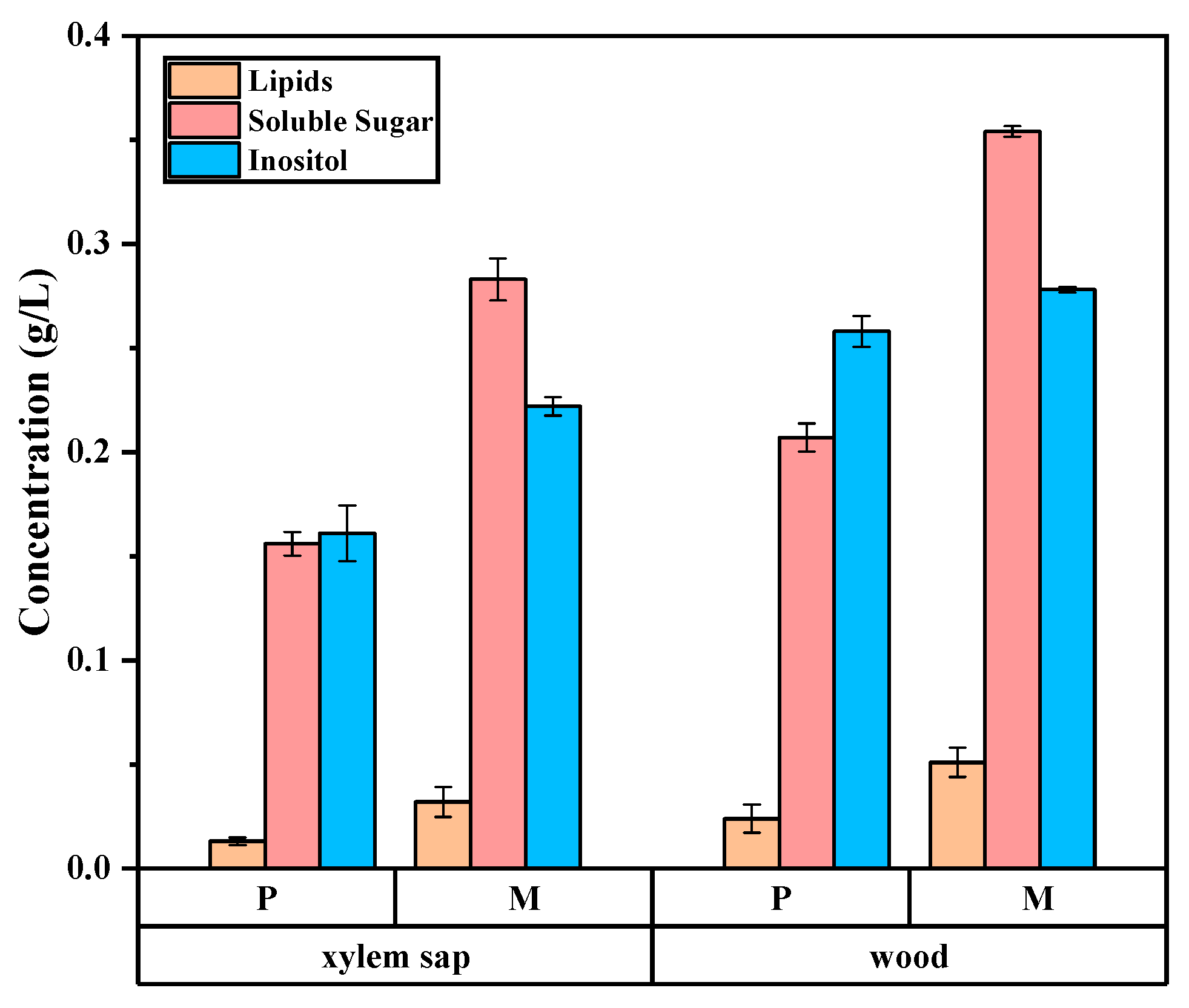
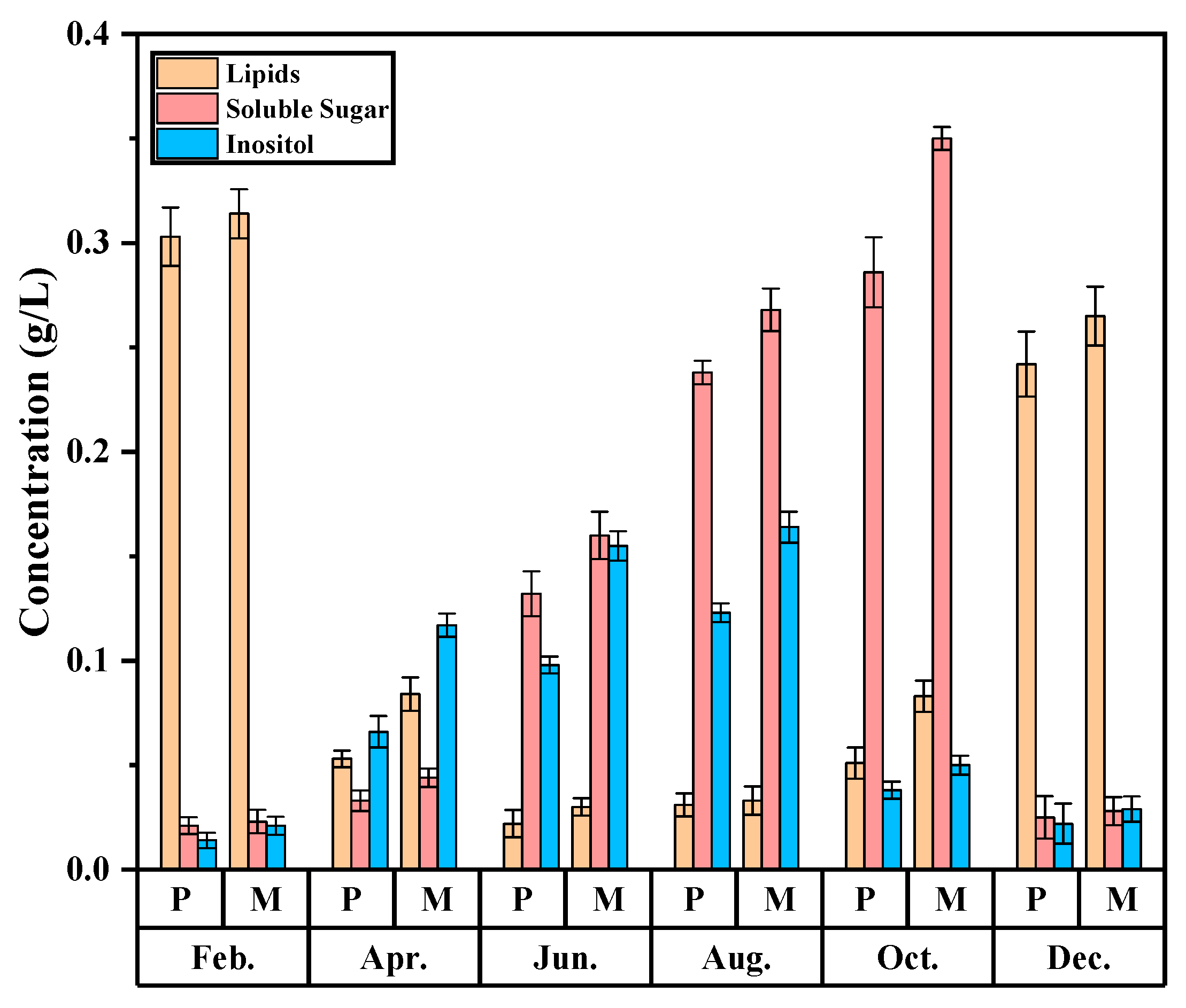
3.6. Monthly Variation of Organic Component
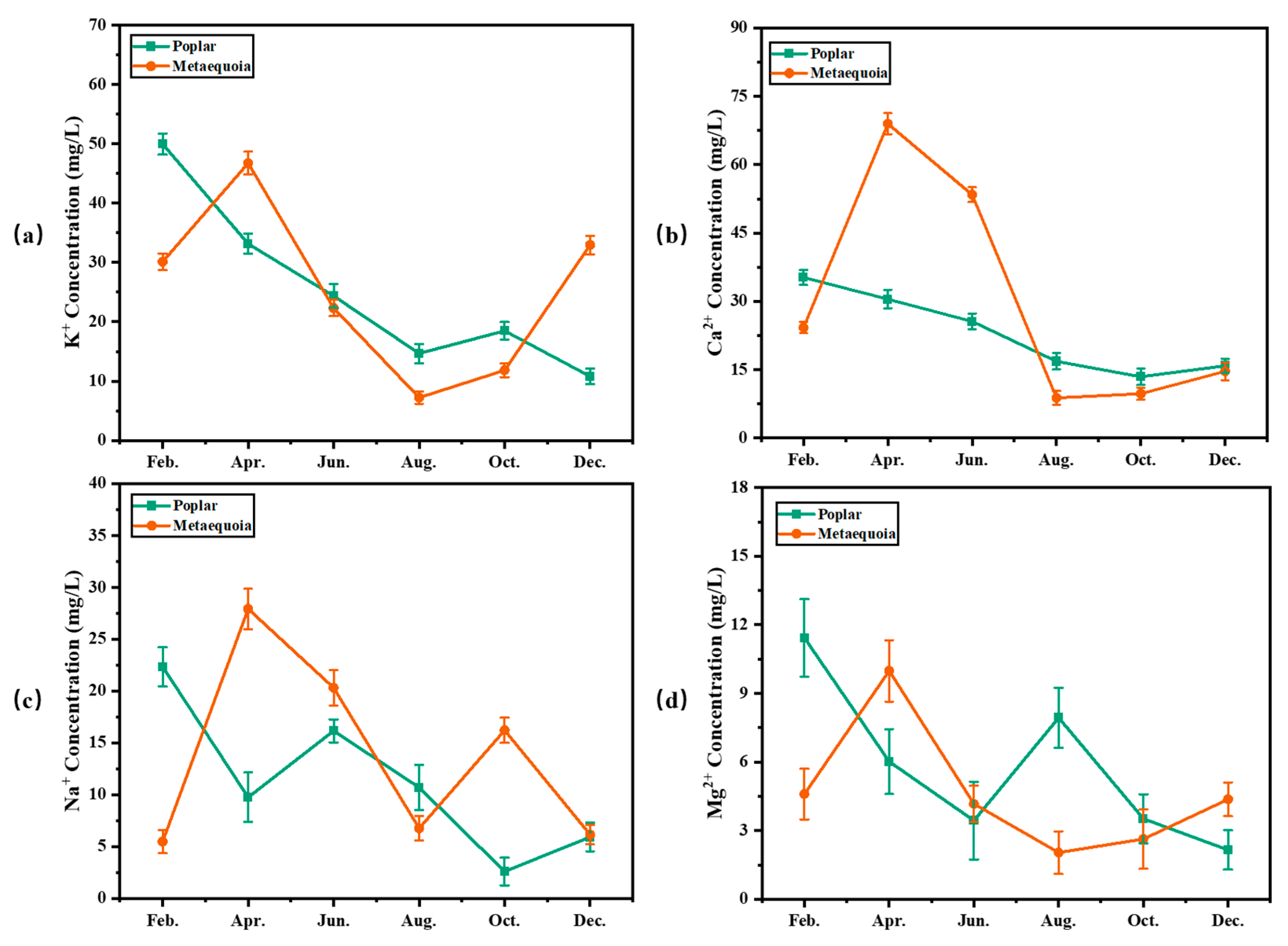
3.7. Monthly Variation of Inorganic Component
4. Discussion
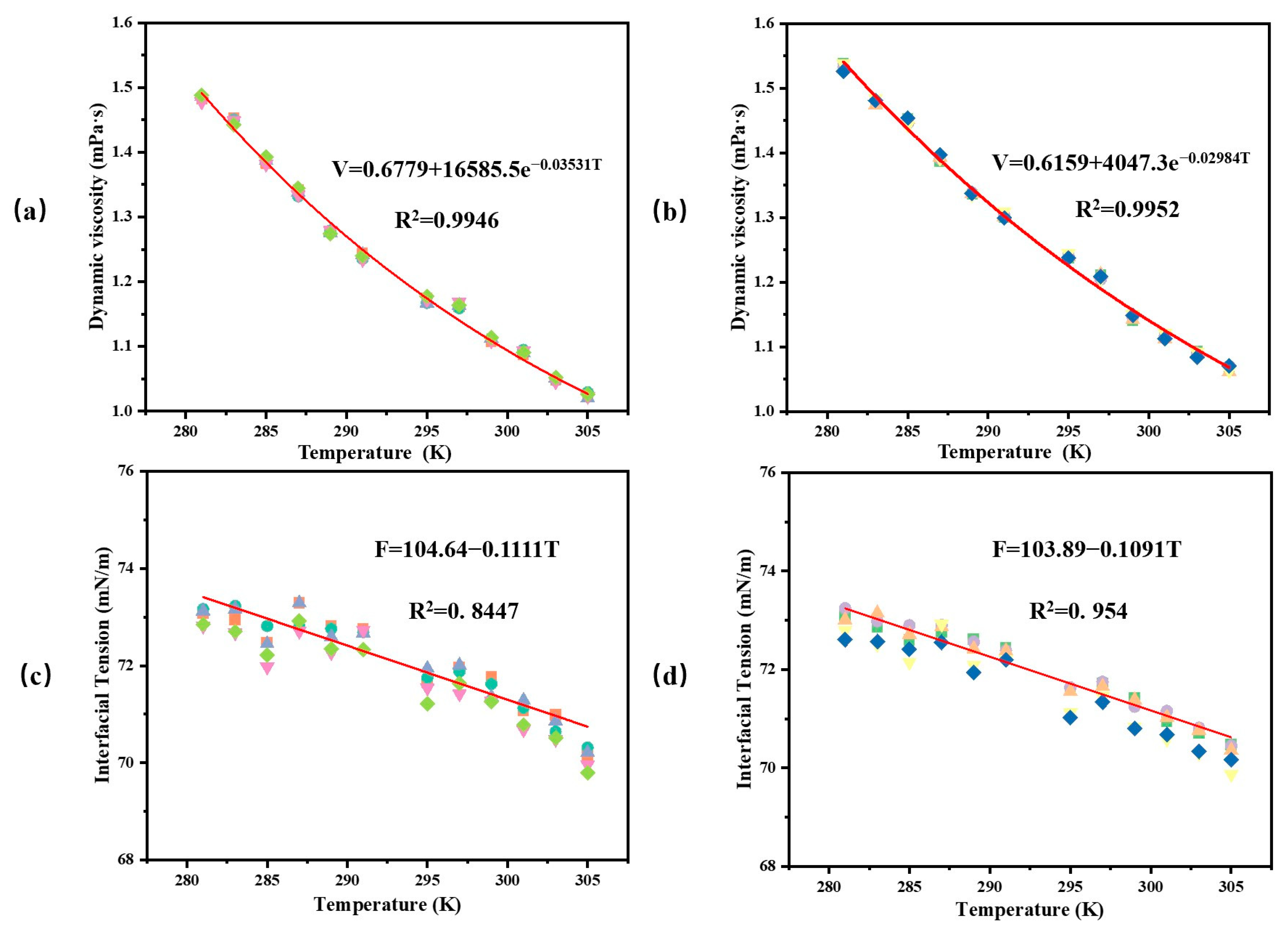
4.1. Effect of Temperature on Dynamic Viscosity and Interfacial Tension of Xylem Sap
4.2. The Dynamic Viscosity Difference between the Xylem Sap of Poplar and Metasequoia in Different Months
4.3. The Interfacial Tension Difference of the Xylem Sap of Poplar and Metasequoia in Different Months
4.4. Correlation between Sap Velocity and the Dynamic Viscosity and Interfacial Tension of Xylem Sap
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Loepfe, L.; Martinez-Vilaltaa, J.; Piñol, J.; Mencuccini, M. The relevance of xylem network structure for plant hydraulic efficiency and safety. J. Theor. Biol. 2007, 247, 788–803. [Google Scholar] [CrossRef] [PubMed]
- De Cannière, S.; Herbst, M.; Vereecken, H.; Defourny, P.; Jonard, F. Constraining water limitation of photosynthesis in a crop growth model with sun-induced chlorophyll fluorescence. Remote Sens. Environ. 2021, 267, 112715–112729. [Google Scholar] [CrossRef]
- Tankari, M.; Wang, C.; Ma, H.; Li, X.; Li, L.; Soothar, R.K.; Cui, N.; Zaman-Allah, M.; Hao, W.; Liu, F.; et al. Drought priming improved water status, photosynthesis and water productivity of cowpea during post-anthesis drought stress. Agric. Water Manag. 2021, 245, 106565–106574. [Google Scholar] [CrossRef]
