Effects of Nitrogen Deposition on Leaf Litter Decomposition and Soil Organic Carbon Density in Arid and Barren Rocky Mountainous Regions: A Case Study of Yimeng Mountain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Leaf Litter Collection and Litter Bag Experiment
2.4. Soil Sample Collection and Laboratory Analysis
2.5. Statistical Analysis
3. Results
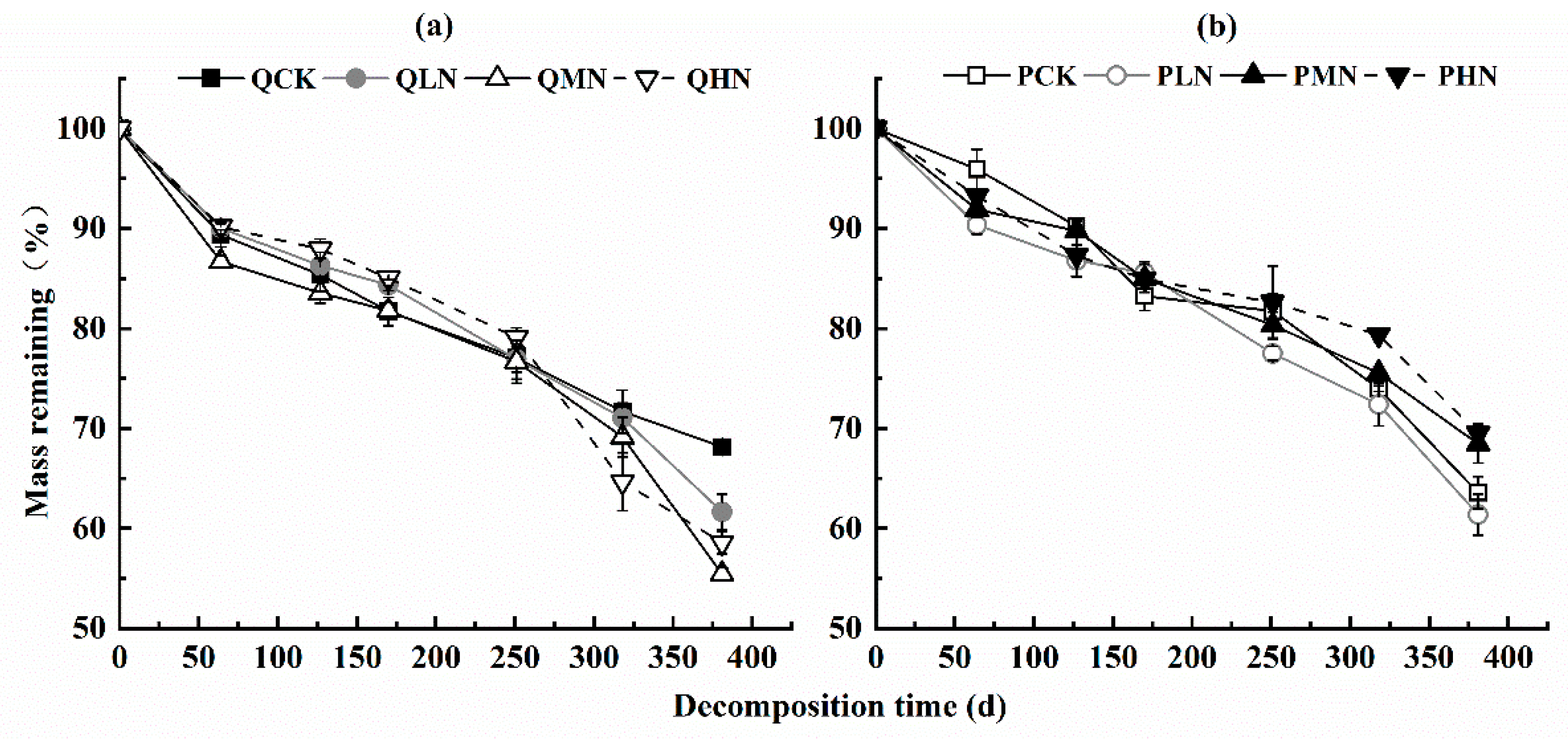
3.1. Leaf Litter Decomposition
3.2. Nutrients Remaining in Leaf Litter
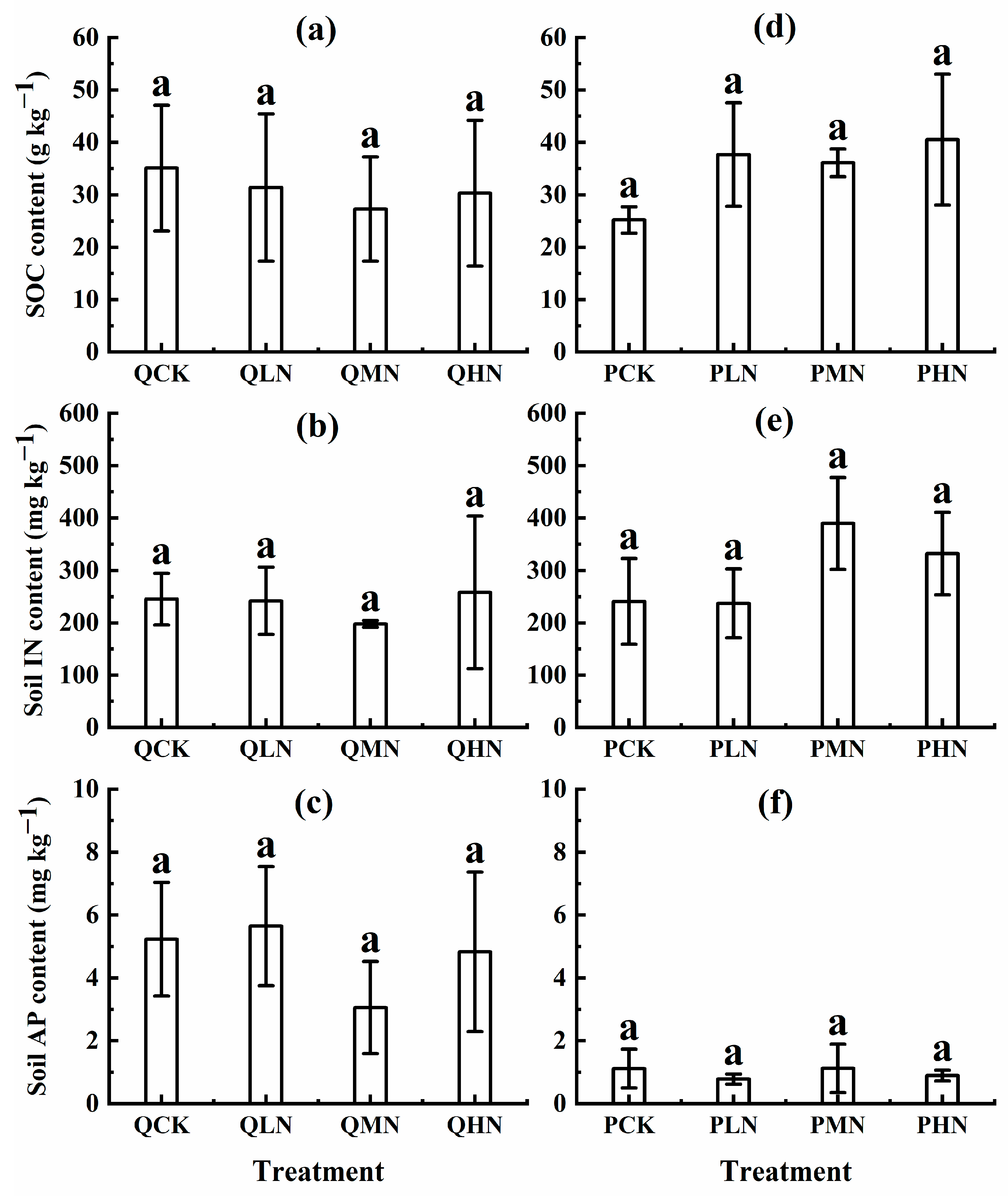
3.3. Soil Available Nutrient and Carbon Density
3.4. Enzyme Activity and Enzyme Stoichiometry
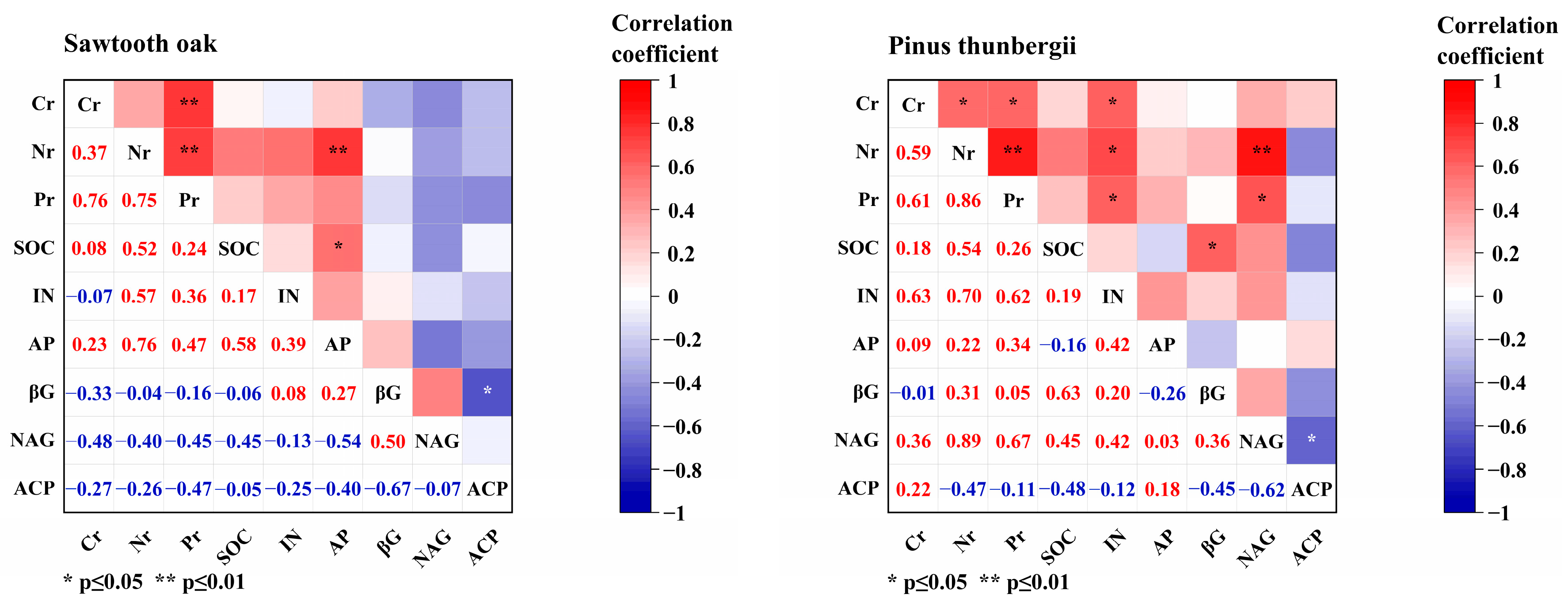
3.5. The Correlation between Leaf Litter, Soil Nutrients and Enzyme Activity
4. Discussion
4.1. Response of Leaf Litter Decomposition to N Addition
4.2. Response of Soil Nutrient Cycling to N Addition
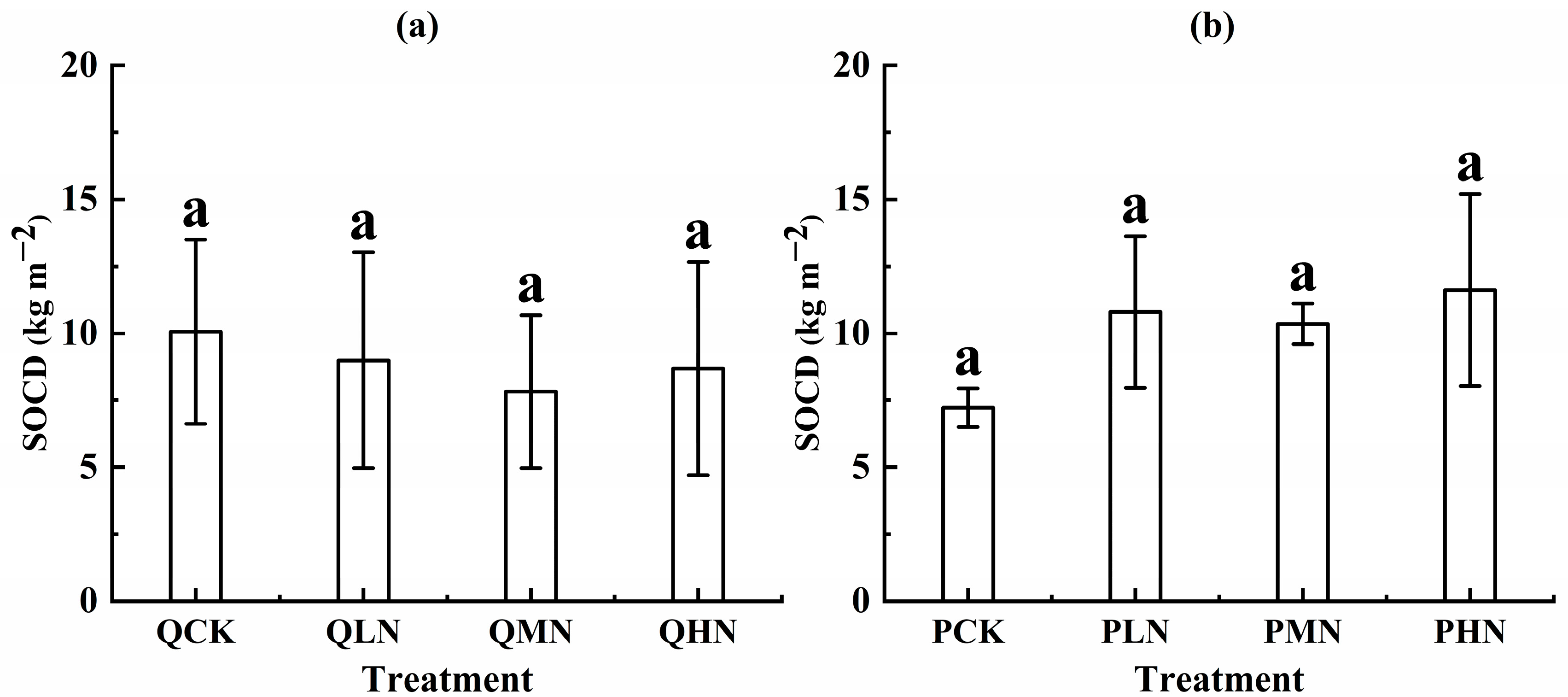
4.3. Response of SOCD to N Addition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, K.; Gao, Y.; Guo, X.; Zhang, J.; Zeng, X.; Ma, M.; Chen, Y.; Luo, K.; Yao, X.; Gao, H. The enhanced role of atmospheric reduced nitrogen deposition in future over East Asia–Northwest Pacific. Sci. Total Environ. 2022, 833, 155146. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A.J.S. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Schichtel, B.A.; Walker, J.T.; Schwede, D.B.; Chen, X.; Lehmann, C.M.B.; Puchalski, M.A.; Gay, D.A.; Collett, J.L. Increasing importance of deposition of reduced nitrogen in the United States. Proc. Natl. Acad. Sci. USA 2016, 113, 5874–5879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleischer, K.; Rebel, K.T.; Van Der Molen, M.K.; Erisman, J.W.; Wassen, M.J.; Van Loon, E.E.; Montagnani, L.; Gough, C.M.; Herbst, M.; Janssens, I.A.; et al. The contribution of nitrogen deposition to the photosynthetic capacity of forests. Glob. Biogeochem. Cycles 2013, 27, 187–199. [Google Scholar] [CrossRef]
