Using Remote Sensing and Climate Data to Map the Extent and Severity of Balsam Woolly Adelgid Infestation in Northern Utah, USA
Abstract
1. Introduction
- Develop an accurate map of current BWA infestation severity for use by land managers, focusing on the relatively recent invasion of northern Utah;
- Compare remote sensing-driven and terrain-/climate-driven approaches to mapping BWA infestation severity using a field-validated quantitative measure of stand-level severity;
- Introduce a new approach for mapping BWA infestation severity that leverages individual strengths and overcomes the individual weaknesses of remote sensing and terrain/climate data;
- Produce a quantitative accounting of landscape-level environmental drivers and identify key geospatial predictors of BWA infestation severity.
2. Materials and Methods
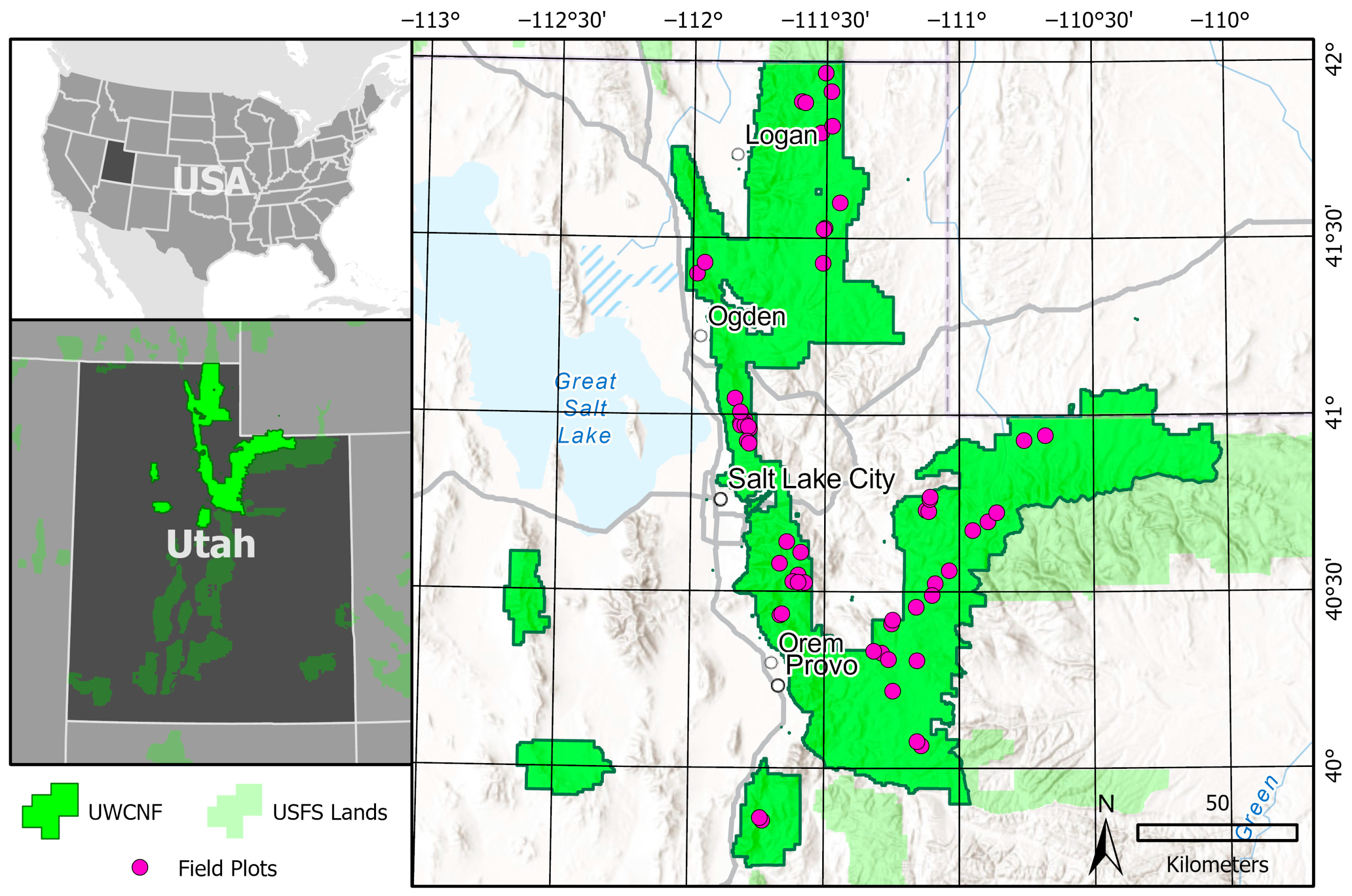
2.1. Study Area
2.2. Field Data
| Metric | Description |
|---|---|
| Species | Tree species |
| Status | Categorical indicator of the tree’s vitality:
|
| DBH | Diameter at breast height in cm |
| Wool Density | Categorical measure of BWA wool density on the lower 6 ft (1.83 m) of the tree bole, measured in wools per ft2 (929 cm2):
|
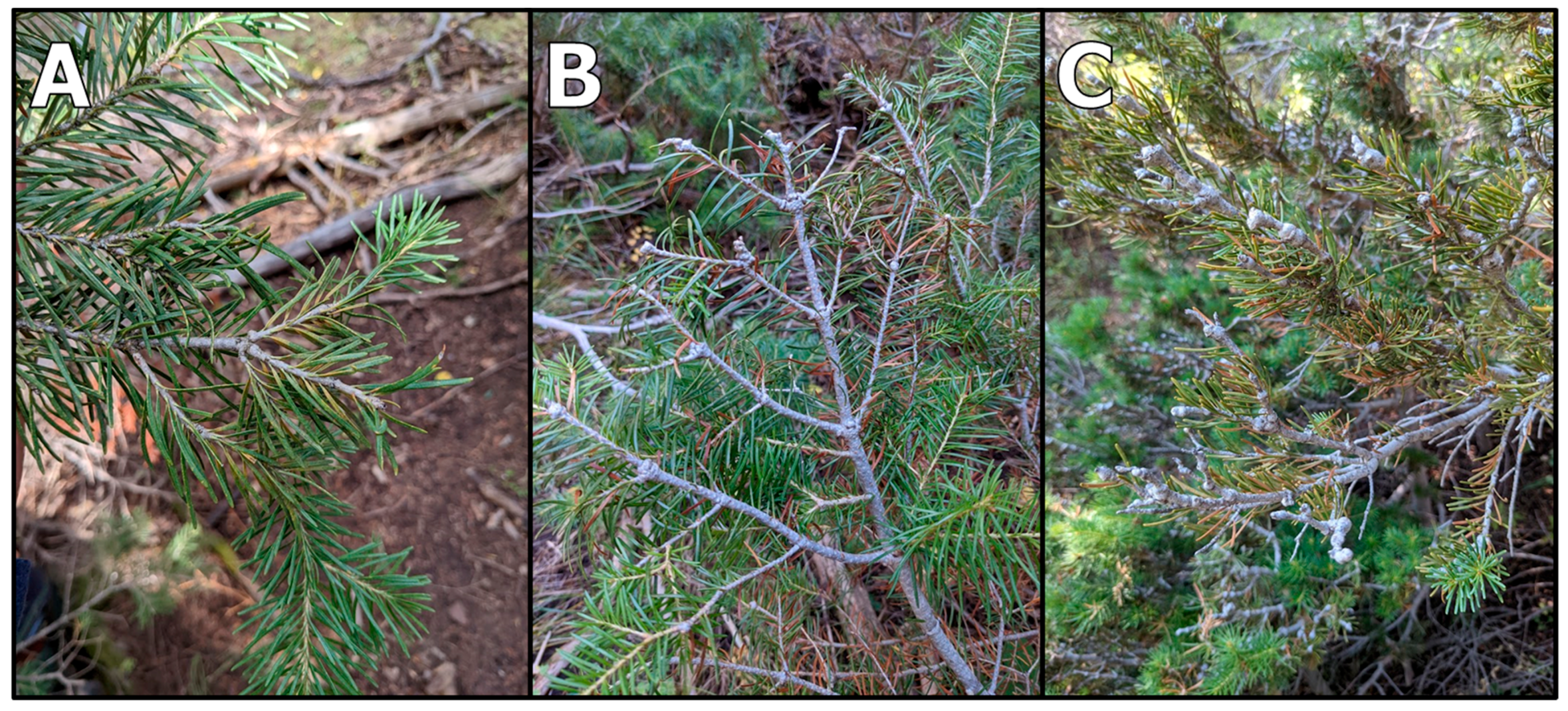