- Baret, M.; Pepin, S.; Pothier, D. Hydraulic limitations in dominant trees as a contributing mechanism to the age-related growth decline of boreal forest stands. For. Ecol. Manag. 2018, 427, 135–142. [Google Scholar] [CrossRef]
- Roig-Oliver, M.; Fullana-Pericas, M.; Bota, J.; Flexas, J. Adjustments in photosynthesis and leaf water relations are related to changes in cell wall composition in Hordeum vulgare and Triticum aestivum subjected to water deficit stress. Plant Sci. 2021, 311, 111015–111022. [Google Scholar] [CrossRef]
- Brodribb, T.J. Xylem hydraulic physiology: The functional backbone of terrestrial plant productivity. Plant Sci. 2009, 177, 245–251. [Google Scholar] [CrossRef]
- Gleason, S.M.; Cooper, M.; Wiggans, D.R.; Bliss, C.A.; Romay, M.C.; Gore, M.A.; Mickelbart, M.V.; Topp, C.N.; Zhang, H.; DeJonge, K.C.; et al. Stomatal conductance, xylem water transport, and root traits underpin improved performance under drought and well-watered conditions across adverse panel of maize inbred lines. Field Crops Res. 2019, 234, 119–128. [Google Scholar] [CrossRef]
- Lens, F.; Tixier, A.; Cochard, H.; Sperry, J.S.; Jansen, S.; Herbette, S. Embolism resistance as a key mechanism to understand adaptive plant strategies. Curr. Opin. Plant Biol. 2013, 16, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Hölttä, T.; Vesala, T.; Sevanto, S.; Perämäki, M.; Nikinmaa, E. Modeling xylem and phloem water flows in trees according to cohesion theory and Münch hypothesis. Trees 2006, 20, 67–78. [Google Scholar] [CrossRef]
- Denny, M. Tree hydraulics: How sap rises. Eur. J. Phys. 2012, 33, 43–53. [Google Scholar] [CrossRef]
