Fertilization and Residue Management Improved Soil Quality of Eucalyptus Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Test Design
2.3. Soil Sampling and Analysis
2.4. Soil Quality Evaluation Method
2.4.1. Construct MDS
2.4.2. Indicator Scores and Weights
2.4.3. Calculating SQI
2.5. Statistical Analysis
3. Results
3.1. Effect of Fertilization on Soil Quality Indicators
3.2. Effect of Residue Management on Soil Quality Indicators
3.3. Effect of Interaction between Fertilization and Residue Management on Soil Quality Indicators
3.4. Soil Quality Assessment
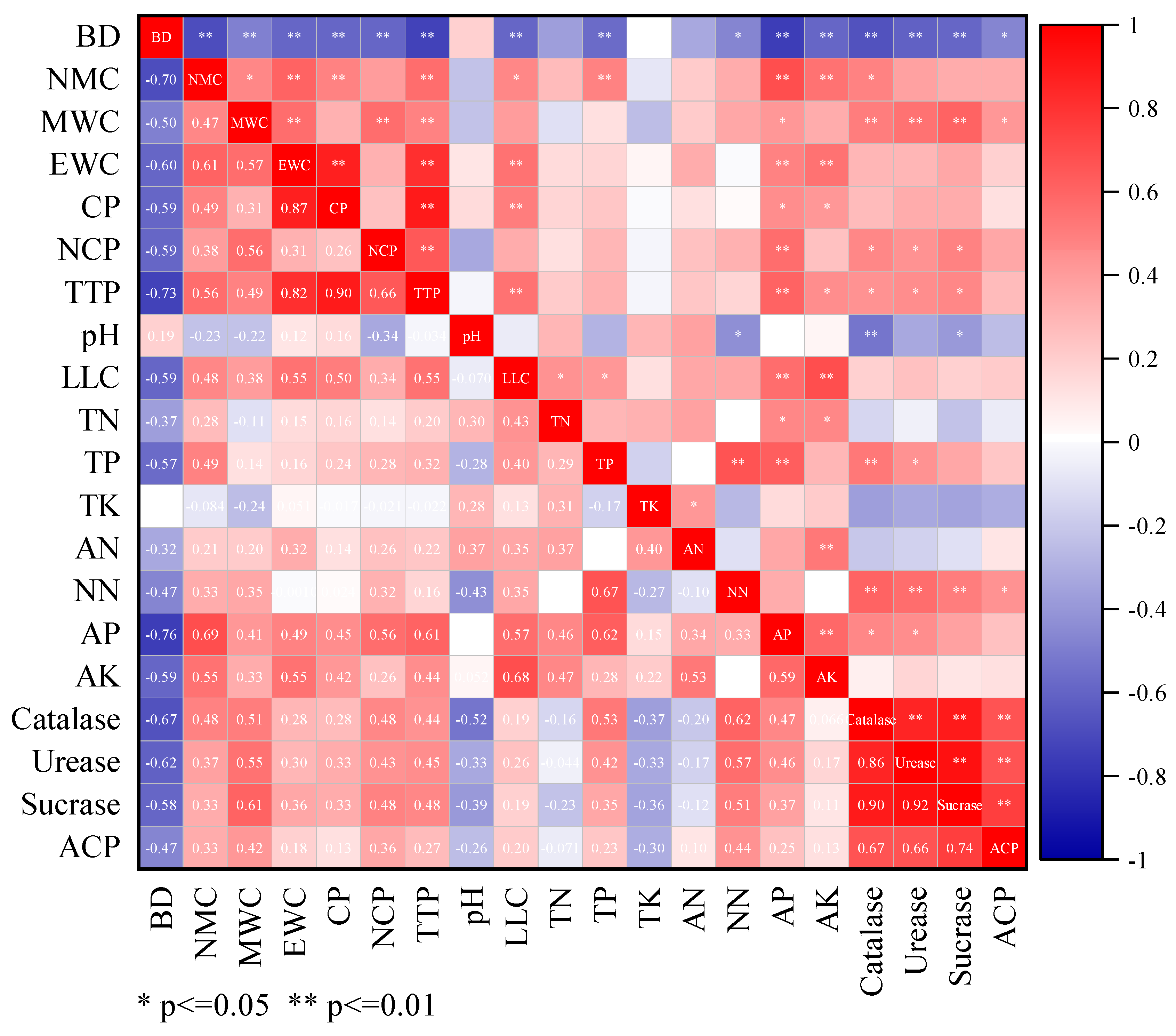
3.4.1. Determining the MDS
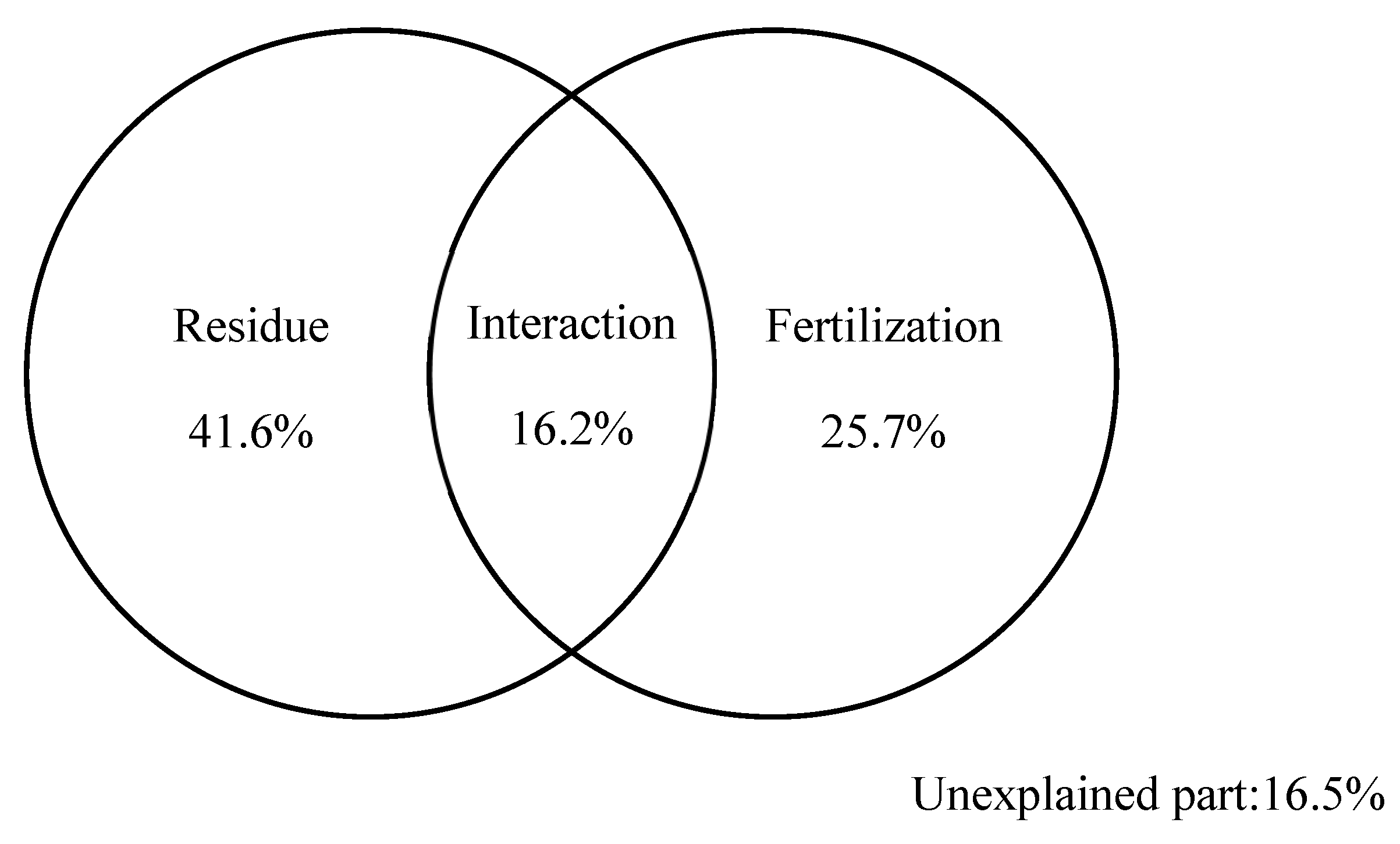
3.4.2. Calculating Soil Quality Index
4. Discussion
4.1. Effects of Fertilization on Soil Physical and Chemical Properties and Enzyme Activities
4.2. Effects of Residue Management on Soil’s Physical and Chemical Properties and Enzyme Activities
4.3. Soil Quality Evaluation of Eucalyptus Plantation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Du, A.; Wang, Z.; Zhu, W.; Li, C.; Wu, L. Effects of different rotation periods of Eucalyptus plantations on soil physiochemical properties, enzyme activities, microbial biomass and microbial community structure and diversity. For. Ecol. Manag. 2020, 456, 117683. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, X.J. Geographical spatial distribution and productivity dynamic change of eucalyptus plantations in China. Sci. Rep. 2021, 11, 19764. [Google Scholar]
- Liu, H.; Li, J. The study of the ecological problems of Eucalyptus plantation and sustainable development in Maoming Xiaoliang. J. Sustain. Dev. 2010, 3, 197. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, X.; Chen, F.; Li, C.; Wu, L. Effects of the successive planting of Eucalyptus urophylla on soil bacterial and fungal community structure, diversity, microbial biomass, and enzyme activity. Land Degrad. Dev. 2019, 30, 636–646. [Google Scholar] [CrossRef]
- Morales, M.; Aroca, G.; Rubilar, R.; Acuña, E.; Mola-Yudego, B.; González-García, S. Cradle-to-gate life cycle assessment of Eucalyptus globulus short rotation plantations in Chile. J. Clean. Prod. 2015, 99, 239–249. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, H.; Wen, Y.; Goodale, U.M.; Zhu, Y.; Yu, S.; Li, C.; Li, X. Intensive management and declines in soil nutrients lead to serious exotic plant invasion in Eucalyptus plantations under successive short-rotation regimes. Land Degrad. Dev. 2020, 31, 297–310. [Google Scholar]
- Gao, Y.; Su, F.; Liang, Y.; Li, S.; Zhu, Y.; Xu, Y.; Tang, J.; Cao, J.; Wu, L. Nutrient input and output in short-rotation Eucalyptus plantations. J. Cent. South Univ. For. Technol. 2021, 41, 56–67. [Google Scholar]
- Molina, A.; Reigosa, M.J.; Carballeira, A. Release of allelochemical agents from litter, throughfall, and topsoil in plantations of Eucalyptus globulus Labill in Spain. J. Chem. Ecol. 1991, 17, 147–160. [Google Scholar]
