Assessing Water Relations and Carbon Dynamics of Pinus taeda Branches Undergoing Shade-Induced Mortality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Study Methods and Design
2.3. Photosynthetic Light–Response Curve Measurements
2.4. Storage Carbohydrate Analysis
2.5. Sap Flow Measurement
2.6. Water Potential Measurement
2.7. Statistical Analysis
3. Results
3.1. Effect of Shade on Parameters of Photosynthetic Light–Response Curve
3.2. Effect of Shade on Storage Carbohydrate
3.3. Effect of Shade on Water Relations
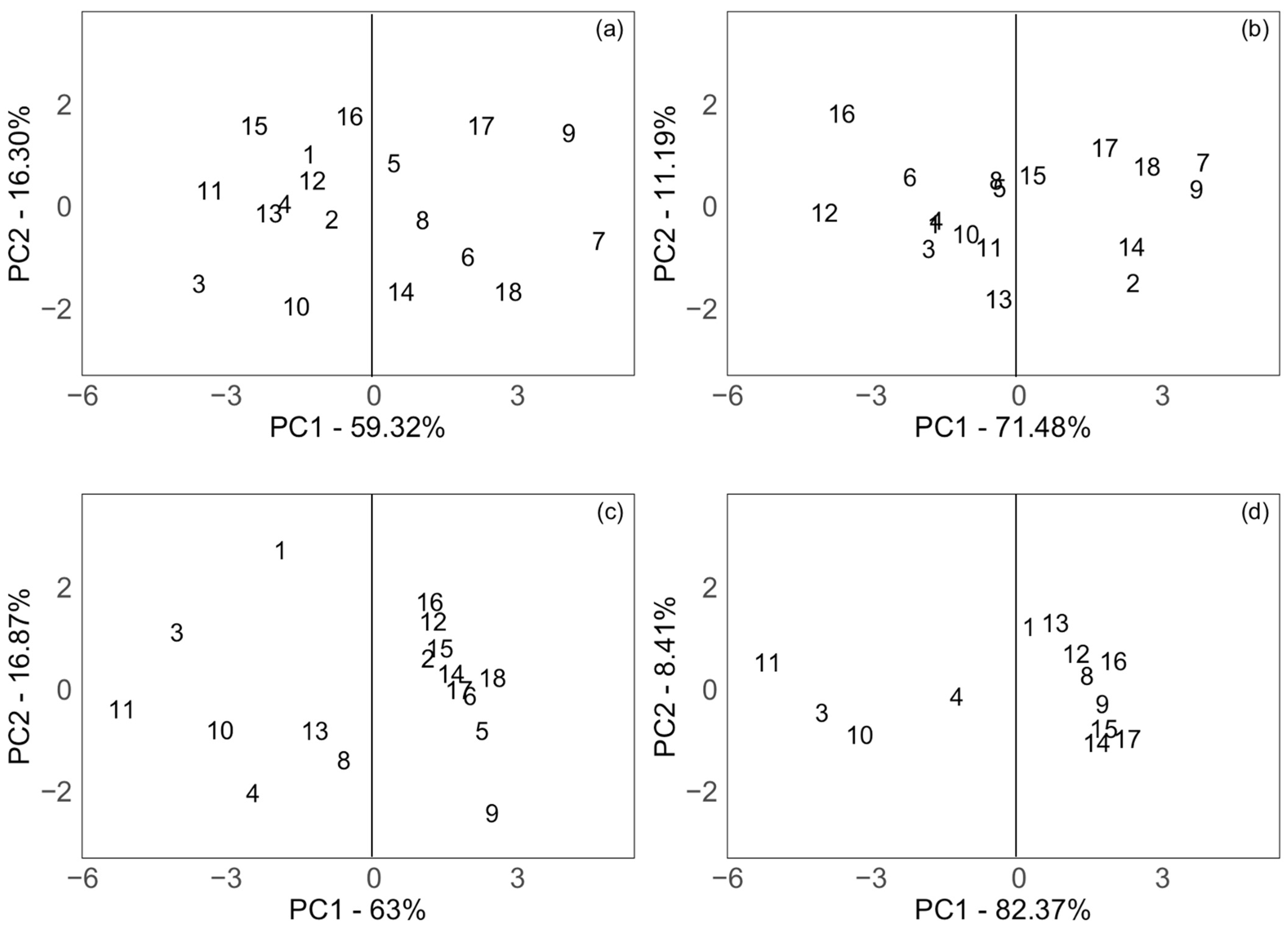
3.4. Principal Component Analysis of Branch Mortality
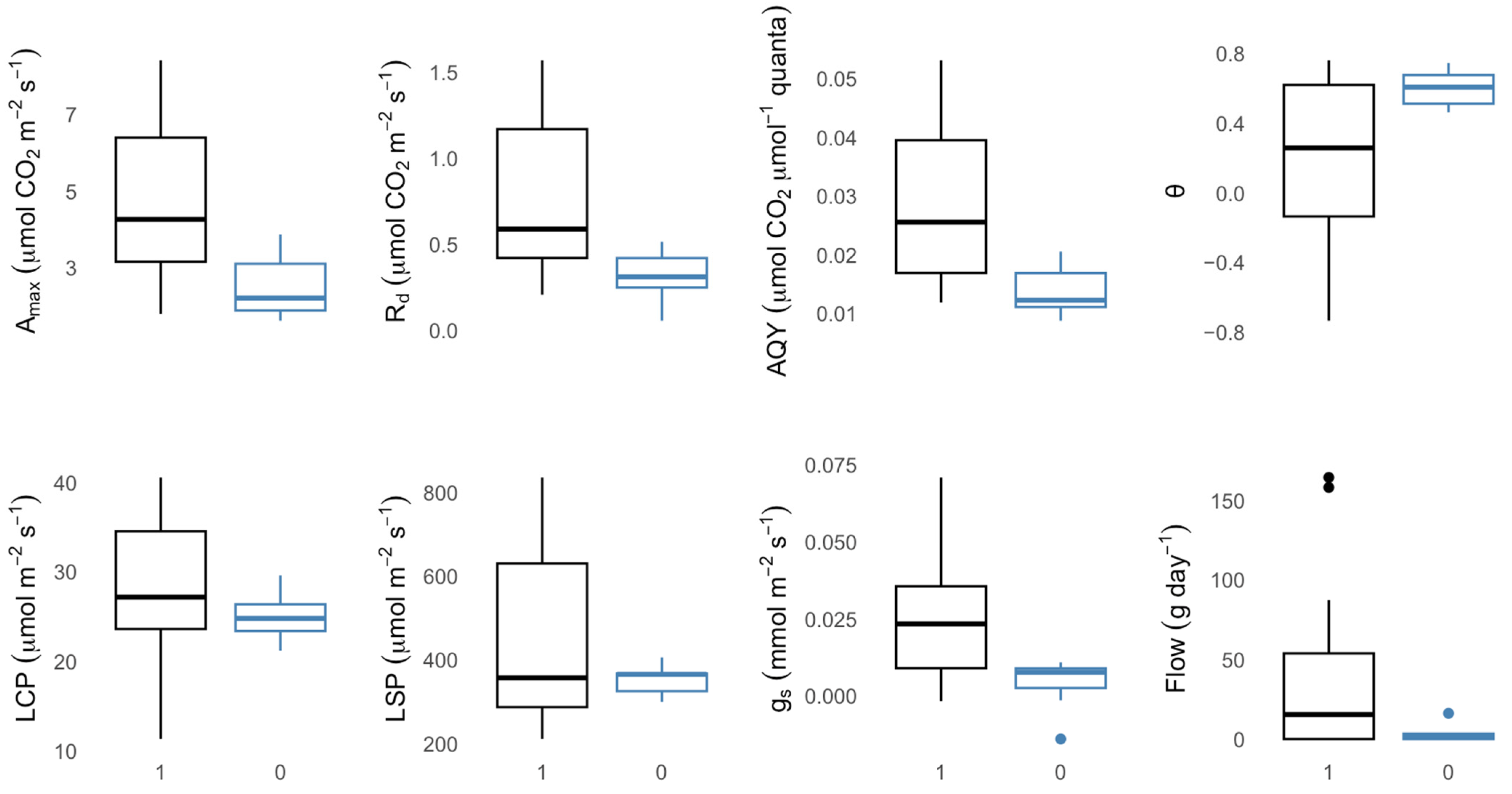
3.5. Comparative Analysis of Photosynthetic and Water Relations Traits between Dying and Surviving Branches
4. Discussion
4.1. Effect of Shade on Parameters of Photosynthetic Light–Response Curve
4.2. Effect of Shade on Storage Carbohydrate
4.3. Effect of Shade on Water Relations
4.4. Principal Component Analysis of Branch Mortality
4.5. Comparative Analysis of Photosynthetic and Water Relations Traits between Dying and Surviving Branches
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gratzer, G.; Darabant, A.; Chhetri, P.B.; Rai, P.B.; Eckmüllner, O. Interspecific Variation in the Response of Growth, Crown Morphology, and Survivorship to Light of Six Tree Species in the Conifer Belt of the Bhutan Himalayas. Can. J. For. Res. 2004, 34, 1093–1107. [Google Scholar] [CrossRef]
- Sugiura, D.; Tateno, M. Concentrative Nitrogen Allocation to Sun-Lit Branches and the Effects on Whole-Plant Growth under Heterogeneous Light Environments. Oecologia 2013, 172, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Li, J.; Zhang, Y.; Korpelainen, H.; Niinemets, Ü.; Li, C. Partial Shading of Lateral Branches Affects Growth, and Foliage Nitrogen-and Water-Use Efficiencies in the Conifer Cunninghamia Lanceolata Growing in a Warm Monsoon Climate. Tree Physiol. 2015, 35, 632–643. [Google Scholar]
- Takenaka, A. Shoot Growth Responses to Light Microenvironment and Correlative Inhibition in Tree Seedlings under a Forest Canopy. Tree Physiol. 2000, 20, 987–991. [Google Scholar] [CrossRef]
- Hennessey, T.; Dougherty, P.; Cregg, B.; Wittwer, R. Annual Variation in Needle Fall of a Loblolly Pine Stand in Relation to Climate and Stand Density. For. Ecol. Manag. 1992, 51, 329–338. [Google Scholar] [CrossRef]