- Elustondo, D. Semi-empirical linear correlation between surface tension and thermodynamics properties of liquid and vapours. Chem. Phys. 2021, 545, 111145–111155. [Google Scholar] [CrossRef]
- Christensen-Dalsgaard, K.K.; Tyree, M.T.; Mussone, P.G. Surface tension phenomena in the xylem sap of three diffuse porous temperate tree species. Tree Physiol. 2011, 31, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Dong, P.; Lu, Y.; Zeng, M.; Zhang, Q. Experimental and theoretical investigation on the surface tension of nano-Lithium Bromide solution. Int. Commun. Heat Mass Transf. 2021, 123, 105231–105238. [Google Scholar] [CrossRef]
- Santos, M.S.C.S.; Reis, J.C.R. Surface tension of liquid mixtures and metal alloys. Thermodynamic conditions for the occurrence of a positive temperature coefficient. J. Alloys Compd. 2021, 864, 158839–158849. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, L.; Wan, X. Frost fatigue and its spring recovery of xylem conduits in ring-porous, diffuse-porous, and coniferous species in situ. Plant Physiol. Biochem. 2021, 146, 177–186. [Google Scholar] [CrossRef]
- De Moraes, D.H.M.; Mesquita, M.; Graciano-Ribeiro, D.; de Araújo, D.S.; Battisti, R.; Flores, R.A.; de Melo, H.C.; Casaroli, D. The effect of xylem vessel diameter on potential hydraulic conductivity in different rice stem longitudinal positions. Flora 2022, 295, 152147–152153. [Google Scholar] [CrossRef]
