Assessment of Summer Branch Drop: A Case Study in Four Portuguese Cities
Abstract
:1. Introduction
2. Materials and Methods
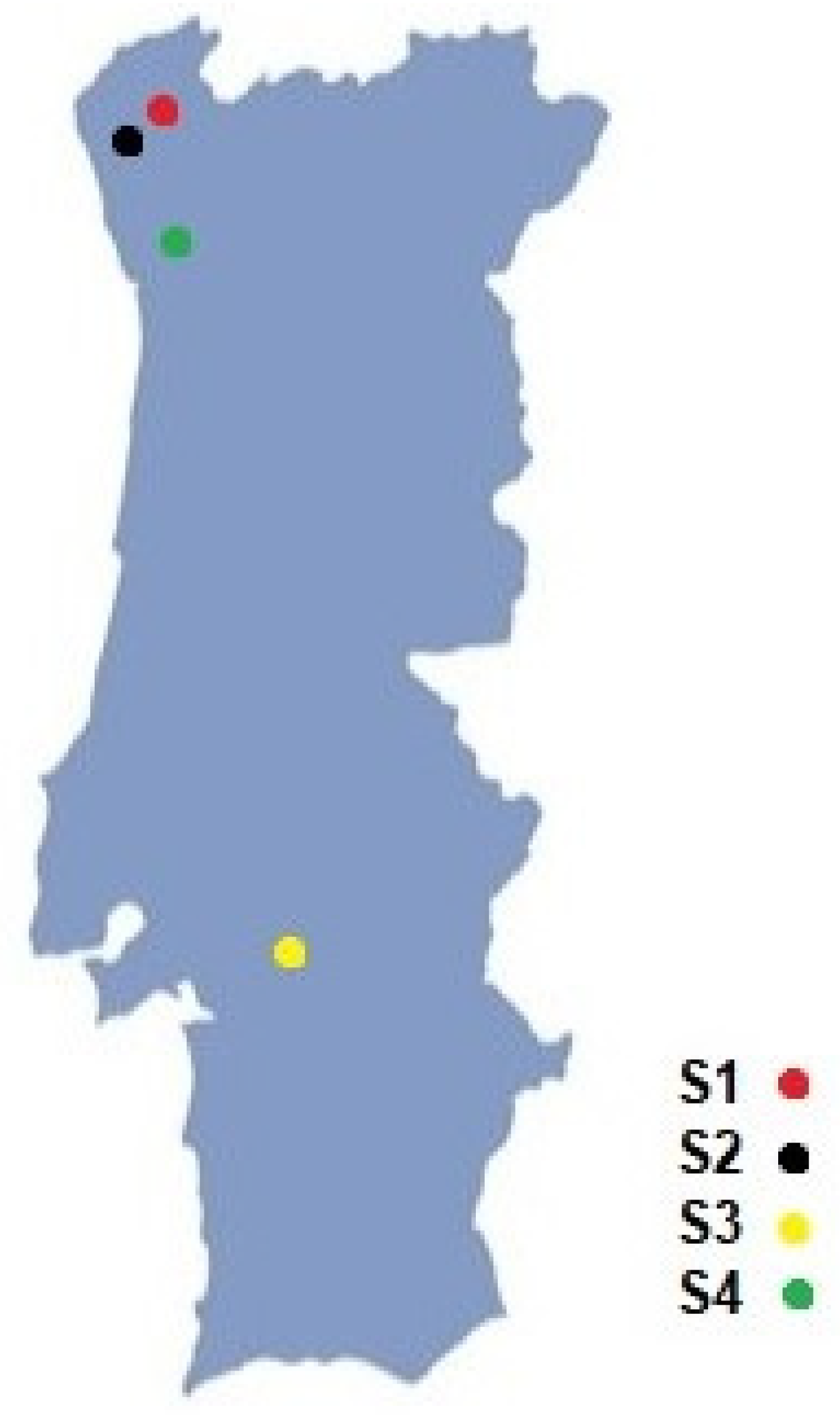
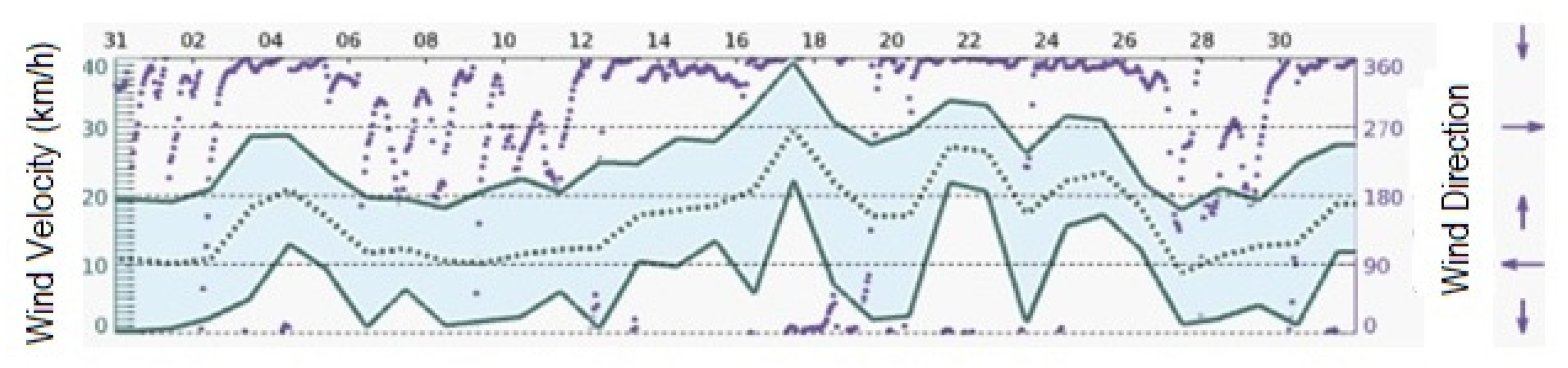
2.1. Study Site
2.2. Dendrometry
2.3. Visual Analysis
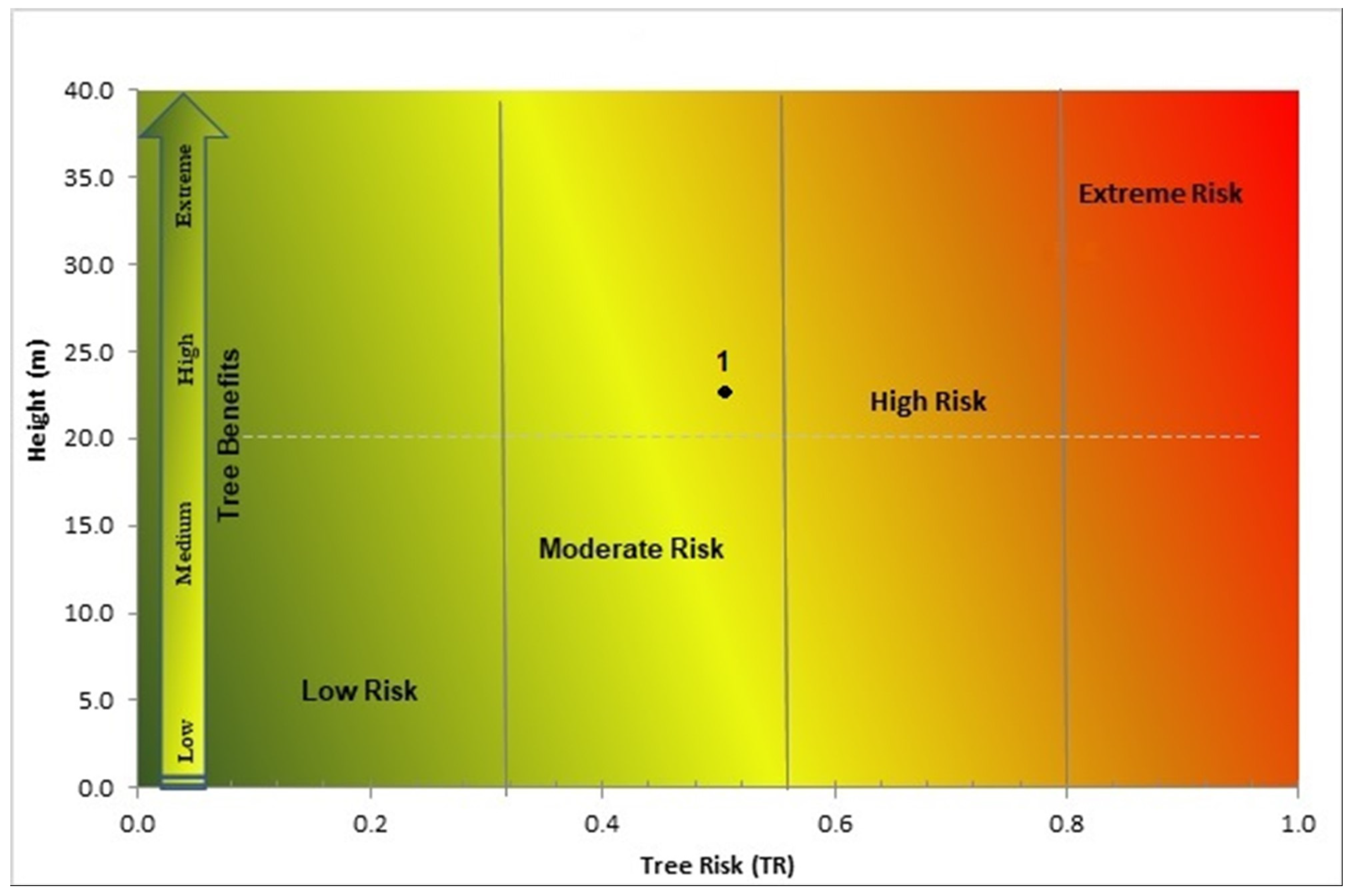
2.4. Risk Matrix
- (a)
- Tree height (0–40 m; 25% weighting);
- (b)