- Hunt, S. Measurements of Photosynthesis and Respiration in Plants. Physiol. Plant. 2003, 117, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Lacointe, A.; Deleens, E.; Ameglio, T.; Saint-Joanis, B.; Lelarge, C.; Vandame, M.; Song, G.; Daudet, F.-A. Testing the Branch Autonomy Theory: A 13C/14C Double-labelling Experiment on Differentially Shaded Branches. Plant Cell Environ. 2004, 27, 1159–1168. [Google Scholar] [CrossRef]
- Gustafsson, C.; Boström, C. Influence of Neighboring Plants on Shading Stress Resistance and Recovery of Eelgrass, Zostera marina L. PLoS ONE 2013, 8, e64064. [Google Scholar]
- Han, Q.; Chiba, Y. Leaf Photosynthetic Responses and Related Nitrogen Changes Associated with Crown Reclosure after Thinning in a Young Chamaecyparis Obtusa Stand. J. For. Res. 2009, 14, 349–357. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and Sink Mechanisms of Nitrogen Transport and Use. New Phytol. 2018, 217, 35–53. [Google Scholar] [PubMed]
- Hikosaka, K. Optimal Nitrogen Distribution within a Leaf Canopy under Direct and Diffuse Light. Plant Cell Environ. 2014, 37, 2077–2085. [Google Scholar] [PubMed]
- Wyckoff, P.H.; Clark, J.S. Predicting Tree Mortality from Diameter Growth: A Comparison of Maximum Likelihood and Bayesian Approaches. Can. J. For. Res. 2000, 30, 156–167. [Google Scholar] [CrossRef]
- Bigler, C.; Gričar, J.; Bugmann, H.; Čufar, K. Growth Patterns as Indicators of Impending Tree Death in Silver Fir. For. Ecol. Manag. 2004, 199, 183–190. [Google Scholar]
- Brodersen, C.R.; McElrone, A.J.; Choat, B.; Lee, E.F.; Shackel, K.A.; Matthews, M.A. In Vivo Visualizations of Drought-Induced Embolism Spread in Vitis Vinifera. Plant Physiol. 2013, 161, 1820–1829. [Google Scholar] [PubMed]
- Nardini, A.; Savi, T.; Losso, A.; Petit, G.; Pacilè, S.; Tromba, G.; Mayr, S.; Trifilò, P.; Lo Gullo, M.A.; Salleo, S. X-ray Microtomography Observations of Xylem Embolism in Stems of Laurus Nobilis Are Consistent with Hydraulic Measurements of Percentage Loss of Conductance. New Phytol. 2017, 213, 1068–1075. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G. Mechanisms of Plant Survival and Mortality during Drought: Why Do Some Plants Survive While Others Succumb to Drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Lone, P.; Khan, N. The Effects of Rate and Timing of N Fertilizer on Growth, Photosynthesis, N Accumulation and Yield of Mustard (Brassica juncea) Subjected to Defoliation. Environ. Exp. Bot. 2007, 60, 318–323. [Google Scholar] [CrossRef]
