Effects of Nitrogen Addition on the Growth and Physiology of Populus deltoides Seedlings under Cd and Mn Pollution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Plant Materials
2.2. Growth and Biomass Determination
2.3. Measurement of Membrane Lipid Peroxidation and Antioxidant Enzyme Activity
2.4. Analysis of Organic Acids in Fine Roots
2.5. Enrichment and Allocation of Cd and Mn in P. deltoides
2.6. Statistical Analysis
3. Results
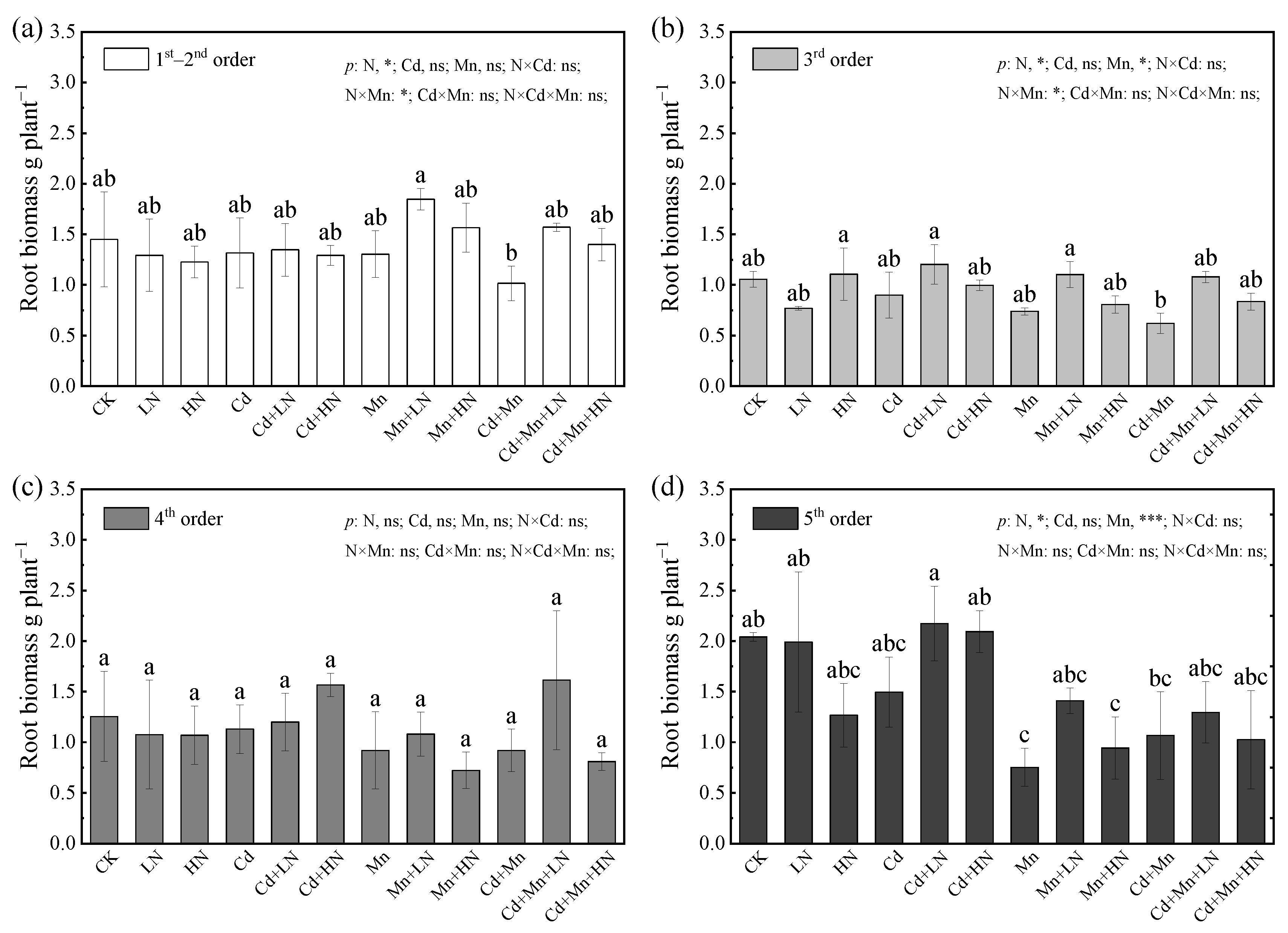
3.1. Effects of N Addition on Growth and Biomass of P. deltoides under Cd and Mn Pollution
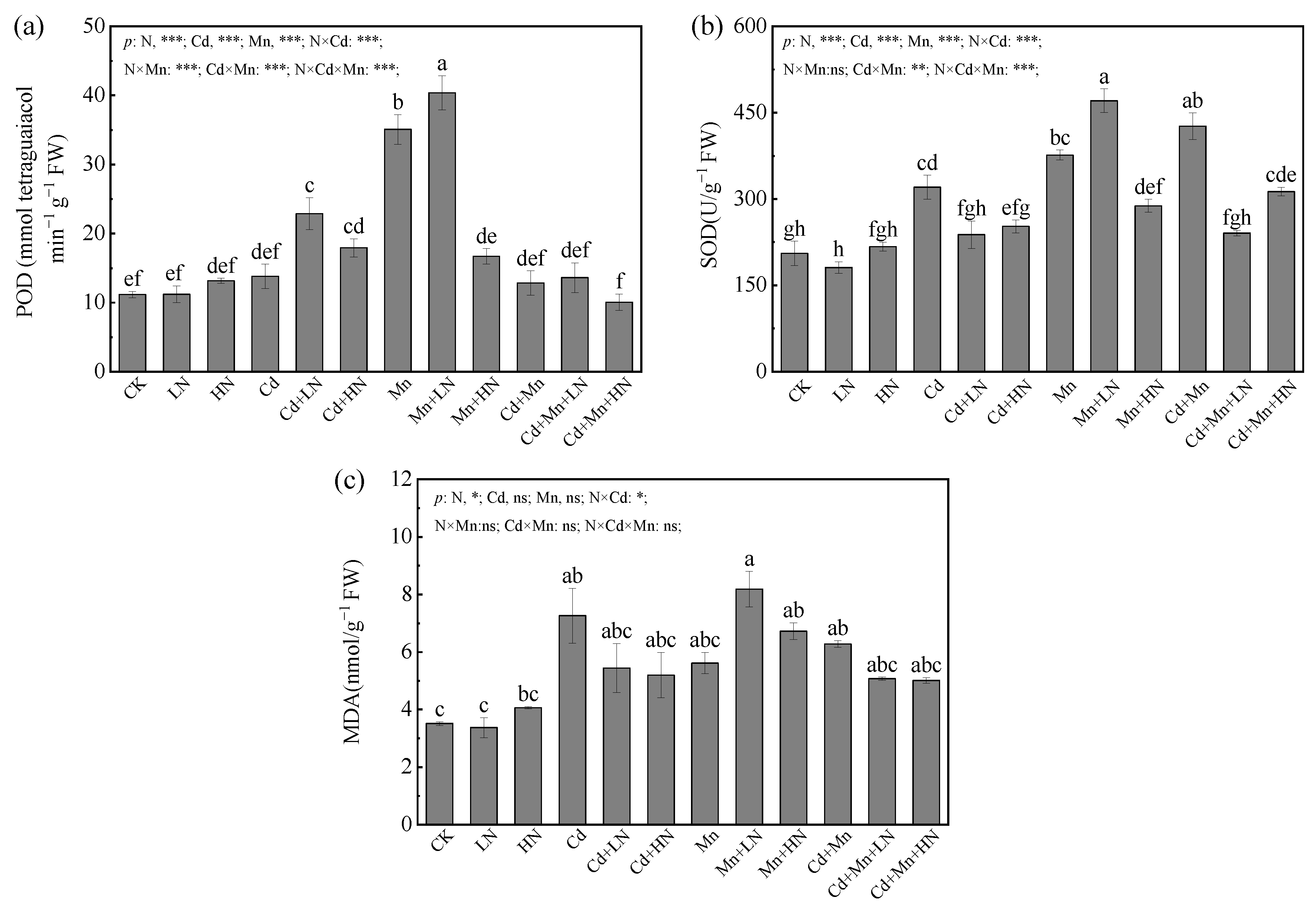
3.2. Effects of N Addition on Activities of Antioxidant Enzymes and MDA of P. deltoides under Cd and Mn Pollution
3.3. Effects of N Addition on Concentrations of Organic Acids in Roots of P. deltoides under Cd and Mn Pollution
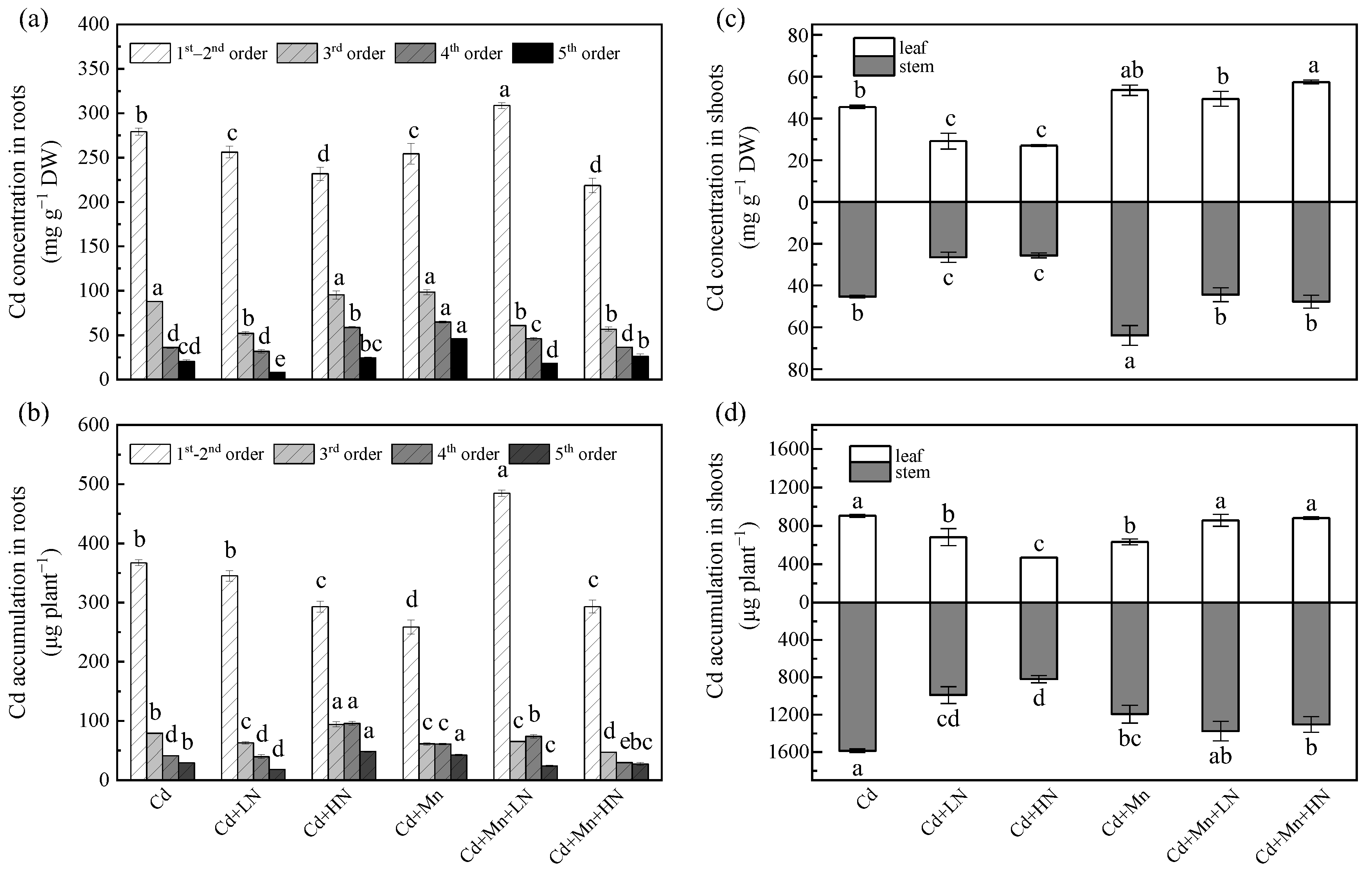
3.4. Effects of N Addition on Cd Concentration in P. deltoides
3.5. Effects of N Addition on Cd Accumulation in P. deltoides
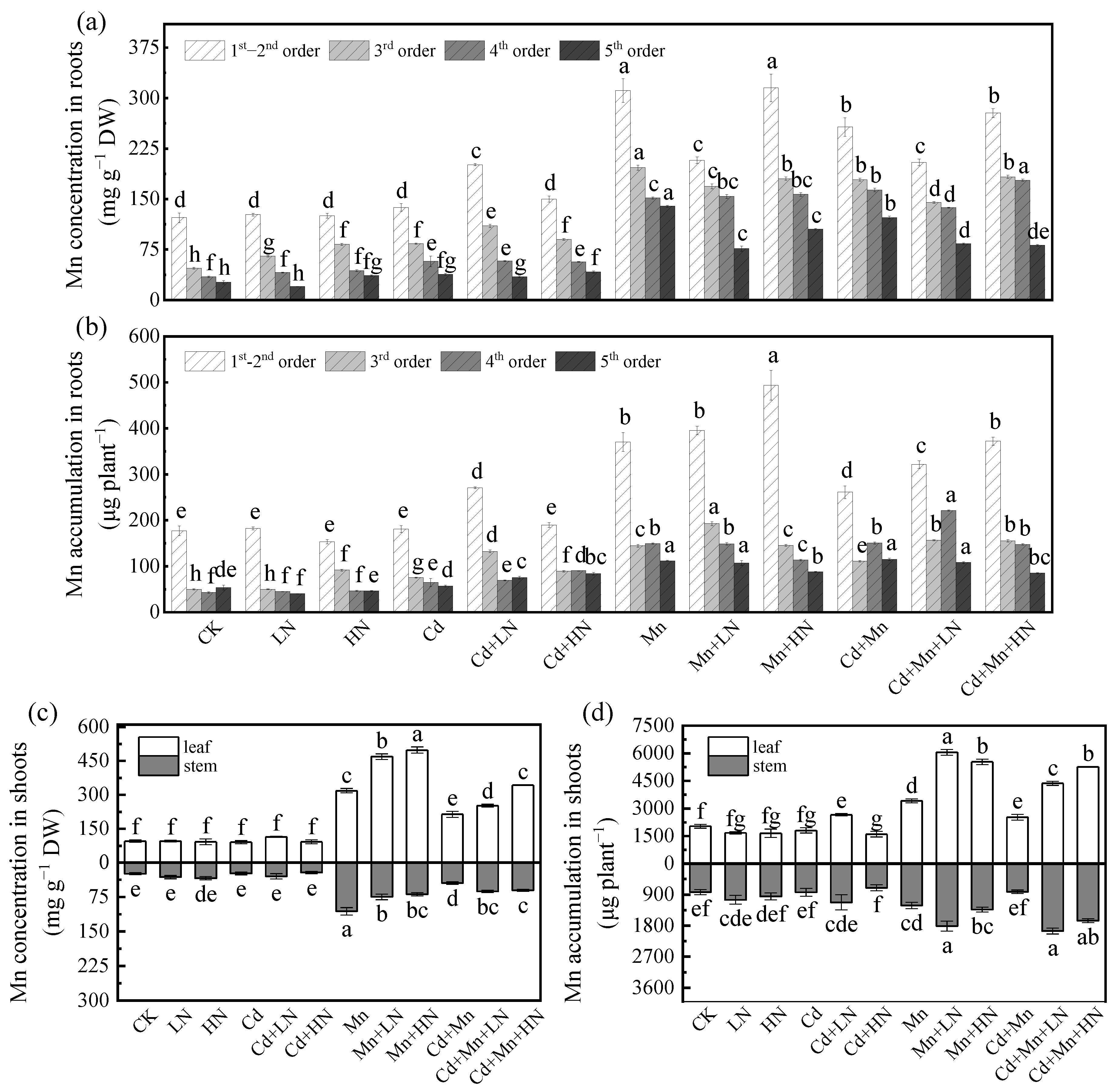
3.6. Effects of N Addition on Mn Concentration in P. deltoides
3.7. Effects of N Addition on Mn Accumulation in P. deltoides
3.8. Effects of N Addition on Enrichment and Allocation of HMs in P. deltoides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hao, L.; Chen, L.; Zhu, P.; Zhang, J.; Zhang, D.; Xiao, J.; Xu, Z.; Zhang, L.; Liu, Y.; Li, H.; et al. Sex-Specific Responses of Populus deltoides to Interaction of Cadmium and Salinity in Root Systems. Ecotoxicol. Environ. Saf. 2020, 195, 110437. [Google Scholar] [CrossRef]
- Bora, M.S.; Gogoi, N.; Sarma, K.P. Tolerance Mechanism of Cadmium in Ceratopteris pteridoides: Translocation and Subcellular Distribution. Ecotoxicol. Environ. Saf. 2020, 197, 110599. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Duan, S.; Wu, Q.; Yu, M.; Shabala, S. Reducing Cadmium Accumulation in Plants: Structure-Function Relations and Tissue-Specific Operation of Transporters in the Spotlight. Plants 2020, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Geszvain, K.; Butterfield, C.; Davis, R.E.; Madison, A.S.; Lee, S.-W.; Parker, D.L.; Soldatova, A.; Spiro, T.G.; Luther, G.W., III; Tebo, B.M. The Molecular Biogeochemistry of Manganese(II) Oxidation. Biochem. Soc. Trans. 2012, 40, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhou, Y.; Gong, J. Physiological Mechanisms of the Tolerance Response to Manganese Stress Exhibited by Pinus massoniana, a Candidate Plant for the Phytoremediation of Mn-Contaminated Soil. Environ. Sci. Pollut. Res. 2021, 28, 45422–45433. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy Metals, Occurrence and Toxicity for Plants: A Review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Tian, Q.; Liu, N.; Bai, W.; Li, L.; Chen, J.C.; Reich, P.B.; Yu, Q.; Guo, D.; Smith, M.D.; Knapp, A.K.; et al. A Novel Soil Manganese Mechanism Drives Plant Species Loss with Increased Nitrogen Deposition in a Temperate Steppe. Ecology 2016, 97, 65–74. [Google Scholar] [CrossRef]
- Sheng, H.; Zeng, J.; Liu, Y.; Wang, X.; Wang, Y.; Kang, H.; Fan, X.; Sha, L.; Zhang, H.; Zhou, Y. Sulfur Mediated Alleviation of Mn Toxicity in Polish Wheat Relates to Regulating Mn Allocation and Improving Antioxidant System. Front. Plant Sci. 2016, 7, 1382. [Google Scholar] [CrossRef]
- Pulford, I. Phytoremediation of Heavy Metal-Contaminated Land by Trees—A Review. Environ. Int. 2003, 29, 529–540. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.; Li, H.; Shi, W.; Polle, A.; Lu, M.; Sun, X.; Luo, Z.-B. Global Poplar Root and Leaf Transcriptomes Reveal Links between Growth and Stress Responses under Nitrogen Starvation and Excess. Tree Physiol. 2015, 35, 1283–1302. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Luo, J.; Ma, C.; Li, S.; Qu, L.; Gai, Y.; Jiang, X.; Janz, D.; Polle, A.; et al. A Transcriptomic Network Underlies Microstructural and Physiological Responses to Cadmium in Populus × canescens. Plant Physiol. 2013, 162, 424–439. [Google Scholar] [CrossRef]
- Marmiroli, M.; Pietrini, F.; Maestri, E.; Zacchini, M.; Marmiroli, N.; Massacci, A. Growth, Physiological and Molecular Traits in Salicaceae Trees Investigated for Phytoremediation of Heavy Metals and Organics. Tree Physiol. 2011, 31, 1319–1334. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gao, S.; Zhu, P.; Liu, Y.; Hu, T.; Zhang, J. Comparative Study of Metal Resistance and Accumulation of Lead and Zinc in Two Poplars. Physiol. Plant. 2014, 151, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Utmazian, M.N.; Wieshammer, G.; Vega, R.; Wenzel, W.W. Hydroponic Screening for Metal Resistance and Accumulation of Cadmium and Zinc in Twenty Clones of Willows and Poplars. Environ. Pollut. 2007, 148, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.J. Nitrogen in the Environment. Science 2019, 363, 578–580. [Google Scholar] [CrossRef]
- Lu, X.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Mo, J. Nitrogen Deposition Contributes to Soil Acidification in Tropical Ecosystems. Glob. Chang. Biol. 2014, 20, 3790–3801. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Zhang, B.; Wu, D.; Shi, Y.; Zhang, W.; Ye, Q.; Yan, J.; Fu, J.; Fang, C.; et al. Canopy and Understory Nitrogen Addition Have Different Effects on Fine Root Dynamics in a Temperate Forest: Implications for Soil Carbon Storage. New Phytol. 2021, 231, 1377–1386. [Google Scholar] [CrossRef]