- Walter, C.A.; Adams, M.B.; Gilliam, F.S.; Peterjohn, W.T. Non-random species loss in a forest herbaceous layer following nitrogen addition. Ecology 2017, 98, 2322–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilliam, F.S.; Burns, D.A.; Driscoll, C.T.; Frey, S.D.; Lovett, G.M.; Watmough, S.A. Decreased atmospheric nitrogen deposition in eastern North America: Predicted responses of forest ecosystems. Environ. Pollut. 2019, 244, 560–574. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, X.; Xu, W.; Liu, X.; Zhang, Y.; Li, Y.; Wei, J.; Lu, X.; Wang, S.; Zhang, W.; et al. Fall of oxidized while rise of reduced reactive nitrogen deposition in China. J. Clean. Prod. 2020, 272, 122875. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Zhang, L.; Chen, Y.F.; Liu, X.J.; Xu, W.; Pan, Y.P.; Duan, L. Atmospheric nitrogen deposition to China: A model analysis on nitrogen budget and critical load exceedance. Atmos. Environ. 2017, 153, 32–40. [Google Scholar] [CrossRef]
- IPCC. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Freschet, G.T.; Cornwell, W.K.; Wardle, D.A.; Elumeeva, T.G.; Liu, W.; Jackson, B.G.; Onipchenko, V.G.; Soudzilovskaia, N.A.; Tao, J.; Cornelissen, J.H. Linking litter decomposition of above- and below-ground organs to plant-soil feedbacks worldwide. J. Ecol. 2013, 101, 943–952. [Google Scholar] [CrossRef]
- Prescott, C.E. Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 2010, 101, 133–149. [Google Scholar] [CrossRef]
- Laiho, R.; Laine, J.; Trettin, C.C.; Finér, L. Scots pine litter decomposition along drainage succession and soil nutrient gradients in peatland forests, and the effects of inter-annual weather variation. Soil Biol. Biochem. 2004, 36, 1095–1109. [Google Scholar] [CrossRef] [Green Version]
- Yin, R.; Liu, Q.; Tian, S.; Potapov, A.; Zhu, B.; Yang, K.; Li, Z.; Zhuang, L.; Tan, B.; Zhang, L.; et al. Nitrogen deposition stimulates decomposition via changes in the structure and function of litter food webs—ScienceDirect. Soil Biol. Biochem. 2022, 166, 108522. [Google Scholar] [CrossRef]
- Song, X.Z.; Zhou, G.M.; Gu, H.H.; Qi, L.H. Management practices amplify the effects of N deposition on leaf litter decomposition of the Moso bamboo forest. Plant Soil 2015, 395, 391–400. [Google Scholar] [CrossRef] [Green Version]