- Barigah, T.S.; Ibrahim, T.; Bogard, A.; Faivre-Vuillin, B.; Lagneau, L.A.; Montpied, P.; Dreyer, E. Irradiance-Induced Plasticity in the Hydraulic Properties of Saplings of Different Temperate Broad-Leaved Forest Tree Species. Tree Physiol. 2006, 26, 1505–1516. [Google Scholar] [CrossRef]
- Trubat, R.; Cortina, J.; Vilagrosa, A. Plant Morphology and Root Hydraulics Are Altered by Nutrient Deficiency in Pistacia lentiscus (L.). Trees 2006, 20, 334–339. [Google Scholar] [CrossRef]
- Boardman, N.K. Comparative Photosynthesis of Sun and Shade Plants. Annu. Rev. Plant Physiol. 1977, 28, 355–377. [Google Scholar] [CrossRef]
- Björkman, O. Responses to Different Quantum Flux Densities. In Physiological Plant Ecology I: Responses to the Physical Environment; Springer: Berlin/Heidelberg, Germany, 1981; pp. 57–107. [Google Scholar]
- Koike, T. Leaf Structure and Photosynthetic Performance as Related to the Forest Succession of Deciduous Broad-Leaved Trees. Plant Species Biol. 1988, 3, 77–87. [Google Scholar] [CrossRef]
- Warrington, I.; Rook, D.; Morgan, D.; Turnbull, H. The Influence of Simulated Shadelight and Daylight on Growth, Development and Photosynthesis of Pinus radiata, Agathis australis and Dacrydium cupressinum. Plant Cell Environ. 1989, 12, 343–356. [Google Scholar] [CrossRef]
- Perry, M.H. Effects of Shading on Photosynthesis and Leaf Morphology of Leucaena Leucocephala (Lam) de Wit Cv K8; University of Hawai‘i at Manoa: Honolulu, HI, USA, 1985; ISBN 9798728258971. [Google Scholar]
- Tsel’niker, Y.L. Resistances to CO2 Uptake at Light Saturation in Forest Tree Seedlings of Different Adaptation to Shade. Photosynthetica 1979, 13, 124–129. [Google Scholar]
- Teskey, R.O.; Shrestha, R.B. A Relationship between Carbon Dioxide, Photosynthetic Efficiency and Shade Tolerance. Physiol. Plant. 1985, 63, 126–132. [Google Scholar] [CrossRef]
- Schmitz, N.; Egerton, J.; Lovelock, C.; Ball, M. Light-dependent Maintenance of Hydraulic Function in Mangrove Branches: Do Xylary Chloroplasts Play a Role in Embolism Repair? New Phytol. 2012, 195, 40–46. [Google Scholar] [PubMed]
