Effects of Soil Microbiological Properties on the Fractional Distribution and Stability of Soil Organic Carbon under Different N Addition Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Soil Sampling
2.3. Analyses of Soil Chemical Properties
2.4. Analyses of Soil Microbiological Properties
2.5. Analyses of Soil C Fractions and CMI
2.6. Statistical Analyses
3. Results
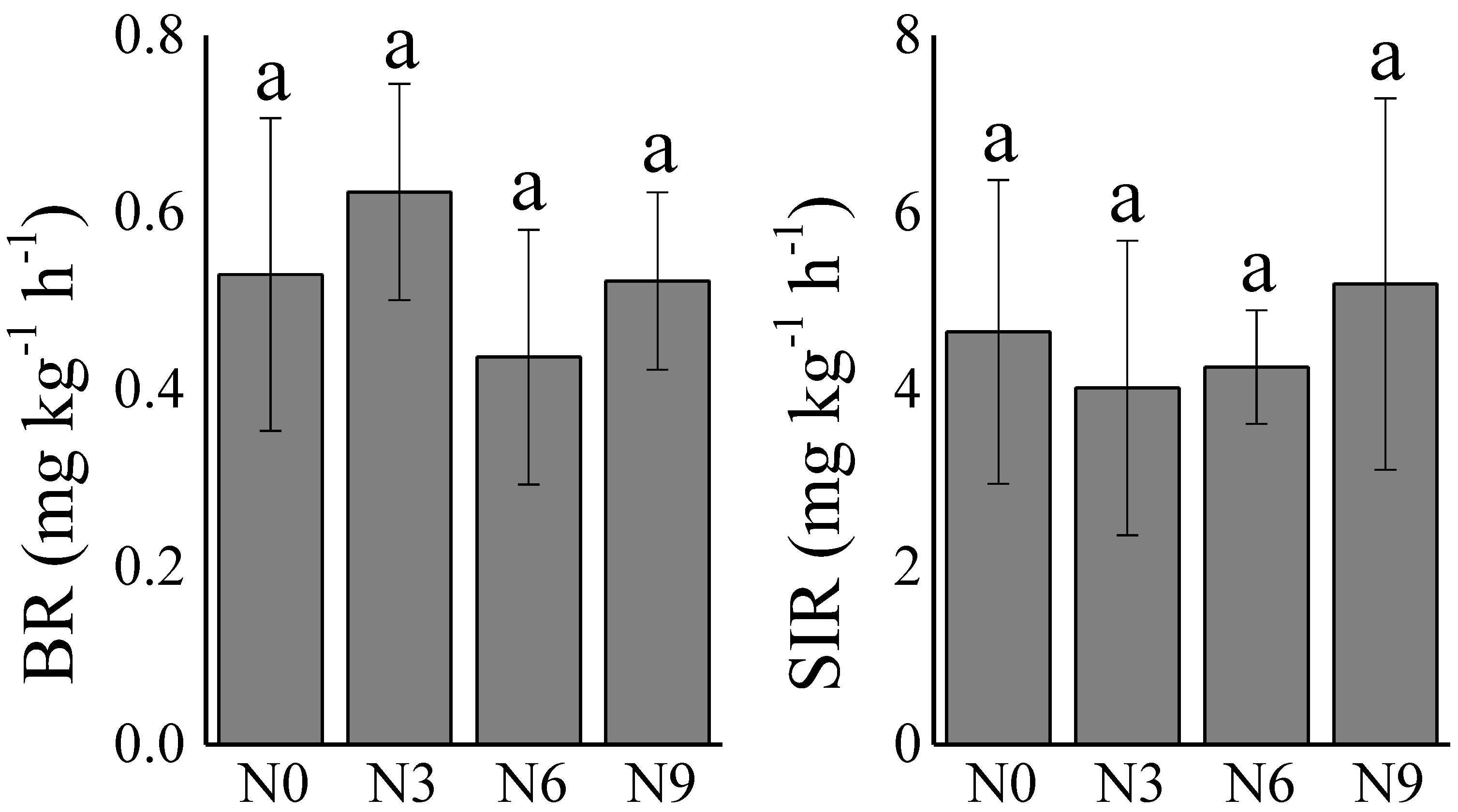
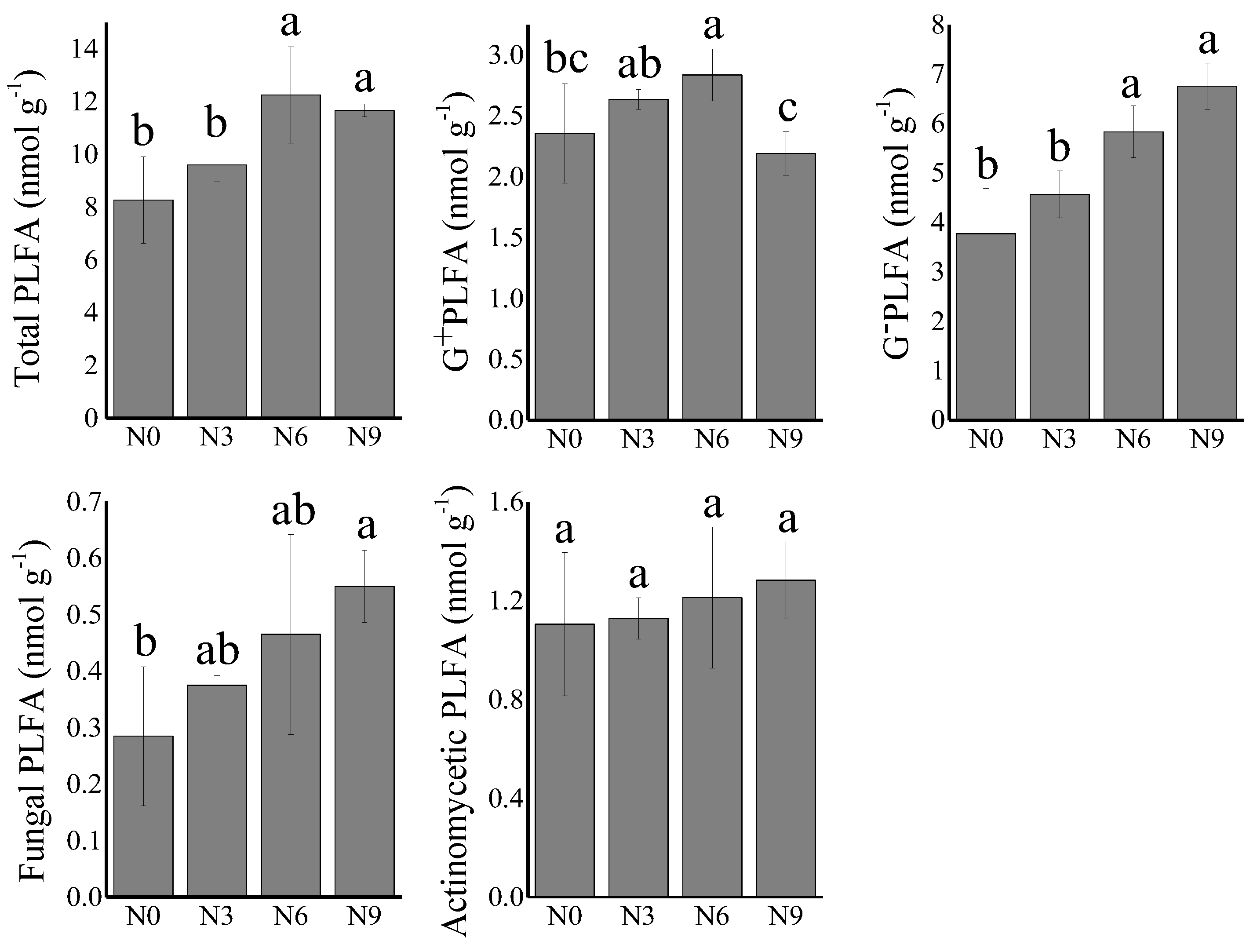
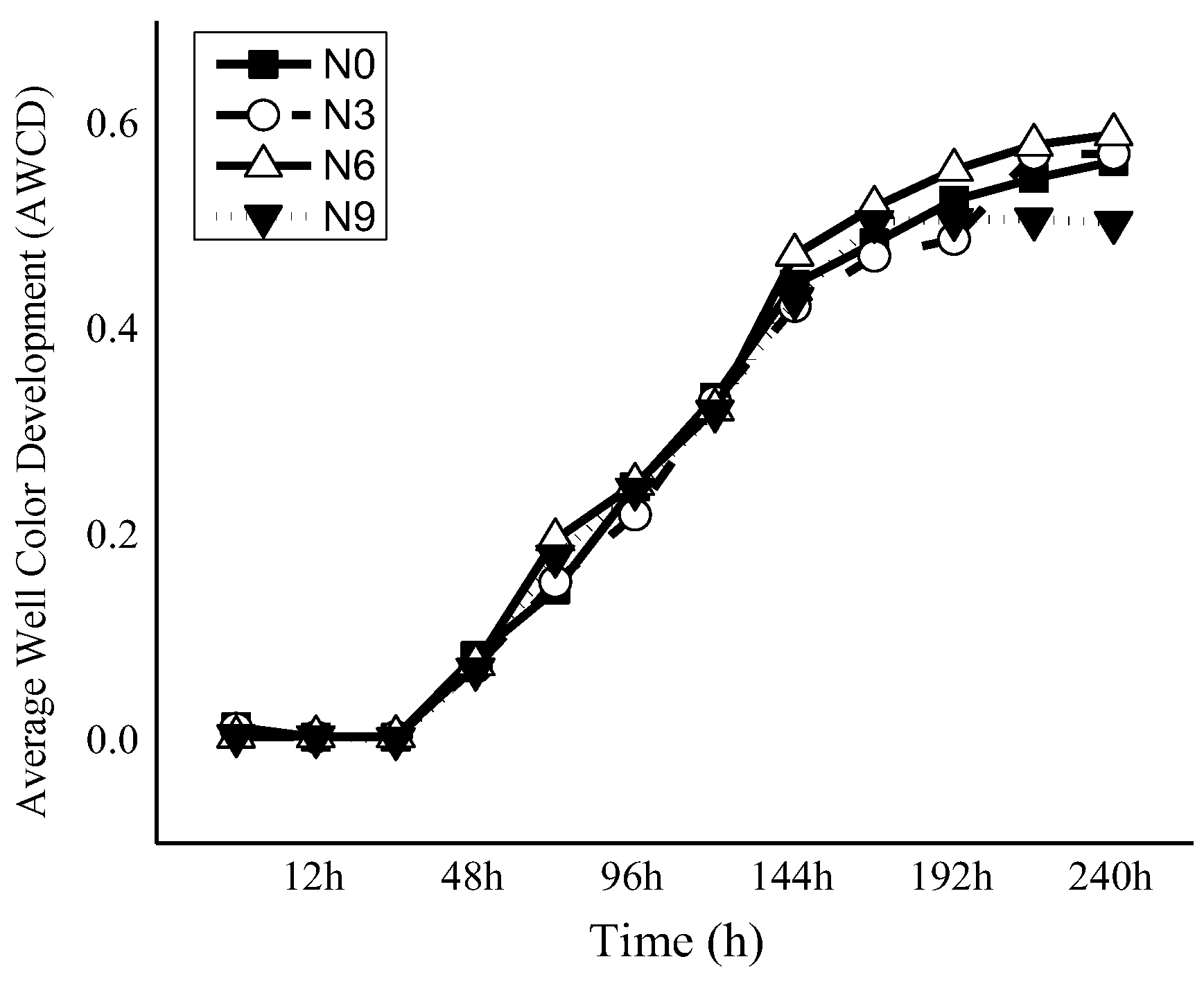
3.1. Soil Microbiological Properties under N Addition
3.1.1. Soil Microbial Activity under N Addition
3.1.2. Soil Microbial PLFAs under N Addition
3.1.3. Soil Microbial Metabolic Diversity under N Addition
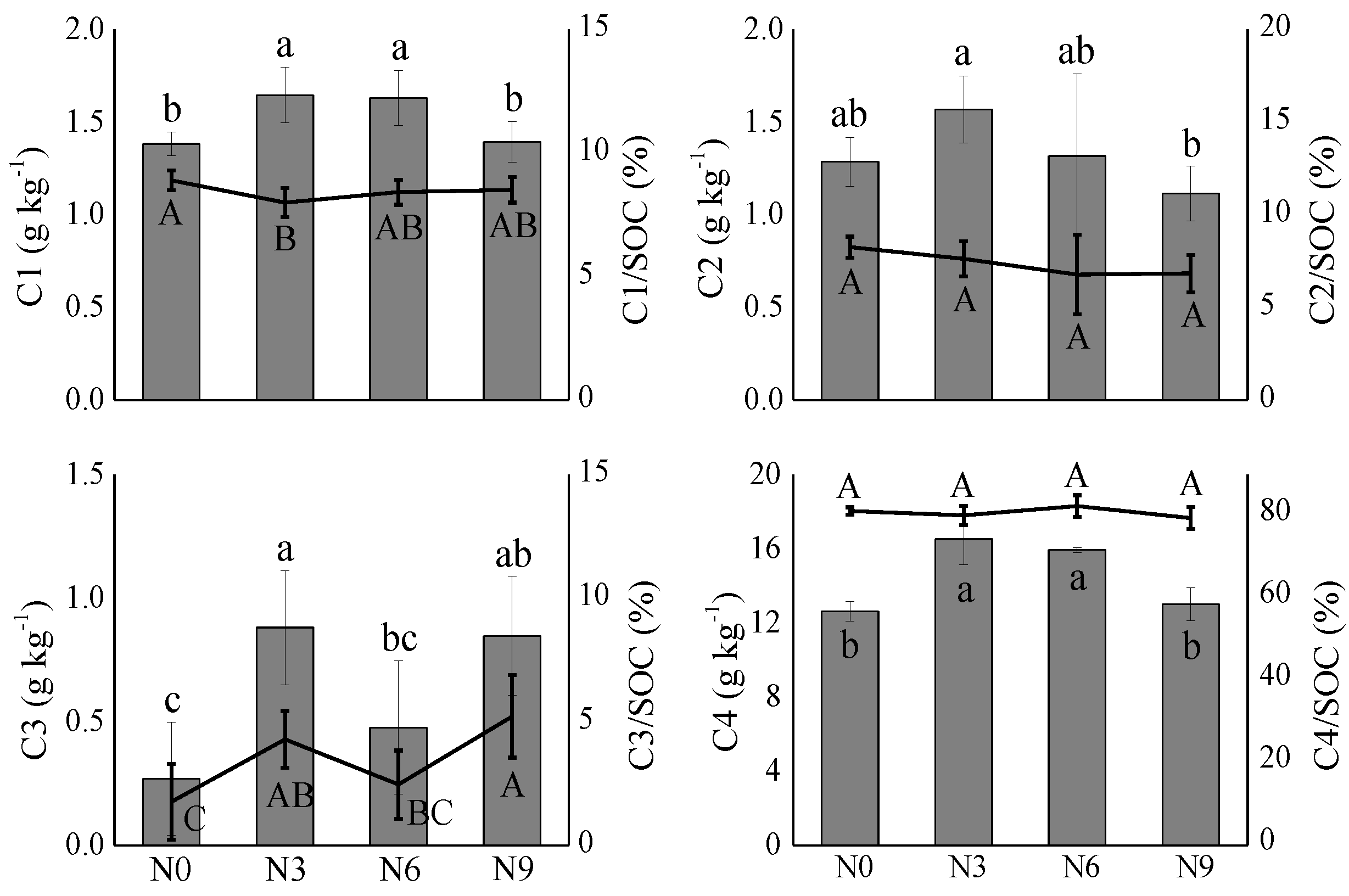
3.2. SOC Fractions under N Addition
3.3. Integrated Effect of Microbiological Properties on SOC Fractions and Stability in Response to N Addition Treatments
4. Discussion
4.1. Response of Soil Microbiological Properties to N Addition
4.2. Response of Different SOC Fractions to N Addition
4.3. Driving Factors of Soil Microbiological Properties on CMI and SOC Stability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Liang, C.; Zhu, X. The soil microbial carbon pump as a new concept for terrestrial carbon sequestration. Sci. China Earth Sci. 2021, 64, 545–558. [Google Scholar] [CrossRef]
- Sanderman, J.; Hengl, T.; Fiske, G.J. Soil carbon debt of 12,000 years of human land use. Proc. Natl. Acad. Sci. USA 2017, 114, 9575–9580. [Google Scholar] [CrossRef]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; Courcelles, V.R.; Singh, K.; et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Gong, S.W.; Zhang, T.; Guo, R.; Cao, H.B.; Shi, L.X.; Guo, J.X.; Sun, W. Response of soil enzyme activity to warming and nitrogen addition in a meadow steppe. Soil Res. 2015, 53, 242–252. [Google Scholar] [CrossRef]