- Probable target relative to the occupation of the space (1–5; 20% weighting);
- (c)
- Visual analysis (0–20; 25% weighting);
- (d)
- Probability of fracture (0–1; 30% weighting).
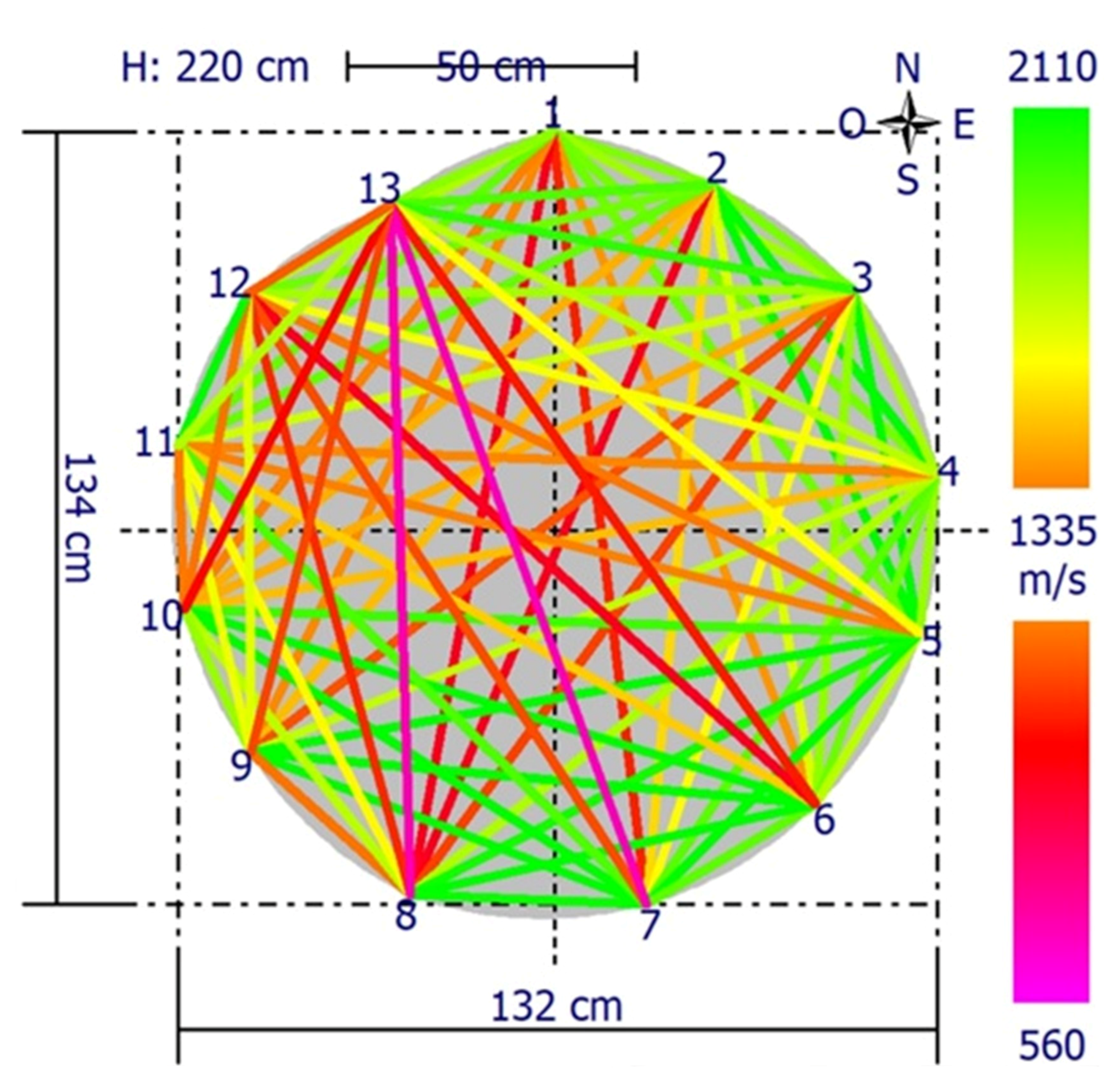
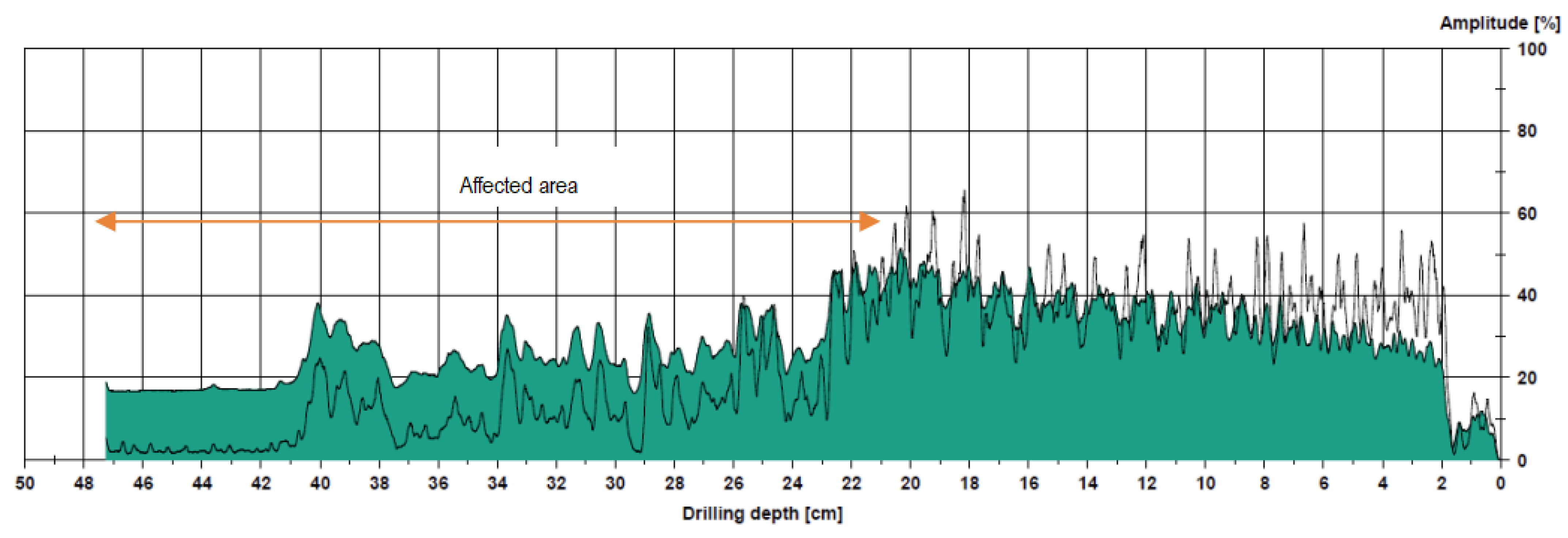
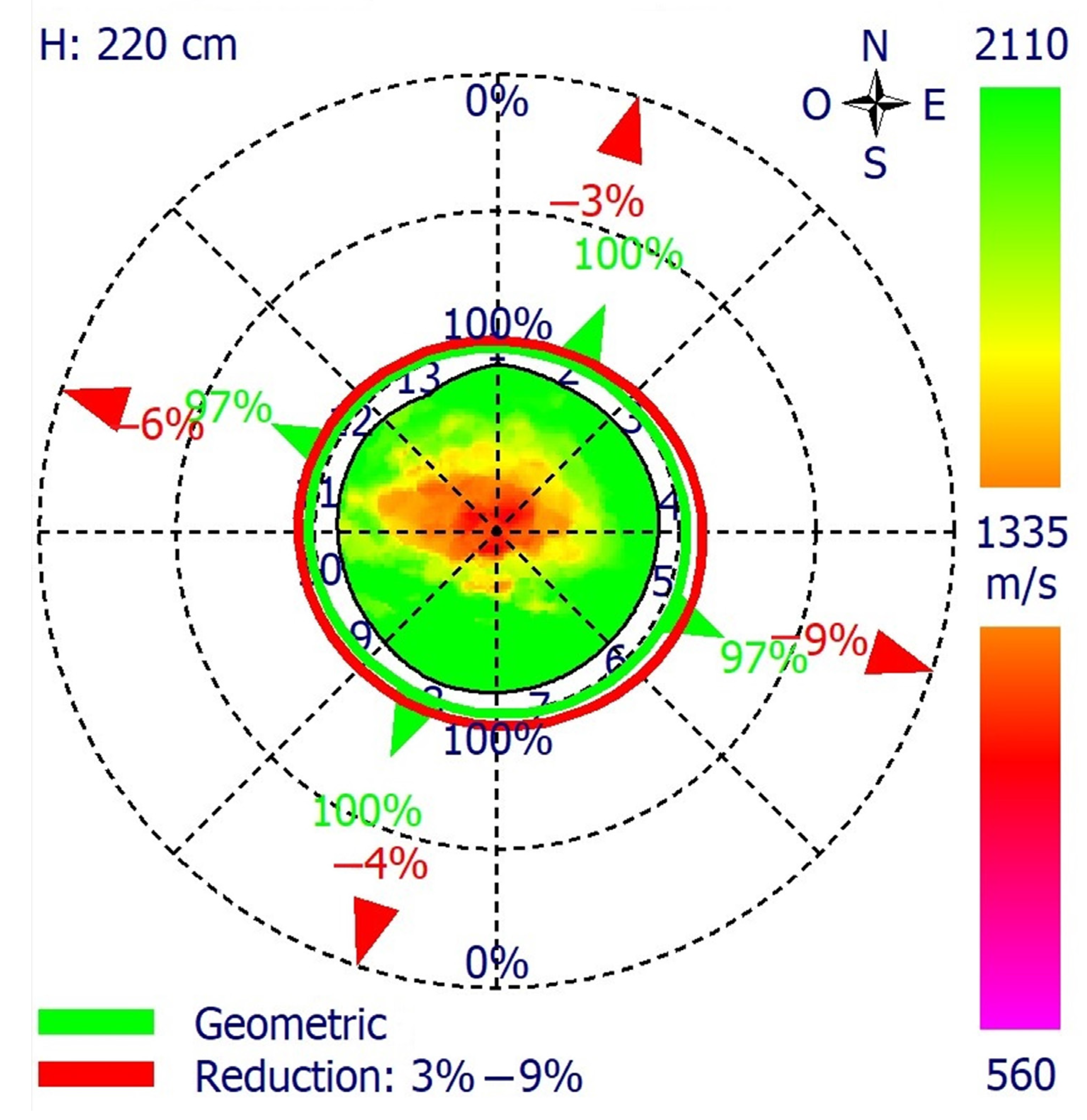
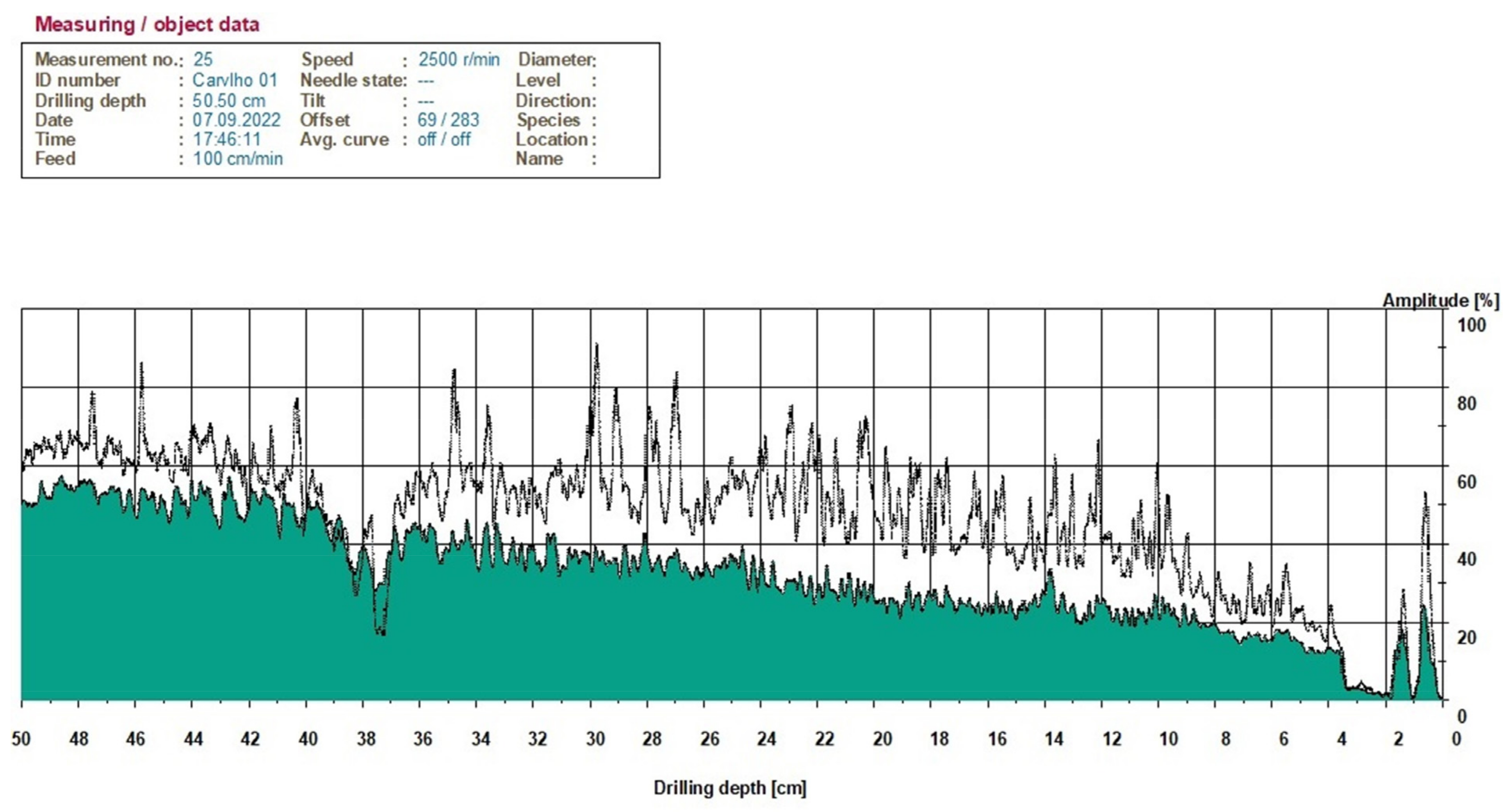
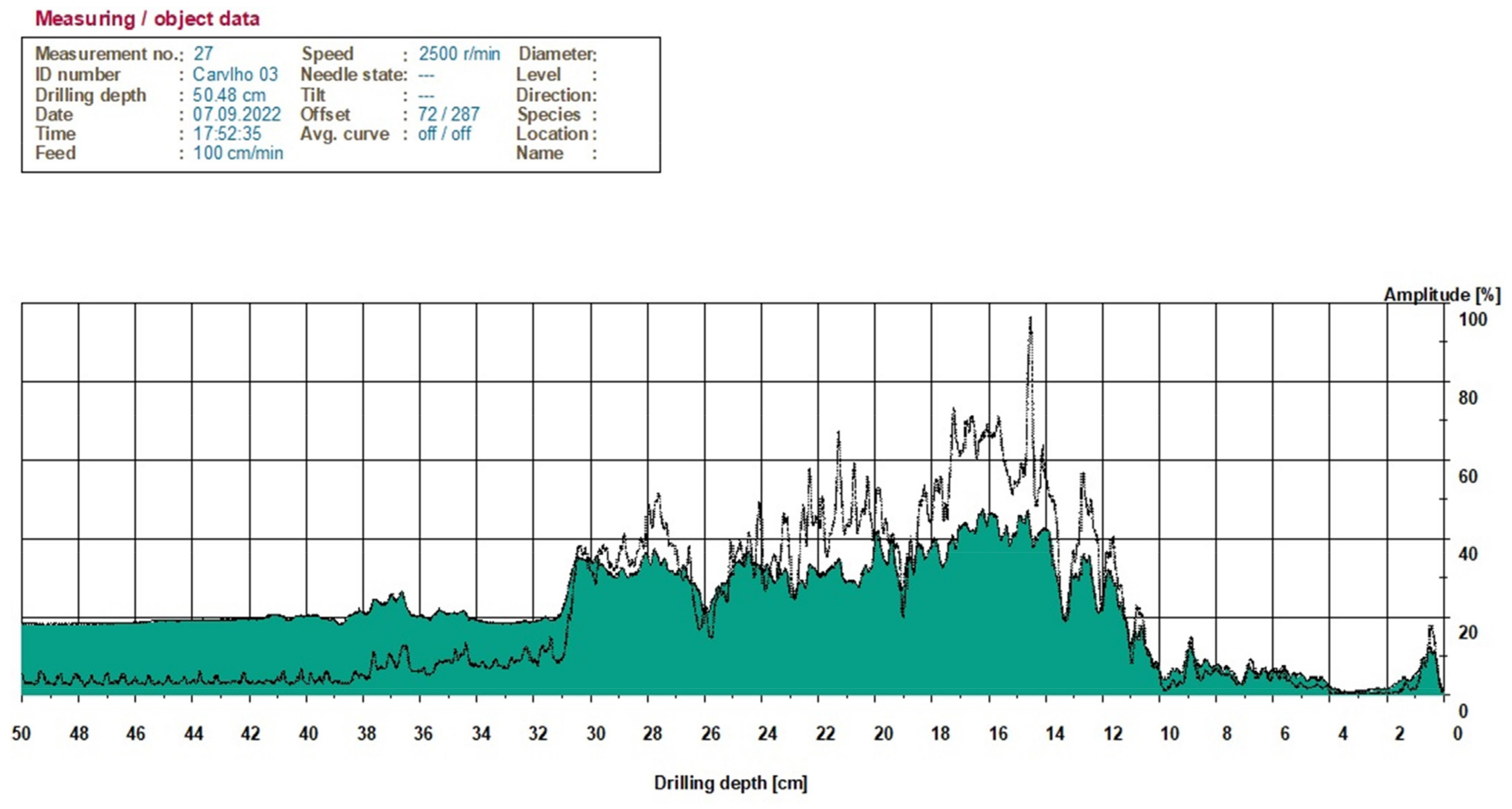
2.5. Instrumental Analysis
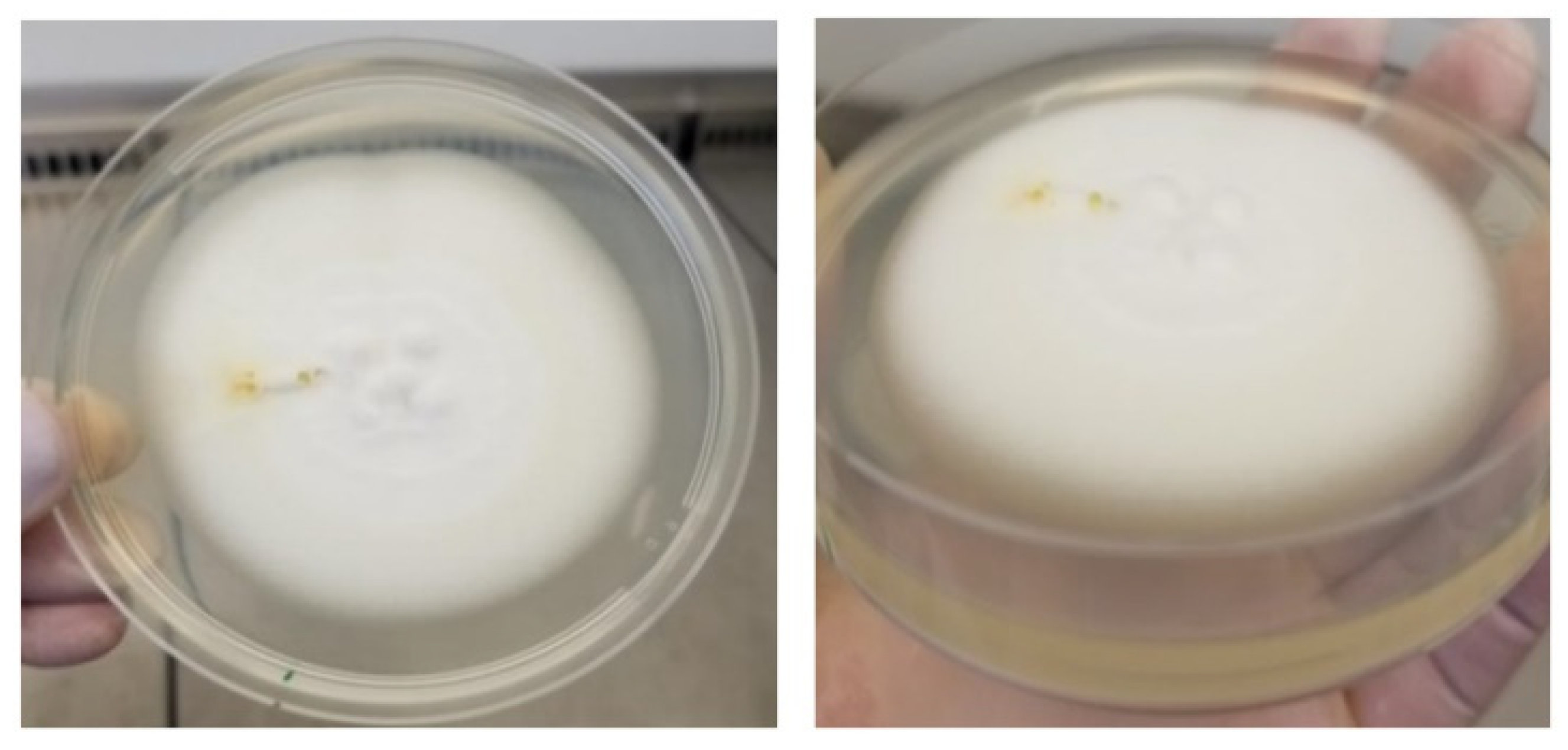