- Cochard, H.; Lemoine, D.; Dreyer, E. The Effects of Acclimation to Sunlight on the Xylem Vulnerability to Embolism in Fagus sylvatica L. Plant Cell Environ. 1999, 22, 101–108. [Google Scholar] [CrossRef]
- Sellin, A.; Õunapuu, E.; Kupper, P. Effects of Light Intensity and Duration on Leaf Hydraulic Conductance and Distribution of Resistance in Shoots of Silver Birch (Betula pendula). Physiol. Plant. 2008, 134, 412–420. [Google Scholar] [CrossRef]
- Daniels, R.F.; Burkhart, H.E. Simulation of Individual Tree Growth and Stand Development in Managed Loblolly Pine Plantations; Virginia Tech. Division of Forestry and Wildlife Resources: Blacksburg, VA, USA, 1975. [Google Scholar]
- Burkhart, H.E.; Walton, S.B. Incorporating Crown Ratio into Taper Equations for Loblolly Pine Trees. For. Sci. 1985, 31, 478–484. [Google Scholar]
- Hann, D.W.; Walters, D.K.; Scrivani, J.A. Incorporating Crown Ratio into Prediction Equations for Douglas-Fir Stem Volume. Can. J. For. Res. 1987, 17, 17–22. [Google Scholar] [CrossRef]
- MacFarlane, D.W.; Green, E.J.; Brunner, A.; Burkhart, H.E. Predicting Survival and Growth Rates for Individual Loblolly Pine Trees from Light Capture Estimates. Can. J. For. Res. 2002, 32, 1970–1983. [Google Scholar] [CrossRef]
- Sharma, R.P.; Vacek, Z.; Vacek, S.; Podrázský, V.; Jansa, V. Modelling Individual Tree Height to Crown Base of Norway Spruce (Picea abies (L.) Karst.) and European Beech (Fagus sylvatica L.). PLoS ONE 2017, 12, e0186394. [Google Scholar]
- Dicus, C.A.; Dean, T.J. Tree-Soil Interactions Affect Production of Loblolly and Slash Pine. For. Sci. 2008, 54, 134–139. [Google Scholar]
- Blazier, M.A.; Tyree, M.C.; Sword Sayer, M.A.; KC, D.; Hood, W.G.; Osbon, B.S. Gas Exchange and Productivity in Temperate and Droughty Years of Four Eastern, Elite Loblolly Pine Genotypes Grown in the Western Gulf Region. Int. J. Agron. 2018, 2018, 3942602. [Google Scholar] [CrossRef]
- Johnson, G.; Murchie, E. Gas Exchange Measurements for the Determination of Photosynthetic Efficiency in Arabidopsis Leaves. In Chloroplast Research in Arabidopsis: Methods and Protocols, Volume II; Jarvis, R.P., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 311–326. ISBN 978-1-61779-237-3. [Google Scholar]