- Chen, L.; Han, Y.; Jiang, H.; Korpelainen, H.; Li, C. Nitrogen Nutrient Status Induces Sexual Differences in Responses to Cadmium in Populus yunnanensis. J. Exp. Bot. 2011, 62, 5037–5050. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, J.; Tao, L.; Cao, Z.; Tang, W.; Zhang, J.; Yu, X.; Fu, G.; Zhang, X.; Lu, Y. Regulatory Mechanisms of Nitrogen (N) on Cadmium (Cd) Uptake and Accumulation in Plants: A Review. Sci. Total Environ. 2020, 708, 135186. [Google Scholar] [CrossRef]
- Yi, L.; Wu, M.; Yu, F.; Song, Q.; Zhao, Z.; Liao, L.; Tong, J. Enhanced Cadmium Phytoremediation Capacity of Poplar Is Associated with Increased Biomass and Cd Accumulation under Nitrogen Deposition Conditions. Ecotoxicol. Environ. Saf. 2022, 246, 114154. [Google Scholar] [CrossRef]
- Sun, J.; Jiao, W.; Wang, Q.; Wang, T.; Yang, H.; Jin, J.; Feng, H.; Guo, J.; Feng, L.; Xu, X.; et al. Potential Habitat and Productivity Loss of Populus deltoides Industrial Forest Plantations Due to Global Warming. For. Ecol. Manag. 2021, 496, 119474. [Google Scholar] [CrossRef]
- Radojčić Redovniković, I.; De Marco, A.; Proietti, C.; Hanousek, K.; Sedak, M.; Bilandžić, N.; Jakovljević, T. Poplar Response to Cadmium and Lead Soil Contamination. Ecotoxicol. Environ. Saf. 2017, 144, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Chen, Y.; Ding, S.; Li, Z.; Shi, W.-G.; Zhang, Y.; Luo, Z.-B. Sulfur Nutrition Stimulates Lead Accumulation and Alleviates Its Toxicity in Populus deltoides. Tree Physiol. 2018, 38, 1724–1741. [Google Scholar] [CrossRef]
- Wang, Q.; Xiong, D.; Zhao, P.; Yu, X.; Tu, B.; Wang, G. Effect of Applying an Arsenic-resistant and Plant Growth-Promoting Rhizobacterium to Enhance Soil Arsenic Phytoremediation by Populus deltoides LH05-17. J. Appl. Microbiol. 2011, 111, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, N.; Zorić, L.; Cvetković, I.; Pajević, S.; Borišev, M.; Orlović, S.; Pilipović, A. Assessment of Cadmium Tolerance and Phytoextraction Ability in Young Populus Deltoides L. and Populus × euramericana Plants through Morpho-Anatomical and Physiological Responses to Growth in Cadmium Enriched Soil. Iforest-Biogeosci. For. 2017, 10, 635. [Google Scholar] [CrossRef]