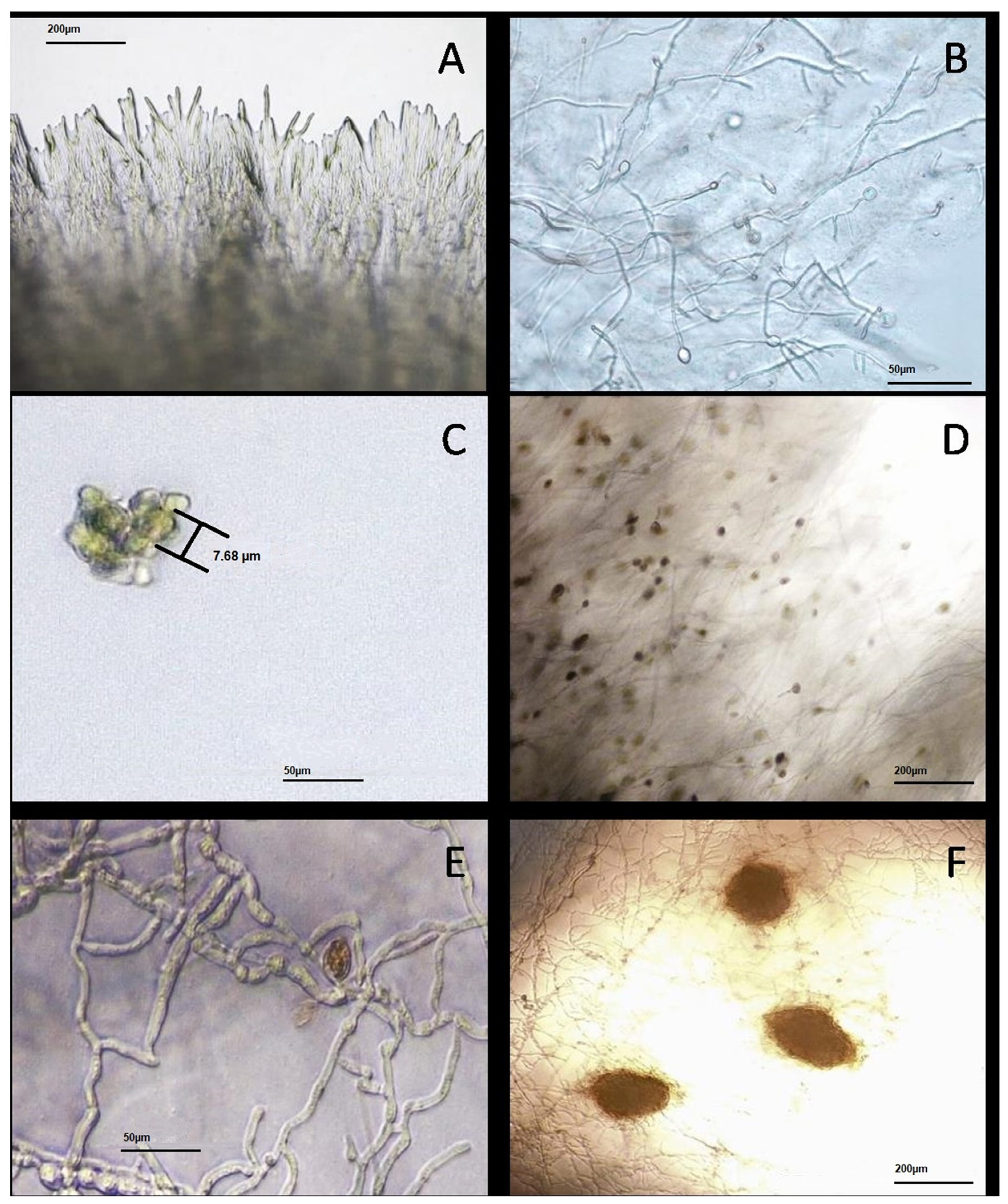
2.6. Laboratory Analysis
- −
- Surface disinfection in 70% alcohol;
- −
- Immersion of the plant material in sterile distilled water;
- −
- Inoculation of the plant material in PDA (potato dextrose agar) medium;
- −
- Incubation of Petri dishes at 30 °C for 36 h;
- −
- Periodic observation and transfer of colonies morphologically resembling typical Ganoderma lucidum colonies from PDA to SNA (Spezieller-Nährstoffarmer agar) medium;
- −
- Incubation at 30 °C;
- −
- Microscopic observation and evaluation of morphological characteristics;
- −
- Periodic observations of fungal colonies with identification based on reproduction structures.
3. Data Analysis and Results
3.1. Dendrometry
3.2. Assessment of SBD in Site 1—Arcos de Valdevez
3.3. Assessment of SBD in Site 2—Ponte de Lima
3.4. Assessment of SBD in Site 3—Montemor-o-Novo
3.5. Assessment of SBD in Site 4—Paços de Ferreira
4. Conclusions
- −
- SBD is mostly related to internal degradation, making it difficult to predict the fall when only considering environmental conditions, but lowered tree defences against internal degradation due to environmental conditions are an element that must be considered.