| Gout Severity | Categorical measure of the degree to which branches and twigs have developed gouts, as approximated based on their noticeability (Figure 2): |
| Crown Deformities | Count of the number of crown deformities observed in the top few meters of the tree. Candidate deformities include stunted growth in the terminal branch (leader), stunted growth in the lateral branches, and a top curl (Figure 3). Values range from 0 to 3. |
| Dead Top | Binary (0: no; 1: yes) indicator of whether or not the uppermost portion of the tree’s crown is dead. |
| Branch Dieback | Proportion of the tree’s retained foliage that is no longer photosynthetically active (yellow, red, or brown), in percentage classes at a 10% interval (e.g., 0%, 10%, …, 100%). |
| Other Damage Agents | Indicator of evidence of any non-BWA agents that have caused damage to the tree’s health, including (but not limited to) fir broom rust, bark beetles, twig beetles, pathogens, mechanical damage, and frost crack. |
| BWA Damage Score (BDS) | Integrated qualitative indicator of our perception of the degree of damage caused by BWA:
|
| Other Agent Damage Score (ODS) | Integrated qualitative indicator of our perception of the degree of damage caused by other agents:
|

2.3. Remote Sensing Data
2.4. Terrain and Climate Data
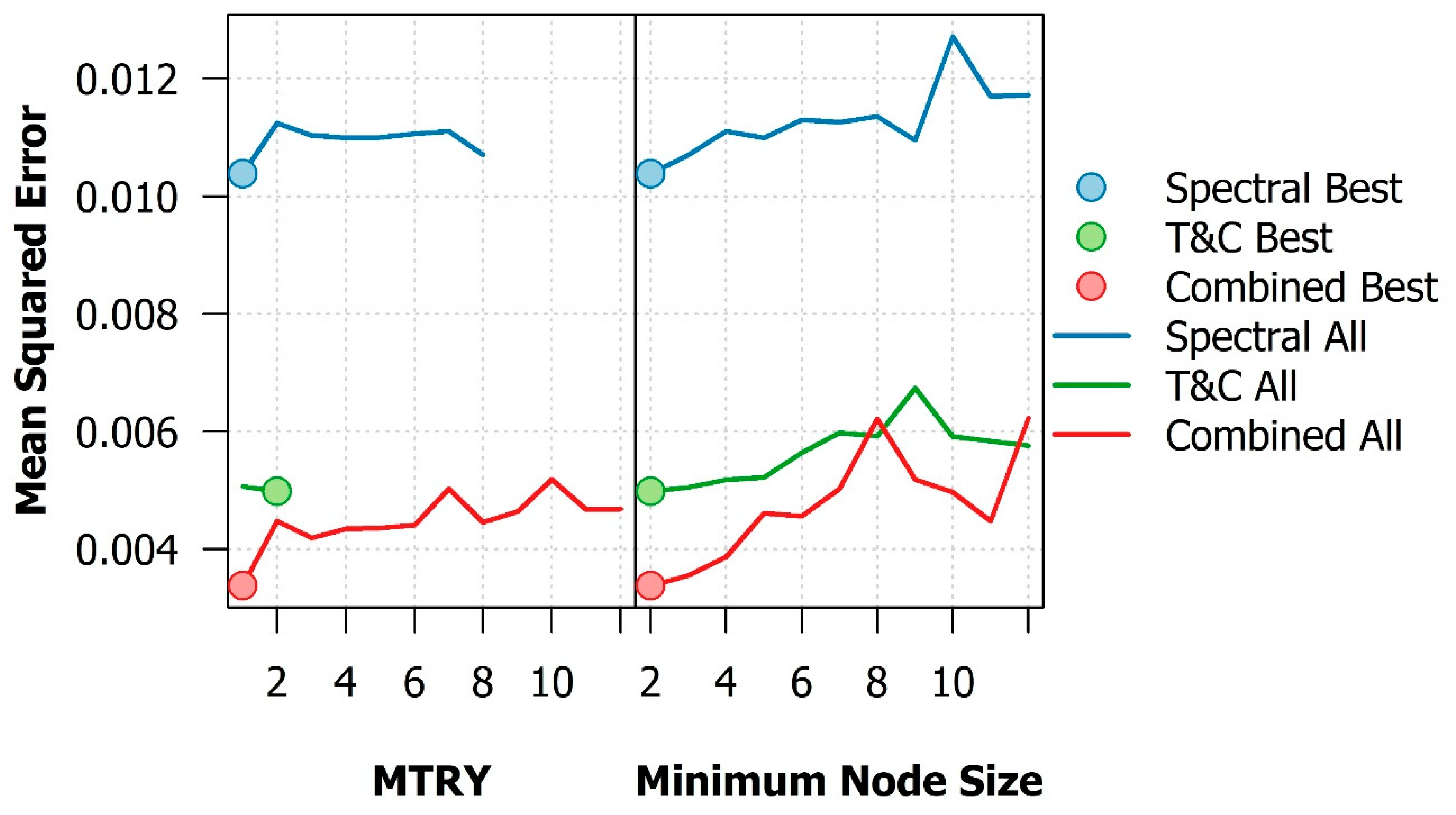
2.5. Modeling and Accuracy Assessment
