Hydraulic Traits in Populus simonii Carr. at Stands of Categorized Ages in a Semi-Arid Area of Western Liaoning, Northeast China
Abstract
:1. Introduction
2. Materials and Methods
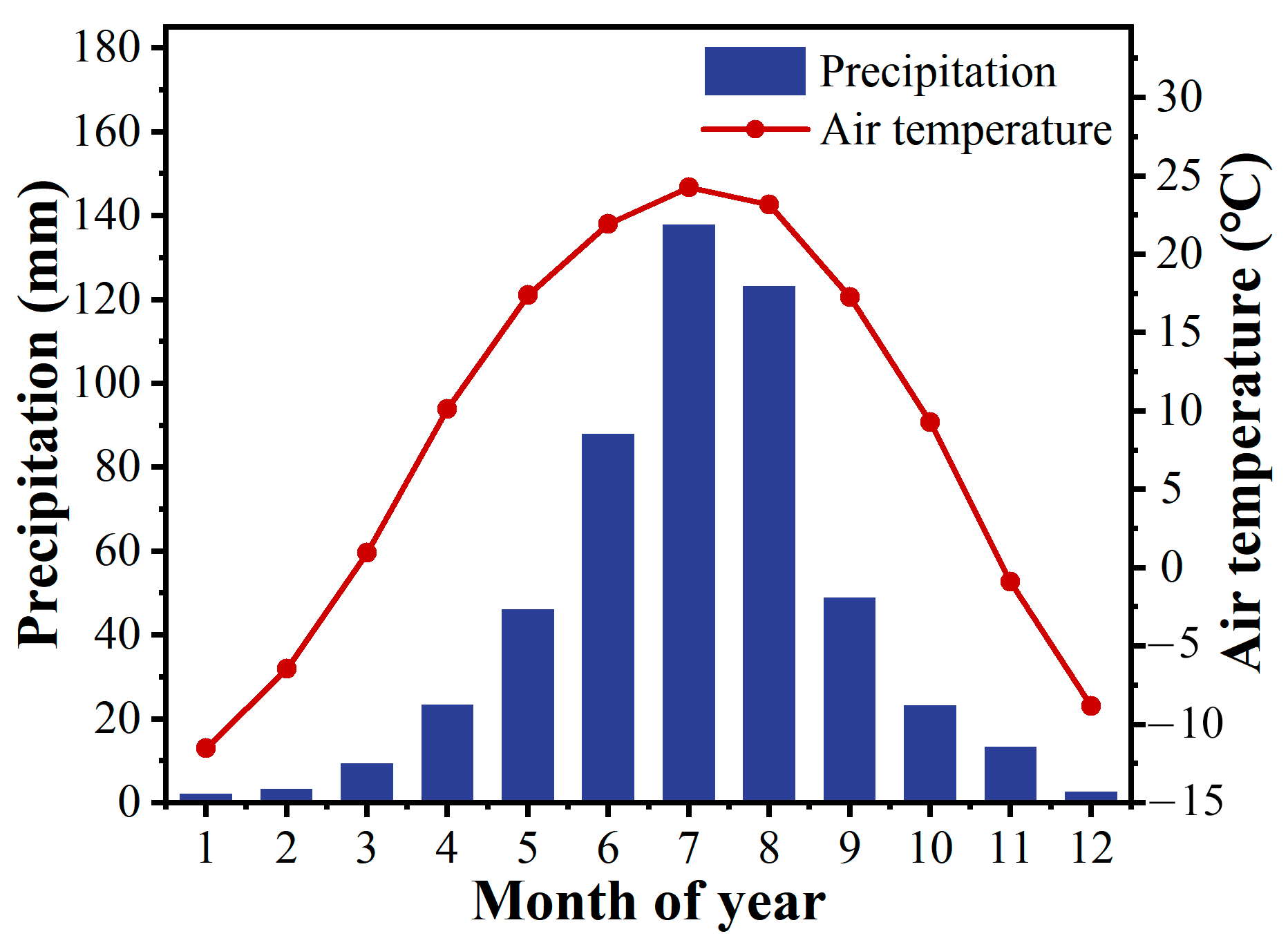
2.1. Site Description
2.2. Experimental Materials
2.3. Leaf Pressure-Volume Curves
2.4. Xylem Embolism Vulnerability Curves
2.5. Hydraulic Structure and Wood Density
2.6. Data Analysis
- (1)
- Determine the positive and negative relationship between each index and environmental adaptability. The index with a positive relationship retained the original data, and the index with a negative relationship divided the original data by 1 as the data of principal component analysis; that is, homogenization. Thus, the consistency of the evaluation indicators (the larger the value, the better) was ensured.
- (2)
- The homogenized data were standardized in SPSS.26 to eliminate the influence of indicators of different properties and dimensions on the results of the comprehensive evaluation process.
- (3)
- Principal component analysis was performed on the standardized data in SPSS.26. Multiple principal components (PC1, PC2, etc.) were selected according to the principle of cumulative contribution rate greater than or equal to 80% and eigenvalue greater than 1. The principal component formula (Equation (5)) can be obtained by multiplying the standardized data with each factor score:where i represented the number of the selected principal component, Ai, Bi, Ci, Di, Ei, Fi, and Gi represented the coefficients of the factor scores in the principal component i, and X1, X2, X3, X4, X5, X6, X7 represented the standardized data values of each index.
