Responses of Physiological, Morphological and Anatomical Traits to Abiotic Stress in Woody Plants
Abstract
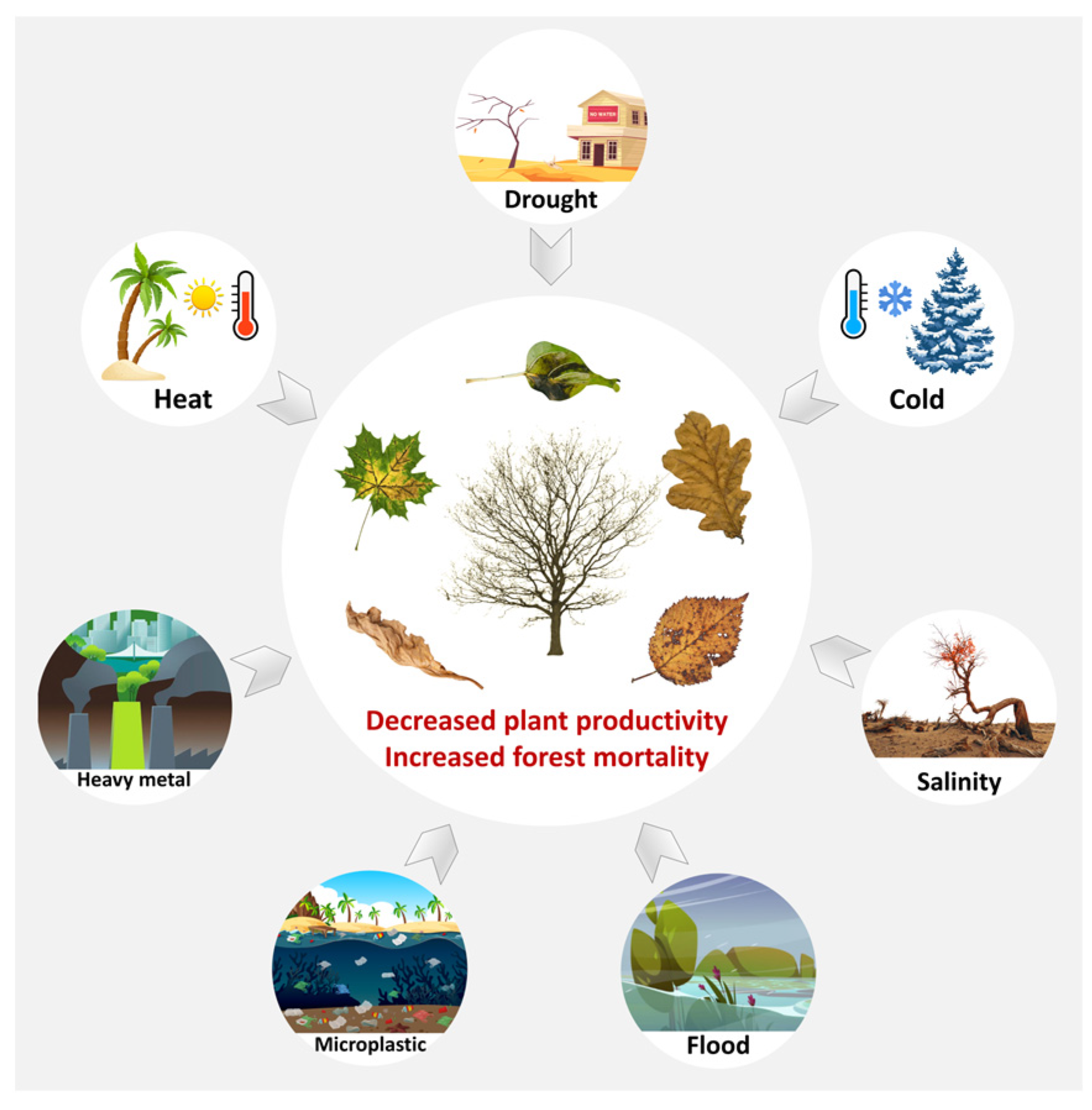
:1. Introduction
2. Response of Woody Plants to a Single Abiotic Stress
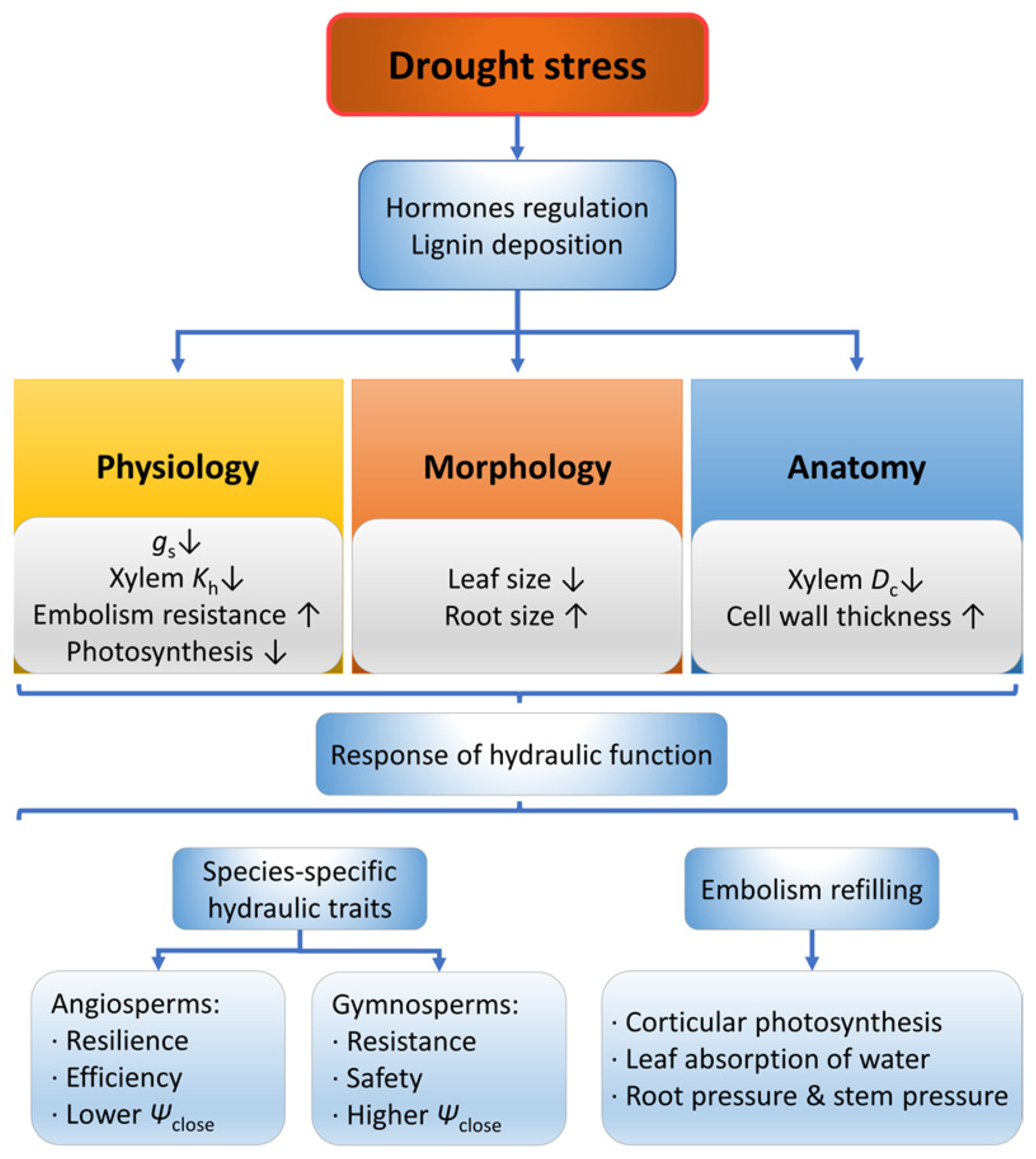
2.1. Drought
2.1.1. Physiological Responses of Plants under Drought
2.1.2. Morphological and Anatomical Responses of Plants under Drought
2.1.3. Xylem Hydraulic Refilling
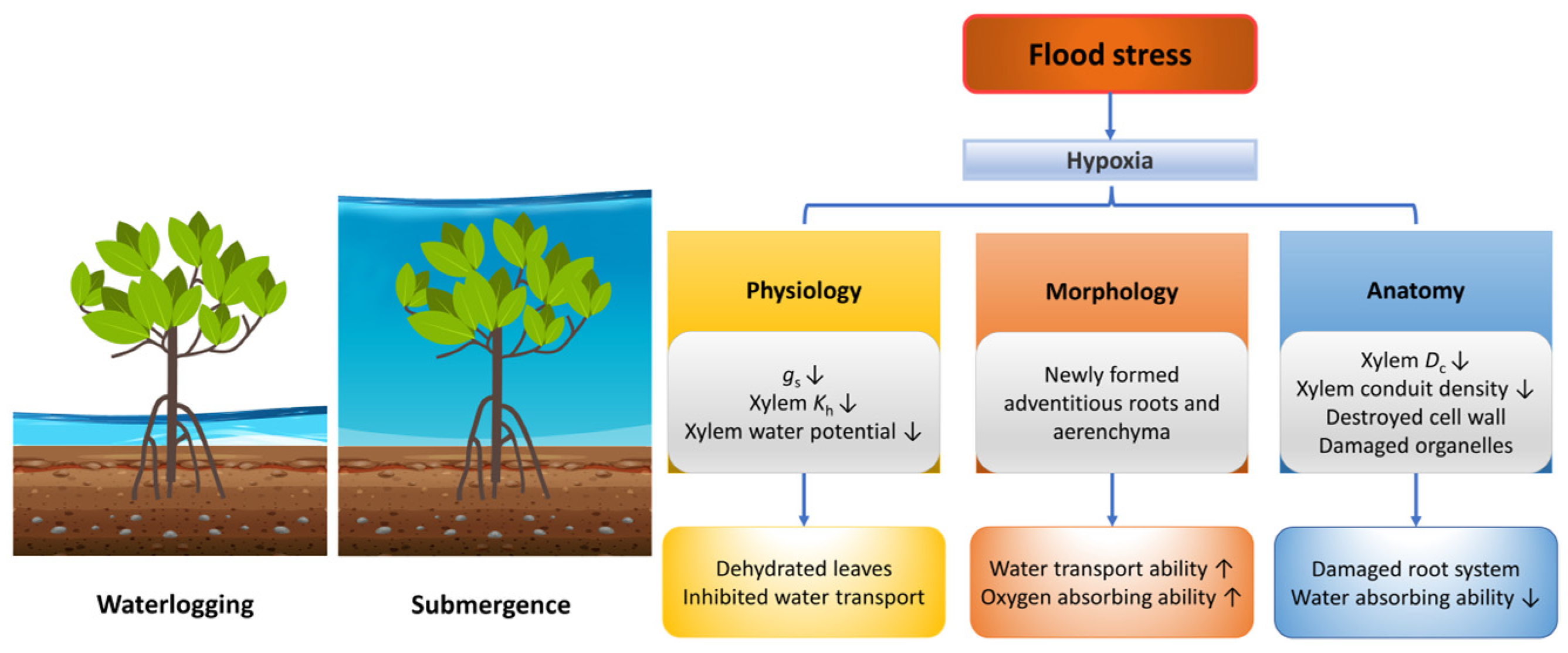
2.2. Flood
2.2.1. Physiological Responses of Plants under Flood Stress
2.2.2. Morphological and Anatomical Responses of Plants under Flood Stress
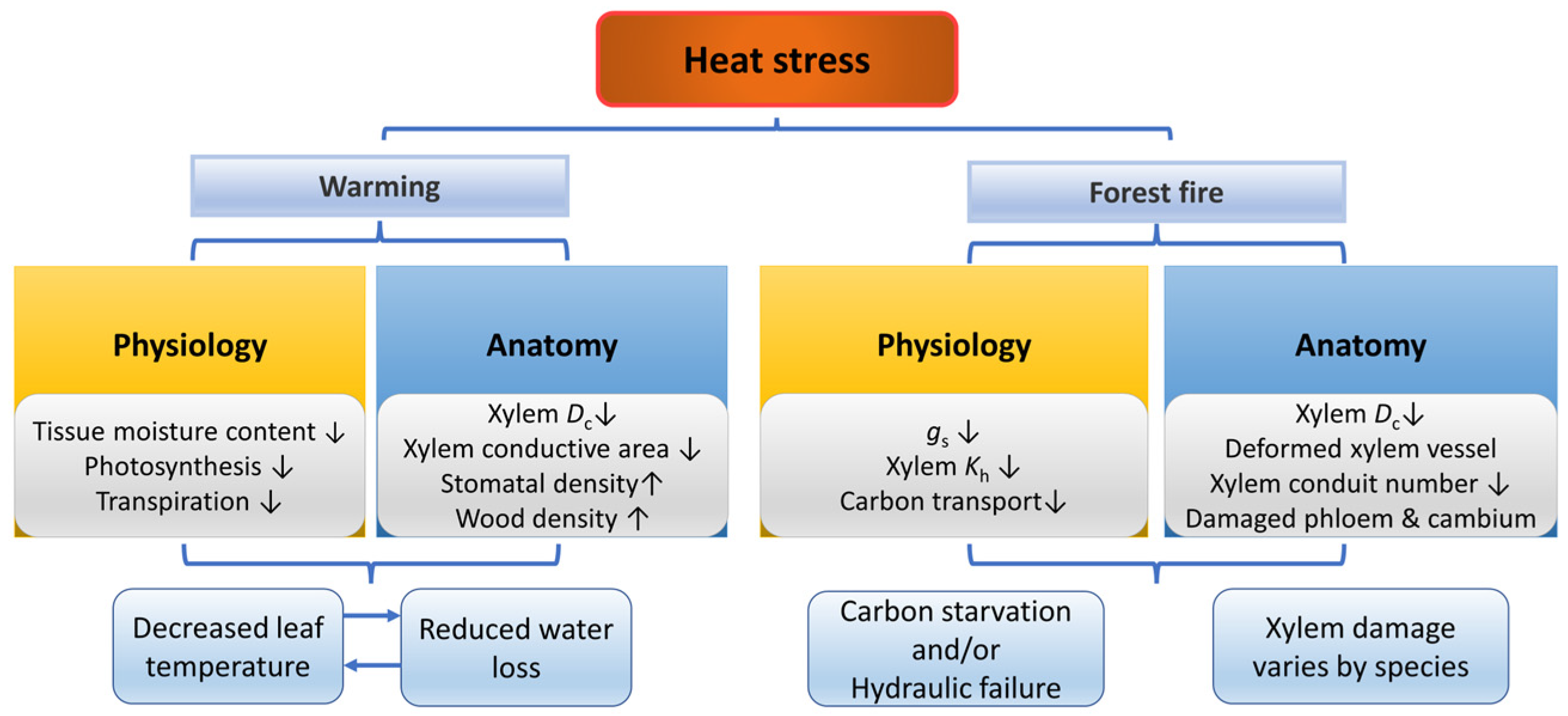
2.3. Extreme Temperature
2.3.1. Heat
Physiological Responses of Plants under Heat Stress
Morphological and Anatomical Responses of Plants under Heat Stress
2.3.2. Cold
Physiological Responses of Plants under Cold Stress
Morphological and Anatomical Responses of Plants under Cold Stress
2.4. Salinity
2.4.1. Physiological Responses of Plants under Salt Stress
2.4.2. Morphological and Anatomical Responses of Plants under Salt Stress
2.5. Heavy Metal
2.5.1. Heavy Metal Absorption and Physiological Responses of Plants
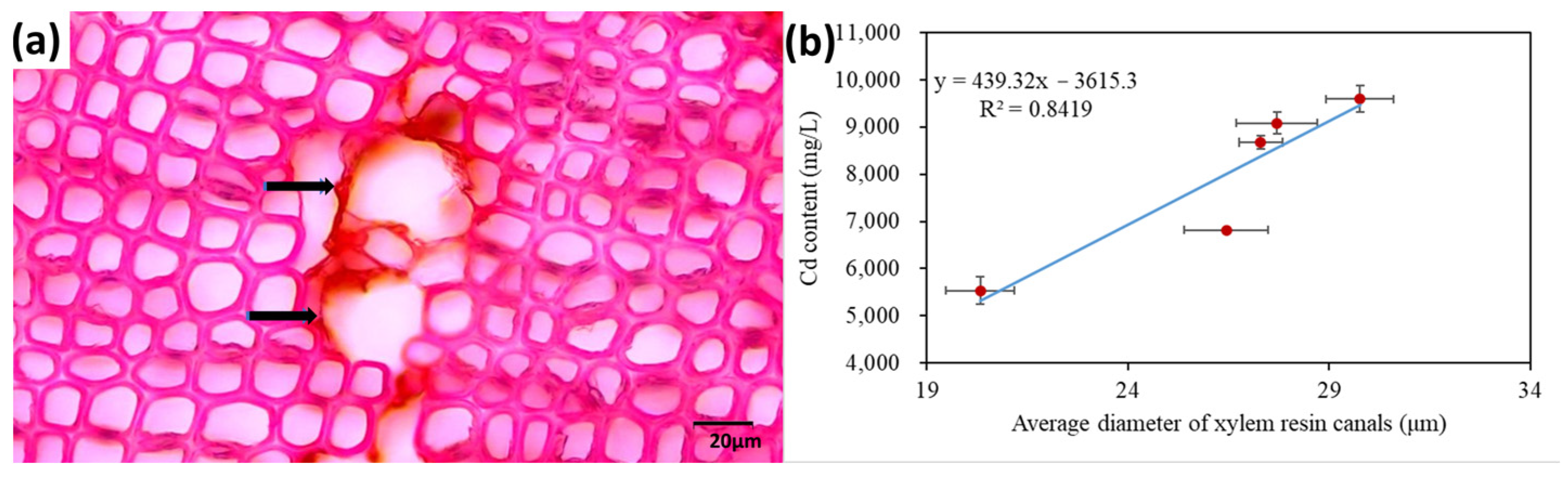
2.5.2. Morphological and Anatomical Responses of Plants under Heavy Metal Stress
3. Response of Woody Plants to Combined Abiotic Stresses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Stott, P. How climate change affects extreme weather events. Science 2016, 352, 1517–1518. [Google Scholar] [CrossRef]
- Salvatierra, A.; Toro, G.; Mateluna, P.; Opazo, I.; Ortiz, M.; Pimentel, P. Keep calm and survive: Adaptation strategies to energy crisis in fruit trees under root hypoxia. Plants 2020, 9, 1108. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Way, D.A.; Sharkey, T.D. Plant heat stress: Concepts directing future research. Plant Cell Environ. 2021, 44, 1992–2005. [Google Scholar] [CrossRef] [PubMed]
- Bowd, E.J.; Blair, D.P.; Lindenmayer, D.B. Prior disturbance legacy effects on plant recovery post-high-severity wildfire. Ecosphere 2021, 12, e03480. [Google Scholar] [CrossRef]
- Knight, M.R.; Knight, H. Low-temperature perception leading to gene expression and cold tolerance in higher plants. New Phytol. 2012, 195, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Ilangumaran, G.; Smith, D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Ghuge, S.A.; Nikalje, G.C.; Kadam, U.S.; Suprasanna, P.; Hong, J.C. Comprehensive mechanisms of heavy metal toxicity in plants, detoxification, and remediation. J. Hazard. Mater. 2023, 450, 131039. [Google Scholar] [CrossRef]