- He, Z.M.; Yu, Z.P.; Huang, Z.Q.; Davis, M.; Yang, Y.S. Litter decomposition, residue chemistry and microbial community structure under two subtropical forest plantations: A reciprocal litter transplant study. Appl. Soil Ecol. 2016, 101, 84–92. [Google Scholar] [CrossRef]
- Frey, S.D.; Ollinger, S.; Nadelhoffer, K.E.; Bowden, R.; Brzostek, E.; Burton, A.; Caldwell, B.A.; Crow, S.; Goodale, C.L.; Grandy, A.S.; et al. Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 2014, 121, 305–316. [Google Scholar] [CrossRef]
- Xia, M.X.; Talhelm, A.F.; Pregitzer, K.S. Chronic nitrogen deposition influences the chemical dynamics of leaf litter and fine roots during decomposition. Soil Biol. Biochem. 2017, 112, 24–34. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Chang. Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Wang, Q.; Kwak, J.H.; Choi, W.J.; Chang, S.X. Decomposition of trembling aspen leaf litter under long-term nitrogen and sulfur deposition: Effects of litter chemistry and forest floor microbial properties. For. Ecol. Manag. 2018, 412, 53–61. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y.J.; Song, S.Y.; Chen, Y.Q.; Chen, G.T.; Tu, L.H. Nitrogen addition slows litter decomposition accompanied by accelerated manganese release: A five-year experiment in a subtropical evergreen broadleaf forest. Soil Biol. Biochem. 2022, 165, 108511. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Jin, Y.H.; Xu, J.W.; He, H.S.; Tao, Y.; Yang, Z.P.; Bai, Y.Y. Effects of exogenous N and endogenous nutrients on alpine tundra litter decomposition in an area of high nitrogen deposition. Sci. Total Environ. 2022, 805, 150388. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.C.; Heathman, G.C.; Wang, Y.Q.; Huang, C.H. Fractal features of soil particle-size distribution as affected by plant communities in the forested region of Mountain Yimeng, China. Geoderma 2009, 154, 123–130. [Google Scholar] [CrossRef]
- Yan, T.T.; Zhao, W.J.; Zhu, Q.K.; Xu, F.J.; Gao, Z.K. Spatial distribution characteristics of the soil thickness on different land use types in the Yimeng Mountain Area, China. Alex. Eng. J. 2021, 60, 511–520. [Google Scholar] [CrossRef]