- Stone, M.M.; Weiss, M.S.; Goodale, C.L.; Adams, M.B.; Fernandez, I.J.; German, D.P.; Allison, S.D. Temperature sensitivity of soil enzyme kinetics under N-fertilization in two temperate forests. Glob. Chang. Biol. 2012, 18, 1173–1184. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Zhong, Y.Q.W.; Yan, W.M.; Shangguan, Z.P. Soil carbon and nitrogen fractions in the soil profile and their response to long-term nitrogen fertilization in a wheat field. Catena 2015, 135, 38–46. [Google Scholar] [CrossRef]
- Chan, K.Y.; Bowman, A.; Oates, A. Oxidizible organic carbon fractions and soil quality changes in an Oxic Paleustalf under different pasture leys. Soil Sci. 2001, 166, 61–67. [Google Scholar] [CrossRef]
- Chen, X.M.; Li, Y.L.; Mo, J.M.; Otieno, D.; Tenhunen, J.; Yan, J.H.; Liu, J.X.; Zhang, D.Q. Effects of nitrogen deposition on soil organic carbon fractions in the subtropical forest ecosystems of S China. J. Plant Nutr. Soil Sci. 2012, 175, 947–953. [Google Scholar] [CrossRef]
- Li, J.; Wen, Y.C.; Li, X.H.; Li, Y.T.; Yang, X.D.; Lin, Z.; Song, Z.Z.; Cooper, J.M.; Zhao, B.Q. Soil labile organic carbon fractions and soil organic carbon stocks as affected by long-term organic and mineral fertilization regimes in the North China Plain. Soil Tillage Res. 2018, 175, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Mtambanengwe, F.; Mapfumo, P. Smallholder farmer management impacts on particulate and labile carbon fractions of granitic sandy soils in Zimbabwe. Nutr. Cycl. Agroecosyst. 2008, 81, 1–15. [Google Scholar] [CrossRef]
- Ghosh, A.; Bhattacharyya, R.; Meena, M.C.; Dwivedi, B.S.; Singh, G.; Agnihotri, R.; Sharma, C. Long-term fertilization effects on soil organic carbon sequestration in an Inceptisol. Soil Tillage Res. 2018, 177, 134–144. [Google Scholar] [CrossRef]