- Águas, A.; Incerti, G.; Saracino, A.; Lanzotti, V.; Silva, J.S.; Rego, F.C.; Mazzoleni, S.; Bonanomi, G. Fire effects on litter chemistry and early development of Eucalyptus globulus. Plant Soil 2018, 422, 495–514. [Google Scholar] [CrossRef]
- Zhao, J.Y. Study on the Effect of Refining Treatment on Soil Properties and Growth of Eucalyptus Urophylla Plantation; Central South University of Forestry & Technology: Changsha, China, 2019. [Google Scholar]
- Mendham, D.S.; O’connell, A.M.; Grove, T.S.; Rance, S.J. Residue management effects on soil carbon and nutrient contents and growth of second rotation eucalypts. For. Ecol. Manag. 2003, 181, 357–372. [Google Scholar] [CrossRef]
- Palviainen, M.; Finér, L.; Kurka, A.M.; Mannerkoski, H.; Piirainen, S.; Starr, M. Decomposition and nutrient release from logging residues after clear-cutting of mixed boreal forest. Plant Soil. 2004, 263, 53–67. [Google Scholar]
- Hernández, J.; Del Pino, A.; Hitta, M.; Lorenzo, M. Management of forest harvest residues affects soil nutrient availability during reforestation of Eucalyptus grandis. Nutr. Cycl. Agroecosyst. 2016, 105, 141–155. [Google Scholar]
- Mendham, D.S.; Sankaran, K.V.; O’Connell, A.M.; Grove, T.S. Eucalyptus globulus harvest residue management effects on soil carbon and microbial biomass at 1 and 5 years after plantation establishment. Soil Biol. Biochem. 2002, 34, 1903–1912. [Google Scholar]
- Parhizkar, M.; Shabanpour, M.; Lucas-Borja, M.E.; Zema, D.A.; Li, S.; Tanaka, N.; Cerdà, A. Effects of length and application rate of rice straw mulch on surface runoff and soil loss under laboratory simulated rainfall. Int. J. Sediment Res. 2021, 36, 468–478. [Google Scholar]
- da Silva, P.H.M.; Poggiani, F.; Libardi, P.L.; Gonçalves, A.N. Fertilizer management of eucalypt plantations on sandy soil in Brazil: Initial growth and nutrient cycling. For. Ecol. Manag. 2013, 301, 67–78. [Google Scholar]
- Battie-Laclau, P.; Delgado-Rojas, J.S.; Christina, M.; Nouvellon, Y.; Bouillet, J.-P.; Piccolo, M.d.C.; Moreira, M.Z.; Gonçalves, J.L.d.M.; Roupsard, O.; Laclau, J.-P. Potassium fertilization increases water-use efficiency for stem biomass production without affecting intrinsic water-use efficiency in Eucalyptus grandis plantations. For. Ecol. Manag. 2016, 364, 77–89. [Google Scholar]
- Li, P.; Zhang, T.; Wang, X.; Yu, D. Development of biological soil quality indicator system for subtropical China. Soil Tillage Res. 2013, 126, 112–118. [Google Scholar]
- Liu, J.; Wu, L.; Chen, D.; Li, M.; Wei, C. Soil quality assessment of different Camellia oleifera stands in mid-subtropical China. Appl. Soil Ecol. 2017, 113, 29–35. [Google Scholar] [CrossRef]
- Chang, X.; Qiu, X.; Liu, X.; Peng, D.; Cheng, S. Soil fertility quality evaluation of pure and mixed Larix principis-rupprechtii forests in Saihanba, Hebei Province of northern China. J. Beijing For. Univ. 2021, 43, 50–59. [Google Scholar]