- Zimmermann, M.H. Xylem Structure and the Ascent of Sap; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Li, S.; Tan, T.; Fan, Y.; Raza, M.A.; Wang, Z.; Wang, B.; Zhang, J.; Tan, X.; Chen, P.; Shafiq, I.; et al. Responses of leaf stomatal and mesophyll conductance to abiotic stress factors. J. Integr. Agric. 2022, 21, 2787–2804. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef]
- Peng, P.; Li, R.; Chen, Z.-H.; Wang, Y. Stomata at the crossroad of molecular interaction between biotic and abiotic stress responses in plants. Front. Plant Sci. 2022, 13, 1031891. [Google Scholar] [CrossRef] [PubMed]
- Karlova, R.; Boer, D.; Hayes, S.; Testerink, C. Root plasticity under abiotic stress. Plant Physiol. 2021, 187, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Gutschick, V.P. Biotic and abiotic consequences of differences in leaf structure. New Phytol. 1999, 143, 3–18. [Google Scholar] [CrossRef]
- Hu, W.; Lu, Z.; Meng, F.; Li, X.; Cong, R.; Ren, T.; Sharkey, T.D.; Lu, J. The reduction in leaf area precedes that in photosynthesis under potassium deficiency: The importance of leaf anatomy. New Phytol. 2020, 227, 1749–1763. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, G.; He, Q.; Zhou, L.; Ji, Y.; Zhou, M. Environmental explanation of maize specific leaf area under varying water stress regimes. Environ. Exp. Bot. 2020, 171, 103932. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, L.; Qi, D. Variation in leaf traits at different altitudes reflects the adaptive strategy of plants to environmental changes. Ecol. Evol. 2020, 10, 8166–8175. [Google Scholar] [CrossRef]
- Ahmad, P.; Prasad, M.N.V. Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Zheng, J.; Zhao, X.; Morris, H.; Jansen, S. Phylogeny best explains latitudinal patterns of xylem tissue fractions for woody angiosperm species across China. Front. Plant Sci. 2019, 10, 556. [Google Scholar] [CrossRef]
- Choat, B.; Cobb, A.R.; Jansen, S. Structure and function of bordered pits: New discoveries and impacts on whole-plant hydraulic function. New Phytol. 2008, 177, 608–626. [Google Scholar] [CrossRef]
- Evert, R.F. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Morris, H.; Plavcová, L.; Cvecko, P.; Fichtler, E.; Gillingham, M.A.F.; Martínez-Cabrera, H.I.; McGlinn, D.J.; Wheeler, E.; Zheng, J.; Ziemińska, K.; et al. A global analysis of parenchyma tissue fractions in secondary xylem of seed plants. New Phytol. 2015, 209, 1553–1565. [Google Scholar] [CrossRef]