- Powlson, D.S.; Bhogal, A.; Chambers, B.J.; Coleman, K.; Whitmore, A.P. The potential to increase soil carbon stocks through reduced tillage or organic material additions in england and wales: A case study. Agric. Ecosyst. Environ. 2012, 146, 1. [Google Scholar] [CrossRef]
- Wang, R.Z.; Dijkstra, F.A.; Liu, H.Y.; Yin, J.F.; Wang, X.; Feng, X.; Xu, Z.W.; Jiang, Y. Response of soil carbon to nitrogen and water addition differs between labile and recalcitrant fractions: Evidence from multi-year data and different soil depths in a semi-arid steppe. Catena 2019, 172, 857–865. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefroy, R.D.B.; Lise, L. Soil Carbon Fractions Based on Their Degree of Oxidation, and the Development of a Carbon Management Index for Agricultural Systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Guan, G.Y.; Fan, Y.M.; Wu, H.Q.; Yan, D.Z. The relationship of soil particle fractal characteristics and organic carbon pool stability under different types of utilization. Acta Agrestia Sin. 2016, 24, 258–262. [Google Scholar]
- Cooper, J.M.; Burton, D.; Daniell, T.J.; Griffiths, B.S.; Zebarth, B.J. Carbon mineralization kinetics and soil biological characteristics as influenced by manure addition in soil incubated at a range of temperatures. Eur. J. Soil Biol. 2011, 47, 392–399. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Hämmerle, I.; Fuchslueger, L.; Hofmhansl, F.; Knoltsch, A.; Schnecker, J.; Takriti, M.; Watzka, M.; Wild, B.; et al. Adjustment of microbial nitrogen use efficiency to carbon:nitrogen imbalance regulates soil nitrogen cycling. Nat. Commun. 2014, 5, 3694. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.J.; Yu, G.R.; Cheng, S.L.; Mo, J.M.; Yan, J.H.; Li, S.G. C-13 abundance, water-soluble and microbial biomass carbon as potential indicators of soil organic carbon dynamics in subtropical forests at different successional stages and subject to different nitrogen loads. Plant Soil 2009, 320, 243–254. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Zak, D.R.; Sinsabaugh, R.L.; Gallo, M.; Lauber, C. Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecol. Appl. 2004, 14, 1172–1177. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, W.; Zhu, W.; Gundersen, P.; Fang, Y.; Li, D.; Wang, H. Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Glob. Chang. Biol. 2008, 14, 403–412. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, E.A.; Dentener, F.J.; Braswell, B.H.; Sulzman, J.M. Contemporary and pre-industrial global reactive nitrogen budgets. Biogeochemistry 1999, 46, 7–43. [Google Scholar] [CrossRef]
- Hok, L.; Sa, J.C.D.; Reyes, M.; Boulakia, S.; Tivet, F.; Leng, V.; Kong, R.; Briedis, C.; Hartman, D.D.; Ferreira, L.A.; et al. Enzymes and C pools as indicators of C build up in short-term conservation agriculture in a savanna ecosystem in Cambodia. Soil Tillage Res. 2018, 177, 125–133. [Google Scholar] [CrossRef]
- Wei, X.; Tong, Y.A.; Qiao, L.; Liu, X.J.; Duan, M.; Li, J. Preliminary estimate of the atmospheric nitrogen deposition in different ecological regions of Shaanxi province. J. Agro Environ. Sci. 2010, 29, 795–800. [Google Scholar]
- Liang, T.; Tong, Y.A.; Liu, X.J.; Qiao, L. Dynamics of Atmospheric Nitrogen Wet Deposition Fluxes in Guanzhong Area, Shaanxi. J. Agro Environ. Sci. 2014, 33, 2389–2394. [Google Scholar]
- Zhang, J.Y.; Ai, Z.M.; Liang, C.T.; Wang, G.L.; Liu, G.B.; Xue, S. How microbes cope with short-term N addition in a Pinus tabuliformis forest-ecological stoichiometry. Geoderma 2019, 337, 630–640. [Google Scholar] [CrossRef]
- Bremner, J.; Mulvaney, C. Nitrogen—Total. Methods of soil analysis Part 2 Chemical and microbiological properties. Soil Sci. Soc. Am. Book Ser. 1982, 5, 595–624. [Google Scholar]