- Midgley, G.F.; Rutherford, M.C.; Davis, G.W.; Bosenberg, J.W. Photosynthetic Responses of Heliophilous Rhus Species to Environmental Modification by Invasive Shrubs. Funct. Ecol. 1992, 6, 334–345. [Google Scholar] [CrossRef]
- Bailey, S.; Walters, R.G.; Jansson, S.; Horton, P. Acclimation of Arabidopsis thaliana to the Light Environment: The Existence of Separate Low Light and High Light Responses. Planta 2001, 213, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, K.; Dyson, B.C.; Webster, R.E.; Johnson, G.N. Dynamic Acclimation of Photosynthesis Increases Plant Fitness in Changing Environments. Plant Physiol. 2010, 152, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Muthuchelian, K.; Paliwal, K.; Gnanam, A. Influence of Shading on Net Photosynthetic and Transpiration Rates, Stomatal Diffusive Resistance, Nitrate Reductase and Biomass Productivity of a Woody Legume Tree Species (Erythrina variegata Lam.). Proc. Plant Sci. 1989, 99, 539–546. [Google Scholar] [CrossRef]
- Kardiman, R.; Ræbild, A. Relationship between Stomatal Density, Size and Speed of Opening in Sumatran Rainforest Species. Tree Physiol. 2018, 38, 696–705. [Google Scholar] [CrossRef]
- Pearcy, R.W.; Sims, D.A. Photosynthetic Acclimation to Changing Light Environments: Scaling from the Leaf to the Whole Plant. In Exploitation of Environmental Heterogeneity by Plants: Ecophysiological Processes Above- and Belowground; Academic Press: Cambridge, MA, USA, 1994; pp. 145–174. [Google Scholar]
- Terashima, I.; Saeki, T. A New Model for Leaf Photosynthesis Incorporating the Gradients of Light Environment and of Photosynthetic Properties of Chloroplasts within a Leaf. Ann. Bot. 1985, 56, 489–499. [Google Scholar] [CrossRef]
- Brooks, J.R.; Sprugel, D.G.; Hinckley, T.M. The Effects of Light Acclimation during and after Foliage Expansion on Photosynthesis of Abies amabilis Foliage within the Canopy. Oecologia 1996, 107, 21–32. [Google Scholar] [CrossRef]
- Temesgen, H.; Weiskittel, A. Leaf Mass per Area Relationships across Light Gradients in Hybrid Spruce Crowns. Trees 2006, 20, 522–530. [Google Scholar]
- Ogren, E. Convexity of the Photosynthetic Light-Response Curve in Relation to Intensity and Direction of Light during Growth. Plant Physiol. 1993, 101, 1013–1019. [Google Scholar] [CrossRef]