- Greenwood, S.; Ruiz-Benito, P.; Martinez-Vilalta, J.; Lloret, F.; Kitzberger, T.; Allen, C.D.; Fensham, R.; Laughlin, D.C.; Kattge, J.; Bonisch, G.; et al. Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecol. Lett. 2017, 20, 539–553. [Google Scholar] [CrossRef]
- Bottero, A.; D’Amato, A.W.; Palik, B.J.; Bradford, J.B.; Fraver, S.; Battaglia, M.A.; Asherin, L.A.; Bugmann, H. Density-dependent vulnerability of forest ecosystems to drought. J. Appl. Ecol. 2017, 54, 1605–1614. [Google Scholar] [CrossRef]
- Nardini, A.; Savi, T.; Trifilò, P.; Lo Gullo, M.A. Drought stress and the recovery from xylem embolism in woody plants. In Progress in Botany Vol. 79; Progress in Botany; Springer: Berlin/Heidelberg, Germany, 2017; pp. 197–231. [Google Scholar]
- Schenk, H.J.; Steppe, K.; Jansen, S. Nanobubbles: A new paradigm for air-seeding in xylem. Trends Plant Sci. 2015, 20, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, J.; Yin, Y.; Li, X.; Deng, L.; Jiang, X.; Chen, Z.; Li, Y. Investigating effects of bordered pit membrane morphology and properties on plant xylem hydraulic functions-A case study from 3d reconstruction and microflow modelling of pit membranes in angiosperm xylem. Plants 2020, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Puglielli, G.; Catoni, R.; Spoletini, A.; Varone, L.; Gratani, L. Short-term physiological plasticity: Trade-off between drought and recovery responses in three Mediterranean cistus species. Ecol. Evol. 2017, 7, 10880–10889. [Google Scholar] [CrossRef]
- Martin-StPaul, N.; Delzon, S.; Cochard, H. Plant resistance to drought depends on timely stomatal closure. Ecol. Lett. 2017, 20, 1437–1447. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Ramachandra Reddy, A.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Wang, J.; Chen, Z.; Lu, S.; Wan, X.; Sun, H.; Wang, L.; Delzon, S.; Cochard, H. Hydraulic traits are coupled with plant anatomical traits under drought-rewatering cycles in Ginkgo biloba L. Tree Physiol. 2021, 42, 1216–1227. [Google Scholar] [CrossRef]
- Mantova, M.; Menezes-Silva, P.E.; Badel, E.; Cochard, H.; Torres-Ruiz, J.M. The interplay of hydraulic failure and cell vitality explains tree capacity to recover from drought. Physiol. Plant 2021, 172, 247–257. [Google Scholar] [CrossRef]
- Scoffoni, C.; Albuquerque, C.; Brodersen, C.R.; Townes, S.V.; John, G.P.; Bartlett, M.K.; Buckley, T.N.; McElrone, A.J.; Sack, L. Outside-xylem vulnerability, not xylem embolism, controls leaf hydraulic decline during dehydration. Plant Physiol. 2017, 173, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, Y.; Li, W.; Ayup, M. Xylem hydraulic conductivity and embolism in riparian plants and their responses to drought stress in desert of Northwest China. Ecohydrology 2013, 6, 984–993. [Google Scholar] [CrossRef]