- Zhang, R.H.; Xia, L.; Heathman, G.C.; Yao, X.Y.; Hu, X.L.; Zhang, G.C. Assessment of soil erosion sensitivity and analysis of sensitivity factors in the Tongbai–Dabie mountainous area of China. Catena 2013, 101, 92–98. [Google Scholar] [CrossRef]
- Lasota, J.; Małek, S.; Jasik, M.; Bońska, E. Effect of planting method on C:N:P stoichiometry in soils, young silver fir (Abies alba Mill.) and stone pine (Pinus cembra L.) in the upper mountain zone of karpaty mountains. Ecol. Indic. 2021, 129, 107905. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Feng, Q.; Adamowski, J.F.; Zhu, M.; Zhang, X.F. Community level response of leaf stoichiometry to slope aspect in a montane environment: A case study from the Central Qilian Mountains, China. Glob. Ecol. Conserv. 2021, 28, e01703. [Google Scholar] [CrossRef]
- Rosinger, C.; Rousk, J.; Sandén, H. Can enzymatic stoichiometry be used to determine growth-limiting nutrients for microorganisms?—A critical assessment in two subtropical soils. Soil Biol. Biochem. 2019, 128, 115–126. [Google Scholar] [CrossRef]
- Xu, M.P.; Jian, J.N.; Wang, J.Y.; Zhang, Z.J.; Yang, G.H.; Han, X.H.; Ren, C.J. Response of root nutrient resorption strategies to rhizosphere soil microbial nutrient utilization along Robinia pseudoacacia plantation chronosequence. For. Ecol. Manag. 2021, 489, 119053. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Zeglin, L.H. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Luo, X.S.; Pan, Y.P.; Zhang, L.; Tang, A.H.; Shen, J.L.; Zhang, Y.; Li, K.H.; Wu, Q.H.; Yang, D.W.; et al. Quantifying atmospheric nitrogen deposition through a nationwide monitoring network across China. Atmos. Chem. Phys. 2015, 15, 12345–12360. [Google Scholar] [CrossRef] [Green Version]
- Deforest, J.L. The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and l -DOPA. Soil Biol. Biochem. 2009, 41, 1180–1186. [Google Scholar] [CrossRef]
- Liu, Q.; Yin, R.; Tan, B.; You, C.M.; Zhang, L.; Zhang, J.; Xu, Z.F.; Schadler, M.; Scheu, S. Nitrogen addition and plant functional type independently modify soil mesofauna effects on litter decomposition. Soil Biol. Biochem. 2021, 160, 108340. [Google Scholar] [CrossRef]