- Olsson, T.; Leverenz, J. Non-uniform Stomatal Closure and the Apparent Convexity of the Photosynthetic Photon Flux Density Response Curve. Plant Cell Environ. 1994, 17, 701–710. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural Carbon in Woody Plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef]
- Maguire, A.J.; Kobe, R.K. Drought and Shade Deplete Nonstructural Carbohydrate Reserves in Seedlings of Five Temperate Tree Species. Ecol. Evol. 2015, 5, 5711–5721. [Google Scholar] [CrossRef]
- Smith, A.M.; Stitt, M. Coordination of Carbon Supply and Plant Growth. Plant Cell Environ. 2007, 30, 1126–1149. [Google Scholar] [PubMed]
- Paul, M.J.; Foyer, C.H. Sink Regulation of Photosynthesis. J. Exp. Bot. 2001, 52, 1383–1400. [Google Scholar] [PubMed]
- Proietti, S.; Paradiso, R.; Moscatello, S.; Saccardo, F.; Battistelli, A. Light Intensity Affects the Assimilation Rate and Carbohydrates Partitioning in Spinach Grown in a Controlled Environment. Plants 2023, 12, 804. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, O.; Ishihara, H.; George, G.M.; Mengin, V.; Flis, A.; Sumner, D.; Arrivault, S.; Feil, R.; Lunn, J.E.; Zeeman, S.C. Leaf Starch Turnover Occurs in Long Days and in Falling Light at the End of the Day. Plant Physiol. 2017, 174, 2199–2212. [Google Scholar] [CrossRef]
- Dayer, S.; Murcia, G.; Prieto, J.A.; Durán, M.; Martínez, L.; Píccoli, P.; Perez Peña, J. Non-structural Carbohydrates and Sugar Export in Grapevine Leaves Exposed to Different Light Regimes. Physiol. Plant. 2021, 171, 728–738. [Google Scholar] [CrossRef]
- Aasamaa, K.; Sõber, A. Stomatal Sensitivities to Changes in Leaf Water Potential, Air Humidity, CO2 Concentration and Light Intensity, and the Effect of Abscisic Acid on the Sensitivities in Six Temperate Deciduous Tree Species. Environ. Exp. Bot. 2011, 71, 72–78. [Google Scholar] [CrossRef]
- Rijkers, T.; Pons, T.; Bongers, F. The Effect of Tree Height and Light Availability on Photosynthetic Leaf Traits of Four Neotropical Species Differing in Shade Tolerance. Funct. Ecol. 2000, 14, 77–86. [Google Scholar] [CrossRef]
- Sellin, A.; Kupper, P. Effects of Light Availability versus Hydraulic Constraints on Stomatal Responses within a Crown of Silver Birch. Oecologia 2005, 142, 388–397. [Google Scholar] [CrossRef]