- Li, X.; Piao, S.; Wang, K.; Wang, X.; Wang, T.; Ciais, P.; Chen, A.; Lian, X.; Peng, S.; Penuelas, J. Temporal trade-off between gymnosperm resistance and resilience increases forest sensitivity to extreme drought. Nat. Ecol. Evol. 2020, 4, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Bauerle, T.L. A global analysis of plant recovery performance from water stress. Oikos 2017, 126, 1377–1388. [Google Scholar] [CrossRef]
- Anderegg, L.D.; HilleRisLambers, J. Drought stress limits the geographic ranges of two tree species via different physiological mechanisms. Glob. Chang. Biol. 2016, 22, 1029–1045. [Google Scholar] [CrossRef]
- Carnicer, J.; Barbeta, A.; Sperlich, D.; Coll, M.; Peñuelas, J. Contrasting trait syndromes in angiosperms and conifers are associated with different responses of tree growth to temperature on a large scale. Front. Plant Sci. 2013, 4, 409. [Google Scholar] [CrossRef]
- Urli, M.; Porte, A.J.; Cochard, H.; Guengant, Y.; Burlett, R.; Delzon, S. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiol. 2013, 33, 672–683. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Cochard, H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol. 2009, 149, 575–584. [Google Scholar] [CrossRef]
- Hammond, W.M.; Yu, K.; Wilson, L.A.; Will, R.E.; Anderegg, W.R.L.; Adams, H.D. Dead or dying? Quantifying the point of no return from hydraulic failure in drought-induced tree mortality. New Phytol. 2019, 223, 1834–1843. [Google Scholar] [CrossRef]
- Stocker, O. Die Abhängigkeit der Transpiration von den Umweltfaktoren; Springer: Berlin/Heidelberg, Germany, 1956. [Google Scholar]
- Tardieu, F.; Simonneau, T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: Modelling isohydric and anisohydric behaviours. J. Exp. Bot. 1998, 49, 419–432. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, Y.; Peng, Q.; Li, K.; Mohammat, A.; Han, W. Nitrogen and phosphorus resorption of desert plants with various degree of propensity to salt in response to drought and saline stress. Ecol. Indic. 2021, 125, 107488. [Google Scholar] [CrossRef]
- Sade, N.; Gebremedhin, A.; Moshelion, M. Risk-taking plants: Anisohydric behavior as a stress-resistance trait. Plant Signal Behav. 2012, 7, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- McAdam, S.A.; Brodribb, T.J. The evolution of mechanisms driving the stomatal response to vapor pressure deficit. Plant Physiol. 2015, 167, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Daszkowska-Golec, A.; Szarejko, I. Open or close the gate-stomata action under the control of phytohormones in drought stress conditions. Front. Plant Sci. 2013, 4, 138. [Google Scholar] [CrossRef]