2.6. Generating a Mapping Mask
2.7. Comparison to Aerial Survey Data
3. Results
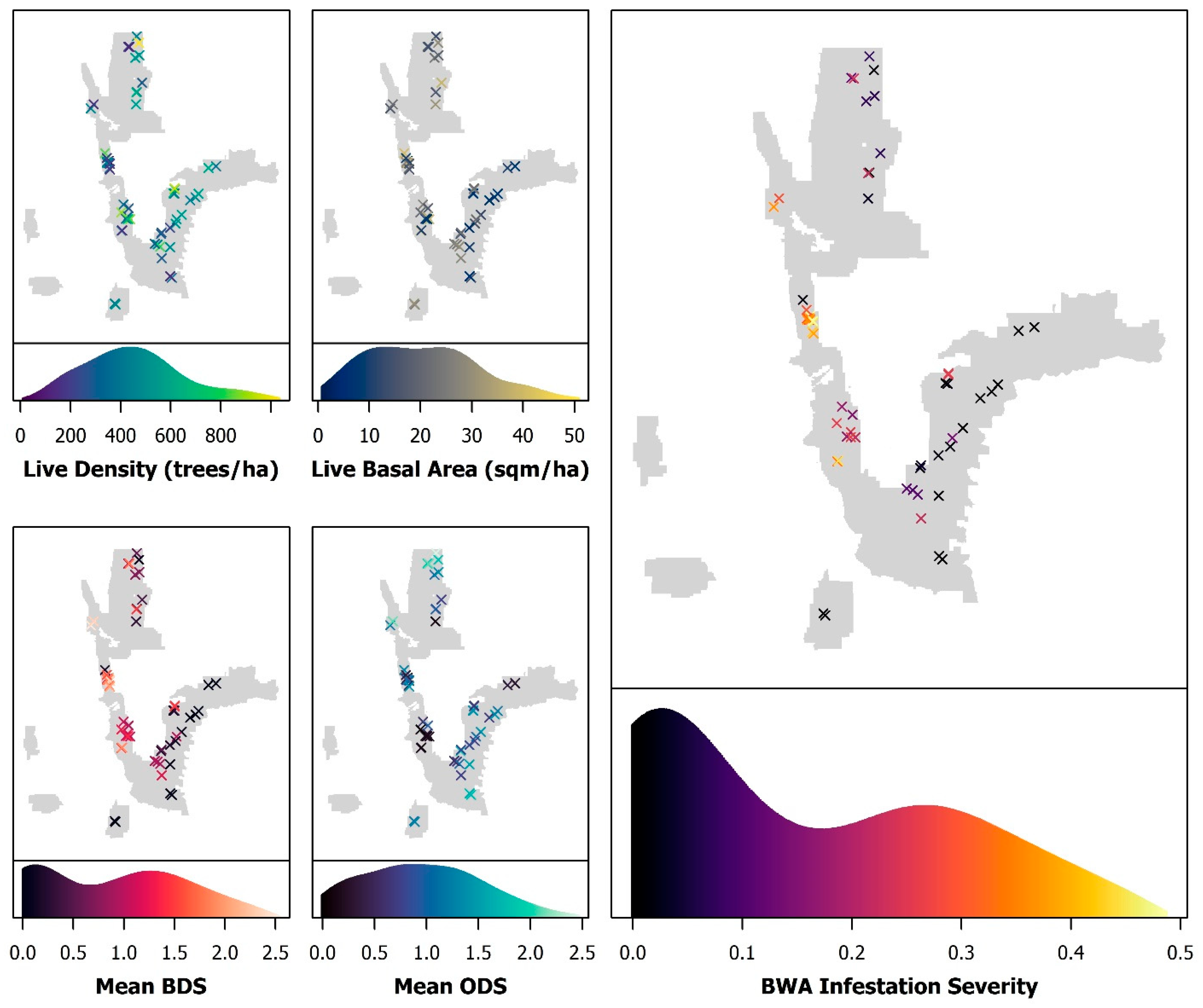
3.1. Field Data
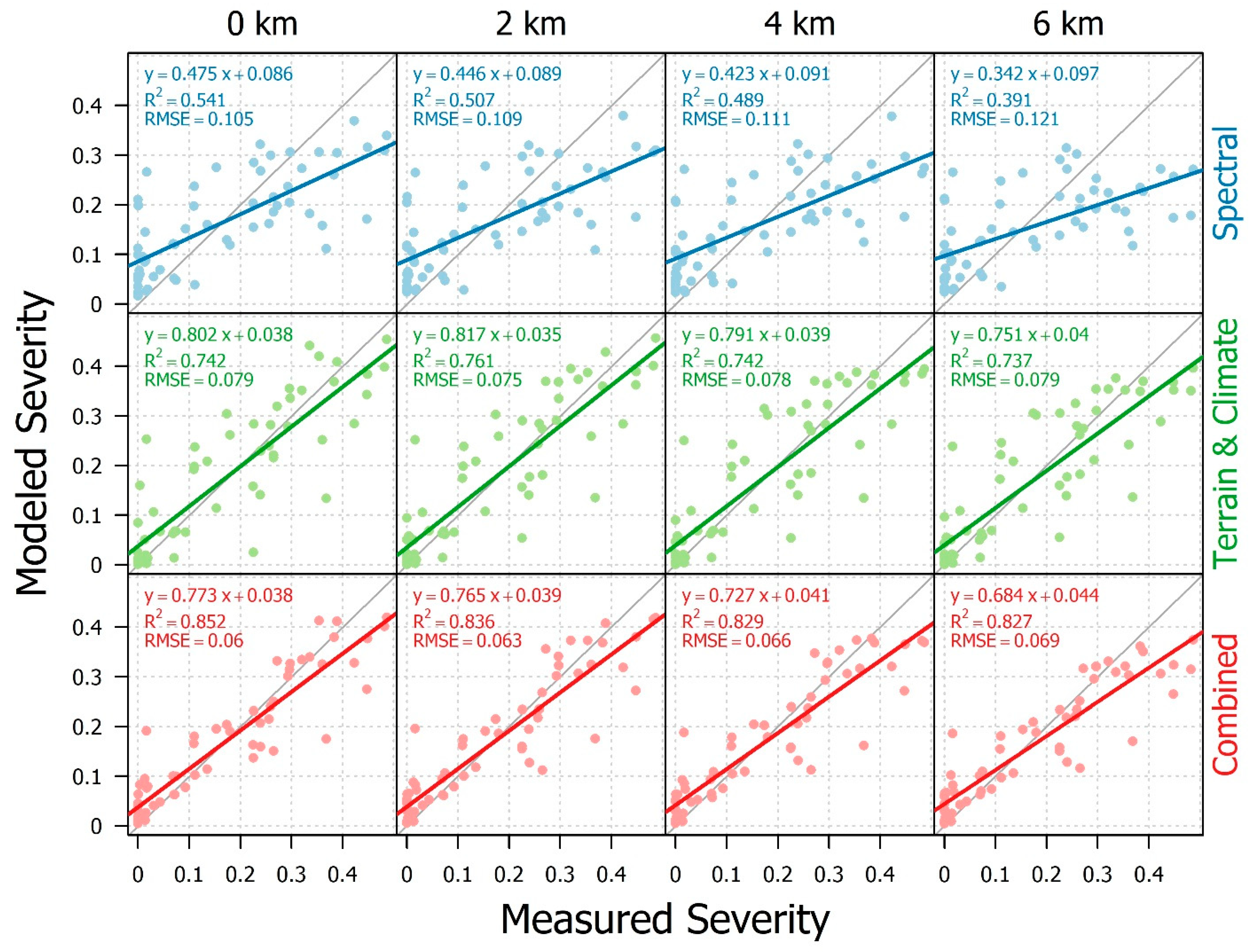
3.2. Model Results
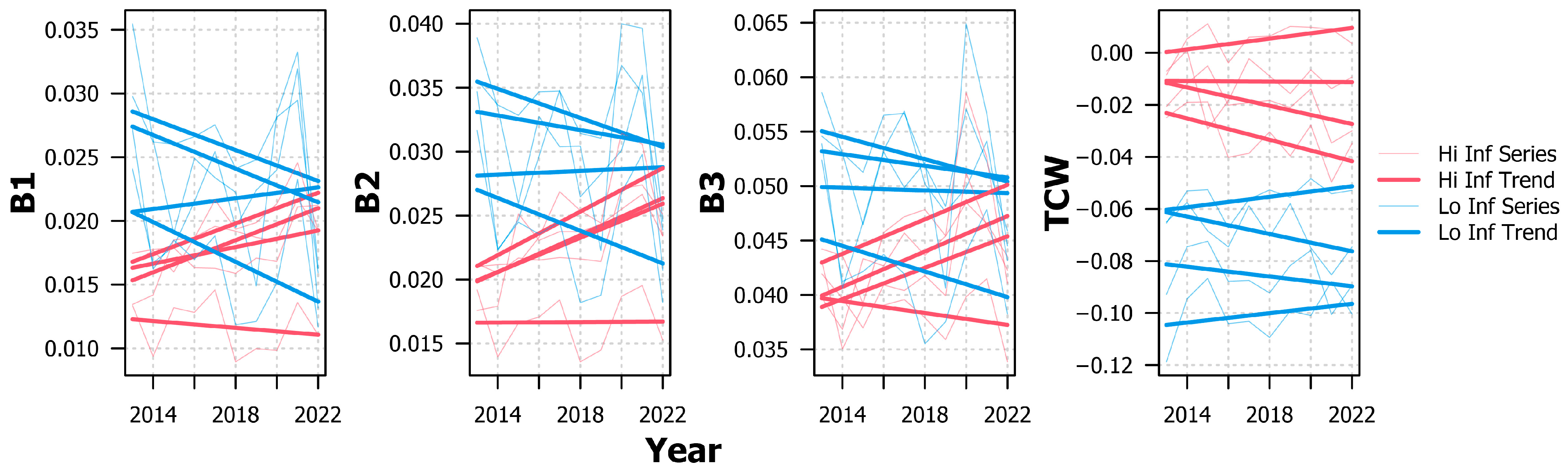
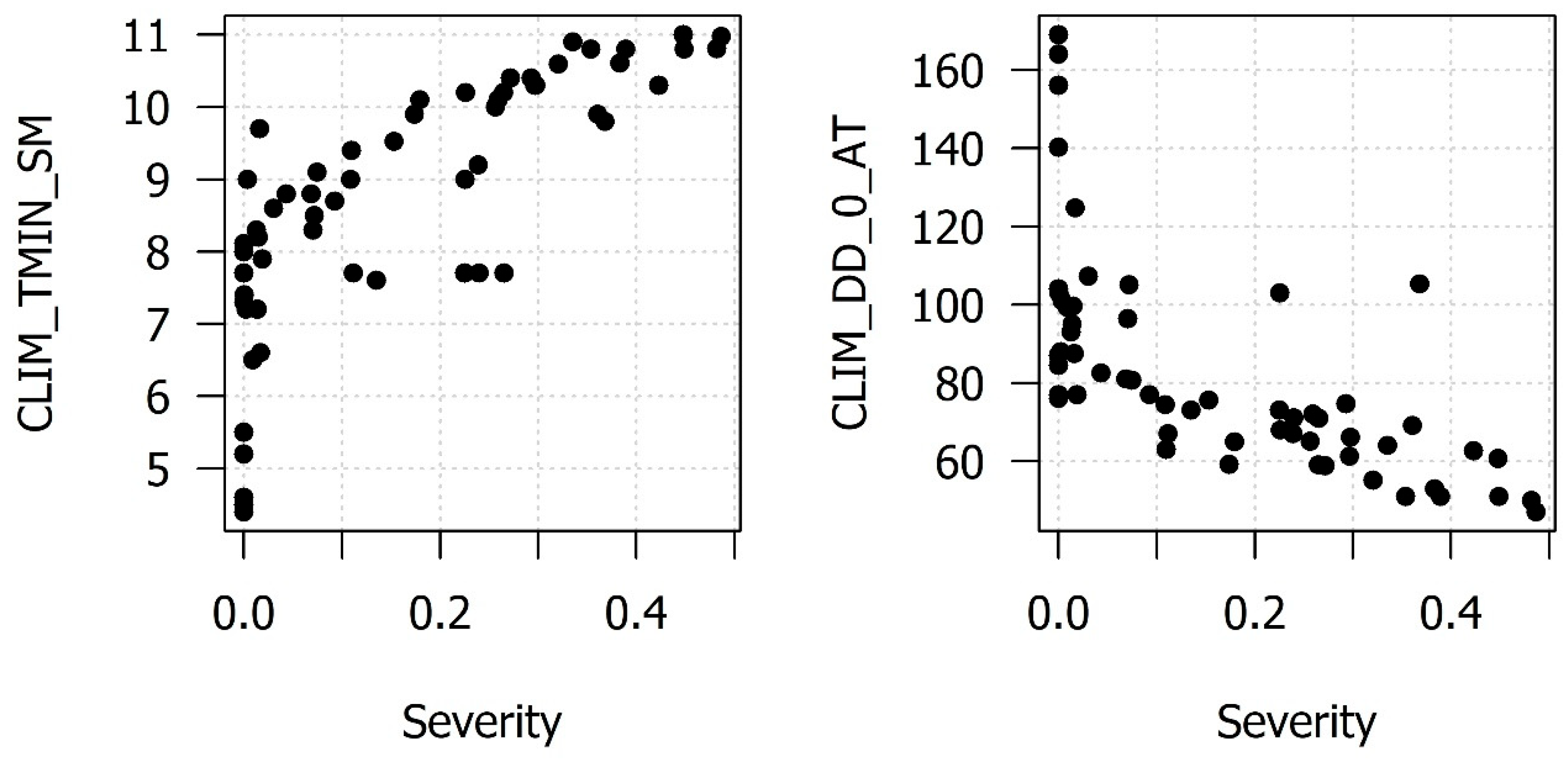
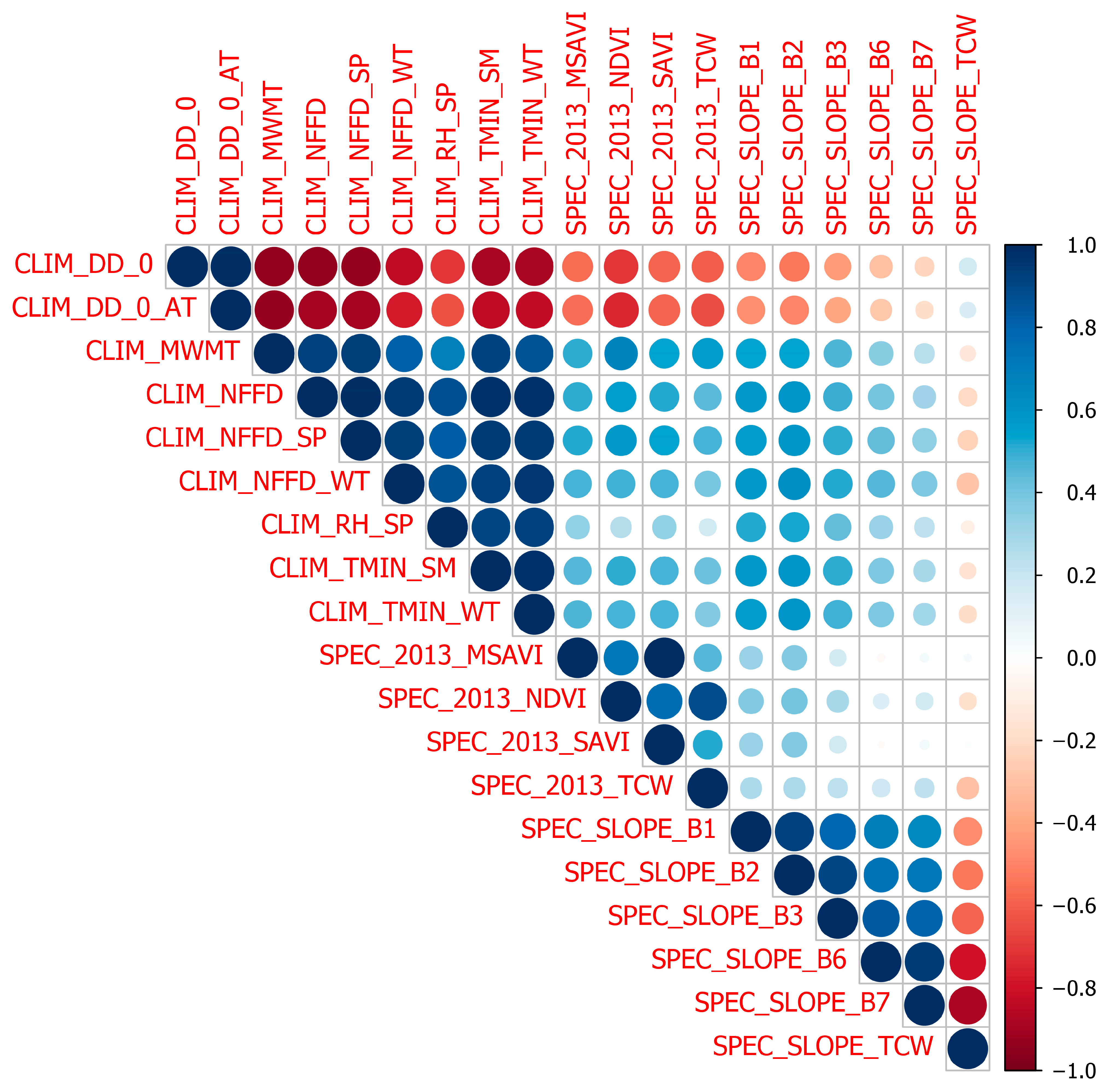
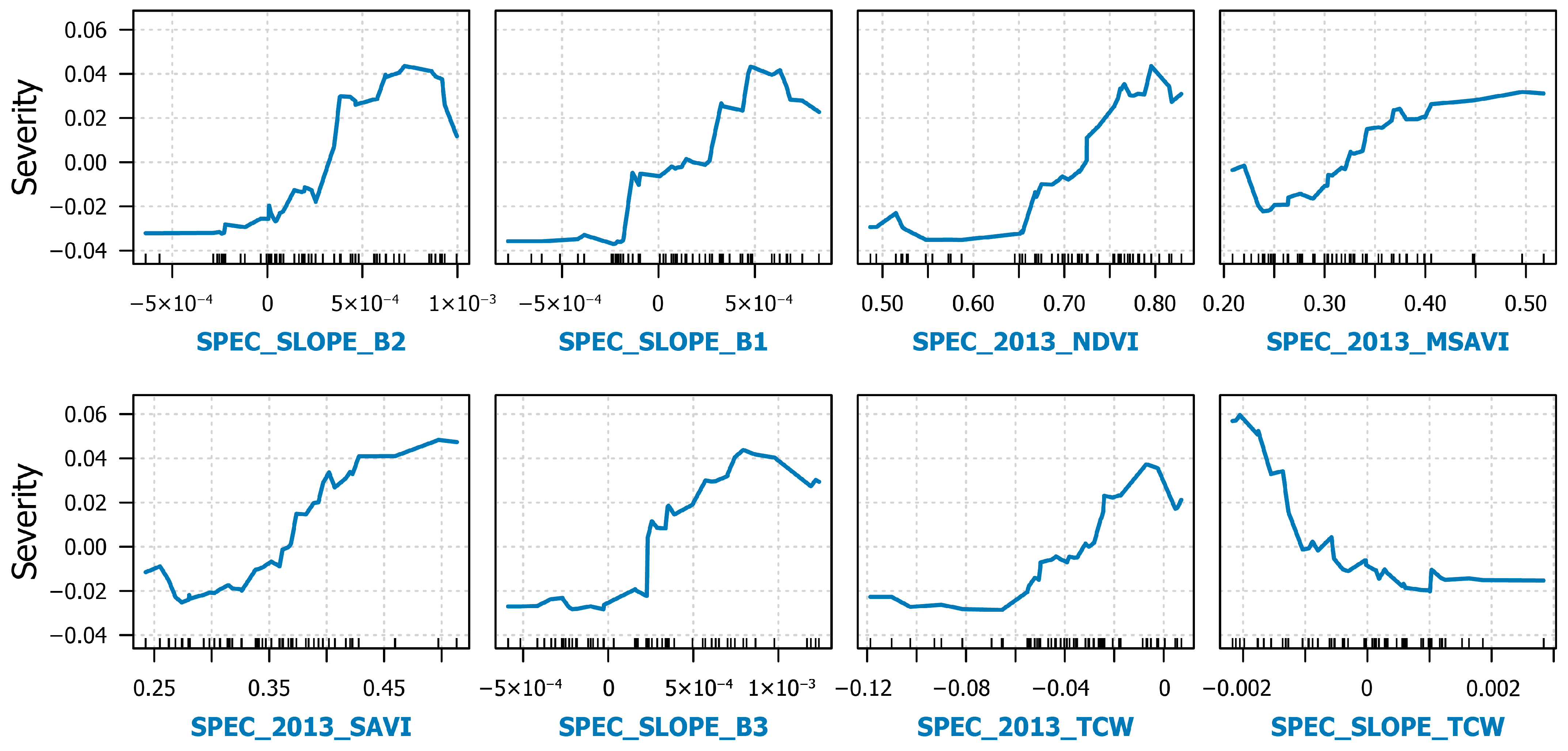
3.3. Evaluating Drivers and Predictors of Infestation Severity
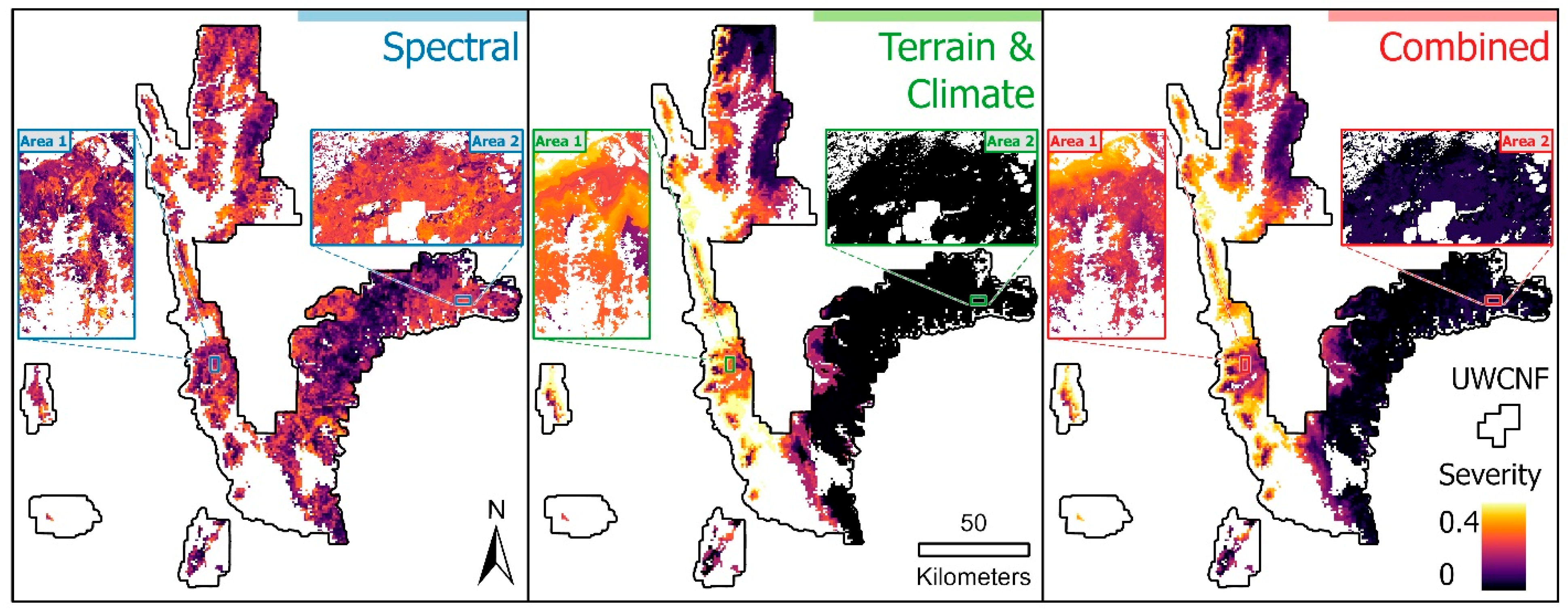
3.4. Map Results
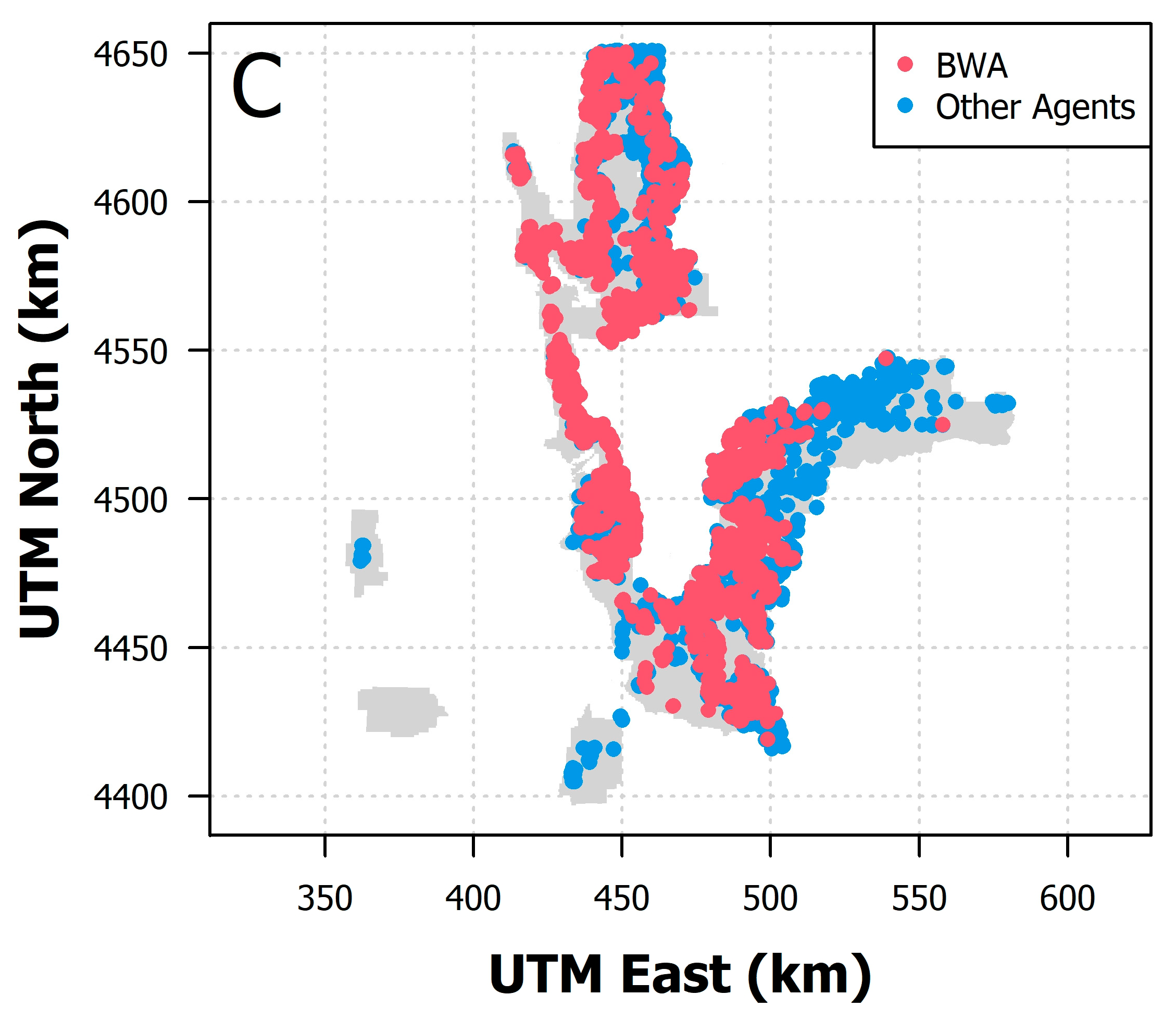
3.5. Comparison to Aerial Survey Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Creation of a Subalpine Fir Map
| Reference Data | ||||
|---|---|---|---|---|
| Presence | Absence | Total | ||
| Predictions | Presence | 170 | 54 | 224 |
| Absence | 59 | 3855 | 3914 | |
| Total | 229 | 3909 | 4138 | |
| Metric | Value |
|---|---|
| Overall accuracy | 0.97 |
| Kappa | 0.74 |
| Sensitivity | 0.74 |
| Specificity | 0.99 |
| Precision | 0.74 |
| Recall | 0.74 |
| F1 | 0.75 |
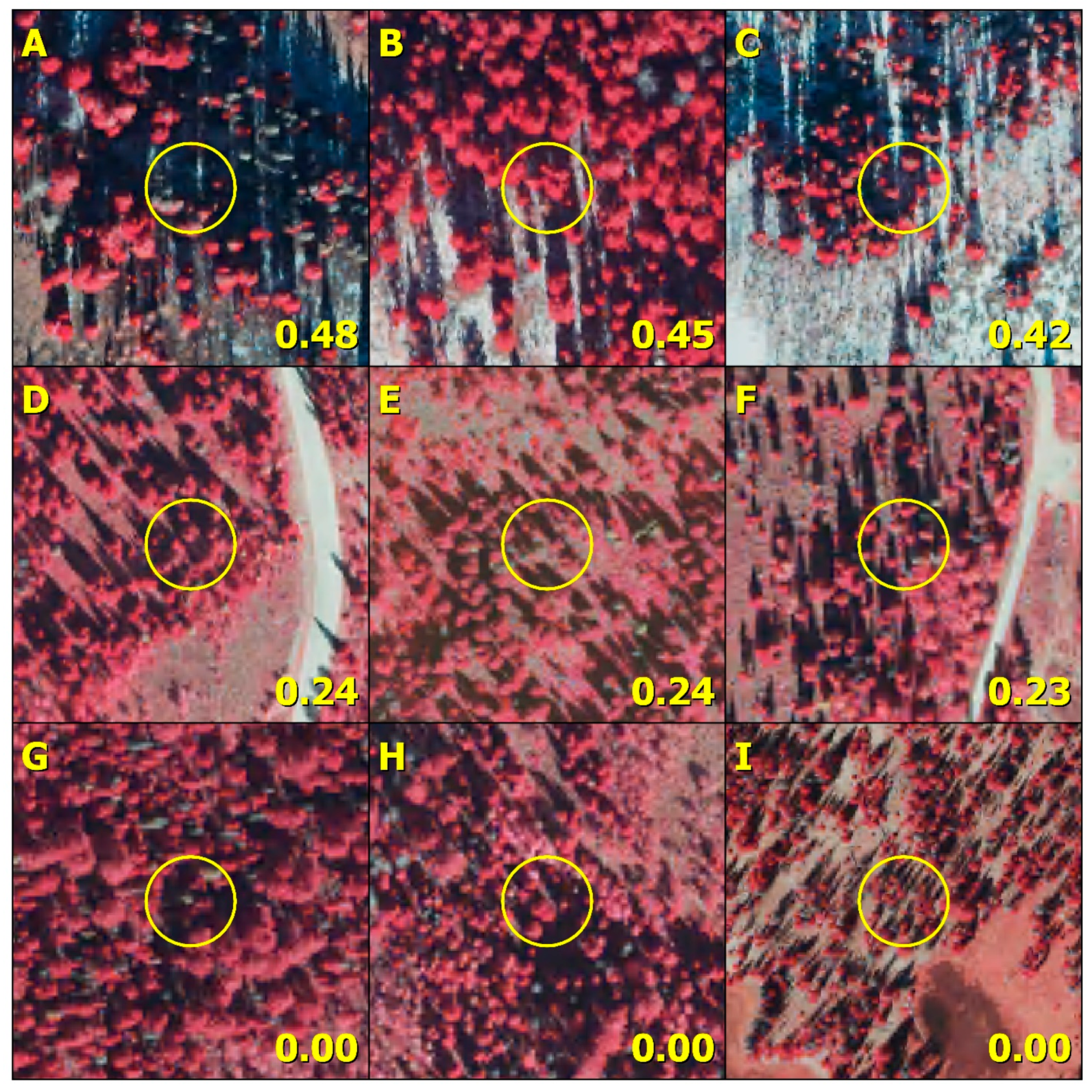
Appendix B. Additional Figures

References
- Davis, G.A.; Lowrey, L.; Eckberg, T.; Hicke, J.A.; Smirnova, E. Characterizing Balsam Woolly Adelgid Infestations and Associated Tree Mortality in Idaho. J. For. 2022, 120, 361–378. [Google Scholar] [CrossRef]