- −
- VTA diagnosis, applied in isolation, could lead to an incorrect prognosis of internal degradation. So, it is important to confirm of the presence of biotic agents using equipment (acoustic tomography and drilling resistance).
- −
- Instrumental diagnostic tests that considering the date of evaluation are very helpful, but for a more complete answer regarding degradation, reducing the subjectivity of the approach to estimating the probability of fracture, a global analysis is necessary, including dendrometric parameters, predisposing and inciting factors, lower tree defences against internal degradation due to environmental conditions, and biotic agents.
- −
- The deconstruction of the natural architecture of the branches seems to be an element that affects the natural processes of decline. So, it is recommended that large branches remain in place.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tallis, M.; Taylor, G.; Sinnett, D.; Freer-Smith, P. Estimating the removal of atmospheric particulate pollution by the urban tree canopy of London, under current and future environments. Landsc. Urban Plan 2011, 103, 129–138. [Google Scholar] [CrossRef]
- Escobedo, F.J.; Nowak, D.J. Spatial heterogeneity and air pollution removal by an urban forest. Landsc. Urban Plan 2009, 90, 102–110. [Google Scholar] [CrossRef]
- Gilbert, E.A.; Smiley, T. Picus Sonic tomography for the quantification of decay in white oak (Quercus alba) and hickory (Carya sp.). J. Arboric. 2004, 30, 277–281. [Google Scholar]
- Wilcox, W.W. Review of literature on the effects of early stages of decay on wood strength. Wood Fiber Sci. 1978, 4, 252–257. [Google Scholar]
- Mattheck, C. Field Guide for Visual Tree Assessment; Forschungzentrum Karlsruhe GmbH: Karlsruhe, Germany, 2007; p. 170. [Google Scholar]
- Linhares, C.; Gonçalves, R.; Yojo, T. Simplified Methodology for the Inference of Drag Coefficient Applied in Species of Tropical Zone. In Proceedings of the 21st International Nondestructive Testing and Evaluation of Wood: Forest Products Society; Forest Research Institute Baden-Württemberg: Freiburg, Germany, 2019; p. 657. [Google Scholar]
- Rinn, F.; Schweingruber, F.H.; Schar, E. Resistograph and x-ray density charts of wood comparative evaluation of drill resistance profiles and x-ray density charts of different wood species. Holzforschung 1996, 50, 303–311. [Google Scholar] [CrossRef]
- Costello, L.R.; Quarles, S.L. Detection of wood decay in blue gum and elm: An evaluation of the RESISTOGRAPH® and the portable drill. J. Arboric. 1999, 25, 311–318. [Google Scholar] [CrossRef]
- Johnstone, D.; Ades, P.K.; Moore, G.M.; Smith, I.W. Predicting wood decay in eucalypts using an expert system and the IMLRESISTOGRAPH® drill. Arboric. Urb. For. 2007, 33, 76–82. [Google Scholar]
- Wang, X.; Allison, R.B. Decay detection in red oak trees using a combination of visual inspection, acoustic testing, and resistance micro drilling. J. Arboric. Urban For. 2008, 34, 1–4. [Google Scholar] [CrossRef]
- Johnstone, D.; Moore, G.; Tausz, T.; Nicolas, M. The measurement of wood decay in landscape trees. Arboric. Urb. For. 2010, 36, 121–127. [Google Scholar] [CrossRef]
- Johnstone, D.; Moore, G.; Tausz, T.; Nicolas, M. Quantifying wood decay in Sydney bluegum (Eucalyptus saligna) trees. Arboric. Urb. For. 2010, 36, 243–253. [Google Scholar] [CrossRef]
- Arciniegas, A.; Prieto, F.; Brancheriau, L.; Lasaygues, P. Literature review of acoustic and ultrasonic tomography in standing trees. Trees 2014, 28, 1559–1567. [Google Scholar] [CrossRef]
- Balázs, M.; Divos, F. Glue Laminated Timber Structure Evaluation by Acoustic Tomography. General Technical Report FPL-GTR-239. In Proceedings of the 19th International Nondestructive Testing and Evaluation of Wood Symposium, Rio de Janeiro, RJ, Brazil, 22–25 September 2015; pp. 462–466. [Google Scholar]
- Trinca, A.J.; Guerra, M.R.; Gonçalves, R. Velocity Variation in Wood as a Function of Defects. General Technical Report FPL-GTR-239. In Proceedings of the 19th International Nondestructive Testing and Evaluation of Wood Symposium, Rio de Janeiro, RJ, Brazil, 22–25 September 2015; pp. 593–599. [Google Scholar]
- Linhares, C.; Trinca, A.; Gonçalves, R. Ultrasonic Tomography in Knots Detection. In Proceedings of the 18th International Nondestructive Testing and Evaluation of Wood Symposium, Madison, WI, USA, 24–27 September 2013; Forest Products Society: Madison, WI, USA, 2013; p. 664. [Google Scholar]
- Sanabria, S.J.; Furrer, R.; Neuenschwander, J.; Niemz, P.; Sennhauser, U. Air-coupled ultrasound inspection of glued laminated timber. Holzforschung 2011, 65, 377–387. [Google Scholar] [CrossRef]
- Batista, F.A.F.; Gonçalves, R.; Cerri, D.G.P.; Secco, C.B. Reprodução da condição interna de peças de madeira através de imagens representativas da propagação de ondas. Madeira Arquitetura E Eng. 2009, 10, 23–32. (In Portuguese) [Google Scholar]
- Brashaw, B.K.; Bucur, V.; Divos, F.; Gonçalves, R.; Lu, J.; Meder, R.; Pellerin, R.F.; Potter, S.; Ross, R.J.; Wang, X.; et al. Nondestructive testing and evaluation of wood: A worldwide research update. For. Prod. J. 2009, 59, 7–14. [Google Scholar]
- Socco, L.V.; Sambuelli, L.; Martinis, R.; Comino, E.; NicolottI, G. Feasibility of ultrasonic tomography for nondestructive testing of decay on living trees. Res. Nondestruct. Eval. 2004, 15, 31–54. [Google Scholar] [CrossRef]
- Lin, C.J.; Huang, Y.H.; Huang, G.S.; Wu, M.L.; Yang, T.H. Detection of termite damage in Hoop pine (Araucaria cunninghamii) trees using nondestructive evaluation techniques. J. Trop. For. Sci. 2016, 28, 79–87. [Google Scholar]
- Matheny, N.; Clark, J. A Photographic Guide to the Evaluation of Hazard Trees in Urban Areas; International Society of Arboriculture: Champaign, IL, USA, 1994; p. 85. [Google Scholar]
- Mattheck, C.; Breloer, H. Field guide for visual tree assessment (VTA). Arbor. J. 1994, 18, 1–23. [Google Scholar] [CrossRef]
- Pokorny, J.D. Urban Tree Risk Management, a Community Guide to Program Design and Implementation; USDA Forest Service North-eastern Area State and Private Forest: Milwaukee, WI, USA, 2003. [Google Scholar]
- Ellison, M.J. Quantified tree risk assessment used in the management of amenity trees. J. Arbor. 2005, 31, 57–65. [Google Scholar] [CrossRef]
- Smiley, E.T.; Matheny, N.; Lilly, S. Best Management Practices: Tree Risk Assessment; International Society of Arboriculture: Champaign, IL, USA, 2011; p. 86. [Google Scholar]
- Manion, P.D. Tree Disease Concepts. In Mycologia; Prentice-Hall, Inc.: Hoboken, NJ, USA, 1991; Volume 83. [Google Scholar] [CrossRef]
- Martins, L. Melhoria da condição de sobreiro no Parque Boticas—Natureza e Biodiversidade. In Proceedings of the 7 o Congresso Florestal Nacional—“Florestas—Conhecimento e Inovação”, Vila Real, Portugal, 5–8 June 2013. [Google Scholar]
- Martins, L.M. New Challenges in Urban Forest. In Proceedings of the Conference in ERASMUS Program, Porto, Portugal, 23–30 May 2015; Università degli Studi di Firenze: Florence, Italy, 2015. [Google Scholar]
- Li, H.; Zhang, X.; Li, Z.; Wen, J.; Tan, X. A review of research on tree risk assessment methods. Forests 2022, 13, 1556. [Google Scholar] [CrossRef]