- Ennajeh, M.; Vadel, A.; Cochard, H.; Khemira, H. Comparative impacts of water stress on the leaf anatomy of a drought-resistant and a drought-sensitive olive cultivar. J. Hortic. Sci. Biotechnol. 2010, 85, 289–294. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K. Drought stress in plants: An overview. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. [Google Scholar]
- Strock, C.F.; Burridge, J.D.; Niemiec, M.D.; Brown, K.M.; Lynch, J.P. Root metaxylem and architecture phenotypes integrate to regulate water use under drought stress. Plant Cell Environ. 2021, 44, 49–67. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Wang, X.; Han, X.; An, Y.; Lin, S.; Shen, C.; Wen, J.; Liu, C.; Yin, W.; et al. Root-specific NF-Y family transcription factor, PdNF-YB21, positively regulates root growth and drought resistance by abscisic acid-mediated indoylacetic acid transport in Populus. New Phytol. 2020, 227, 407–426. [Google Scholar] [CrossRef]
- Rais, A.; van de Kuilen, J.-W.G.; Pretzsch, H. Growth reaction patterns of tree height, diameter, and volume of Douglas-fir (Pseudotsuga menziesii [Mirb.] Franco) under acute drought stress in Southern Germany. Eur. J. For. Res. 2014, 133, 1043–1056. [Google Scholar] [CrossRef]
- Yang, B.; Shan, L.; Ma, J.; Xie, T.; Yang, J.; Wei, C. Response of growth and root morphological characteristics of Reaumuria soongorica seedlings to drought-rehydration. Arid. Zone Res. 2021, 38, 469–478. [Google Scholar]
- Sasani, N.; Paques, L.E.; Boulanger, G.; Singh, A.P.; Gierlinger, N.; Rosner, S.; Brendel, O. Physiological and anatomical responses to drought stress differ between two larch species and their hybrid. Trees 2021, 35, 1467–1484. [Google Scholar] [CrossRef]
- Thangthong, N.; Jogloy, S.; Jongrungklang, N.; Kvien, C.K.; Dodd, I.C.; Vorasoot, N. Changes in root xylem anatomy of peanut genotypes with different drought resistance levels under early-season drought. J. Agron. Crop Sci. 2021, 207, 803–813. [Google Scholar] [CrossRef]
- Jing, L.; Yubao, G.; Zhirong, Z.; Zenglu, G. Hydraulic architecture of three Caragana species and its relationship with environmental factors in different habitats of the Inner Mongolian Plateau, China. Acta Ecol. Sin. 2007, 27, 837–845. [Google Scholar] [CrossRef]