- Olson, J.S. Energy Storage and the Balance of Producers and Decomposers in Ecological Systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Deforest, J.L. Chronic phosphorus enrichment and elevated pH suppresses Quercus spp. leaf litter decomposition in a temperate forest—ScienceDirect. Soil Biol. Biochem. 2019, 135, 206–212. [Google Scholar] [CrossRef]
- Wu, J.P.; Liu, W.F.; Zhang, W.X.; Shao, Y.H.; Duan, H.L.; Chen, B.D.; Wei, X.H.; Fan, H.B. Long-term nitrogen addition changes soil microbial community and litter decomposition rate in a subtropical forest. Appl. Soil Ecol. 2019, 142, 43–51. [Google Scholar] [CrossRef]
- Wu, C.S.; Shu, C.J.; Zhang, Z.J.; Zhang, Y.; Liu, Y.Q. Effect of N deposition on the home-field advantage of wood decomposition in a subtropical forest. Ecol. Indic. 2022, 140, 109043. [Google Scholar] [CrossRef]
- Vivanco, L.; Austin, A.T. Nitrogen addition stimulates forest litter decomposition and disrupts species interactions in Patagonia, Argentina. Clobal Chang. Biol. 2011, 17, 1963–1974. [Google Scholar] [CrossRef]
- Wu, A.; Yin, R.; Xu, Z.; Zhang, L.; You, C.; Liu, Y.; Li, H.; Wang, L.; Liu, S.; Zhang, Y.; et al. Forest gaps slow lignin and cellulose degradation of fir (Abies faxoniana) twig litter in an alpine forest. Geoderma 2019, 424, 116010. [Google Scholar] [CrossRef]
- Pei, G.T.; Liu, J.; Peng, B.; Gao, D.C.; Wang, C.; Dai, W.W.; Jiang, P.; Bai, E. Nitrogen, lignin, C/N as important regulators of gross nitrogen release and immobilization during litter decomposition in a temperate forest ecosystem. For. Ecol. Manag. 2019, 440, 61–69. [Google Scholar] [CrossRef]
- Frey, S.D.; Knorr, M.; Parrent, J.L.; Simpson, R.T. Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. For. Ecol. Manag. 2004, 196, 159–171. [Google Scholar] [CrossRef]
- Micks, P.; Downs, M.R.; Magill, A.H.; Nadelhoffer, K.J.; Aber, J.D. Decomposing litter as a sink for 15N-enriched additions to an oak forest and a red pine plantation. For. Ecol. Manag. 2004, 196, 71–87. [Google Scholar] [CrossRef]
- Knorr, M.; Frey, S.D.; Curtis, P.S. Nitrogen additions and litter decomposition: A meta-analysis. Ecology 2005, 86, 3252–3257. [Google Scholar] [CrossRef] [Green Version]