- Kotinsky, J. The European fir trunk bark louse (Chermes (Dreyfusia) piceae Ratz.) apparently long established in the United States. Proc. Entomol. Soc. Wash. 1916, 18, 14–16. [Google Scholar]
- Annand, P.N. A Contribution toward a Monograph of the Adelginae (Phylloxeridae) of North America; Stanford University Publications: Redwood City, CA, USA, 1928; Volume 6. [Google Scholar]
- Keen, F.P. Insect Enemies of Western Forests; Miscellaneous Publication, United States Deptartment of Agriculture, Bureau of Entomology and Plant Quarantine: Washington, DC, USA, 1938.
- Johnson, N.E.; Wright, K.H. The Balsam Woolly Aphid Problem in Oregon and Washington; US Department of Agriculture, Forest Service, Pacific Northwest Forest: Washington, DC, USA, 1957.
- Livingston, R.; Dewey, J.; Beckman, D.; Stipe, L. Distribution of the Balsam Woolly Adelgid in Idaho. West. J. Appl. For. 2000, 15, 227–231. [Google Scholar] [CrossRef]
- Davis, G.; Lowrey, L.; Eckberg, T.; Gannon, A.; Malesky, D.; Havill, N. Notes on balsam woolly adelgid, Adelges piceae (Ratzeburg, 1844) (Hemiptera: Adelgidae), range expansion in Idaho, Montana and Utah. Pan-Pacific Èntomol. 2020, 96, 129–133. [Google Scholar] [CrossRef]
- Amman, G.D. Effects of Temperature and Humidity on Development and Hatching of Eggs of Adelges piceae. Ann. Èntomol. Soc. Am. 1968, 61, 1606–1611. [Google Scholar] [CrossRef]