- Cunha, A.F.A.; Rodrigues, P.H.D.; Anghinoni, A.C.; de Paiva, V.J.; Pinheiro, D.G.S.; Campos, M.L. Mechanical wounding impacts the growth versus defense balance in tomato (Solanum lycopersicum). Plant Sci. 2023, 329, 111601–111608. [Google Scholar] [CrossRef]
- Schenk, H.J.; Michaud, J.M.; Mocko, K.; Espino, S.; Melendres, T.; Roth, M.R.; Welti, R.; Kaack, L.; Jansen, S. Lipids in xylem sap of woody plants across the angiosperm phylogeny. Plant J. 2021, 1056, 1477–1494. [Google Scholar] [CrossRef]
- Xu, S.; Wang, X.; Han, S.; Mei, J.; Cui, H.; Li, J.; Zhou, W.; Guo, G.; Guo, J.; Zhang, J. GB/T 22235-2008; Determination of Liquid Viscosity. National Standardization Administration Committee: Beijing, China, 2008. [Google Scholar]
- Sun, L.; Ma, L.; Gao, S.; Zhao, H.; Zhu, S. Research Achievement on Sugar Metabolism in Deciduous Fruit Trees During Dormancy Period. North. Fruits 2019, 2, 1–4. [Google Scholar]
- Yang, N. Expression and Function of Inositol Metabolism Key Enzyme Gene in Poplar; Ludong University: Yantai, China, 2017. [Google Scholar]
- Losso, A.; Nardini, A.; Dämon, B.; Mayr, S. Xylem sap chemistry: Seasonal changes in timberline conifers Pinus cembra, Picea abies, and Larix decidua. Biol. Plant. 2018, 62, 157–165. [Google Scholar] [CrossRef]
- Anschütz, U.; Becker, D.; Shabala, S. Going beyond nutrition: Regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 2014, 171, 670–687. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, S.A.; Dodd, I.C. Xylem sap calcium concentrations do not explain liming-induced inhibition of legume gas exchange. Plant Soil 2014, 382, 17–30. [Google Scholar] [CrossRef]