- (4)
- The comprehensive scoring model (Equation (6)) was constructed as follows:where a, b, and z represent the proportion of the contribution rate of each principal component in the cumulative contribution rate. The higher the composite score value, the better the environmental adaptability.
3. Results
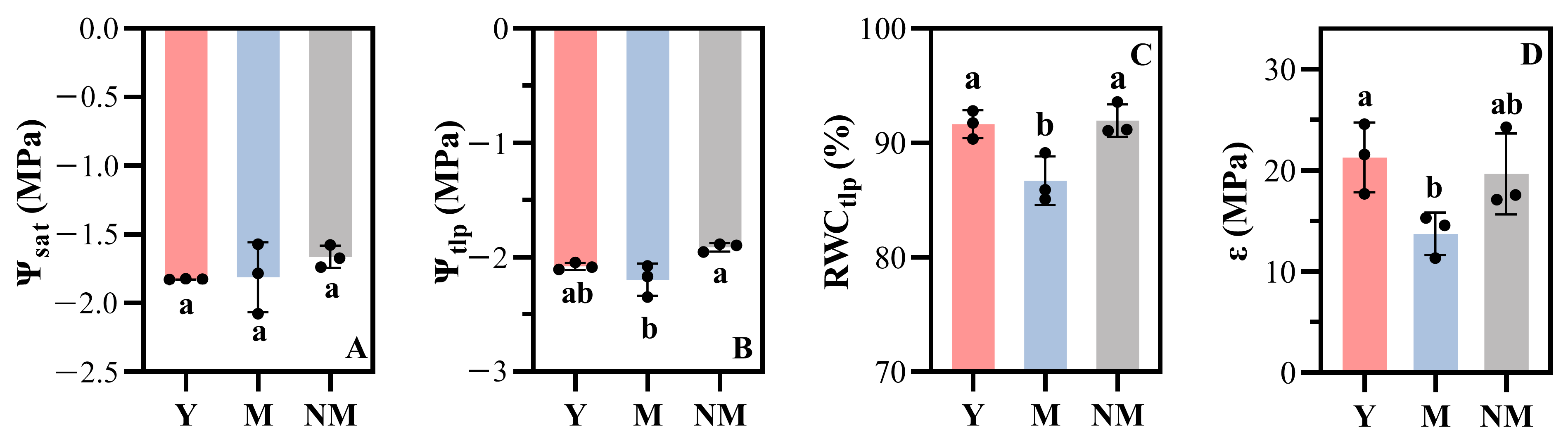
3.1. Pressure-Volume Curves
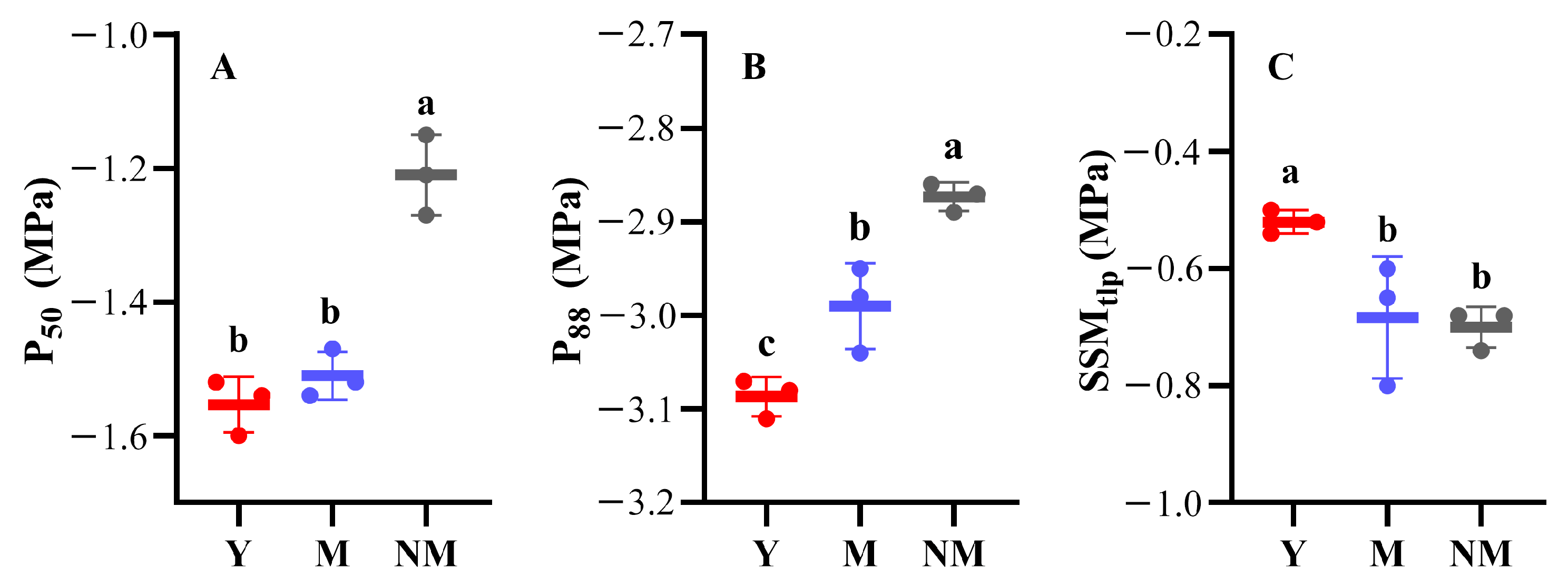
3.2. Xxylem Embolism Vulnerability Curves
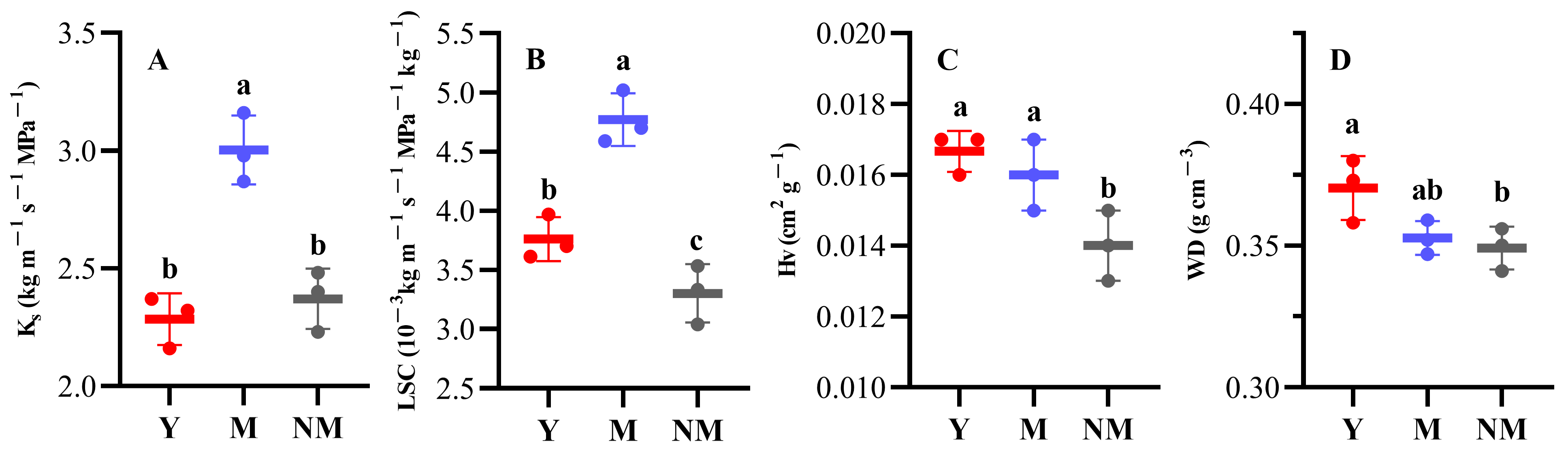
3.3. Hydraulic Structure and Wood Density
3.4. Tradeoff of Drought Tolerance-Safety-Efficiency
3.5. Comprehensive Evaluation of Environmental Adaptability
4. Discussion
4.1. Drought Tolerance and Xylem Embolism Resistance
4.2. Tradeoff of Drought Tolerance-Safety-Efficiency
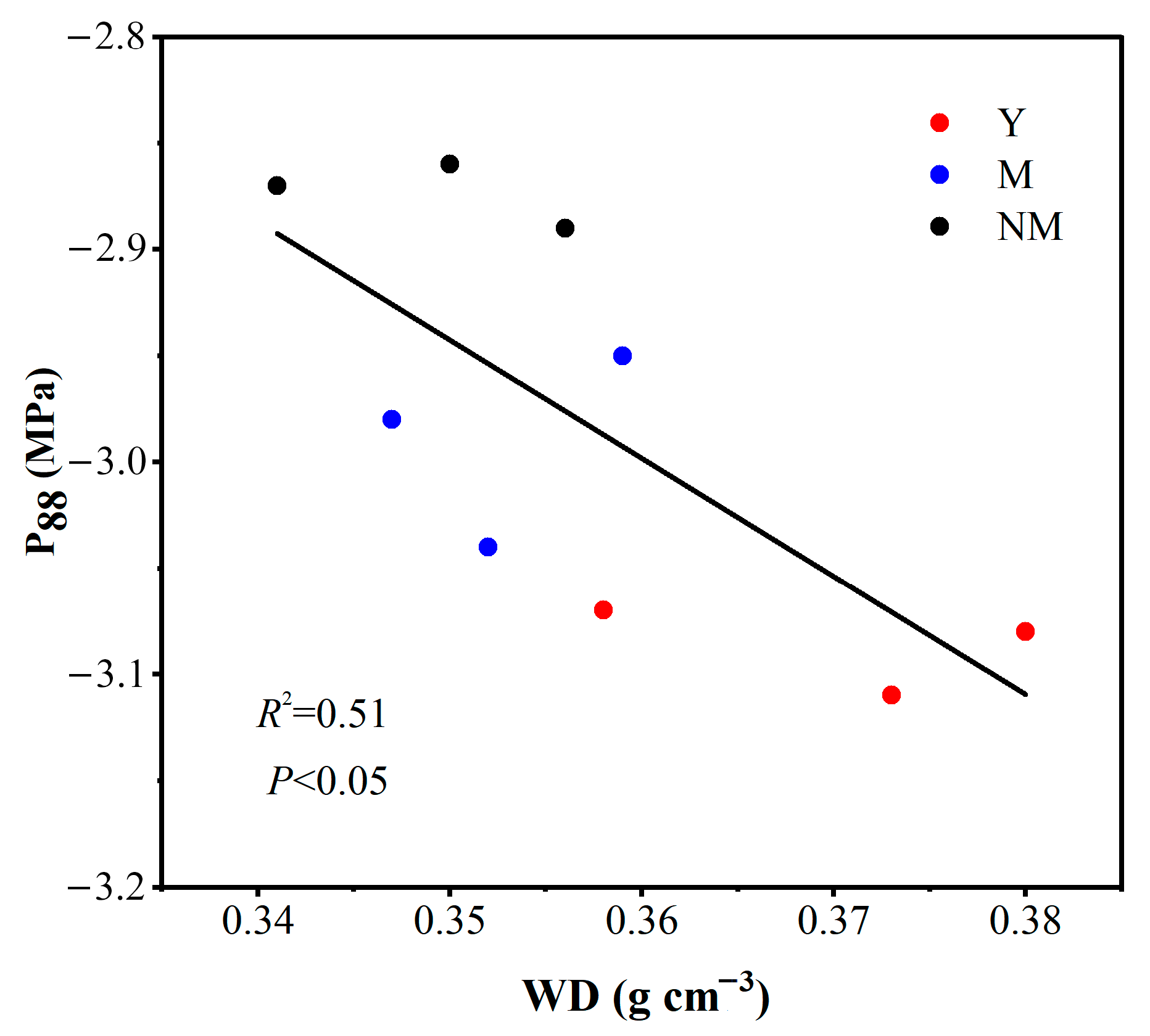
4.3. Analysis of WD as a Proxy Indicator of Hydraulic Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiao, Y.; Jiang, Y.; Zhang, C. Contribution of karst ecological restoration engineering to Vegetation Greening in Southwest China during recent decade. Ecol. Indic. 2021, 121, 107081. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Dai, A.G.; Schrier, G.V.D.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Anderegg, L.D.L.; Kerr, K.L.; Trugman, A.T. Widespread drought-induced tree mortality at dry range edges indicates that climate stress exceeds species’ compensating mechanisms. Glob. Chang. Biol. 2019, 25, 3793–3802. [Google Scholar] [CrossRef]
- Bauman, D.; Fortunel, C.; Delhaye, G.; Malhi, Y.; Cernusak, L.A.; Bentley, L.P.; Rifai, S.W.; Aguirre-Gutierrez, J.; Menor, I.O.; Phillips, O.L.; et al. Tropical tree mortality has increased with rising atmospheric water stress. Nature 2022, 608, 528–533. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Song, H.Q.; Chen, Y.J.; Fu, P.L.; Zhang, J.L.; Cao, K.F.; Zhu, S.D. Hydraulic determinants of drought-induced tree mortality and changes in tree abundance between two tropical forests with different water availability. Agric. For. Meteorol. 2023, 331, 109329. [Google Scholar] [CrossRef]
- Poplars and Other Fast-Growing Trees—Renewable Resources for Future Green Economies. Available online: http://www.fao.org/forestry/ipc2016/en (accessed on 15 April 2023).
- Kumar, R.; Bhatnagar, P.R.; Kakade, V.; Dobhal, S. Tree Plantation and soil water conservation enhances climate resilience and carbon sequestration of agro ecosystem in semi-arid degraded ravine lands. Agric. For. Meteorol. 2020, 282, 107857. [Google Scholar] [CrossRef]
- National Forestry and Grassland Administration. China Forest Resources Report; China Standard Press: Beijing, China, 2019. [Google Scholar]
- Xi, B.Y.; Clothier, B.; Coleman, M.; Duan, J.; Hu, W.; Li, D.D.; Di, N.; Liu, Y.; Fu, J.Y.; Li, J.S.; et al. Irrigation management in poplar (Populus spp.) plantations: A review. For. Ecol. Manag. 2021, 494, 119330. [Google Scholar] [CrossRef]
- Sun, S.J.; He, C.X.; Qiu, L.F.; Li, C.Y.; Zhang, J.S.; Meng, P. Stable isotope analysis reveals prolonged drought stress in Poplar plantation mortality of the three-north shelter forest in northern China. Agric. For. Meteorol. 2018, 252, 39–48. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Xu, Z.Q.; Ma, C.M.; Sun, S.J.; Yan, T.F. The factors influencing the poplar shelterbelt degradation in the Bashang Plateau of northwest Hebei Province. For. Resour. Manag. 2018, 9–15. [Google Scholar] [CrossRef]