- Bryant, D.G. A Review of the Taxonomy, Biology and Importance of the Adelgid Pests of True Firs; Newfoundland Forest Research Centre: St. John’s, NL, Canada, 1974; p. 50. [Google Scholar]
- Greenbank, D.O. Climate and the ecology of the balsam woolly aphid. Can. Èntomol. 1970, 102, 546–578. [Google Scholar] [CrossRef]
- Mitchell, R.G.; Buffam, P.E. Patterns of Long-Term Balsam Woolly Adelgid Infestations and Effects in Oregon and Washington. West. J. Appl. For. 2001, 16, 121–126. [Google Scholar] [CrossRef]
- McRoberts, R.E. The Enhanced Forest Inventory and Analysis Program—National Sampling Design and Estimation Procedures; Bechtold, W.A., Patterson, P.L., Eds.; General Technical Report; USDA Forest Service, Southern Research Station: Asheville, NC, USA, 2005.
- Werstack, C.E., Jr.; Shaw, J.D.; Goeking, S.A.; Witt, C.; Menlove, J.; Thompson, M.T.; DeRose, R.J.; Amacher, M.C.; Jovan, S.; Morgan, T.A.; et al. Utah’s Forest Resources, 2003–2012; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2016; Volume 20, p. 159. [CrossRef]
- Mitchell, R.G. Infestation Characteristics of the Balsam Woolly Aphid in the Pacific Northwest; Research Papers; Pacific Northwestern Forest and Range Experiment Station: Portland, OR, USA, 1966.