- Nazmabadi, M.; Shirdast, A.; Sharif, A.; Aalaie, J. Aqueous/brine solutions viscosity and surface properties of hydrophobically modified scleroglucans: Role of grafted chain length. Carbohydr. Polym. 2020, 229, 115519–115525. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wang, C.; Zhu, X. Estimation of the kinematic viscosities of bio-oil/alcohol blends: Kinematic viscosity-temperature formula and mixing rules. Fuel 2019, 254, 115687–115695. [Google Scholar] [CrossRef]
- Abdullah, A.M.; Chowdhury, A.R.; Yang, Y.; Vasquez, H.; Moore, H.J.; Parsons, J.G.; Lozano, K.; Gutierrez, J.J.; Martirosyan, K.S.; Uddin, M.J. Tailoring the viscosity of water and ethylene glycol based TiO2 nanofluids. J. Mol. Liq. 2020, 297, 111982–111993. [Google Scholar] [CrossRef]
- Benìtez, E.I.; Genovese, D.B.; Lozano, J.E. Effect of typical sugars on the viscosity and colloidal stability of apple juice. Food Hydrocoll. 2009, 232, 519–525. [Google Scholar] [CrossRef]
- Qi, Z.; Gu, G. Effect of dissolved impurities on water viscosity. Guangxi Phys. 2006, 272, 32–33. [Google Scholar]
- Gössi, A.; Riedl, W.; Schuur, B. Mass transfer analysis and kinetic modeling for process design of countercurrent membrane supported reactive extraction of carboxylic acids. Chem. Eng. Sci. 2022, 13, 100119–100130. [Google Scholar] [CrossRef]
- Duan, X. The Influence of Different Sucrose Solution Concentration on Surface Tension. J. Shanxi Datong Univ. Nat. Sci. 2019, 35, 13–14+73. [Google Scholar]