- Luo, H.; Chen, J.; He, J.; Kang, W. Melaleuca alternifolia (Maiden & Betche) Cheel Residues Affect the Biomass and Soil Quality of Plantation. Forests 2022, 13, 2134. [Google Scholar]
- Pang, S.; Ou, Z.; Shen, W.; Hou, Y.; Huang, X.; Zheng, W. Edaphic characteristics of different regeneration patterns in karst mountainous areas of Guangxi. J. Cent. South Univ. For. Technol. 2016, 36, 60–66. [Google Scholar]
- Askari, M.S.; Holden, N.M. Indices for quantitative evaluation of soil quality under grassland management. Geoderma 2014, 230, 131–142. [Google Scholar] [CrossRef]
- Gonçalves, J.L.M.; Wichert, M.C.P.; Gava, J.L.; Serrano, M.I.P. Soil fertility and growth of Eucalyptus grandis in Brazil under different residue management practices. South. Hemisph. For. J. 2007, 69, 95–102. [Google Scholar]
- Zhao, J.; Wan, S.; Fu, S.; Wang, X.; Wamg, M.; Liang, C.; Chen, Y.; Zhu, X. Effects of understory removal and nitrogen fertilization on soil microbial communities in Eucalyptus plantations. For. Ecol. Manag. 2013, 310, 80–86. [Google Scholar] [CrossRef]
- Williamson, J.R.; Neilsen, W.A. The effect of soil compaction, profile disturbance and fertilizer application on the growth of eucalypt seedlings in two glasshouse studies. Soil Tillage Res. 2003, 71, 95–107. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Z.; Wu, Q.; Huang, K.; Wen, J.; Li, H.; Zhu, L.; Tang, Y.; Chen, L.; Wu, L. Soil qualities and change rules of Eucalyptus grandis × Eucalyptus urophylla plantation with different slash disposals. Sci. Rep. 2022, 12, 20988. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, J.; Liu, X.; Zhang, D.; Li, L.; Li, W.; Sheng, L. Effects of long-term application of organic fertilizer on improving organic matter content and retarding acidity in red soil from China. Soil Tillage Res. 2019, 195, 104382. [Google Scholar] [CrossRef]
- Gutser, R.; Ebertseder, T.; Weber, A.; Schraml, M.; Schmidhalter, U. Short-term and residual availability of nitrogen after long-term application of organic fertilizers on arable land. J. Plant Nutr. Soil Sci. 2005, 168, 439–446. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar]
- Li, Q.; Zhang, D.; Song, Z.; Ren, L.; Jin, X.; Fang, W.; Yan, D.; Li, Y.; Wang, Q.; Cao, A. Organic fertilizer activates soil beneficial microorganisms to promote strawberry growth and soil health after fumigation. Environ. Pollut. 2022, 295, 118653. [Google Scholar]
- Chang, E.H.; Chung, R.S.; Tsai, Y.H. Effect of different application rates of organic fertilizer on soil enzyme activity and microbial population. Soil Sci. Plant Nutr. 2007, 53, 132–140. [Google Scholar] [CrossRef]
- Rocha, J.H.T.; de Moraes Goncalves, J.L.; Brandani, C.B.; de Vicente Ferraz, A.; Franci, A.F.; Marques, E.R.G.; Junior, J.C.A.; Hubner, A. Forest residue removal decreases soil quality and affects wood productivity even with high rates of fertilizer application. For. Ecol. Manag. 2018, 430, 188–195. [Google Scholar]