- Ji, Y.H.; Zhou, G.S.; Li, Z.S.; Wang, S.D.; Zhou, H.L.; Song, X.Y. Triggers of widespread dieback and mortality of poplar (Populus spp.) plantations across northern China. J. Arid. Environ. 2020, 174, 104076. [Google Scholar] [CrossRef]
- Fang, L.D.; Ning, Q.R.; Guo, J.J.; Gong, X.W.; Zhu, J.J.; Hao, G.Y. Hydraulic limitation underlies the dieback of Populus pseudo-simonii trees in water-limited areas of northern China. For. Ecol. Manag. 2021, 483, 118764. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.Y.; Ye, Q.; Liu, H.; Brodribb, T.J. Wood density predicts mortality threshold for diverse trees. New Phytol. 2021, 229, 3053–3057. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; McAdam, S.A.; Carins, M.M.R. Xylem and stomata, coordinated through time and space. Plant Cell Environ. 2017, 40, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Xi, B.Y.; Wang, Y.; Feng, J.C.; Li, X.M.; Tissue, D.T. Lack of phenotypic plasticity in leaf hydraulics for 10 woody species common to urban forests of North China. Tree Physiol. 2022, 42, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Rowland, L.; da Costa, A.C.; Galbraith, D.R.; Oliveira, R.S.; Binks, O.J.; Oliveira, A.A.R.; Pullen, A.M.; Doughty, C.E.; Metcalfe, D.B.; Vasconcelos, S.S.; et al. Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature 2015, 528, 119–122. [Google Scholar] [CrossRef]
- Liu, Z.H.; Jia, G.D.; Yu, X.X.; Lu, W.W.; Sun, L.B.; Wang, Y.S.; Baheti, Z. Morphological trait as a determining factor for Populus simonii Carr. to survive from drought in semi-arid region. Agric. Water Manag. 2021, 253, 106943. [Google Scholar] [CrossRef]
- Giardina, F.; Konings, A.G.; Kennedy, D.; Alemohammad, S.H.; Oliveira, R.S.; Uriarte, M.; Gentine, P. Tall Amazonian forests are less sensitive to precipitation variability. Nat. Geosci. 2018, 11, 405–409. [Google Scholar] [CrossRef]
- Klockow, P.A.; Edgar, C.B.; Moore, G.W.; Vogel, J.G. Southern pines are resistant to mortality from an exceptional drought in east Texas. Front. For. Glob. Chang. 2020, 3, 23. [Google Scholar] [CrossRef]
- Bennett, A.C.; McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.J. Larger trees suffer most during drought in forests worldwide. Nat. Plants 2015, 1, 15139. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Bose, A.K.; Andivia, E.; Candel-Perez, D.; Plaza-Alvarez, P.A.; Linares, J.C. Assessing Tree Drought Resistance and Climate-Growth Relationships under Different Tree Age Classes in a Pinus nigra Arn. ssp. salzmannii Forest. Forests 2021, 12, 1161. [Google Scholar] [CrossRef]
- Ma, T.X.; Liang, Y.; Li, Z.Y.; Liu, Z.H.; Liu, B.; Wu, M.M.; Lau, M.K.; Fang, Y.T. Age-related patterns and climatic driving factors of drought-induced forest mortality in Northeast China. Agric. For. Meteorol. 2023, 332, 109360. [Google Scholar] [CrossRef]