- Cai, J.; Tyree, M.T. The impact of vessel size on vulnerability curves: Data and models for within-species variability in saplings of aspen, Populus tremuloides Michx. Plant Cell Environ. 2010, 33, 1059–1069. [Google Scholar] [CrossRef]
- Hacke, U.G.; Spicer, R.; Schreiber, S.G.; Plavcová, L. An ecophysiological and developmental perspective on variation in vessel diameter. Plant Cell Environ. 2017, 40, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Hacke, U.G.; Lachenbruch, B.; Pittermann, J.; Mayr, S.; Domec, J.-C.; Schulte, P.J. The hydraulic architecture of conifers. In Functional and Ecological Xylem Anatomy; Springer: Berlin/Heidelberg, Germany, 2015; pp. 39–75. [Google Scholar]
- Li, L.; Li, Z.; Jing, C.; Han, Z.; Yongqin, C.; Zaimin, J. Hydraulic characteristics and embolism repair of Populus alba × P. glandulosa after drought stress and rehydration. J. Beijing For. Univ. 2021, 43, 22–30. [Google Scholar]
- Montwé, D.; Spiecker, H.; Hamann, A. An experimentally controlled extreme drought in a Norway spruce forest reveals fast hydraulic response and subsequent recovery of growth rates. Trees 2014, 28, 891–900. [Google Scholar] [CrossRef]
- Bouche, P.S.; Delzon, S.; Choat, B.; Badel, E.; Brodribb, T.J.; Burlett, R.; Cochard, H.; Charra-Vaskou, K.; Lavigne, B.; Li, S. Are needles of Pinus pinaster more vulnerable to xylem embolism than branches? New insights from X-ray computed tomography. Plant Cell Environ. 2016, 39, 860–870. [Google Scholar] [CrossRef]
- Guerin, M.; von Arx, G.; Martin-Benito, D.; Andreu-Hayles, L.; Griffin, K.L.; McDowell, N.G.; Pockman, W.; Gentine, P. Distinct xylem responses to acute vs prolonged drought in pine trees. Tree Physiol. 2020, 40, 605–620. [Google Scholar] [CrossRef]
- Jupa, R.; Krabickova, D.; Plichta, R.; Mayr, S.; Gloser, V. Do angiosperm tree species adjust intervessel lateral contact in response to soil drought? Physiol. Plant 2021, 172, 2048–2058. [Google Scholar] [CrossRef]
- Soro, A.; Lenz, P.; Roussel, J.R.; Larochelle, F.; Bousquet, J.; Achim, A. The phenotypic and genetic effects of drought-induced stress on apical growth, ring width, wood density and biomass in white spruce seedlings. New For. 2022, 54, 789–811. [Google Scholar] [CrossRef]
- Balducci, L.; Deslauriers, A.; Giovannelli, A.; Beaulieu, M.; Delzon, S.; Rossi, S.; Rathgeber, C.B.K. How do drought and warming influence survival and wood traits of Picea mariana saplings? J. Exp. Bot. 2015, 66, 377–389. [Google Scholar] [CrossRef]
- Rosner, S.; Světlík, J.; Andreassen, K.; Børja, I.; Dalsgaard, L.; Evans, R.; Karlsson, B.; Tollefsrud, M.M.; Solberg, S. Wood density as a screening trait for drought sensitivity in Norway spruce. Can. J. For. Res. 2014, 44, 154–161. [Google Scholar] [CrossRef]