- Nambiar, E.K.S.; Harwood, C.E. Productivity of acacia and eucalypt plantations in Southeast Asia. 1. Bio-physical determinants of production: Opportunities and challenges. Int. For. Rev. 2014, 16, 225–248. [Google Scholar]
- Fernández, C.; Vega, J.A.; Bará, S.; Beloso, C.; Alonso, M.; Fonturbel, T. Nitrogen mineralization after clearcutting and residue managementin a second rotation Eucalyptus globulus Labill. stand in Galicia (NW Spain). Ann. For. Sci. 2009, 66, 807. [Google Scholar] [CrossRef][Green Version]
- Rocha, J.H.T.; Gonçalves, J.L.D.M.; Gava, J.L.; Godinho, T.D.O.; Melo, E.A.; Bazani, J.H.; Hubner, A.; Junior, J.C.A.; Wichert, M.P. Forest residue maintenance increased the wood productivity of a Eucalyptus plantation over two short rotations. For. Ecol. Manag. 2016, 379, 1–10. [Google Scholar]
- Laclau, J.P.; Levillain, J.; Deleporte, P.; de Dieu Nzila, J.; Bouillet, J.P.; André, L.S.; Versini, A.; Mareschal, L.; Nouvellon, Y.; M’Bou, A.T.; et al. Organic residue mass at planting is an excellent predictor of tree growth in Eucalyptus plantations established on a sandy tropical soil. For. Ecol. Manag. 2010, 260, 2148–2159. [Google Scholar] [CrossRef]
- O’connell, A.M.; Grove, T.S.; Mendham, D.S.; Rance, S.J. Impact of harvest residue management on soil nitrogen dynamics in Eucalyptus globulus plantations in south western Australia. Soil Biol. Biochem. 2004, 36, 39–48. [Google Scholar]
- Corbeels, M.; O’Connell, A.; Grove, T.; Mendham, D.; Rance, S. Nitrogen release from eucalypt leaves and legume residues as influenced by their biochemical quality and degree of contact with soil. Plant Soil 2003, 250, 15–28. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Huang, K.; Li, H.; Tang, Y.; Zhu, L.; Tang, J.; Shi, Y.; Lin, H.; Wang, Y. Seasonal dynamic response of soil bacterial community of Eucalyptus plantations to different non-slash-burning woodland renewal models. J. Cent. South Univ. For. Technol. 2022, 42, 39–52. [Google Scholar]
- Qiu, X.; Peng, D.; Wang, H.; Wang, Z.; Cheng, S. Minimum data set for evaluation of stand density effects on soil quality in Larix principis-rupprechtii plantations in North China. Ecol. Indic. 2019, 103, 236–247. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M. Soil quality indicators: Critical tools in ecosystem restoration. Curr. Opin. Environ. Sci. Health 2018, 5, 47–52. [Google Scholar]
- Paul, G.C.; Saha, S.; Ghosh, K.G. Assessing the soil quality of Bansloi river basin, eastern India using soil-quality indices (SQIs) and Random Forest machine learning technique. Ecol. Indic. 2020, 118, 106804. [Google Scholar] [CrossRef]
- Legaz, B.V.; De Souza, D.M.; Teixeira, R.; Antón, A.; Putman, B.; Sala, S. Soil quality, properties, and functions in life cycle assessment: An evaluation of models. J. Clean. Prod. 2017, 140, 502–515. [Google Scholar]

| Indicator | Numerical Value | Indicator | Numerical Value |