- Akram, M.A.; Zhang, Y.; Wang, X.; Shrestha, N.; Malik, K.; Khan, I.; Ma, W.; Sun, Y.; Li, F.; Ran, J.; et al. Phylogenetic independence in the variations in leaf functional traits among different plant life forms in an arid environment. J. Plant Physiol. 2022, 272, 153671. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.A.; Wang, X.; Shrestha, N.; Zhang, Y.; Sun, Y.; Yao, S.; Li, J.; Hou, Q.; Hu, W.; Ran, J.; et al. Variations and driving factors of leaf functional traits in the dominant desert plant species along an environmental gradient in the drylands of China. Sci. Total Environ. 2023, 897, 165394. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Powell, T.L.; Wheeler, J.K.; de Oliveira, A.A.R.; da Costa, A.C.L.; Saleska, S.R.; Meir, P.; Moorcroft, P.R. Differences in xylem and leaf hydraulic traits explain differences in drought tolerance among mature Amazon rainforest trees. Glob. Chang. Biol. 2017, 23, 4280–4293. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Powers, J.; Cochard, H.; Choat, B. Hanging by a thread? Forests and drought. Science 2020, 368, 261–266. [Google Scholar] [CrossRef]
- Meir, P.; Mencuccini, M.; Dewar, R.C. Drought-related tree mortality: Addressing the gaps in understanding and prediction. New Phytol. 2015, 207, 28–33. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Klein, T.; Bartlett, M.; Sack, L.; Pellegrini, A.F.A.; Choat, B.; Jansen, S. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Natl. Acad. Sci. USA 2016, 113, 5024–5029. [Google Scholar] [CrossRef]
- Venturas, M.D.; MacKinnon, E.D.; Dario, H.L.; Jacobsen, A.L.; Pratt, R.B.; Davis, S.D. Chaparral Shrub Hydraulic Traits, Size, and Life History Types Relate to Species Mortality during California’s Historic Drought of 2014. PLoS ONE 2016, 11, e0159145. [Google Scholar] [CrossRef]
- Ferriz, M.; Martin-Benito, D.; Canellas, I.; Gea-Izquierdo, G. Sensitivity to water stress drives differential decline and mortality dynamics of three co-occurring conifers with different drought tolerance. For. Ecol. Manag. 2021, 486, 118964. [Google Scholar] [CrossRef]
- Zhu, S.D.; Chen, Y.J.; Ye, Q.; He, P.C.; Liu, H.; Li, R.H.; Fu, P.L.; Jiang, G.F.; Cao, K.F. Leaf turgor loss point is correlated with drought tolerance and leaf carbon economics traits. Tree Physiol. 2018, 38, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.B.; Reese Næsborg, R.; Dawson, T.E. Coping with gravity: The foliar water relations of giant sequoia. Tree Physiol. 2017, 37, 1312–1326. [Google Scholar] [CrossRef] [PubMed]
- Pivovaroff, A.L.; Cook, V.M.W.; Santiago, L.S. Stomatal behavior and stem xylem traits are coordinated for woody plant species under exceptional drought conditions. Plant Cell Environ. 2018, 41, 2617–2626. [Google Scholar] [CrossRef] [PubMed]
- Markesteijn, L.; Poorter, L.; Paz, H.; Sack, L.; Bongers, F. Ecological differentiation in xylem cavitation resistance is associated with stem and leaf structural traits. Plant Cell Environ. 2011, 34, 137–148. [Google Scholar] [CrossRef]
- Skelton, R.P.; West, A.G.; Dawson, T.E. Predicting plant vulnerability to drought in biodiverse regions using functional traits. Proc. Natl. Acad. Sci. USA 2015, 112, 5744–5749. [Google Scholar] [CrossRef]