- Zou, J.; Hu, W.; Li, Y.; Zhu, H.; He, J.; Wang, Y.; Meng, Y.; Chen, B.; Zhao, W.; Wang, S.; et al. Leaf anatomical alterations reduce cotton’s mesophyll conductance under dynamic drought stress conditions. Plant J. 2022, 111, 391–405. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Su, M.X.; Han, Z.J.; Shi, J.Y. Evaluation of salt and drought tolerances of Populus talassica × Populus euphratica seedlings using leaf anatomical structures and physiological processes. Pak. J. Bot. 2023, 55, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Cao, L. Eco-Physiology Responses of Camellia oleifera to the Drought Stress. Master’s Thesis, Chinese Academy of Forestry, Beijing, China, 2017. [Google Scholar]
- Dickison, W.C. Integrative Plant Anatomy; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Little, C.; Savidge, R. The role of plant growth regulators in forest tree cambial growth. In Hormonal Control of Tree Growth; Springer: Berlin/Heidelberg, Germany, 1987; pp. 137–169. [Google Scholar]
- Abe, H.; Nakai, T.; Utsumi, Y.; Kagawa, A. Temporal water deficit and wood formation in Cryptomeria japonica. Tree Physiol. 2003, 23, 859–863. [Google Scholar] [CrossRef]
- Popko, J.; Hansch, R.; Mendel, R.R.; Polle, A.; Teichmann, T. The role of abscisic acid and auxin in the response of poplar to abiotic stress. Plant Biol. 2010, 12, 242–258. [Google Scholar] [CrossRef]
- Zhao, D.; Luan, Y.; Shi, W.; Zhang, X.; Meng, J.; Tao, J. A Paeonia ostii caffeoyl-CoA O-methyltransferase confers drought stress tolerance by promoting lignin synthesis and ROS scavenging. Plant Sci. 2021, 303, 110765. [Google Scholar] [CrossRef]
- Donaldson, L.A. Abnormal lignin distribution in wood from severely drought stressed Pinus radiata trees. IAWA J. 2002, 23, 161–178. [Google Scholar] [CrossRef]
- Niu, Z.; Li, G.; Hu, H.; Lv, J.; Zheng, Q.; Liu, J.; Wan, D. A gene that underwent adaptive evolution, LAC2 (LACCASE), in Populus euphratica improves drought tolerance by improving water transport capacity. Hortic. Res. 2021, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Linker, R.; Gepstein, S.; Tanimoto, E.; Yamamoto, R.; Neumann, P.M. Progressive inhibition by water deficit of cell wall extensibility and growth along the elongation zone of maize roots is related to increased lignin metabolism and progressive stelar accumulation of wall phenolics. Plant Physiol. 2006, 140, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Hura, T.; Hura, K.; Dziurka, K.; Ostrowska, A.; Bączek-Kwinta, R.; Grzesiak, M. An increase in the content of cell wall-bound phenolics correlates with the productivity of triticale under soil drought. J. Plant Physiol. 2012, 169, 1728–1736. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef] [PubMed]