- Rubino, M.; Dungait, J.A.J.; Evershed, R.P.; Bertolini, T.; De Angelis, P.; D’Onofrio, A.; Lagomarsino, A.; Lubritto, C.; Merola, A.; Terrasi, F.; et al. Carbon input belowground is the major C flux contributing to leaf litter mass loss: Evidences from a 13C labelled-leaf litter experiment. Soil Biol. Biochem. 2010, 42, 1009–1016. [Google Scholar] [CrossRef]
- Lin, H.; He, Z.H.; Hao, J.W.; Tian, K.; Jia, X.Q.; Kong, X.S.; Akbar, S.; Bei, Z.L.; Tian, X.J. Effect of N addition on home-field advantage of litter decomposition in subtropical forests—ScienceDirect. For. Ecol. Manag. 2017, 398, 216–225. [Google Scholar] [CrossRef]
- Duan, P.P.; Xiao, K.C.; Wang, K.L.; Li, D.J. Responses of soil respiration to nitrogen addition are mediated by topography in a subtropical karst forest. Catena 2023, 221, 106759. [Google Scholar] [CrossRef]
- Marshall, J.D.; Peichl, M.; Tarvainen, L.; Lim, H.; Lundmark, T.; Nasholm, T.; Oquist, M.; Linder, S. A carbon-budget approach shows that reduced decomposition causes the nitrogen-induced increase in soil carbon in a boreal forest. For. Ecol. Manag. 2021, 502, 119750. [Google Scholar] [CrossRef]
- Ngaba, M.J.Y.; Uwiragiye, Y.; Bol, R.; Vries, W.D.; Zhou, J.B. Low-level nitrogen and short-term addition increase soil carbon sequestration in Chinese forest ecosystems. Catena 2014, 215, 106333. [Google Scholar] [CrossRef]
- Dias, T.; Oakley, S.; Alarcón-Gutiérrez, E.; Ziarelli, F.; Trindade, H.; Martins, M.A.; Sheppard, L.; Ostle, N.; Cruz, C. N-driven changes in a plant community affect leaf-litter traits and may delay organic matter decomposition in a Mediterranean maquis. Soil Biol. Biochem. 2013, 58, 163–171. [Google Scholar] [CrossRef]
- Chomel, M.; Baldy, V.; Guittonny-Larchevque, M. Effect of mixing herbaceous litter with tree litters on decomposition and N release in boreal plantations. Plant Soil 2016, 398, 229–241. [Google Scholar] [CrossRef]
- Bai, X.J.; Dippold, M.A.; An, S.S.; Wang, B.R.; Zhang, H.X.; Loeppmann, S. Extracellular enzyme activity and stoichiometry: The effect of soil microbial element limitation during leaf litter decomposition. Ecol. Indic. 2021, 121, 107200. [Google Scholar] [CrossRef]