- Chen, Z.C.; Li, S.; Luan, J.W.; Zhang, Y.T.; Zhu, S.D.; Wan, X.C.; Liu, S.R. Prediction of temperate broadleaf tree species mortality in arid limestone habitats with stomatal safety margins. Tree Physiol. 2019, 39, 1428–1437. [Google Scholar] [CrossRef]
- Li, X.; Blackman, C.J.; Choat, B.; Duursma, R.A.; Rymer, P.D.; Medlyn, B.E. Tree hydraulic traits are coordinated and strongly linked to climate-of-origin across a rainfall gradient. Plant Cell Environ. 2018, 41, 646–660. [Google Scholar] [CrossRef]
- Xi, B.Y.; Li, G.D.; Bloomberg, M.; Jia, L.M. The effects of subsurface irrigation at different soil water potential thresholds on the growth and transpiration of Populus tomentosa in the North China Plain. Aust. For. 2014, 77, 159–167. [Google Scholar] [CrossRef]
- Sabine, R. Wood density as a proxy for vulnerability to cavitation: Size matters. J. Plant Hydraul. 2017, 4, e001. [Google Scholar] [CrossRef]
- Savi, T.; Tintner, J.; Da Sois, L.; Grabner, M.; Petit, G.; Rosner, S. The potential of Mid-Infrared spectroscopy for prediction of wood density and vulnerability to embolism in woody angiosperms. Tree Physiol. 2019, 39, 503–510. [Google Scholar] [CrossRef]
- De Guzman, M.E.; Acosta-Rangel, A.; Winter, K.; Meinzer, F.C.; Bonal, D.; Santiago, L.S. Hydraulic traits of Neotropical canopy liana and tree species across a broad range of wood density: Implications for predicting drought mortality with models. Tree Physiol. 2021, 41, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, S.; Ruiz-Benito, P.; Martínez-Vilalta, J.; Lloret, F.; Kitzberger, T.; Allen, C.D.; Fensham, R.; Laughlin, D.C.; Kattge, J.; Bonisch, G.; et al. Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecol. Lett. 2017, 20, 539–553. [Google Scholar] [CrossRef] [PubMed]
- National Forestry Administration. Age-Class and Age-Group of Main Tree Species; China Standard Press: Beijng, China, 2017. [Google Scholar]
- Tyree, M.T.; Hammel, H.T. The Measurement of the Turgor Pressure and the Water Relations of Plants by the Pressure-bomb Technique. J. Exp. Bot. 1972, 23, 267–282. [Google Scholar] [CrossRef]
- Sperry, J.S.; Donnelly, J.R.; Tyree, M.T. A method for measuring hydraulic conductivity and embolism in xylem. Plant Cell Environ. 1988, 11, 35–40. [Google Scholar] [CrossRef]
- Cochard, H.; Badel, E.; Herbette, S.; Delzon, S.; Choat, B.; Jansen, S. Methods for measuring plant vulnerability to cavitation: A critical review. J. Exp. Bot. 2013, 64, 4779–4791. [Google Scholar] [CrossRef]
- Unawong, W.; Yaemphum, S.; Nathalang, A.; Chen, Y.; Domec, J.C.; Tor-Ngern, P. Variations in leaf water status and drought tolerance of dominant tree species growing in multi-aged tropical forests in Thailand. Sci. Rep. 2022, 12, 6882. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.K.; Huggett, B.A.; Tofte, A.N.; Rockwell, F.E.; Holbrook, N.M. Cutting xylem under tension or supersaturated with gas can generate PLC and the appearance of rapid recovery from embolism. Plant Cell Environ. 2013, 36, 1938–1949. [Google Scholar] [CrossRef]
- Pammenter, N.W.; Vander Willigen, C. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiol. 1998, 18, 589–593. [Google Scholar] [CrossRef]
- Martin-StPaul, N.; Delzon, S.; Cochard, H. Plant resistance to drought depends on timely stomatal closure. Ecol. Lett. 2017, 20, 1437–1447. [Google Scholar] [CrossRef]