- Schade, J.D.; Kyle, M.; Hobbie, S.E.; Fagan, W.F.; Elser, J.J. Stoichiometric tracking of soil nutrients by a desert insect herbivore. Ecol. Lett. 2003, 6, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Veen, G.F.; Olff, H.; Duyts, H.; van der Putten, W.H. Vertebrate herbivores influence soil nematodes by modifying plant communities. Ecology 2010, 91, 828–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michel, K.; Matzner, E. Response of enzyme activities to nitrogen addition in forest floors of different C-to-N ratios. Biol. Fertil. Soils 2003, 38, 102–109. [Google Scholar] [CrossRef]
- Wang, Q.K.; Wang, S.L.; Liu, Y. Responses to N and P fertilization in a young Eucalyptus dunnii plantation: Microbial properties, enzyme activities and dissolved organic matter. Appl. Soil Ecol. 2008, 40, 484–490. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Shah, J. Ecoenzymatic Stoichiometry and Ecological Theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 313–343. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.C.; Zhang, S.T. Nitrogen addition shapes soil enzyme activity patterns by changing pH rather than the composition of the plant and microbial communities in an alpine meadow soil. Plant Soil 2019, 440, 11–24. [Google Scholar] [CrossRef]
- Song, Y.Y.; Song, C.C.; Tao, B.X.; Wang, J.Y.; Zhu, X.Y.; Wang, X.W. Short-term responses of soil enzyme activities and carbon mineralization to added nitrogen and litter in a freshwater marsh of Northeast China. Eur. J. Soil Biol. 2014, 61, 72–79. [Google Scholar] [CrossRef]
- Kang, H.; Lee, D. Inhibition of extracellular enzyme activities in a forest soil by additions of inorganic nitrogen. Commun. Soil Sci. Plant Anal. 2005, 36, 2129–2135. [Google Scholar] [CrossRef]