- Riehm, D.A.; Rokke, D.J.; Paul, P.G.; Lee, H.S.; Vizanko, B.S.; McCormick, A.V. Dispersion of oil into water using lecithin-Tween 80 blends: The role of spontaneous emulsification. J. Colloid Interface Sci. 2017, 487, 52–59. [Google Scholar] [CrossRef]
- Pierantozzi, M.; Nicola, G.D. Surface tension correlation of carboxylic acids from liquid viscosity data. Fluid Phase Equilibria 2019, 482, 118–125. [Google Scholar] [CrossRef]
- Soultani, S.; Ognier, S.; Engasser, J.M.; Ghoul, M. Comparative study of some surface active properties of fructose esters and commercial sucrose esters. Colloids Surf. A Physicochem. Eng. Asp. 2003, 227, 35–44. [Google Scholar] [CrossRef]
- Chen, L.; Cao, Y.; Zhang, Z.; Liu, X.; Teramage, M.T.; Zhang, X.; Sun, X. Characteristics of chemical components in the trunk xylem sap of pine trees by means of a centrifugation collection method. Plant Physiol. Biochem. 2019, 142, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Ieperen, W. Ion-mediated changes of xylem hydraulic resistance in planta: Fact or fiction. Trends Plant Sci. 2007, 124, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Meitern, A.; Õunapuu-Pikas, E.; Sellin, A. Circadian patterns of xylem sap properties and their covariation with plant hydraulic traits in hybrid aspen. J. Plant Physiol. 2017, 213, 148–156. [Google Scholar] [CrossRef]
- Trifilò, P.; Lo Gullo, M.A.; Salleo, S.; Callea, K.; Nardini, A. Xylem embolism alleviated by ion-mediated increase in hydraulic conductivity of functional xylem: Insights from field measurements. Tree Physiol. 