- Mitchell, R.G.; Johnson, N.E.; Rudinsky, J.A. Seasonal History of the Balsam Woolly Aphid in the Pacific Northwest. Can. Èntomol. 1961, 93, 794–798. [Google Scholar] [CrossRef]
- Lass, L.W.; Cook, S.P.; Shafii, B.; Prather, T.S. Development of a Dispersal Model for Balsam Woolly Adelgid, Adelges piceaeRatzeburg (Hemiptera: Adelgidae), to Facilitate Landscape-Level Management Planning. Int. J. For. Res. 2014, 2014, e519010. [Google Scholar] [CrossRef]
- Johnson, N.E. Pienus Infestation on True Firs in Western Washington. J. Econ. Èntomol. 1959, 52, 828–829. [Google Scholar] [CrossRef]
- Underwood, G.R.; Balch, R.E. A New Species of Pineus (Homoptera: Adelgidae) on Abies. Can. Èntomol. 1964, 96, 522–528. [Google Scholar] [CrossRef]
- Havill, N.P.; Griffin, B.P.; Andersen, J.C.; Foottit, R.G.; Justesen, M.J.; Caccone, A.; D’Amico, V.; Elkinton, J.S. Species delimitation and invasion history of the balsam woolly adelgid, Adelges(Dreyfusia) piceae (Hemiptera: Aphidoidea: Adelgidae), species complex. Syst. Èntomol. 2020, 46, 186–204. [Google Scholar] [CrossRef]
- Balch, R.E. Studies on the Balsam Woolly Aphid (Adelges piceae Ratz.) and Its Effects on Balsam Fir, Abies balsamea (L.) Mill; Department of Agriculture: Ottawa, ON, Canada, 1952; pp. 1–76.
- Doerksen, A.H.; Mitchell, R.G. Effects of the Balsam Woolly Aphid upon Wood Anatomy of Some Western True Firs. For. Sci. 1965, 11, 181–188. [Google Scholar]
- El-Ghany, N.M.A.; El-Aziz, S.E.A.; Marei, S.S. A review: Application of remote sensing as a promising strategy for insect pests and diseases management. Environ. Sci. Pollut. Res. 2020, 27, 33503–33515. [Google Scholar] [CrossRef]
- Chen, G.; Meentemeyer, R.K. Remote Sensing of Forest Damage by Diseases and Insects. In Remote Sensing for Sustainability; CRC Press: Boca Raton, FL, USA, 2016; pp. 145–162. [Google Scholar]
- Hall, R.; Castilla, G.; White, J.; Cooke, B.; Skakun, R. Remote sensing of forest pest damage: A review and lessons learned from a Canadian perspective. Can. Èntomol. 2016, 148, S296–S356. [Google Scholar] [CrossRef]
- Senf, C.; Seidl, R.; Hostert, P. Remote sensing of forest insect disturbances: Current state and future directions. Int. J. Appl. Earth Obs. Geoinf. 2017, 60, 49–60. [Google Scholar] [CrossRef]
- Bright, B.C.; Hudak, A.T.; Meddens, A.J.; Egan, J.M.; Jorgensen, C.L. Mapping Multiple Insect Outbreaks across Large Regions Annually Using Landsat Time Series Data. Remote Sens. 2020, 12, 1655. [Google Scholar] [CrossRef]
- Coops, N.C.; Waring, R.H.; Wulder, M.A.; White, J.C. Prediction and assessment of bark beetle-induced mortality of lodgepole pine using estimates of stand vigor derived from remotely sensed data. Remote Sens. Environ. 2009, 113, 1058–1066. [Google Scholar] [CrossRef]
- Fernandez-Carrillo, A.; Patočka, Z.; Dobrovolný, L.; Franco-Nieto, A.; Revilla-Romero, B. Monitoring Bark Beetle Forest Damage in Central Europe. A Remote Sensing Approach Validated with Field Data. Remote Sens. 2020, 12, 3634. [Google Scholar] [CrossRef]
- Gomez, D.F.; Ritger, H.M.; Pearce, C.; Eickwort, J.; Hulcr, J. Ability of Remote Sensing Systems to Detect Bark Beetle Spots in the Southeastern US. Forests 2020, 11, 1167. [Google Scholar] [CrossRef]
- Meddens, A.J.; Hicke, J.A.; Vierling, L.A.; Hudak, A.T. Evaluating methods to detect bark beetle-caused tree mortality using single-date and multi-date Landsat imagery. Remote Sens. Environ. 2013, 132, 49–58. [Google Scholar] [CrossRef]
- Meigs, G.W.; Kennedy, R.E.; Cohen, W.B. A Landsat time series approach to characterize bark beetle and defoliator impacts on tree mortality and surface fuels in conifer forests. Remote Sens. Environ. 2011, 115, 3707–3718. [Google Scholar] [CrossRef]
- Cook, S.P.; Humes, K.S.; Hruska, R.; Fraley, G.; Williams, C.J. Identifying Subalpine Fir (Abies lasiocarpa) Attacked by the Balsam Woolly Adelgid (Adelges piceae) Using Spectral Measurements of the Foliage. Int. J. For. Res. 2010, 2010, 1–8. [Google Scholar] [CrossRef]
- Shrimpton, G. The Balsam Woolly Adelgid and Pine Needle Mite. In Proceedings of the Target Seedling Symposium Proceedings, Combined Meeting of the Western Forest Nursery Associations, Roseburg, OR, USA, 13–17 August 1990; US Department of Agriculture, Forest Service, Rocky Mountain Forest: Washington, DC, USA, 1990; Volume 200, p. 181. [Google Scholar]
- Franklin, S.E.; Bowers, W.W.; Ghitter, G. Discrimination of adelgid-damage on single balsam fir trees with aerial remote sensing data. Int. J. Remote Sens. 1995, 16, 2779–2794. [Google Scholar] [CrossRef]
- Alonso, K.; Bachmann, M.; Burch, K.; Carmona, E.; Cerra, D.; Reyes, R.D.L.; Dietrich, D.; Heiden, U.; Hölderlin, A.; Ickes, J.; et al. Data Products, Quality and Validation of the DLR Earth Sensing Imaging Spectrometer (DESIS). Sensors 2019, 19, 4471. [Google Scholar] [CrossRef]
- Galeazzi, C.; Sacchetti, A.; Cisbani, A.; Babini, G. The PRISMA Program. In Proceedings of the IGARSS 2008—2008 IEEE International Geoscience and Remote Sensing Symposium, Boston, MA, USA, 8–11 July 2008; Volume 4, pp. IV-105–IV-108. [Google Scholar]
- Hutten, K.M. Landscape Vegetation Change, Pattern Detection, and Interpretation in a Subalpine Fir Forest Infested with Bal-sam Woolly Adelgid. Ph.D. Thesis, Washington of University, Washington, DC, USA, 2015. [Google Scholar]
- Hain, F.P. The Balsam Woolly Adelgid in North America. In Dynamics of Forest Insect Populations: Patterns, Causes, Implications; Berryman, A.A., Ed.; Population Ecology; Springer: Boston, MA, USA, 1988; pp. 87–109. ISBN 978-1-4899-0789-9. [Google Scholar]
- Hrinkevich, K.H.; Progar, R.A.; Shaw, D.C. Climate Risk Modelling of Balsam Woolly Adelgid Damage Severity in Subalpine Fir Stands of Western North America. PLoS ONE 2016, 11, e0165094. [Google Scholar] [CrossRef]
- Kanoti, A.M. Relationship between Balsam Woolly Adelgid Damage, Radial Growth, Climate and Stand Characteristics in Eastern Maine. Master’s Thesis, The University of Maine, Orono, ME, USA, 2006. [Google Scholar]
- McManamay, R.H.; Resler, L.M.; Campbell, J.B.; McManamay, R.A. Assessing the Impacts of Balsam Woolly Adelgid (Adelges piceae Ratz.) and Anthropogenic Disturbance on the Stand Structure and Mortality of Fraser Fir [Abies fraseri (Pursh) Poir.] in the Black Mountains, North Carolina. Castanea 2011, 76, 1–19. [Google Scholar] [CrossRef]
- Quiring, D.; Ostaff, D.; Hartling, L.; Lavigne, D.; Moore, K.; DeMerchant, I. Temperature and plant hardiness zone influence distribution of balsam woolly adelgid damage in Atlantic Canada. For. Chron. 2008, 84, 558–562. [Google Scholar] [CrossRef]
- PRISM Climate Group, Oregon State University 30-Year Normals. Available online: http://prism.oregonstate.edu/ (accessed on 28 January 2019).
- Zhang, Y.; Woodcock, C.E.; Chen, S.; Wang, J.A.; Sulla-Menashe, D.; Zuo, Z.; Olofsson, P.; Wang, Y.; Friedl, M.A. Mapping causal agents of disturbance in boreal and arctic ecosystems of North America using time series of Landsat data. Remote Sens. Environ. 2022, 272, 112935. [Google Scholar] [CrossRef]
- Abdi, O. Climate-Triggered Insect Defoliators and Forest Fires Using Multitemporal Landsat and TerraClimate Data in NE Iran: An Application of GEOBIA TreeNet and Panel Data Analysis. Sensors 2019, 19, 3965. [Google Scholar] [CrossRef]
- Minasny, B.; McBratney, A.B. A conditioned Latin hypercube method for sampling in the presence of ancillary information. Comput. Geosci. 2006, 32, 1378–1388. [Google Scholar] [CrossRef]
- Hrinkevich, K.H.; Progar, R.A.; Shaw, D.C. A Severity Rating System for Evaluating Stand-Level Balsam Woolly Adelgid (Hemiptera: Adelgidae) Damage in Two Abies Species in Western North America. For. Sci. 2016, 62, 181–189. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Campbell, M.J.; Dennison, P.E.; Kerr, K.L.; Brewer, S.C.; Anderegg, W.R. Scaled biomass estimation in woodland ecosystems: Testing the individual and combined capacities of satellite multispectral and lidar data. Remote Sens. Environ. 2021, 262, 112511. [Google Scholar] [CrossRef]
- Rouse, J.W. Monitoring vegetation systems in the Great Plains with ERTS. NASA Spec. Publ. 1974, 351, 309. [Google Scholar]
- Liu, H.Q.; Huete, A. A feedback based modification of the NDVI to minimize canopy background and atmospheric noise. IEEE Trans. Geosci. Remote Sens. 1995, 33, 457–465. [Google Scholar] [CrossRef]
- Badgley, G.; Field, C.B.; Berry, J.A. Canopy near-infrared reflectance and terrestrial photosynthesis. Sci. Adv. 2017, 3, e1602244. [Google Scholar] [CrossRef]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Hardisky, M.; Klemas, V.; Smart, R. Close The influence of soil salinity, growth form and leaf moisture on the spectral radiance of Spartina alterniflora canopies. Photogramm. Eng. Remote Sens. 1983, 49, 77–83. [Google Scholar]
- Key, C.H.; Benson, N.C. The Normalized Burn Ratio (NBR): A Landsat TM Radiometric Measure of Burn Severity; United States Geological Survey, Northern Rocky Mountain Science Center: Bozeman, MT, USA, 1999.