|---|---|---|---|
| BD/g·cm−3 | 1.18 (0.17) | TP/g·kg−1 | 0.75 (0.18) |
| NMC/% | 16.2 (3.4) | TK/g·kg−1 | 1.94 (0.52) |
| TTP/% | 38.4 (5.3) | AN/mg·kg−1 | 20.3 (5.5) |
| pH | 4.61 (0.22) | NN/mg·kg−1 | 3.94 (0.89) |
| LLC | 73.9 (21.5) | AP/mg·kg−1 | 1.85 (0.43) |
| TN/g·kg−1 | 1.51 (0.32) | AK/mg·kg−1 | 9.4 (1.7) |
| Fertilization | Residue | Orthogonal Group | Total Fertilization Mass/kg·ha−1 | Altitude/m | Slope/° |
|---|---|---|---|---|---|
| In-O | T | In-O × T | inorganic fertilizer, 3500 | 377 | 15 |
| R | In-O × R | 382 | 12 | ||
| S | In-O × S | 367 | 15 | ||
| O | T | O × T | organic fertilizer, 3500 | 375 | 10 |
| R | O × R | 370 | 15 | ||
| S | O × S | 364 | 14 | ||
| M | T | M × T | mixed fertilizer, 3500 | 374 | 15 |
| R | M × R | 382 | 10 | ||
| S | M × S | 382 | 12 |
| Element | C/g·kg−1 | N/g·kg−1 | P/g·kg−1 | K/g·kg−1 |
|---|---|---|---|---|
| Content | 514 (67) | 7.15 (1.44) | 0.83 (0.15) | 6.33 (0.22) |
| Indicator | Unit | Method [18,19] |
|---|---|---|
| Bulk density (BD) | g·cm−3 | Soil core method |
| Natural moisture content (NMC) | % | Soil core method |
| Mass water-holding capacity (MWC) | % | Soil core immersion method |
| Effective water-holding capacity (EWC) | % | Soil core immersion method |
| Capillary porosity (CP) | % | Soil core immersion method |
| Non-capillary porosity (NCP) | % | Soil core immersion method |
| The total porosity (TTP) | % | Soil core immersion method |
| pH | Potentiometry (soil: H2O = 1:2.5) | |
| Leaching liquid conductivity (LLC) | Leaching, potentiometry | |
| Total nitrogen (TN) | g·kg−1 | Semi-micro Kjeldahl method |
| Total phosphorus (TP) | g·kg−1 | Alkali fusion-interrupt analyzer |
| Total kalium (TK) | g·kg−1 | Alkali fusion-flame photometer method |
| Ammonia nitrogen (AN) | mg·kg−1 | KCl-interrupt analyzer |
| Nitrate nitrogen (NN) | mg·kg−1 | CaSO4-interrupt analyzer |
| Available phosphorus (AP) | mg·kg−1 | Double acid extraction-molybdenum antimony anti-colorimetric method |
| Available kalium (AK) | mg·kg−1 | Ammonium acetate extraction-flame photometer method |
| Catalase | nmol·g−1 | Potassium permanganate titration |
| Urease | nmol·g−1 | Phenol-sodium hypochlorite colorimetry |
| Sucrase | nmol·g−1 | 3,5-Dinitrosalicylic acid colorimetry |
| Acid phosphatase | 10−3 nmol·g−1 | Phosphoric acid-disodium benzene colorimetry |
| Indicator | Fertilization Method | Minimum | Maximum | Variation Coefficient | ||
|---|---|---|---|---|---|---|
| Group In-O | Group O | Group M | ||||
| BD | 1.19 (0.14) | 1.17 (0.12) | 1.22 (0.08) | 0.96 | 1.40 | 0.100 |
| NMC | 17.9 (2.8) | 19.1 (3.3) | 16.2 (3.0) | 11.8 | 26.0 | 0.184 |
| MWC | 32.5 (3.4) b | 36.8 (4.0) a | 34.8 (4.6) ab | 28.0 | 45.1 | 0.119 |
| EWC | 29.3 (3.6) | 30.1 (3.0) | 28.7 (2.9) | 22.9 | 37.8 | 0.123 |
| CP | 35.3 (4.9) | 36.4 (2.6) | 34.7 (4.8) | 26.1 | 41.3 | 0.122 |
| NCP | 7.9 (2.5) | 7.5 (2.9) | 8.5 (2.3) | 3.4 | 12.0 | 0.328 |