- Petruzzellis, F.; Nardini, A.; Savi, T.; Tonet, V.; Castello, M.; Bacaro, G. Less safety for more efficiency: Water relations and hydraulics of the invasive tree Ailanthus altissima (Mill.) Swingle compared with native Fraxinus ornus L. Tree Physiol. 2019, 39, 76–87. [Google Scholar] [CrossRef]
- Zuo, L.X. The Water Use Traits and Simulation for Differentage of Populus simonii in Loess Plateau. Master’s Thesis, Northwest A&F University, Xianyang, China, 2013. [Google Scholar]
- Ryan, M.G.; Phillips, N.; Bond, B.J. The hydraulic limitation hypothesis revisited. Plant Cell Environ. 2006, 29, 367–381. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Zhang, Y.; Kreidler, N.; Sun, S.W.; Ardy, R.; Cao, K.F.; Sack, L. Global analysis of plasticity in turgor loss point, a key drought tolerance trait. Ecol. Lett. 2014, 17, 1580–1590. [Google Scholar] [CrossRef]
- Marechaux, I.; Bartlett, M.K.; Sack, L.; Baraloto, C.; Engel, J.; Joetzjer, E.; Chave, J. Drought tolerance as predicted by leaf water potential at turgor loss point varies strongly across species within an Amazonian forest. Funct. Ecol. 2015, 29, 1268–1277. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Scoffoni, C.; Ardy, R.; Zhang, Y.; Sun, S.W.; Cao, K.F.; Sack, L. Rapid determination of comparative drought tolerance traits: Using an osmometer to predict turgor loss point. Methods Ecol. Evol. 2012, 3, 880–888. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Scoffoni, C.; Sack, L. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: A global meta-analysis. Ecol. Lett. 2012, 15, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; Lopez, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Hacke, U. Functional and Ecological Xylem Anatomy, 1st ed.; Springer: Cham, Switzerland, 2015; p. 112. [Google Scholar]
- Bittencourt, P.R.L.; Oliveira, R.S.; da Costa, A.C.L.; Giles, A.L.; Coughlin, I.; Costa, P.B.; Bartholomew, D.C.; Ferreira, L.V.; Vasconcelos, S.S.; Barros, F.V.; et al. Amazonia trees have limited capacity to acclimate plant hydraulic properties in response to long-term drought. Glob. Chang. Biol. 2020, 26, 3569–3584. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.C.; Miniat, C.F.; Oish, A.C.; Denham, S.O.; Domec, J.C.; Johnson, D.M.; Missik, J.E.; Phillips, R.P.; Wood, J.D.; Novick, K.A. The xylem of anisohydric Quercus alba L. is more vulnerable to embolism than isohydric codominants. Plant Cell Environ. 2022, 45, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, U.; Rockwell, F.E.; Holbrook, N.M.; Cochard, H. Iso/Anisohydry: A Plant-Environment Interaction Rather Than a Simple Hydraulic Trait. Trends Plant Sci. 2018, 23, 112–120. [Google Scholar] [CrossRef]
- McGregor, I.R.; Helcoski, R.; Kunert, N.; Tepley, A.J.; GonzalezAkre, E.B.; Herrmann, V.; Zailaa, J.; Stovall, A.E.L.; Bourg, N.A.; McShea, W.J.; et al. Tree height and leaf drought tolerance traits shape growth responses across droughts in a temperate broadleaf forest. New Phytol. 2021, 231, 601–616. [Google Scholar] [CrossRef]
- Ryan, M.G. Tree mortality: Large trees losing out to drought. Nat. Plants 2015, 1, 15150. [Google Scholar] [CrossRef]