- Hawbaker, T.J.; Vanderhoof, M.K.; Schmidt, G.L.; Beal, Y.-J.; Picotte, J.J.; Takacs, J.D.; Falgout, J.T.; Dwyer, J.L. The Landsat Burned Area algorithm and products for the conterminous United States. Remote Sens. Environ. 2020, 244, 111801. [Google Scholar] [CrossRef]
- Baig, M.H.A.; Zhang, L.; Shuai, T.; Tong, Q. Derivation of a tasselled cap transformation based on Landsat 8 at-satellite reflectance. Remote Sens. Lett. 2014, 5, 423–431. [Google Scholar] [CrossRef]
- Kennedy, R.E.; Yang, Z.; Cohen, W.B. Detecting trends in forest disturbance and recovery using yearly Landsat time series: 1. LandTrendr—Temporal segmentation algorithms. Remote Sens. Environ. 2010, 114, 2897–2910. [Google Scholar] [CrossRef]
- Zhu, Z.; Woodcock, C.E. Continuous change detection and classification of land cover using all available Landsat data. Remote Sens. Environ. 2014, 144, 152–171. [Google Scholar] [CrossRef]
- Rodman, K.C.; Andrus, R.A.; Veblen, T.T.; Hart, S.J. Disturbance detection in landsat time series is influenced by tree mortality agent and severity, not by prior disturbance. Remote Sens. Environ. 2020, 254, 112244. [Google Scholar] [CrossRef]
- Peckham, S.D. Profile, plan and streamline curvature: A simple derivation and applications. Proc. Geomorphometry 2011, 4, 27–30. [Google Scholar]
- Ironside, K.E.; Mattson, D.J.; Arundel, T.; Theimer, T.; Holton, B.; Peters, M.; Edwards, T.C., Jr.; Hansen, J. Geomorphometry in Landscape Ecology: Issues of scale, physiography, and application. Environ. Ecol. Res. 2018, 6, 397–412. [Google Scholar] [CrossRef]
- Iverson, L.R.; Prasad, A.M. A GIS-derived integrated moisture index. In Characteristics of Mixed Oak Forest Ecosystems in Southern Ohio Prior to the Reintroduction of Fire; Sutherland, E.K., Hutchinson, T.F., Eds.; US Department of Agriculture, Forest Service, Northeastern Research Station: Newtown Square, PA, USA, 2003; Volume 299, pp. 29–41. [Google Scholar]
- Weiss, A. Topographic position and landforms analysis. In Proceedings of the Poster Presentation, ESRI User Conference, San Diego, CA, USA, 9–13 July 2001; Volume 200. [Google Scholar]
- Sørensen, R.; Zinko, U.; Seibert, J. On the calculation of the topographic wetness index: Evaluation of different methods based on field observations. Hydrol. Earth Syst. Sci. 2006, 10, 101–112. [Google Scholar] [CrossRef]
- Wang, T.; Hamann, A.; Spittlehouse, D.; Carroll, C. Locally Downscaled and Spatially Customizable Climate Data for Historical and Future Periods for North America. PLoS ONE 2016, 11, e0156720. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Campbell, M.J.; Dennison, P.E.; Tune, J.W.; Kannenberg, S.A.; Kerr, K.L.; Codding, B.F.; Anderegg, W.R. A multi-sensor, multi-scale approach to mapping tree mortality in woodland ecosystems. Remote Sens. Environ. 2020, 245, 111853. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Randomforest: Breiman and Cutler’s Random Forests for Classification and Regression. R Pack. Vers. 2018, 4, 4–6. [Google Scholar]
- Genuer, R.; Poggi, J.-M.; Tuleau-Malot, C. VSURF: An R Package for Variable Selection Using Random Forests. R J. 2015, 7, 19–33. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Bivand, R.; Pebesma, E.; Sumner, M.D. Terra: Spatial Data Analysis. 2023. Available online: https://cran.microsoft.com/snapshot/2023-03-06/web/packages/terra/terra.pdf (accessed on 1 June 2023).
- Pebesma, E.; Bivand, R.; Racine, E.; Sumner, M.; Cook, I.; Keitt, T.; Lovelace, R.; Wickham, H.; Ooms, J.; Müller, K.; et al. sf: Simple Features for R 2022. Available online: https://r-spatial.github.io/sf/ (accessed on 1 June 2023).
- R Core Team. R: A language and environment for statistical computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Probst, P.; Wright, M.N.; Boulesteix, A. Hyperparameters and tuning strategies for random forest. WIREs Data Min. Knowl. Discov. 2019, 9, e1301. [Google Scholar] [CrossRef]
- Ploton, P.; Mortier, F.; Réjou-Méchain, M.; Barbier, N.; Picard, N.; Rossi, V.; Dormann, C.; Cornu, G.; Viennois, G.; Bayol, N.; et al. Spatial validation reveals poor predictive performance of large-scale ecological mapping models. Nat. Commun. 2020, 11, 4540. [Google Scholar] [CrossRef]
- Apley, D.W.; Zhu, J. Visualizing the Effects of Predictor Variables in Black Box Supervised Learning Models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2020, 82, 1059–1086. [Google Scholar] [CrossRef]
- Johnson, E.W.; Wittwer, D. Aerial detection surveys in the United States. Aust. For. 2008, 71, 212–215. [Google Scholar] [CrossRef]
- Coleman, T.W.; Graves, A.D.; Heath, Z.; Flowers, R.W.; Hanavan, R.P.; Cluck, D.R.; Ryerson, D. Accuracy of aerial detection surveys for mapping insect and disease disturbances in the United States. For. Ecol. Manag. 2018, 430, 321–336. [Google Scholar] [CrossRef]
- Johnson, E.W.; Ross, J. Quantifying error in aerial survey data. Aust. For. 2008, 71, 216–222. [Google Scholar] [CrossRef]
- Hanavan, R.P.; Kamoske, A.G.; Schaaf, A.N.; Eager, T.; Fisk, H.; Ellenwood, J.; Warren, K.; Asaro, C.; Vanderbilt, B.; Hutten, K.; et al. Supplementing the Forest Health National Aerial Survey Program with Remote Sensing during the COVID-19 Pandemic: Lessons Learned from a Collaborative Approach. J. For. 2021, 120, 125–132. [Google Scholar] [CrossRef]
- Belitz, K.; Stackelberg, P. Evaluation of six methods for correcting bias in estimates from ensemble tree machine learning regression models. Environ. Model. Softw. 2021, 139, 105006. [Google Scholar] [CrossRef]
- Ringard, J.; Seyler, F.; Linguet, L. A Quantile Mapping Bias Correction Method Based on Hydroclimatic Classification of the Guiana Shield. Sensors 2017, 17, 1413. [Google Scholar] [CrossRef] [PubMed]
- Pastick, N.J.; Jorgenson, M.T.; Goetz, S.J.; Jones, B.M.; Wylie, B.K.; Minsley, B.J.; Genet, H.; Knight, J.F.; Swanson, D.K.; Jorgenson, J.C. Spatiotemporal remote sensing of ecosystem change and causation across Alaska. Glob. Chang. Biol. 2018, 25, 1171–1189. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, T.A.; Schleeweis, K.G.; Moisen, G.G.; Toney, C.; Cohen, W.B.; Freeman, E.A.; Yang, Z.; Huang, C. Testing a Land-sat-based approach for mapping disturbance causality in US forests. Remote Sens. Environ. 2017, 195, 230–243. [Google Scholar] [CrossRef]
- Dennison, P.E.; Brunelle, A.R.; Carter, V.A. Assessing canopy mortality during a mountain pine beetle outbreak using GeoEye-1 high spatial resolution satellite data. Remote Sens. Environ. 2010, 114, 2431–2435. [Google Scholar] [CrossRef]
- Meddens, A.J.; Hicke, J.A.; Vierling, L.A. Evaluating the potential of multispectral imagery to map multiple stages of tree mortality. Remote Sens. Environ. 2011, 115, 1632–1642. [Google Scholar] [CrossRef]
- Minařík, R.; Langhammer, J.; Lendzioch, T. Automatic Tree Crown Extraction from UAS Multispectral Imagery for the Detection of Bark Beetle Disturbance in Mixed Forests. Remote Sens. 2020, 12, 4081. [Google Scholar] [CrossRef]
- Oumar, Z.; Mutanga, O. Using WorldView-2 bands and indices to predict bronze bug (Thaumastocoris peregrinus) damage in plantation forests. Int. J. Remote Sens. 2012, 34, 2236–2249. [Google Scholar] [CrossRef]
- Williams, J.P.; Hanavan, R.P.; Rock, B.N.; Minocha, S.C.; Linder, E. Influence of hemlock woolly adelgid infestation on the physiological and reflectance characteristics of eastern hemlock. Can. J. For. Res. 2016, 46, 410–426. [Google Scholar] [CrossRef]
- Wadoux, A.M.-C.; Heuvelink, G.B.; de Bruin, S.; Brus, D.J. Spatial cross-validation is not the right way to evaluate map accuracy. Ecol. Model. 2021, 457, 109692. [Google Scholar] [CrossRef]
- Hicke, J.A.; Davis, G.; Lowrey, L.; Xu, B.; Smirnova, E.; Kalachev, L. An evaluation of climate influences on balsam woolly adelgid infestations in Idaho. For. Ecol. Manag. 2023, 534, 120849. [Google Scholar] [CrossRef]
- Speiser, J.L.; Miller, M.E.; Tooze, J.; Ip, E. A comparison of random forest variable selection methods for classification prediction modeling. Expert Syst. Appl. 2019, 134, 93–101. [Google Scholar] [CrossRef]
- Boonprong, S.; Cao, C.; Chen, W.; Bao, S. Random Forest Variable Importance Spectral Indices Scheme for Burnt Forest Recovery Monitoring—Multilevel RF-VIMP. Remote Sens. 2018, 10, 807. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Wallace, J.; Campbell, E. Estimating annual runoff in response to forest change: A statistical method based on random forest. J. Hydrol. 2020, 589, 125168. [Google Scholar] [CrossRef]
- Lindner, T.; Puck, J.; Verbeke, A. Beyond addressing multicollinearity: Robust quantitative analysis and machine learning in international business research. J. Int. Bus. Stud. 2022, 53, 1307–1314. [Google Scholar] [CrossRef]
- Oukawa, G.Y.; Krecl, P.; Targino, A.C. Fine-scale modeling of the urban heat island: A comparison of multiple linear regression and random forest approaches. Sci. Total Environ. 2022, 815, 152836. [Google Scholar] [CrossRef] [PubMed]
- Triscowati, D.W.; Sartono, B.; Kurnia, A.; Dirgahayu, D.; Wijayanto, A.W. Classification of rice-plant growth phase using supervised random forest method based on landsat-8 multitemporal data. Int. J. Remote Sens. Earth Sci. (IJReSES) 2020, 16, 187–196. [Google Scholar] [CrossRef]
- Zhan, Y.; Luo, Y.; Deng, X.; Zhang, K.; Zhang, M.; Grieneisen, M.L.; Di, B. Satellite-Based Estimates of Daily NO2 Exposure in China Using Hybrid Random Forest and Spatiotemporal Kriging Model. Environ. Sci. Technol. 2018, 52, 4180–4189. [Google Scholar] [CrossRef]
- Hicke, J.A.; Xu, B.; Meddens, A.J.; Egan, J.M. Characterizing recent bark beetle-caused tree mortality in the western United States from aerial surveys. For. Ecol. Manag. 2020, 475, 118402. [Google Scholar] [CrossRef]
- Lalande, B.M.; Hughes, K.; Jacobi, W.R.; Tinkham, W.T.; Reich, R.; Stewart, J.E. Subalpine fir mortality in Colorado is associated with stand density, warming climates and interactions among fungal diseases and the western balsam bark beetle. For. Ecol. Manag. 2020, 466, 118133. [Google Scholar] [CrossRef]
- Reich, R.M.; Lundquist, J.E.; Hughes, K. Host-environment mismatches associated with subalpine fir decline in Colorado. J. For. Res. 2016, 27, 1177–1189. [Google Scholar] [CrossRef]
- USDA Forest Service FIA DataMart 2.0: Home. Available online: https://apps.fs.usda.gov/fia/datamart/datamart.html (accessed on 3 February 2023).