| TTP | 43.2 (6.4) | 44.0 (3.2) | 43.2 (6.6) | 31.3 | 53.3 | 0.131 |
| pH | 4.54 (0.13) | 4.34 (0.16) | 4.42 (0.09) | 4.07 | 4.80 | 0.035 |
| LLC | 89.1 (28.5) | 86.4 (21.2) | 79.6 (12.2) | 58.4 | 134.0 | 0.259 |
| TN | 2.00 (0.30) a | 1.49 (0.14) b | 1.50 (0.25) b | 1.08 | 2.60 | 0.204 |
| TP | 0.92 (0.23) | 0.96 (0.25) | 0.87 (0.09) | 0.55 | 1.49 | 0.227 |
| TK | 2.12 (0.57) | 1.84 (0.48) | 1.95 (0.19) | 1.02 | 3.93 | 0.271 |
| AN | 28.0 (6.19) | 21.4 (2.79) | 26.9 (5.47) | 17.7 | 38.2 | 0.228 |
| NN | 3.70 (0.80) | 5.05 (1.86) | 4.24 (0.82) | 2.55 | 8.73 | 0.318 |
| AP | 3.54 (1.21) | 2.52 (1.30) | 2.80 (0.99) | 1.03 | 3.93 | 0.271 |
| AK | 12.1 (2.74) | 10.5 (1.93) | 10.1 (2.31) | 6.50 | 17.62 | 0.232 |
| Catalase | 4.05 (1.04) b | 6.82 (2.73) a | 5.80 (1.38) ab | 2.27 | 11.71 | 0.394 |
| Urease | 0.053 (0.012) c | 0.081 (0.030) a | 0.064 (0.021) b | 0.034 | 0.147 | 0.391 |
| Sucrase | 2.45 (0.60) b | 3.90 (1.19) a | 3.49 (0.92) a | 1.53 | 6.19 | 0.342 |
| ACP | 2.34 (0.67) b | 3.47 (0.94) a | 3.36 (0.93) a | 1.49 | 5.58 | 0.327 |
| Indicator | Residue Management Mode | Minimum | Maximum | Variation Coefficient | ||
|---|---|---|---|---|---|---|
| Group T | Group R | Group S | ||||
| BD | 1.06 (0.07) c | 1.31 (0.07) a | 1.21 (0.03) b | 0.96 | 1.40 | 0.100 |
| NMC | 20.7 (2.9) a | 15.2 (2.1) c | 17.7 (2.1) b | 11.8 | 26.0 | 0.184 |
| MWC | 39.2 (3.3) a | 33.4 (3.2) b | 32.9 (2.6) b | 28.0 | 45.1 | 0.119 |
| EWC | 32.3 (2.7) a | 27.1 (3.2) b | 28.5 (2.8) b | 22.9 | 37.8 | 0.123 |
| CP | 37.8 (2.8) a | 32.1 (4.4) b | 35.9 (3.6) ab | 26.1 | 41.3 | 0.122 |
| NCP | 10.2 (1.4) a | 5.7 (2.1) c | 7.7 (2.1) b | 3.4 | 12.0 | 0.328 |
| TTP | 48.0 (3.3) a | 37.7 (4.1) c | 43.7 (3.9) b | 31.3 | 53.3 | 0.131 |
| pH | 4.40 (0.21) | 4.46 (0.06) | 4.43 (0.15) | 4.07 | 4.80 | 0.035 |
| LLC | 105.2 (21.9) a | 78.6 (12.4) b | 71.2 (13.5) b | 58.4 | 134.0 | 0.259 |
| TN | 1.80 (0.44) | 1.55 (0.26) | 1.65 (0.22) | 1.08 | 2.60 | 0.204 |
| TP | 1.07 (0.22) a | 0.79 (0.13) b | 0.89 (0.15) ab | 0.55 | 1.49 | 0.227 |
| TK | 2.11 (0.57) | 2.25 (0.68) | 1.90 (0.46) | 1.02 | 3.93 | 0.271 |
| AN | 28.9 (6.7) | 25.0 (4.6) | 22.4 (3.8) | 17.7 | 38.2 | 0.228 |
| NN | 5.16 (1.83) a | 3.79 (0.77) b | 4.04 (0.82) b | 2.55 | 8.73 | 0.318 |
| AP | 4.32 (0.49) a | 1.75 (0.38) c | 2.80 (1.00) b | 1.03 | 3.93 | 0.271 |
| AK | 13.2 (2.3) a | 9.8 (1.8) b | 9.6 (1.6) b | 6.50 | 17.62 | 0.232 |
| Catalase | 7.46 (2.26) a | 3.54 (0.67) c | 5.67 (1.08) b | 2.27 | 11.71 | 0.394 |
| Urease | 0.088 (0.029) a | 0.046 (0.008) c | 0.064 (0.014) b | 0.034 | 0.147 | 0.391 |
| Sucrase | 4.25 (1.06) a | 2.42 (0.60) c | 3.17 (0.76) b | 1.53 | 6.19 | 0.342 |
| ACP | 3.80 (1.08) a | 2.64 (0.85) b | 2.73 (0.53) ab | 1.49 | 5.58 | 0.327 |
| Indicator | Source of Variation | df | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| BD | Fertilization | 2 | 0.006 | 1.867 | 0.183 |
| Residue | 2 | 0.145 | 42.102 | 0.000 ** | |
| Fertilization × Residue | 4 | 0.005 | 1.456 | 0.257 | |