- Tu, L.H.; Hu, T.X.; Zhang, J.; Li, X.W.; Hu, H.L.; Liu, L.; Xiao, Y.L. Nitrogen addition stimulates different components of soil respiration in a subtropical bamboo ecosystem. Soil Biol. Biochem. 2013, 58, 255–264. [Google Scholar] [CrossRef]
- Samuelson, L.; Mathew, R.; Stokes, T.; Feng, Y.C.; Aubrey, D.; Coleman, M. Soil and microbial respiration in a loblolly pine plantation in response to seven years of irrigation and fertilization. For. Ecol. Manag. 2009, 258, 2431–2438. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Nitrogen fertilization inhibits soil microbial respiration regardless of the form of nitrogen applied. Soil Biol. Biochem. 2010, 42, 2336–2338. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Townsend, A.R. Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc. Natl. Acad. Sci. USA 2006, 103, 10316–10321. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.G.; Law, B.E. Interpreting, measuring, and modeling soil respiration. Biogeochemistry 2005, 73, 3–27. [Google Scholar] [CrossRef]
- Zhong, W.H.; Cai, Z.C. Long-term effects of inorganic fertilizers on microbial biomass and community functional diversity in a paddy soil derived from quaternary red clay. Appl. Soil Ecol. 2007, 36, 84–91. [Google Scholar] [CrossRef]
- Fang, H.J.; Cheng, S.L.; Yu, G.R.; Xu, M.J.; Wang, Y.S.; Li, L.S.; Dang, X.S.; Wang, L.; Li, Y.N. Experimental nitrogen deposition alters the quantity and quality of soil dissolved organic carbon in an alpine meadow on the Qinghai-Tibetan Plateau. Appl. Soil Ecol. 2014, 81, 1–11. [Google Scholar] [CrossRef]
- He, Y.T.; Qi, Y.C.; Dong, Y.S.; Xiao, S.S.; Peng, Q.; Liu, X.C.; Sun, L.J. Effects of Nitrogen Fertilization on Soil Microbial Biomass and Community Functional Diversity in Temperate Grassland in Inner Mongolia, China. Clean Soil Air Water 2013, 41, 1216–1221. [Google Scholar] [CrossRef]