- Koeser, A.K.; Smiley, E.T. Impact of assessor on tree risk assessment ratings and prescribed mitigation measures. Urban For. Urban Gree 2017, 24, 109–115. [Google Scholar] [CrossRef]
- Dandy, N. The Social and Cultural Values, and Governance, of Street Trees. Climate Change & Street Trees Project: Social Research Report; Forestry Commission: Surrey, UK, 2010; Volume olume 39. [Google Scholar]
- Williamson, T.; Dubb, S.; Alperovitz, G. Climate Change, Community Stability, and the Next 150 Million Americans; The Democracy Collaborative: College Park, MD, USA, 2010. [Google Scholar]
- Rinn, F. Shell-wall thickness and breaking safety of mature trees. West. Arborist 2013, 39, 40–44. [Google Scholar]
- Gonçalves, R.; Trinca, A.J.; Cerri, D.G.P. Comparison of Elastic Constants of Wood Determined by Ultrasonic Wave Propagation and Static Compression Testing. Wood Fiber Sci. 2011, 43, 64–75. [Google Scholar]
- Linhares, C.F.; Gonçalves, R.; Martins, L.M.; Knapic, S. Structural stability of urban trees using visual and instrumental techniques: A review. Forests 2021, 12, 1752. [Google Scholar] [CrossRef]
- Bucur, V. Ultrasonic techniques for nondestructive testing of standing trees. Ultrasonics 2005, 43, 237–239. [Google Scholar] [CrossRef]
- Divos, F.; Szalai, L. Tree Evaluation by Acoustic Tomography. In Proceedings of the 13th International Symposium on Nondestructive Testing of Wood, Berkeley, CA, USA, 19–21 August 2002; Forest Products Society: Madison, WI, USA; pp. 251–256. [Google Scholar]
- Du, X.; Li, S.; Li, G.; Feng, H.; Chen, S. Stress wave tomography of wood internal defects using ellipse-based spatial interpolation and velocity compensation. BioResources 2015, 10, 3948–3962. [Google Scholar] [CrossRef]
- Maurer, H.; Schubert, S.I.; Bachle, F.; Clauss, S.; Gsell, D.; Dual, J.; Niemz, P. A simple anisotropy correction procedure for acoustic wood tomography. Holzforschung 2006, 60, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Stefan, H.; Gottfried, S. Detection of Butt Rot. In Proceedings of the International Symposium on Nondestructive Testing of Wood, Sopron, Hungary, 13–15 September 2000. [Google Scholar]
- Bucur, V. Acoustics of Wood (Second Edition). J. Acoust. Soc. Am. 2006, 119, 3506. [Google Scholar] [CrossRef]
- Wang, X.; Divos, F.; Pilon, C.; Brashaw, B.K.; Ross, R.J.; Pellerin, R.F. Assessment of Decay in Standing Timber Using Stress Wave Timing Nondestructive Evaluation Tools: A Guide for Use and Interpretation; Technical Report FPL-GTR-147; US Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2004; Volume 12, p. 47. [Google Scholar]
- Zhu, J.; Tadooka, N.; Takata, K.; Koizumi, A. Growth and wood quality of sugi (Cryptomeria japonica) planted in Akita prefecture (II). Juvenile/mature wood determination of aged trees. J. Wood Sci. 2005, 51, 95–101. [Google Scholar] [CrossRef]
- Kijidani, Y.; Kitahara, R. Variation of wood properties with height position in the stems of Obi-sugi [Cryptomeria japonica] cultivars. J. Jpn. Wood Res. Soc. 2009, 55, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Bucur, V. High resolution imaging of wood. In Proceedings of the 13th International Nondestructive Testing and Evaluation of Wood Symposium, Berkeley, CA, USA, 19–21 August 2002. [Google Scholar]
- Trinca, A.J.; Gonçalves, R. Effect of the transversal section dimensions and transducer frequency on ultrasound wave propagation velocity in wood. Revista Árvore 2009, 33, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Palma, S.S.A.; Gonçalves, R.; Trinca, A.J.; Costa, C.P.; Guerra, M.N.R.; Martins, G.A. Interference from knots, wave propagation direction and effect of juvenile and reaction wood on velocities in ultrasound tomography. BioResources 2018, 13, 2834–2845. [Google Scholar] [CrossRef]
- Brancheriau, L.; Ghodrati, A.; Gallet, P.; Thaunay, P.; Lasaygues, P. Application of ultrasonic tomography to characterize the mechanical state of standing trees (Picea abies). J. Phys. Conf. Ser. 2012, 353, 012007. [Google Scholar] [CrossRef]
- Feng, H.; Li, G.; Fu, S.; Wang, X. Tomographic image reconstruction using an interpolation method for tree decay detection. BioResources 2014, 9, 3248–3263. [Google Scholar] [CrossRef]
- Lin, C.J.; Kao, Y.C.; Lin, T.T.; Tsai, M.J.; Wang, S.Y.; Lin, L.D.; Chan, M.H. Application of an ultrasonic tomographic technique for detecting defects in standing trees. Int. Biodeter. Biodegr. 2008, 62, 434–441. [Google Scholar] [CrossRef]
- Strobel, J.R.A.; de Carvalho, M.A.G.; Gonçalves, R.; Pedroso, C.B.; dos Reis, M.N.; Martins, P.S. Quantitative image analysis of acoustic tomography in woods. Eur. J. Wood Wood Prod. 2018, 76, 1379–1389. [Google Scholar] [CrossRef]
- Palma, S.S.A.; Gonçalves, R. Tomographic images of tree trunks generated using ultrasound and post-processed images: Influence of the number of measurements points. BioResources 2022, 14, 6638–6655. [Google Scholar] [CrossRef]
- Kubus, M. The Evaluation of Using Resistograph when Specifying the Health Condition of a Monumental Tree. Not. Bot. Horti. Agrobo. 2009, 37, 157–164. [Google Scholar]
- Bodensee, B.T.; Pietruschinski, M. Example Arbotom Report; ICT international: Freiburg, Germany, 2012. [Google Scholar]
- Rinn, F. A new method for direct measuring of wood density of broadleaf trees and conifers. Dendrochronologia 1989, 7, 159–168. [Google Scholar]
- Brashaw, B.K.; Vatalaro, R.; Ross, R.J.; Wang, X.; Schmieding, S.; Okstad, W. Historic Log Cabin structural condition assessment and rehabilitation—A case study. In Proceedings of the 17th International Nondestructive Testing and Evaluation of Wood Symposium, Sopron, Hungary, 14–16 September 2011; pp. 505–512. [Google Scholar]
- Rinn, F. Basics of Micro-Resistance-Drilling for Timber Inspection. Holztechnologie 2012, 53, 24–29. [Google Scholar]
- Wang, X.; Wiedenbeck, J.; Liang, S. Acoustic tomography for decay detection in black cherry trees. Wood Fiber Sci. 2009, 41, 127–137. [Google Scholar]
- Dos Reis, M.N.; Gonçalves, R.; Lopes Garcia, G.H.; Manes, L. Profiles of a Non-Calibrated Resistance Drill Compared with Deteriorated Stem Cross Sections. Arbor. Urb. For. 2019, 45, 1–9. [Google Scholar] [CrossRef]
- Tannert, T.; Anthony, R.W.; Kasal, B.; Kloiber, M.; Piazza, M.; Riggio, M.; Rinn, F.; Widman, N.R.; Yamaguchi, N. In situ assessment of structural timber using semi-destructive techniques. Mater. Struct. 2014, 47, 767–785. [Google Scholar] [CrossRef]
- Martins, L.; Amaral, J.G. Floresta urbana: O paradoxo da naturalização em contexto artificial. Ingenium 2021, 173, 84–87. [Google Scholar]
- Martins, L.; Macedo, F.; Saraiva, S. Avaliação Da Condição Das Árvores Dos Parques Do Porto Com Apoio Da Aplicação Idtree Em Appsheet®. In Proceedings of the 2º Simpósio SCAP de Proteção das Plantas, Santarém, Portugal, 26–27 October 2017. [Google Scholar]
- Mattheck, C.; Kubler, H. Wood the Internal Optimization of Trees; Springer Verlag: Berlin, Germany; Scientific Research Publishing: Wuhan, China, 1995. [Google Scholar]
- Shigo, A. Modern Arboriculture. 1991. Available online: https://www.amazon.com/Modern-Arboriculture-AlexShigo-1991-12-31/dp/B019TLQFX (accessed on 15 March 2023).