- Song, L.; Liu, X.; Skiba, U.; Zhu, B.; Zhang, X.; Liu, M.; Twigg, M.; Shen, J.; Dore, A.; Reis, S.; et al. Ambient Concentrations and Deposition Rates of Selected Reactive Nitrogen Species and Their Contribution to PM2.5 Aerosols at Three Locations with Contrasting Land Use in Southwest China. Environ. Pollut. 2018, 233, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Pregitzer, K.S. Tree Root Architecture: Form and Function. New Phytol. 2008, 180, 562–564. [Google Scholar] [CrossRef]
- Komárková, M.; Chromý, J.; Pokorná, E.; Soudek, P.; Máchová, P. Physiological and Transcriptomic Response of Grey Poplar (Populus × canescens Aiton Sm.) to Cadmium Stress. Plants 2020, 9, 1485. [Google Scholar] [CrossRef]
- Romè, C.; Romeo, S.; Francini, A.; Andreucci, A.; Sebastiani, L. Leaves Position in Populus alba Villafranca Clone Reveals a Strategy towards Cadmium Uptake Response. Plant Growth Regul. 2016, 79, 355–366. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, K.; Tian, X.; Korpelainen, H.; Li, C. Effect of Mn Toxicity on Morphological and Physiological Changes in Two Populus cathayana Populations Originating from Different Habitats. Trees 2007, 21, 569–580. [Google Scholar] [CrossRef]
- Li, X.; Cui, X.; Zhang, X.; Liu, W.; Cui, Z. Combined Toxicity and Detoxification of Lead, Cadmium and Arsenic in Solanum nigrum L. J. Hazard. Mater. 2020, 389, 121874. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Hou, J.; Chen, C.; Xiong, J.; Wei, R.; Wang, M.; Tan, W. Quantitative Analysis of Dose Interval Effect of Pb-Cd Interaction on Oryza sativa L. Root. Ecotoxicol. Environ. Saf. 2023, 252, 114622. [Google Scholar] [CrossRef] [PubMed]
- Yotsova, E.; Dobrikova, A.; Stefanov, M.; Misheva, S.; Bardáčová, M.; Matušíková, I.; Žideková, L.; Blehová, A.; Apostolova, E. Effects of Cadmium on Two Wheat Cultivars Depending on Different Nitrogen Supply. Plant Physiol. Biochem. 2020, 155, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, D.; Zhang, Y.; Han, J.; Zhang, W.; Yang, Q.; Gessler, A.; Li, M.-H.; Xu, M.; Guan, X.; et al. Investment of Needle Nitrogen to Photosynthesis Controls the Nonlinear Productivity Response of Young Chinese Fir Trees to Nitrogen Deposition. Sci. Total Environ. 2022, 840, 156537. [Google Scholar] [CrossRef]
- Yang, L.P.; Zhu, J.; Wang, P.; Zeng, J.; Tan, R.; Yang, Y.Z.; Liu, Z.M. Effect of Cd on Growth, Physiological Response, Cd Subcellular Distribution and Chemical Forms of Koelreuteria paniculata. Ecotoxicol. Environ. Saf. 2018, 160, 10–18. [Google Scholar] [CrossRef]
- Zornoza, P.; Sánchez-Pardo, B.; Carpena, R.O. Interaction and Accumulation of Manganese and Cadmium in the Manganese Accumulator Lupinus albus. J. Plant Physiol. 2010, 167, 1027–1032. [Google Scholar] [CrossRef]