- Zhang, Y.; Shengzhe, E.; Wang, Y.; Su, S.; Bai, L.; Wu, C.; Zeng, X. Long-term manure application enhances the stability of aggregates and aggregate-associated carbon by regulating soil physicochemical characteristics. Catena 2021, 203, 105342. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, C.; Wang, Y.Q.; Cheng, H.; An, S.S.; Chang, S.X. Soil extracellular enzyme stoichiometry reflects the shift from P- to N-limitation of microorganisms with grassland restoration. Soil Biol. Biochem. 2020, 149, 107928. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.R.; An, S.S. Ecological stoichiometry in leaves, roots, litters and soil among different plant communities in a desertified region of Northern China. Catena 2018, 166, 328–338. [Google Scholar] [CrossRef]
- Li, J.W.; Liu, Y.L.; Hai, X.Y.; Shangguan, Z.P.; Deng, L. Dynamics of soil microbial C:N:P stoichiometry and its driving mechanisms following natural vegetation restoration after farmland abandonment. Sci. Total Environ. 2019, 693, 133613. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, Y.; Lu, Z.; Wang, F.; Lambers, H.; Zhang, J.; Qin, G.; Zhou, J.; Wu, J.; Zhang, L.; et al. Nitrogen deposition in low-phosphorus tropical forests benefits soil C sequestration but not stabilization. Ecol. Indic. 2023, 146, 109761. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Wang, J.; You, Y.; Yang, Y.; Shi, Z.; Huang, X.; Zheng, L.; Li, Z.; Ming, A.; et al. Mixed-species plantation with Pinus massoniana and Castanopsis hystrix accelerates C loss in recalcitrant coniferous litter but slows C loss in labile broadleaf litter in southern China. For. Ecol. Manag. 2018, 422, 207–213. [Google Scholar] [CrossRef]


| Tree Species | C (%) | N (%) | P (%) | Lignin (%) | Cellulose (%) | C:N | Lignin:N |
|---|---|---|---|---|---|---|---|
| Quercus acutissima Carruth. | 47.60 ± 2.79 a | 0.81 ± 0.02 a | 0.12 ± 0.01 b | 6.39 ± 0.44 a | 14.29 ± 1.59 b | 59.17 ± 4.08 b | 7.93 ± 0.52 a |
| Pinus thunbergii Parl. | 48.91 ± 2.99 a | 0.77 ± 0.01 b | 0.13 ± 0.01 a | 5.92 ± 0.22 b | 18.14 ± 2.08 a | 63.75 ± 3.81 a | 7.72 ± 0.28 a |