- Fraedrich, B.R. Compartmentalization of Decay in Trees; Technical Report TR-18; Bartlett Tree Research Laboratories: Charlotte, NC, USA, 1999. [Google Scholar]
- Reis, M.N.D.; Gonçalves, R.; Brazolin, S.; de Assis Palma, S.S. Strength Loss Inference Due to Decay or Cavities in Tree Trunks Using Tomographic Imaging Data Applied to Equations Proposed in the Literature. Forests 2022, 13, 596. [Google Scholar] [CrossRef]
- Väänänen, P.J.; Osem, Y.; Cohen, S.; Grünzweig, J.M. Differential drought resistance strategies of co-existing woodland species enduring the long rainless Eastern Mediterranean summer. Tree Physiol. 2020, 40, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Condo, T.K.; Reinhardt, K. Large variation in branch and branch-tip hydraulic functional traits in Douglas-fir (Pseudotsuga menziesii) approaching lower treeline. Tree Physiol. 2019, 39, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Wagner, Y.; Pozner, E.; Bar-On, P.; Ramon, U.; Raveh, E.; Neuhaus, E.; Cohen, S.; Grünzweig, J.; Klein, T. Rapid stomatal response in lemon saves trees and their fruit yields under summer desiccation but fails under recurring droughts. Agric. For. Meteorol. 2021, 307, 108487. [Google Scholar] [CrossRef]
- Aydın, T.Y. Temperature Influenced Anisotropic Elastic Parameters of Red Pine. Russ. J. Nondestruct. Test. 2022, 58, 548–562. [Google Scholar] [CrossRef]
- Chuste, P.A.; Massonnet, C.; Gérant, D.; Zeller, B.; Levillain, J.; Hossann, C.; Angeli, N.; Wortemann, R.; Bréda, N.; Maillard, P. Short-term nitrogen dynamics are impacted by defoliation and drought in Fagus sylvatica L. branches. Tree Physiol. 2019, 39, 792–804. [Google Scholar] [CrossRef]
- Velisevich, S.N.; Bender, O.G.; Goroshkevich, S.N. The influence of scion donor tree age on the growth and morphogenesis of Siberian stone pine grafts. New For. 2021, 52, 473–491. [Google Scholar] [CrossRef]















| Predisposing Factors | Inciting Factors | Contributing Factor |
|---|---|---|
| Urban environment | Drought | Biotic agents |
| Soil compaction | Floods | |
| Genetic potential | Severe pruning | |
| Age | Excavation | |
| Lack of light | Air pollution | |
| Sea proximity | Overwatering | |
| Root cutting |
| Root | Cavity; Injury; Surface |
| Stem | Cavity; Injury; Codominance; Inclined; Adventitious; Spheroplast; Included bark; Protuberance |
| Branch | Cavity; Wound; Codominance; Dense; Dry: Large; Slender |
| Twig | Adventitious; Dry; Low; Dense |
| Leaf | Dry; Necrosis; Chlorosis; Small; Large; Perforated; Epinasty |
| Crown | Dead; Dieback; Transparent; Inclined; High; Low; Unbalanced; Dense |
| Biotic Agents | White rot; Brown rot; Soft rot; Fumagine; Aphids; Anthracnose; Xanthogaleruca luteola; Termite; Other phytopathogeneses |
| Lesion | Height; Width; Depth; Height above the ground; Distance to the back wall; Thickness of the front wall; Tree diameter at lesion entrance |
| SITE | SPECIE | PBH (cm) | DBH (cm) | ACD (m) | HBC (m) | H (m) | A (years) |
|---|---|---|---|---|---|---|---|
| S1 | Tilia tomentosa | 73.2 | 12.0 | 3.2 | 27.0 | 41—50 | |
| S1 | Populus nigra | 91.4 | 22.2 | 3.9 | 30.7 | 31—40 | |
| S2 | Platanus x hispanica | 357.8 | 113.9 | 19.6 | 7.2 | 28.5 | 130 |
| S2 | Fagus sylvatica | 149.3 | 15.0 | 5.8 | 22.1 | ||
| S3 | Ulmus carpinifolia | 188.5 | 60.0 | 14.4 | 2.5 | 17.4 | 60—70 |
| S3 | Tilia cordata | 58.5 | 8.1 | 2.4 | 11.2 | 30—40 | |
| S4 | Quercus robur | 391.1 | 124.5 | 22.3 | 1.8 | 18.0 | >150 |
| Specie | Stem | Branch | Twig |
|---|---|---|---|
| Fagus sylvatica | Cavities | Codominant | Dry |
| Predisposing Factor | Biotic Agent | Numerical Condition | Global Qualitative |
|---|---|---|---|
| Age | Ganoderma sp. | 10 | Reasonable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linhares, C.S.F.; Gonçalves, R.; Dias, A.; Knapic, S.; Martins, L.M. Assessment of Summer Branch Drop: A Case Study in Four Portuguese Cities. Forests 2023, 14, 1398. https://doi.org/10.3390/f14071398
Linhares CSF, Gonçalves R, Dias A, Knapic S, Martins LM. Assessment of Summer Branch Drop: A Case Study in Four Portuguese Cities. Forests. 2023; 14(7):1398. https://doi.org/10.3390/f14071398
Chicago/Turabian StyleLinhares, Camila S. F., Raquel Gonçalves, Alfredo Dias, Sofia Knapic, and Luis M. Martins. 2023. "Assessment of Summer Branch Drop: A Case Study in Four Portuguese Cities" Forests 14, no. 7: 1398. https://doi.org/10.3390/f14071398