| NMC | Fertilization | 2 | 19.864 | 3.439 | 0.054 * |
| Residue | 2 | 67.242 | 11.643 | 0.001 ** | |
| Fertilization × Residue | 4 | 3.477 | 0.602 | 0.666 | |
| MWC | Fertilization | 2 | 18.998 | 1.803 | 0.193 |
| Residue | 2 | 110.459 | 10.481 | 0.001 ** | |
| Fertilization × Residue | 4 | 6.817 | 0.647 | 0.636 | |
| EWC | Fertilization | 2 | 4.484 | 0.612 | 0.553 |
| Residue | 2 | 64.449 | 8.801 | 0.002 ** | |
| Fertilization × Residue | 4 | 20.902 | 2.854 | 0.054 | |
| CP | Fertilization | 2 | 8.982 | 0.888 | 0.429 |
| Residue | 2 | 76.491 | 7.566 | 0.004 ** | |
| Fertilization × Residue | 4 | 38.572 | 3.815 | 0.02 * | |
| NCP | Fertilization | 2 | 2.176 | 0.516 | 0.605 |
| Residue | 2 | 44.87 | 10.644 | 0.001 ** | |
| Fertilization × Residue | 4 | 3.001 | 0.712 | 0.594 | |
| TTP | Fertilization | 2 | 4.972 | 0.312 | 0.736 |
| Residue | 2 | 239.435 | 15.005 | 0.000 ** | |
| Fertilization × Residue | 4 | 22.123 | 1.386 | 0.278 | |
| pH | Fertilization | 2 | 0.089 | 4.852 | 0.021 * |
| Residue | 2 | 0.009 | 0.466 | 0.635 | |
| Fertilization × Residue | 4 | 0.03 | 1.612 | 0.215 | |
| LLC | Fertilization | 2 | 214.721 | 1.952 | 0.171 |
| Residue | 2 | 2878.221 | 26.168 | 0.000 ** | |
| Fertilization × Residue | 4 | 1239.382 | 11.268 | 0.000 ** | |
| TN | Fertilization | 2 | 0.767 | 14.381 | 0.000 ** |
| Residue | 2 | 0.145 | 2.722 | 0.093 | |
| Fertilization × Residue | 4 | 0.083 | 1.551 | 0.23 | |
| TP | Fertilization | 2 | 0.021 | 0.722 | 0.499 |
| Residue | 2 | 0.185 | 6.485 | 0.008 ** | |
| Fertilization × Residue | 4 | 0.064 | 2.228 | 0.107 | |
| TK | Fertilization | 2 | 1.187 | 4.174 | 0.032 * |
| Residue | 2 | 0.188 | 0.662 | 0.528 | |
| Fertilization × Residue | 4 | 0.219 | 0.772 | 0.558 | |
| AN | Fertilization | 2 | 111.729 | 10.775 | 0.001 ** |
| Residue | 2 | 98.177 | 9.468 | 0.002 ** | |
| Fertilization × Residue | 4 | 75.417 | 7.273 | 0.001 ** | |
| NN | Fertilization | 2 | 4.16 | 8.741 | 0.002 ** |
| Residue | 2 | 4.758 | 9.998 | 0.001 ** | |
| Fertilization × Residue | 4 | 6.212 | 13.053 | 0.000 ** | |
| AP | Fertilization | 2 | 2.484 | 16.958 | 0.000 ** |
| Residue | 2 | 15.092 | 103.016 | 0.000 ** | |
| Fertilization × Residue | 4 | 1.114 | 7.605 | 0.001 ** | |
| AK | Fertilization | 2 | 11.241 | 3.582 | 0.049 * |
| Residue | 2 | 36.677 | 11.686 | 0.001 ** | |
| Fertilization × Residue | 4 | 4.961 | 1.581 | 0.222 | |
| Catalase | Fertilization | 2 | 17.82 | 36.59 | 0.000 ** |
| Residue | 2 | 34.665 | 71.176 | 0.000 ** | |
| Fertilization × Residue | 4 | 4.011 | 8.237 | 0.001 ** | |
| Urease | Fertilization | 2 | 0.002 | 6.262 | 0.009 ** |
| Residue | 2 | 0.004 | 14.167 | 0.000 ** | |
| Fertilization × Residue | 4 | 0.001 | 5.178 | 0.015 * | |
| Sucrase | Fertilization | 2 | 5.048 | 13.328 | 0.000 ** |
| Residue | 2 | 7.684 | 20.289 | 0.000 ** | |
| Fertilization × Residue | 4 | 0.424 | 1.119 | 0.379 | |
| ACP | Fertilization | 2 | 3.535 | 6.411 | 0.008 ** |