- Tognetti, R.; Michelozzi, M.; Giovannelli, A. Geographical variation in water relations, hydraulic architecture and terpene composition of Aleppo pine seedlings from Italian provenances. Tree Physiol. 1997, 17, 241–250. [Google Scholar] [CrossRef]
- Santiago, L.S.; De Guzman, M.E.; Baraloto, C.; Vogenberg, J.E.; Brodie, M.; Herault, B.; Fortunel, C.; Bonal, D. Coordination and trade-offs among hydraulic safety, efficiency and drought avoidance traits in Amazonian rainforest canopy tree species. New Phytol. 2018, 218, 1015–1024. [Google Scholar] [CrossRef]
- Sperry, J.S.; Hacke, U.G.; Pittermann, J. Size and function in conifer tracheids and angiosperm vessels. Am. J. Bot. 2006, 93, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Zanne, A.E.; Westoby, M.; Falster, D.S.; Ackerly, D.D.; Loarie, S.R.; Arnold, S.E.J.; Coomes, D.A. Angiosperm wood structure: Global patterns in vessel anatomy and their relation to wood density and potential conductivity. Am. J. Bot. 2010, 97, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Nardini, A.; Salleo, S.; Jansen, S. More than just a vulnerable pipeline: Xylem physiology in the light of ion-mediated regulation of plant water transport. J. Exp. Bot. 2011, 62, 4701–4718. [Google Scholar] [CrossRef]
- Avila, R.T.; Kane, C.N.; Batz, T.A.; Trabi, C.; Damatta, F.M.; Jansen, S.; McAdam, S.A.M. The relative area of vessels in xylem correlates with stem embolism resistance within and between genera. Tree Physiol. 2023, 43, 75–87. [Google Scholar] [CrossRef]
- De Guzman, M.E.; Santiago, L.S.; Schnitzer, S.A.; Alvarez-Cansino, L. Trade-offs between water transport capacity and drought resistance in neotropical canopy liana and tree species. Tree Physiol. 2017, 37, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Pivovaroff, A.L.; Pasquini, S.C.; De Guzman, M.E.; Alstad, K.P.; Stemke, J.; Santiago, L.S. Multiple strategies for drought survival among woody plant species. Funct. Ecol. 2016, 30, 517–526. [Google Scholar] [CrossRef]
- Sanchez-Martinez, P.; Martínez-Vilalta, J.; Dexter, K.G.; Segovia, R.A.; Mencuccini, M. Adaptation and coordinated evolution of plant hydraulic traits. Ecol. Lett. 2020, 23, 1599–1610. [Google Scholar] [CrossRef]
- Peters, J.M.R.; Lopez, R.; Nolf, M.; Hutley, L.B.; Wardlaw, T.; Cernusak, L.A.; Choat, B. Living on the edge: A continental-scale assessment of forest vulnerability to drought. Glob. Chang. Biol. 2021, 27, 3620–3641. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, B.O.; Gloor, M.; Fauset, S.; Fyllas, N.M.; Galbraith, D.R.; Baker, T.R.; Kruijt, B.; Rowland, L.; Fisher, R.A.; Binks, O.J.; et al. Linking hydraulic traits to tropical forest function in a size-structured and trait-driven model (TFS v. 1-Hydro). Geosci. Model Dev. 2016, 9, 4227–4255. [Google Scholar] [CrossRef]
- Carvalho, E.C.D.; Souza, B.C.; Silva, M.S.; Menezes, B.S.; Martins, F.R.; Araujo, F.S.; Soares, A.A. Xylem anatomical traits determine the variation in wood density and water storage of plants in tropical semiarid climate. Flora 2023, 298, 152185. [Google Scholar] [CrossRef]