- Qin, S.; Liu, H.; Nie, Z.; Rengel, Z.; Gao, W.; Li, C.; Zhao, P. Toxicity of Cadmium and Its Competition with Mineral Nutrients for Uptake by Plants: A Review. Pedosphere 2020, 30, 168–180. [Google Scholar] [CrossRef]
- Song, J.; Finnegan, P.M.; Liu, W.; Li, X.; Yong, J.W.H.; Xu, J.; Zhang, Q.; Wen, Y.; Qin, K.; Guo, J.; et al. Mechanisms Underlying Enhanced Cd Translocation and Tolerance in Roots of Populus euramericana in Response to Nitrogen Fertilization. Plant Sci. 2019, 287, 110206. [Google Scholar] [CrossRef]
- Li, Y.; Liu, K.; Zhu, J.; Jiang, Y.; Huang, Y.; Zhou, Z.; Chen, C.; Yu, F. Manganese Accumulation and Plant Physiology Behavior of Camellia Oleifera in Response to Different Levels of Nitrogen Fertilization. Ecotoxicol. Environ. Saf. 2019, 184, 109603. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular Mechanisms for Heavy Metal Detoxification and Tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Li, J.; Jia, Y.; Dong, R.; Huang, R.; Liu, P.; Li, X.; Wang, Z.; Liu, G.; Chen, Z. Advances in the Mechanisms of Plant Tolerance to Manganese Toxicity. Int. J. Mol. Sci. 2019, 20, 5096. [Google Scholar] [CrossRef]
- Yan, L.; Riaz, M.; Liu, J.; Yu, M.; Cuncang, J. The Aluminum Tolerance and Detoxification Mechanisms in Plants; Recent Advances and Prospects. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1491–1527. [Google Scholar] [CrossRef]
- Guo, B.-Y.; Peng, Z.-L.; Han, F.; Shan, X.-Q.; Lin, J.-M. Study of Low-Molecular Weight Organic Acids in Maize Roots under the Stress of Cadmium Using Capillary Zone Electrophoresis. J. Sep. Sci. 2007, 30, 2742–2747. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Weiss, D.J.; Weng, B.; Liu, J.; Lu, H.; Yan, C. The Short-Term Effect of Cadmium on Low Molecular Weight Organic Acid and Amino Acid Exudation from Mangrove (Kandelia obovata (S., L.) Yong) Roots. Environ. Sci. Pollut. Res. 2013, 20, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhao, X.; Fang, J.; Xiao, Y. Physiological Responses and Metal Uptake of Miscanthus under Cadmium/Arsenic Stress. Environ. Sci. Pollut. Res. 2018, 25, 28275–28284. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Chen, J.; Zhang, S.; Xu, J.; Han, X.; Feng, Y.; Chen, Y.; Zhang, X.; Dong, G.; et al. Xylem Development, Cadmium Bioconcentration, and Antioxidant Defense in Populus × euramericana Stems under Combined Conditions of Nitrogen and Cadmium. Environ. Exp. Bot. 2019, 164, 1–9. [Google Scholar] [CrossRef]