- Homer, C.; Huang, C.; Yang, L.; Wylie, B.; Coan, M. Michael Michael Coan 1SAIC Corporation Development of a 2001 National Land-Cover Database for the United States. Photogramm. Eng. Remote Sens. 2004, 70, 829–840. [Google Scholar] [CrossRef]
- Rollins, M.G. LANDFIRE: A nationally consistent vegetation, wildland fire, and fuel assessment. Int. J. Wildland Fire 2009, 18, 235–249. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef]
- Potapov, P.; Li, X.; Hernandez-Serna, A.; Tyukavina, A.; Hansen, M.C.; Kommareddy, A.; Pickens, A.; Turubanova, S.; Tang, H.; Silva, C.E.; et al. Mapping global forest canopy height through integration of GEDI and Landsat data. Remote Sens. Environ. 2020, 253, 112165. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J.; Freidank, M.; Cai, J.; Protivinsky, T. Corrplot: Visualization of a Corre-lation Matrix. 2021. Available online: https://cran.r-project.org/web/packages/corrplot/corrplot.pdf (accessed on 1 June 2023).




| Index | Abbreviation | Formula | Source |
|---|---|---|---|
| Normalized Difference Vegetation Index | NDVI | [50] | |
| Enhanced Vegetation Index | EVI | [51] | |
| Near Infrared Reflectance of Vegetation | NIRV | [52] | |
| Soil Adjusted Vegetation Index | SAVI | [53] | |
| Modified Soil Adjusted Vegetation Index | MSAVI | [54] | |
| Normalized Difference Moisture Index | NDMI | [55] | |
| Normalized Burn Ratio | NBR | [56] | |
| Normalized Burn Ratio 2 | NBR2 | [57] | |
| Tasseled Cap Brightness | TCB | [58] | |
| Tasseled Cap Greenness | TCG | [58] | |
| Tasseled Cap Wetness | TCW | [58] |
| Abbreviation | Description |
|---|---|
| ASPECT_COS | Cosine of the direction of steepest decline, representing north–south-ness |
| ASPECT_SIN | Sine of the direction of steepest terrain decline, representing east–west-ness |
| CURV_PLAN | Curvature of the terrain in the perpendicular direction to the slope [62] |
| CURV_PROF | Curvature in the terrain in the parallel direction to the slope [62] |
| ELEV | Raw elevation data from DEM |
| HLI | Measure of solar radiation that incorporates slope, aspect, and latitude [63] |
| IMI | Wetness measure that incorporates accumulation of water flow, local curvature, and exposure to solar radiation [64] |
| SD_x | Standard deviation of elevation within a circular focal area with a radius x for x in 10, 20, 30, 40, and 50 pixels |
| SIE | Measure of solar radiation that incorporates slope and aspect [63] |
| SLOPE | Angle of steepest terrain decline |
| SLOPE_ASPECT_COS | Cosine of aspect multiplied by the slope |
| SLOPE_ASPECT_SIN | Sine of aspect multiplied by the slope |
| SLOPE_DERIV | First derivative of slope, representing the rate of change of slope |
| TPI_x | Difference between elevation at a given location and mean elevation of an annulus surrounding that location with an outer radius of x for x in 10, 20, 30, 40, and 50 pixels, where the inner radius is equal to x/2 [65] |
| TRAI | Measure of solar radiation that only incorporates aspect [63] |
| TWI | Wetness measure that incorporates slope, direction of water flow, accumulation of water flow, and upslope contributing drainage basin size [66] |
| Abbreviation | Description |
|---|---|
| AHM | Annual heat-moisture index (MAT + 10)/(MAP/1000) |
| BFFP | The day of the year on which FFP begins |
| CMD | Hargreaves climatic moisture deficit (mm), annual |
| CMD_x | Hargreaves climatic moisture deficit (mm) for each season x in (SP, SM, AT, and WT |
| CMI | Hogg’s climate moisture index (mm), annual |
| CMI_x | Hogg’s climate moisture index (mm) for each season x in SP, SM, AT, and WT |
| DD1040 | Degree days above 10 °C and below 40 °C |
| DD18 | Degree days above 18 °C, cooling degree days, annual |
| DD18_x | Degree days above 18 °C, cooling degree days for each season x in SP, SM, AT, and WT |
| DD_18 | Degree days below 18 °C, heating degree days, annual |
| DD_18_x | Degree days below 18 °C, heating degree days for each season x in SP, SM, AT, and WT |
| DD5 | Degree days above 5 °C, growing degree days, annual |
| DD5_x | Degree days above 5 °C, growing degree days for each season x in SP, SM, AT, and WT |
| DD_0 | Degree days below 0 °C, chilling degree days, annual |
| DD_0_x | Degree days below 0 °C, chilling degree days for each season x in SP, SM, AT, and WT |
| EFFP | The day of the year on which FFP ends |
| EMT | Extreme minimum temperature over 10 years (°C) |
| EREF | Hargreaves reference evaporation (mm), annual |
| EREF_x | Hargreaves reference evaporation (mm) for each season x in SP, SM, AT, and WT |
| EXT | Extreme maximum temperature over 10 years (°C) |
| FFP | Frost-free period |
| MAP | Mean precipitation (mm), annual |
| MAT | Mean temperature (°C), annual |
| MCMT | Mean coldest month temperature (°C) |
| MSP | Mean summer precipitation (mm) |
| MWMT | Mean warmest month temperature (°C) |
| NFFD | The number of frost-free days, annual |
| NFFD_x | The number of frost-free days for each season x in SP, SM, AT, and WT |
| PAS | Precipitation as snow (mm), annual |
| PAS_x | Precipitation as snow (mm) for each season x in SP, SM, AT, and WT |
| PPT_x | Mean precipitation for each season x in SP, SM, AT, and WT |
| RH | Mean relative humidity (%), annual |
| RH_x | Mean relative humidity (%) for each season x in SP, SM, AT, and WT |
| SHM | Summer heat-moisture index (MWMT)/(MSP/1000) |
| TAVE_x | Mean average temperature for each season x in SP, SM, AT, and WT |
| TD | Temperature difference between MWMT and MCMT, or continentality (°C) |
| TMAX_x | Maximum average temperature for each season x in SP, SM, AT, and WT |
| TMIN_x | Minimum average temperature for each season x in SP, SM, AT, and WT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, M.J.; Williams, J.P.; Berryman, E.M. Using Remote Sensing and Climate Data to Map the Extent and Severity of Balsam Woolly Adelgid Infestation in Northern Utah, USA. Forests 2023, 14, 1357. https://doi.org/10.3390/f14071357
Campbell MJ, Williams JP, Berryman EM. Using Remote Sensing and Climate Data to Map the Extent and Severity of Balsam Woolly Adelgid Infestation in Northern Utah, USA. Forests. 2023; 14(7):1357. https://doi.org/10.3390/f14071357
Chicago/Turabian StyleCampbell, Michael J., Justin P. Williams, and Erin M. Berryman. 2023. "Using Remote Sensing and Climate Data to Map the Extent and Severity of Balsam Woolly Adelgid Infestation in Northern Utah, USA" Forests 14, no. 7: 1357. https://doi.org/10.3390/f14071357
APA StyleCampbell, M. J., Williams, J. P., & Berryman, E. M. (2023). Using Remote Sensing and Climate Data to Map the Extent and Severity of Balsam Woolly Adelgid Infestation in Northern Utah, USA. Forests, 14(7), 1357. https://doi.org/10.3390/f14071357