- Prober, S.M.; Leff, J.W.; Bates, S.T.; Borer, E.T.; Firn, J.; Harpole, W.S.; Lind, E.M.; Seabloom, E.W.; Adler, P.B.; Bakker, J.D.; et al. Plant diversity predicts beta but not alpha diversity of soil microbes across grasslands worldwide. Ecol. Lett. 2015, 18, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.M.; Zhang, H.F.; Zhao, J.N.; Wang, H.; Qin, J.; Yang, D.L.; Zhang, N.Q. Effect of nitrogen addition on labile soil organic pool and carbon pool management index of Stipa baicalensis steppe in Inner Mongolia, China. Acta Pratacult. Sin. 2020, 29, 18–26. [Google Scholar]
- Frostegard, A.; Tunlid, A.; Baath, E. Phospholipid fatty-acid composition, biomass, and activity of microbial communities from 2 soil types experimentally exposed to different heavy-metals. Appl. Environ. Microbiol. 1993, 59, 3605–3617. [Google Scholar] [CrossRef]
- Garland, J.L.; Mills, A.L. Classification and Characterization of Heterotrophic Microbial Communities on the Basis of Patterns of Community-Level Sole-Carbon-Source Utilization. Appl. Environ. Microbiol. 1991, 57, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Hueso, S.; Hernandez, T.; Garcia, C. 2011. Resistance and resilience of the soil microbial biomass to severe drought in semiarid soils: The importance of organic amendments. Appl. Soil Ecol. 2011, 50, 27–36. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Zelles, L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: A review. Biol. Fertil. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Zhang, J.; Ai, Z.; Liang, C.; Wang, G.; Xue, S. Response of soil microbial communities and nitrogen thresholds of Bothriochloa ischaemum to short-term nitrogen addition on the Loess Plateau. Geoderma 2017, 308, 112–119. [Google Scholar] [CrossRef]



| Treatment a | HPLFA | EPLFA | HBIOLOG | EBIOLOG |
|---|---|---|---|---|
| N0 | 3.15 ± 0.12 a b | 0.93 ± 0.01 a | 2.78 ± 0.05 a | 0.82 ± 0.01 ab |