- Guo, Y.-Y.; Wang, J.-J.; Kong, D.-L.; Wang, W.; Guo, D.-L.; Wang, Y.-B.; Xie, Q.-L.; Liu, Y.-S.; Zeng, H. Fine Root Branch Orders Contribute Differentially to Uptake, Allocation, and Return of Potentially Toxic Metals. Environ. Sci. Technol. 2013, 47, 11465–11472. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Yamaji, N.; Yokosho, K.; Ma, J.F. Nramp5 Is a Major Transporter Responsible for Manganese and Cadmium Uptake in Rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef] [PubMed]
- Socha, A.; Guerinot, M.L. Mn-Euvering Manganese: The Role of Transporter Gene Family Members in Manganese Uptake and Mobilization in Plants. Front. Plant Sci. 2014, 5, 106. [Google Scholar] [CrossRef]
- Ahmad, A.; Hadi, F.; Ali, N.; Jan, A.U. Enhanced Phytoremediation of Cadmium Polluted Water through Two Aquatic Plants Veronica anagallis-Aquatica and Epilobium laxum. Environ. Sci. Pollut. Res. 2016, 23, 17715–17729. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, C.; Hu, P.; Luo, Y.; Wu, L.; Sale, P.; Tang, C. Influence of Nitrogen Form on the Phytoextraction of Cadmium by a Newly Discovered Hyperaccumulator Carpobrotus rossii. Environ. Sci. Pollut. Res. 2016, 23, 1246–1253. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Basal Diameter (mm) | Plant Height (cm) | Leaf Area (dm2) | Specific Leaf Area (cm2 g−1) | Leaf Biomass (g) | Stem Biomass (g) | Root Biomass (g) | Total Biomass (g) | Root–Shoot Ratio |
|---|---|---|---|---|---|---|---|---|---|
| CK | 1.08 ± 0.08 abc | 169.53 ± 8.82 a | 46.53 ± 1.22 a | 216.64 ± 1.72 ab | 20.00 ± 2.16 abc | 35.02 ± 2.7 ab | 5.80 ± 0.01 abc | 60.82 ± 4.88 ab | 0.10 ± 0.02 b |
| LN | 1.20 ± 0.08 ab | 177.90 ± 5.50 a | 40.04 ± 4.57 ab | 227.78 ± 13.60 a | 21.88 ± 0.74 ab | 33.93 ± 1.40 ab | 5.30 ± 0.79 ab | 61.11 ± 0.13 ab | 0.10 ± 0.02 b |
| HN | 1.11 ± 0.05 abc | 175.33 ± 9.50 a | 39.42 ± 5.76 ab | 220.45 ± 6.26 ab | 18.55 ± 0.96 abcde | 33.02 ± 0.98 abc | 4.84 ± 0.81 abc | 56.41 ± 0.82 abc | 0.11 ± 0.04 b |
| Cd | 1.07 ± 0.06 abcd | 171.00 ± 4.60 a | 37.57 ± 2.32 abc | 188.33 ± 8.77 b | 17.93 ± 3.06 abcd | 27.80 ± 3.62 bc | 4.67 ± 1.00 abc | 50.40 ± 5.45 bc | 0.09 ± 0.01 b |
| Cd + LN | 1.26 ± 0.06 a | 175.77 ± 4.35 a | 47.59 ± 5.76 a | 203.31 ± 10.94 ab | 23.36 ± 1.77 a | 37.50 ± 2.75 a | 5.92 ± 0.27 a | 66.79 ± 4.75 a | 0.10 ± 0.00 b |
| Cd + HN | 1.14 ± 0.09 abc | 163.50 ± 4.92 a | 36.96 ± 3.63 abc | 211.99 ± 9.08 ab | 17.42 ± 0.97 abcde | 31.97 ± 3.05 abc | 5.86 ± 0.01 ab | 55.24 ± 4.03 abc | 0.12 ± 0.01 ab |
| Mn | 0.85 ± 0.07 d | 99.83 ± 10.77 c | 24.39 ± 4.74 bc | 226.02 ± 11.37 a | 10.75 ± 1.56 e | 11.49 ± 2.67 f | 3.72 ± 0.87 bc | 25.95 ± 5.09 e | 0.17 ± 0.01 a |
| Mn + LN | 0.94 ± 0.12 cd | 121.27 ± 11.04 bc | 26.05 ± 1.28 bc | 201.51 ± 0.97 ab | 12.93 ± 0.57 cde | 24.26 ± 0.57 cde | 5.42 ± 0.40 abc | 42.61 ± 0.39 cd | 0.15 ± 0.01 ab |