- Carrer, M.; Von, A.G.; Castagneri, D.; Petit, G. Distilling allometric and environmental information from time series of conduit size: The standardization issue and its relationship to tree hydraulic architecture. Tree Physiol. 2015, 35, 27–33. [Google Scholar] [CrossRef]
- Rosell, J.A.; Olson, M.E.; Anfodillo, T. Scaling of Xylem Vessel Diameter with Plant Size: Causes, Predictions, and Outstanding Questions. Curr. For. Rep. 2017, 3, 46–59. [Google Scholar] [CrossRef]
- Lachenbruch, B.; McCulloh, K.A. Traits, properties, and performance: How woody plants combine hydraulic and mechanical functions in a cell, tissue, or whole plant. New Phytol. 2014, 204, 747–764. [Google Scholar] [CrossRef]
- Hoeber, S.; Leuschner, C.; Kohler, L.; Arias-Aguilar, D.; Schuldt, B. The importance of hydraulic conductivity and wood density to growth performance in eight tree species from a tropical semi-dry climate. For. Ecol. Manag. 2014, 330, 126–136. [Google Scholar] [CrossRef]
- Janssen, T.A.J.; Holtta, T.; Fleischer, K.; Naudts, K.; Dolman, H. Wood allocation trade-offs between fiber wall, fiber lumen, and axial parenchyma drive drought resistance in neotropical trees. Plant Cell Environ. 2020, 43, 965–980. [Google Scholar] [CrossRef]



| Stand Age | Age (yr) | Height (m) | DBH (cm) | P (m) | SWC (%) |
|---|---|---|---|---|---|
| Y | 9 | 9.50 ± 0.19 | 18.14 ± 0.56 | 4.04 ± 0.71 | 8.02 ± 0.77 |
| M | 17 | 13.29 ± 0.60 | 28.17 ± 0.38 | 5.56 ± 0.58 | 8.12 ± 1.82 |
| NM | 29 | 17.28 ± 1.07 | 40.76 ± 0.85 | 7.52 ± 0.94 | 7.76 ± 1.13 |
| Indicators | Stand Age | ||
|---|---|---|---|
| Y | M | NM | |
| Ψsat (MPa) | −1.82 ± 0.00 | −1.81 ± 0.26 | −1.66 ± 0.08 |
| Ψtlp (MPa) | 2.07 ± 0.03 | −2.19 ± 0.14 | −1.91 ± 0.04 |
| RWCtlp (%) | 91.64 ± 1.22 | 86.71 ± 2.13 | 91.94 ± 1.43 |
| ε (MPa) | 21.27 ± 3.44 | 13.75 ± 2.10 | 19.65 ± 3.99 |
| P50 (MPa) | −1.55 ± 0.04 | −1.51 ± 0.04 | −1.21 ± 0.06 |
| P88 (MPa) | −3.09 ± 0.02 | −2.99 ± 0.05 | −2.87 ± 0.02 |
| SSMtlp (MPa) | −0.52 ± 0.02 | −0.68 ± 0.10 | −0.70 ± 0.03 |
| Ks (kg m−1 s−1 MPa−1) | 2.28 ± 0.11 | 3.00 ± 0.15 | 2.37 ± 0.13 |
| LSC (10−3 kg m−1 s−1 MPa−1 kg−1) | 3.80 ± 0.187 | 4.80 ± 0.223 | 3.30 ± 0.246 |
| Hv (cm2 g−1) | 0.0170 ± 0.00 | 0.0160 ± 0.00 | 0.0140 ± 0.00 |
| WD (g cm−3) | 0.370 ± 0.011 | 0.353 ± 0.006 | 0.349 ± 0.008 |
| Stand Age | PC1 | PC2 | PC | Comprehensive Ranking |
|---|---|---|---|---|
| Y | 1.920 | 2.683 | 2.244 | 1 |
| M | 2.380 | −2.317 | 0.388 | 2 |
| NM | −4.301 | −0.365 | −2.631 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; He, W.; Wei, H.; Hu, S.; Zhou, Y.; Wang, Y. Hydraulic Traits in Populus simonii Carr. at Stands of Categorized Ages in a Semi-Arid Area of Western Liaoning, Northeast China. Forests 2023, 14, 1759. https://doi.org/10.3390/f14091759
Liu P, He W, Wei H, Hu S, Zhou Y, Wang Y. Hydraulic Traits in Populus simonii Carr. at Stands of Categorized Ages in a Semi-Arid Area of Western Liaoning, Northeast China. Forests. 2023; 14(9):1759. https://doi.org/10.3390/f14091759
Chicago/Turabian StyleLiu, Ping, Wenting He, Hongxu Wei, Shiyu Hu, Yiming Zhou, and Yutao Wang. 2023. "Hydraulic Traits in Populus simonii Carr. at Stands of Categorized Ages in a Semi-Arid Area of Western Liaoning, Northeast China" Forests 14, no. 9: 1759. https://doi.org/10.3390/f14091759