| N3 | 3.24 ± 0.10 a | 0.92 ± 0.01 a | 2.71 ± 0.05 a | 0.80 ± 0.02 b |
| N6 | 2.94 ± 0.05 b | 0.88 ± 0.04 b | 2.78 ± 0.11 a | 0.85 ± 0.03 a |
| N9 | 2.91 ± 0.16 b | 0.87 ± 0.02 b | 2.77 ± 0.10 a | 0.81 ± 0.03 ab |
| Treatment a | CPI | Lability (%) | Lability Index | CMI | SI b (L) (%) | SI c (C1) (%) | SI (C2) (%) | SI (C3) (%) |
|---|---|---|---|---|---|---|---|---|
| N0 | 1 b | 23.29 ± 1.36 ab d | 1ab | 100 | 0 ab | 0 b | 0 ab | 0 c |
| N3 | 1.32 ± 0.09 a | 24.92 ± 3.55 ab | 1.07 ± 0.15 ab | 141.35 ± 19.77 a | 7.00 ± 15.26 ab | 19.06 ± 10.87 a | 21.91 ± 14.18 a | 227.57 ± 85.80 a |
| N6 | 1.24 ± 0.04 a | 21.49 ± 2.27 b | 0.92 ± 0.10 b | 115.01 ± 15.71 b | −7.73 ± 9.77 b | 17.88 ± 10.77 a | 2.48 ± 23.43 ab | 77.21 ± 100.75 bc |
| N9 | 1.05 ± 0.05 b | 25.97 ± 2.70 a | 1.12 ± 0.12 a | 117.00 ± 9.64 b | 11.53 ± 11.59 a | 0.68 ± 7.93 b | −13.20 ± 11.57 b | 215.14 ± 89.88 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Huang, H.; Liu, H.; Wu, H.; Zhang, Z.; Wang, G.; Xue, S.; Liu, G. Effects of Soil Microbiological Properties on the Fractional Distribution and Stability of Soil Organic Carbon under Different N Addition Treatments. Forests 2023, 14, 1540. https://doi.org/10.3390/f14081540
Zhang J, Huang H, Liu H, Wu H, Zhang Z, Wang G, Xue S, Liu G. Effects of Soil Microbiological Properties on the Fractional Distribution and Stability of Soil Organic Carbon under Different N Addition Treatments. Forests. 2023; 14(8):1540. https://doi.org/10.3390/f14081540
Chicago/Turabian StyleZhang, Jiaoyang, Hui Huang, Hongfei Liu, Hongmiao Wu, Zhen Zhang, Guoliang Wang, Sha Xue, and Guobin Liu. 2023. "Effects of Soil Microbiological Properties on the Fractional Distribution and Stability of Soil Organic Carbon under Different N Addition Treatments" Forests 14, no. 8: 1540. https://doi.org/10.3390/f14081540