| Mn + HN | 0.97 ± 0.05 bcd | 119.17 ± 6.93 bc | 23.38 ± 2.88 c | 211.96 ± 14.14 ab | 11.12 ± 2.08 e | 19.34 ± 2.68 def | 3.93 ± 0.29 bc | 34.39 ± 4.75 de | 0.13 ± 0.01 ab |
| Cd + Mn | 1.02 ± 0.10 bcd | 163.60 ± 6.22 a | 26.49 ± 4.95 bc | 225.08 ± 7.57 a | 11.82 ± 2.57 de | 18.30 ± 0.59 ef | 3.50 ± 0.51 c | 33.62 ± 2.71 de | 0.12 ± 0.01 ab |
| Cd + Mn + LN | 0.95 ± 0.10 cd | 136.60 ± 13.09 b | 36.88 ± 0.20 abc | 213.62 ± 21.08 ab | 17.35 ± 1.62 abcde | 31.02 ± 0.24 abc | 5.56 ± 0.54 abc | 53.93 ± 0.84 abc | 0.12 ± 0.01 ab |
| Cd + Mn + HN | 0.97 ± 0.05 cd | 135.83 ± 4.25 b | 34.40 ± 9.64 abc | 223.82 ± 2.00 ab | 15.35 ± 4.17 bcde | 27.33 ± 2.01 bcd | 4.07 ± 0.86 abc | 46.75 ± 7.04 bcd | 0.09 ± 0.01 b |
| Acid | Lactic Acid | Succinic Acid | Fumaric Acid | Malic Acid | Citric Acid | Malonic Acid | Glucuronic Acid | Pantothenic Acid | Niacin | L-Pyroglutamic Acid | 3-Hydroxy-3-Methylglutamic Acid |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CK | 72.90 + 0.52 d | 8.69 + 0.13 e | 11.31 + 0.19 d | 166.34 + 3.26 de | 220.63 + 1.00 c | 0.93 + 0.07 d | 1.42 + 0.21 g | 98.03 + 9.49 fg | 0.24 + 0.01 c | 7.49 + 0.05 e | 0.70 + 0.01 de |
| LN | 85.47 + 6.53 d | 10.33 + 0.96 cde | 13.80 + 1.06 c | 221.64 + 9.56 c | 191.73 + 14.84 d | 1.10 + 0.07 cde | 1.58 + 0.11 g | 121.33 + 7.1 def | 0.30 + 0.05 bc | 8.00 + 0.56 e | 1.01 + 0.12 bc |
| HN | 119.30 + 3.05 c | 12.07 + 1.26 bcd | 15.88 + 0.81 b | 215.46 + 15.00 c | 184.73 + 12.13 d | 1.14 + 0.08 bcd | 1.94 + 0.08 efg | 300.16 + 11.74 b | 0.36 + 0.05 abc | 17.41 + 1.34 a | 1.19 + 0.06 b |
| Cd | 158.87 + 3.43 a | 12.23 + 0.32 bcd | 16.91 + 0.26 b | 287.78 + 3.19 b | 322.98 + 2.5 b | 1.15 + 0.04 bcd | 2.43 + 0.22 cde | 142.39 + 3.39 de | 0.27 + 0.05 bc | 16.55 + 0.98 ab | 1.55 + 0.06 a |
| Cd + LN | 76.26 + 3.19 d | 12.52 + 0.71 ab | 20.53 + 0.99 a | 373.04 + 16.14 a | 375.22 + 10.09 a | 1.31 + 0.15 abc | 2.58 + 0.18 bcd | 254.34 + 32.05 c | 0.28 + 0.06 bc | 15.44 + 1.28 abc | 1.54 + 0.08 a |
| Cd + HN | 79.95 + 4.00 d | 14.33 + 0.27 a | 19.85 + 0.61 a | 275.02 + 11.45 b | 230.79 + 9.48 c | 1.36 + 0.09 abc | 2.58 + 0.25 bcd | 454.19 + 8.98 a | 0.25 + 0.02 bc | 15.73 + 1.19 abc | 1.60 + 0.05 a |
| Mn | 114.12 + 8.42 c | 10.17 + 0.08 de | 5.45 + 0.19 ef | 81.18 + 2.86 fg | 77.18 + 1.14 ef | 1.46 + 0.03 a | 3.07 + 0.13 b | 140.28 + 5.41 de | 0.34 + 0.07 abc | 13.35 + 0.47 cd | 0.75 + 0.01 d |
| Mn + LN | 74.06 + 6.53 d | 10.44 + 1.01 cde | 5.10 + 0.59 f | 102.91 + 4.58 f | 81.26 + 6.61 ef | 1.41 + 0.07 ab | 2.82 + 0.18 bc | 160.89 + 3.12 d | 0.34 + 0.08 abc | 14.66 + 1.05 bc | 0.73 + 0.10 d |