- Blenkinsop, P.G.; Dale, J.E. The Effects of Shade Treatment and Light Intensity on Rihulose-1,5-Diphosphate Carboxylase Activity and Fraction I Protein Level in the First Leaf of Barley. J. Exp. Bot. 1974, 25, 899–912. [Google Scholar] [CrossRef]
- Choi, D.; Jang, W.; Toda, H.; Yoshikawa, M. Differences in Characteristics of Photosynthesis and Nitrogen Utilization in Leaves of the Black Locust (Robinia pseudoacacia L.) According to Leaf Position. Forests 2021, 12, 348. [Google Scholar] [CrossRef]
- Hanstein, S.; de Beer, D.; Felle, H.H. Miniaturised Carbon Dioxide Sensor Designed for Measurements within Plant Leaves. Sens. Actuators B Chem. 2001, 81, 107–114. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M. Photosynthesis and Drought: Can We Make Metabolic Connections from Available Data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar]
- Kobe, R.K. Carbohydrate Allocation to Storage as a Basis of Interspecific Variation in Sapling Survivorship and Growth. Oikos 1997, 80, 226–233. [Google Scholar] [CrossRef]
- Sevanto, S.; Mcdowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W.T. How Do Trees Die? A Test of the Hydraulic Failure and Carbon Starvation Hypotheses. Plant Cell Environ. 2014, 37, 153–161. [Google Scholar] [CrossRef]



| Shade | South | North |
|---|---|---|
| 0% | S01, S02, S04 | N01, N02, N05 |
| 30% | S302, S304, S305 | N301, N303, N304 |
| 60% | S601, S603, S605 | N602, N603, N604 |
| Treatment | Mean | Std Error |
|---|---|---|
| Assimilation at Light Saturation (μmol CO2 m−2 s−1) | ||
| 0 percent | 7.1593 a | 0.5915 |
| 30 percent | 5.6694 a,b | 0.6029 |
| 60 percent | 4.3087 b | 0.6142 |
| Dark Respiration (μmol CO2 m−2 s−1) | ||
| 0 percent | 1.3268 a | 0.1174 |
| 30 percent | 0.9777 b | 0.1196 |
| 60 percent | 0.6638 b | 0.1218 |
| Apparent Quantum Yield (μmol CO2 μmol −1 quanta) | ||
| 0 percent | 0.0427 a | 0.0045 |
| 30 percent | 0.0383 a,b | 0.0046 |
| 60 percent | 0.0261 b | 0.0046 |
| Theta | ||
| 0 percent | 0.0891 a | 0.1174 |
| 30 percent | 0.0651 a | 0.1189 |
| 60 percent | 0.4876 b | 0.1209 |
| Light Compensation Point (μmol m−2 s−1) | ||
| 0 percent | 37.7372 a | 1.9196 |
| 30 percent | 30.7806 b | 1.9491 |
| 60 percent | 27.1304 b | 1.9836 |
| Light Saturation Point (μmol m−2 s−1) | ||
| 0 percent | 605.3300 a | 45.5826 |
| 30 percent | 536.9100 a | 46.2257 |
| 60 percent | 390.3500 b | 47.0563 |
| Treatment | Mean | Std Error |