| Treatment | Decomposition Equation | Decomposition Constant (K Value) | 50% Decomposition Age (a) | 95% Decomposition Age (a) | Correlation Coefficient |
|---|---|---|---|---|---|
| QCK | y = e−0.386x | 0.386 | 1.796 | 7.761 | 0.967 |
| QLN | y = e−0.421x | 0.421 | 1.646 | 7.116 | 0.968 |
| QMN | y = e−0.484x | 0.484 | 1.432 | 6.190 | 0.913 |
| QHN | y = e−0.46x | 0.460 | 1.507 | 6.512 | 0.931 |
| PCK | y = e−0.367x | 0.367 | 1.889 | 8.163 | 0.937 |
| PLN | y = e−0.416x | 0.416 | 1.666 | 7.201 | 0.958 |
| PMN | y = e−0.34x | 0.340 | 2.039 | 8.811 | 0.982 |
| PHN | y = e−0.315x | 0.315 | 2.200 | 9.510 | 0.940 |
| Treatment | Enzyme C:N Ratio | Enzyme C:P Ratio | Enzyme N:P Ratio |
|---|---|---|---|
| QCK | 0.67 ± 0.22 b | 0.34 ± 0.09 c | 0.52 ± 0.05 b |
| QLN | 1.37 ± 0.03 a | 0.95 ± 0.06 a | 0.70 ± 0.05 a |
| QMN | 0.63 ± 0.09 b | 0.34 ± 0.01 c | 0.54 ± 0.06 b |
| QHN | 1.27 ± 0.10 a | 0.79 ± 0.02 b | 0.62 ± 0.06 ab |
| PCK | 1.18 ± 0.11 b | 0.67 ± 0.01 c | 0.58 ± 0.05 c |
| PLN | 1.35 ± 0.03 a | 0.86 ± 0.02 a | 0.64 ± 0.01 b |
| PMN | 1.20 ± 0.02 b | 0.79 ± 0.02 b | 0.66 ± 0.02 b |
| PHN | 1.08 ± 0.04 b | 0.84 ± 0.03 a | 0.78 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, B.; Zhou, J.; Qi, L.; Jiao, S.; Ma, L.; Geng, W.; Zhao, Y.; Gao, T.; Gong, J.; Li, K.; et al. Effects of Nitrogen Deposition on Leaf Litter Decomposition and Soil Organic Carbon Density in Arid and Barren Rocky Mountainous Regions: A Case Study of Yimeng Mountain. Forests 2023, 14, 1351. https://doi.org/10.3390/f14071351
Kong B, Zhou J, Qi L, Jiao S, Ma L, Geng W, Zhao Y, Gao T, Gong J, Li K, et al. Effects of Nitrogen Deposition on Leaf Litter Decomposition and Soil Organic Carbon Density in Arid and Barren Rocky Mountainous Regions: A Case Study of Yimeng Mountain. Forests. 2023; 14(7):1351. https://doi.org/10.3390/f14071351
Chicago/Turabian StyleKong, Baishu, Jilei Zhou, Liguo Qi, Shuying Jiao, Lujie Ma, Wenwen Geng, Yuhao Zhao, Ting Gao, Jie Gong, Kun Li, and et al. 2023. "Effects of Nitrogen Deposition on Leaf Litter Decomposition and Soil Organic Carbon Density in Arid and Barren Rocky Mountainous Regions: A Case Study of Yimeng Mountain" Forests 14, no. 7: 1351. https://doi.org/10.3390/f14071351