| Mn + HN | 87.15 + 2.49 d | 5.36 + 0.13 f | 4.99 + 0.13 f | 60.82 + 0.20 g | 43.68 + 1.25 g | 1.16 + 0.05 bcd | 1.75 + 0.15 fg | 113.91 + 2.52 ef | 0.41 + 0.05 ab | 6.36 + 0.04 e | 0.40 + 0.01 f |
| Cd + Mn | 166.22 + 6.45 a | 9.26 + 0.10 e | 11.55 + 0.28 d | 154.05 + 2.66 e | 87.92 + 0.17 e | 1.44 + 0.10 a | 2.30 + 0.13 cde | 235.34 + 3.17 c | 0.48 + 0.07 a | 11.36 + 0.17 d | 1.07 + 0.03 b |
| Cd + Mn + LN | 138.49 + 6.24 b | 9.02 + 0.37 e | 11.48 + 1.06 d | 184.11 + 10.19 d | 83.5 + 7.22 ef | 1.55 + 0.13 a | 4.15 + 0.28 a | 317.31 + 22.39 b | 0.47 + 0.04 a | 11.55 + 1.02 d | 0.84 + 0.09 cd |
| Cd + Mn + HN | 155.88 + 7.74 a | 6.16 + 0.25 f | 7.15 + 0.14 e | 86.41 + 1.48 fg | 60.07 + 2.82 fg | 0.93 + 0.10 d | 2.18 + 0.05 def | 62.60 + 1.48 g | 0.40 + 0.04 abc | 11.85 + 0.28 d | 0.51 + 0.05 ef |
| Treatment | BCFCd | TFCd | BCFMn | TFMn |
|---|---|---|---|---|
| CK | / | / | 0.48 ± 0.07 h | 0.51 ± 0.01 cd |
| LN | / | / | 0.52 ± 0.01 h | 0.51 ± 0.01 cd |
| HN | / | / | 0.54 ± 0.01 h | 0.45 ± 0.06 d |
| Cd | 14.50 ± 0.14 d | 0.21 ± 0.02 c | 0.63 ± 0.02 g | 0.36 ± 0.03 e |
| Cd + LN | 12.26 ± 0.04 e | 0.16 ± 0.02 d | 0.83 ± 0.02 e | 0.36 ± 0.03 e |
| Cd + HN | 15.29 ± 0.04 c | 0.13 ± 0.01 d | 0.68 ± 0.02 f | 0.33 ± 0.01 e |
| Mn | / | / | 1.42 ± 0.03 a | 0.52 ± 0.02 c |
| Mn + LN | / | / | 0.93 ± 0.02 d | 0.89 ± 0.02 a |
| Mn + HN | / | / | 1.18 ± 0.06 b | 0.75 ± 0.01 cb |
| Cd + Mn | 19.34 ± 0.05 a | 0.25 ± 0.01 b | 1.10 ± 0.03 c | 0.37 ± 0.02 e |
| Cd + Mn + LN | 18.94 ± 0.05 b | 0.22 ± 0.02 bc | 0.82 ± 0.02 e | 0.55 ± 0.02 c |
| Cd + Mn + HN | 14.63 ± 0.09 d | 0.31 ± 0.04 a | 1.14 ± 0.04 bc | 0.56 ± 0.02 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Hao, L.; Mi, J.; Yin, M.; Xing, C.; Wan, X.; Zhang, F.; Yang, H.; He, F.; Hu, H.; et al. Effects of Nitrogen Addition on the Growth and Physiology of Populus deltoides Seedlings under Cd and Mn Pollution. Forests 2023, 14, 1707. https://doi.org/10.3390/f14091707
Wang X, Hao L, Mi J, Yin M, Xing C, Wan X, Zhang F, Yang H, He F, Hu H, et al. Effects of Nitrogen Addition on the Growth and Physiology of Populus deltoides Seedlings under Cd and Mn Pollution. Forests. 2023; 14(9):1707. https://doi.org/10.3390/f14091707
Chicago/Turabian StyleWang, Xue, Linting Hao, Jiaxuan Mi, Man Yin, Cailan Xing, Xueqin Wan, Fan Zhang, Hanbo Yang, Fang He, Hongling Hu, and et al. 2023. "Effects of Nitrogen Addition on the Growth and Physiology of Populus deltoides Seedlings under Cd and Mn Pollution" Forests 14, no. 9: 1707. https://doi.org/10.3390/f14091707