|---|---|---|
| Stomatal conductance (mmol m−2 s−1) | ||
| 0 percent | 0.0423 a | 0.0046 |
| 30 percent | 0.0321 a,b | 0.0048 |
| 60 percent | 0.0250 b | 0.0049 |
| Sap flow (g/day) | ||
| 0 percent | 220.4600 a | 24.5518 |
| 30 percent | 130.2300 b | 25.8615 |
| 60 percent | 62.4087 b | 27.1485 |
| Pre-dawn water potential (MPa) | ||
| 0 percent | −1.0642 a | 0.1656 |
| 30 percent | −1.1700 a | 0.1425 |
| 60 percent | −1.3423 a | 0.1687 |
| Mid-day water potential (MPa) | ||
| 0 percent | −1.9017 a | 0.2306 |
| 30 percent | −2.1921 a | 0.2328 |
| 60 percent | −2.3391 a | 0.2953 |
| Classification by Month | ||||||
|---|---|---|---|---|---|---|
| No. | Branch ID | Status | August | September | October | November |
| 1 | N01 | Alive | ✓ | ✓ | ✓ | ✗ |
| 2 | N02 | Dead | ✗ | ✓ | ✓ | * |
| 3 | N05 | Alive | ✓ | ✓ | ✓ | ✓ |
| 4 | N301 | Alive | ✓ | ✓ | ✓ | ✓ |
| 5 | N303 | Dead | ✓ | ✗ | ✓ | * |
| 6 | N304 | Dead | ✓ | ✗ | ✓ | * |
| 7 | N602 | Dead | ✓ | ✓ | * | * |
| 8 | N603 | Alive | ✗ | ✓ | ✓ | ✗ |
| 9 | N604 | Dead | ✓ | ✓ | ✓ | ✓ |
| 10 | S01 | Alive | ✓ | ✓ | ✓ | ✓ |
| 11 | S02 | Alive | ✓ | ✓ | ✓ | ✓ |
| 12 | S04 | Alive | ✓ | ✓ | ✗ | ✗ |
| 13 | S302 | Alive | ✓ | ✓ | ✓ | ✗ |
| 14 | S304 | Alive | ✗ | ✗ | ✗ | ✗ |
| 15 | S305 | Alive | ✓ | ✗ | ✗ | ✗ |
| 16 | S601 | Alive | ✓ | ✓ | ✗ | ✗ |
| 17 | S603 | Dead | ✓ | ✓ | ✓ | ✓ |
| 18 | S605 | Dead | ✓ | ✓ | ✓ | * |
| Accuracy (%) | 83.3 | 77.8 | 76.5 | 46.2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majekobaje, A.R.; Dean, T.J. Assessing Water Relations and Carbon Dynamics of Pinus taeda Branches Undergoing Shade-Induced Mortality. Forests 2023, 14, 1704. https://doi.org/10.3390/f14091704
Majekobaje AR, Dean TJ. Assessing Water Relations and Carbon Dynamics of Pinus taeda Branches Undergoing Shade-Induced Mortality. Forests. 2023; 14(9):1704. https://doi.org/10.3390/f14091704
Chicago/Turabian StyleMajekobaje, Adelodun R., and Thomas J. Dean. 2023. "Assessing Water Relations and Carbon Dynamics of Pinus taeda Branches Undergoing Shade-Induced Mortality" Forests 14, no. 9: 1704. https://doi.org/10.3390/f14091704
APA StyleMajekobaje, A. R., & Dean, T. J. (2023). Assessing Water Relations and Carbon Dynamics of Pinus taeda Branches Undergoing Shade-Induced Mortality. Forests, 14(9), 1704. https://doi.org/10.3390/f14091704