2008, 2810, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudvand, M.; Javadi, A.; Pourafshary, P.; Vatanparast, H.; Bahramian, A. Effects of cation salinity on the dynamic interfacial tension and viscoelasticity of a water-oil system. J. Pet. Sci. Eng. 2021, 206, 108970–108979. [Google Scholar] [CrossRef]
- Olesena, K.B.; Foganga, L.T.; Palm-Henriksena, G.; Alyafeib, N.; Sølling, T.I. How a range of metal ions influence the interfacial tension of n-decane/carboxylic acid/water systems: The impact of concentration.; molecular- and electronic structure. J. Pet. Sci. Eng. 2019, 182, 106307–106313. [Google Scholar] [CrossRef]

| Age | DBH (cm) | Height (m) | |
|---|---|---|---|
| poplar | 45 | 34.91 ± 1.59 | 12.16 ± 0.35 |
| metasequoia | 50 | 36.18 ± 1.44 | 14.26 ± 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, L.; Wang, X.; Xu, Q.; Tong, J.; Zhao, W.; Zhang, Y.; Lv, J.; Cai, L.; Xia, C. Impacting Factors of Changes in Dynamic Viscosity and Interfacial Tension of Wood Xylem Sap. Forests 2023, 14, 1344. https://doi.org/10.3390/f14071344
Wen L, Wang X, Xu Q, Tong J, Zhao W, Zhang Y, Lv J, Cai L, Xia C. Impacting Factors of Changes in Dynamic Viscosity and Interfacial Tension of Wood Xylem Sap. Forests. 2023; 14(7):1344. https://doi.org/10.3390/f14071344
Chicago/Turabian StyleWen, Liang, Xuan Wang, Qin Xu, Jiewei Tong, Wanwan Zhao, Yaoli Zhang, Jianxiong Lv, Liping Cai, and Changlei Xia. 2023. "Impacting Factors of Changes in Dynamic Viscosity and Interfacial Tension of Wood Xylem Sap" Forests 14, no. 7: 1344. https://doi.org/10.3390/f14071344