| Residue | 2 | 3.753 | 6.806 | 0.006 ** | |
| Fertilization × Residue | 4 | 0.636 | 5.153 | 0.014 * |
| PC1 | PC2 | PC3 | PC4 | |
|---|---|---|---|---|
| Eigenvalue | 8.060 | 3.630 | 1.784 | 1.222 |
| Percent | 44.73 | 20.15 | 9.90 | 6.78 |
| Cumulative percent | 44.73 | 64.88 | 74.78 | 81.56 |
| Eigenvector | ||||
| Sucrase | 0.880 | 0.282 | 0.051 | −0.197 |
| Catalase | 0.839 | 0.180 | 0.262 | −0.313 |
| Urease | 0.798 | 0.242 | 0.214 | −0.236 |
| ACP | 0.768 | 0.073 | 0.080 | 0.022 |
| MWC | 0.659 | 0.449 | −0.044 | 0.200 |
| NCP | 0.649 | 0.264 | 0.137 | 0.292 |
| NN | 0.619 | −0.241 | 0.546 | −0.122 |
| PH | −0.564 | 0.224 | −0.102 | 0.328 |
| CP | 0.034 | 0.932 | 0.217 | −0.070 |
| EWC | 0.166 | 0.894 | 0.166 | 0.161 |
| TTP | 0.306 | 0.842 | 0.264 | 0.073 |
| TP | 0.295 | −0.006 | 0.826 | −0.240 |
| AP | 0.318 | 0.341 | 0.698 | 0.269 |
| TN | −0.234 | 0.181 | 0.686 | 0.221 |
| LLC | 0.157 | 0.391 | 0.599 | 0.297 |
| BD | −0.522 | −0.459 | −0.582 | −0.178 |
| NMC | 0.290 | 0.453 | 0.569 | 0.048 |
| AK | 0.050 | 0.392 | 0.541 | 0.491 |
| AN | −0.009 | 0.144 | 0.168 | 0.874 |
| TK | −0.300 | −0.069 | 0.059 | 0.634 |
| Item | Sucrase | CP | TP | AN |
|---|---|---|---|---|
| In-O × T | 0.334 (0.048) | 0.921 (0.088) | 0.617 (0.119) | 0.866 (0.164) |
| In-O × R | 0.052 (0.050) | 0.265 (0.121) | 0.213 (0.109) | 0.219 (0.116) |
| In-O × S | 0.207 (0.071) | 0.533 (0.271) | 0.367 (0.277) | 0.423 (0.017) |
| O × T | 0.807 (0.149) | 0.612 (0.132) | 0.754 (0.181) | 0.142 (0.076) |
| O × R | 0.239 (0.083) | 0.736 (0.172) | 0.228 (0.179) | 0.258 (0.076) |
| O × S | 0.480 (0.078) | 0.698 (0.158) | 0.346 (0.025) | 0.146 (0.016) |
| M × T | 0.612 (0.133) | 0.777 (0.158) | 0.309 (0.142) | 0.635 (0.094) |
| M × R | 0.279 (0.102) | 0.190 (0.159) | 0.333 (0.047) | 0.592 (0.195) |
| M × S | 0.370 (0.177) | 0.729 (0.179) | 0.383 (0.067) | 0.115 (0.010) |
| Weights | 0.549 | 0.247 | 0.121 | 0.083 |
| Indicator | Source of Variation | df | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| SQI | Fertilization | 2 | 0.072 | 10.785 | 0.007 ** |
| Residue | 2 | 0.295 | 26.189 | 0.000 ** | |
| Fertilization × Residue | 4 | 0.002 | 8.256 | 0.013 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Wu, L. Fertilization and Residue Management Improved Soil Quality of Eucalyptus Plantations. Forests 2023, 14, 1570. https://doi.org/10.3390/f14081570
Zhu Z, Wu L. Fertilization and Residue Management Improved Soil Quality of Eucalyptus Plantations. Forests. 2023; 14(8):1570. https://doi.org/10.3390/f14081570
Chicago/Turabian StyleZhu, Zhiyuan, and Lichao Wu. 2023. "Fertilization and Residue Management Improved Soil Quality of Eucalyptus Plantations" Forests 14, no. 8: 1570. https://doi.org/10.3390/f14081570
APA StyleZhu, Z., & Wu, L. (2023). Fertilization and Residue Management Improved Soil Quality of Eucalyptus Plantations. Forests, 14(8), 1570. https://doi.org/10.3390/f14081570