- Creek, D.; Blackman, C.J.; Brodribb, T.J.; Choat, B.; Tissue, D.T. Coordination between leaf, stem, and root hydraulics and gas exchange in three arid-zone angiosperms during severe drought and recovery. Plant Cell Environ. 2018, 41, 2869–2881. [Google Scholar] [CrossRef]
- Hasan, M.M.; Gong, L.; Nie, Z.-F.; Li, F.-P.; Ahammed, G.J.; Fang, X.-W. ABA-induced stomatal movements in vascular plants during dehydration and rehydration. Environ. Exp. Bot. 2021, 186, 104436. [Google Scholar] [CrossRef]
- Bi, M.H.; Jiang, C.; Brodribb, T.; Yang, Y.J.; Yao, G.Q.; Jiang, H.; Fang, X.W. Ethylene constrains stomatal reopening in Fraxinus chinensis post moderate drought. Tree Physiol. 2023, 43, 883–892. [Google Scholar] [CrossRef]
- Klein, T.; Zeppel, M.J.; Anderegg, W.R.; Bloemen, J.; De Kauwe, M.G.; Hudson, P.; Ruehr, N.K.; Powell, T.L.; von Arx, G.; Nardini, A. Xylem embolism refilling and resilience against drought-induced mortality in woody plants: Processes and trade-offs. Ecol. Res. 2018, 33, 839–855. [Google Scholar] [CrossRef]
- Liu, J.; Gu, L.; Yu, Y.; Huang, P.; Wu, Z.; Zhang, Q.; Qian, Y.; Wan, X.; Sun, Z. Corticular photosynthesis drives bark water uptake to refill embolized vessels in dehydrated branches of Salix matsudana. Plant Cell Environ. 2019, 42, 2584–2596. [Google Scholar] [CrossRef]
- Tomasella, M.; Natale, S.; Petruzzellis, F.; Di Bert, S.; D’Amico, L.; Tromba, G.; Nardini, A. No evidence for light-induced embolism repair in cut stems of drought-resistant mediterranean species under soaking. Plants 2022, 11, 307. [Google Scholar] [CrossRef]
- Fuenzalida, T.I.; Blacker, M.J.; Turner, M.; Sheppard, A.; Ball, M.C. Foliar water uptake enables embolism removal in excised twigs of Avicennia marina. New Phytol. 2023, 237, 1136–1145. [Google Scholar] [CrossRef]
- Singh, S. Root pressure: Getting to the root of pressure. In Progress in Botany 77; Progress in Botany; Springer: Berlin/Heidelberg, Germany, 2016; pp. 105–150. [Google Scholar]
- Ewers, F.W.; Cochard, H.; Tyree, M.T. A survey of root pressures in vines of a tropical lowland forest. Oecologia 1997, 110, 191–196. [Google Scholar] [CrossRef]
- Wang, L.; Dai, Y.; Zhang, J.; Meng, P.; Wan, X. Xylem structure and hydraulic characteristics of deep roots, shallow roots and branches of walnut under seasonal drought. BMC Plant Biol. 2022, 22, 440. [Google Scholar] [CrossRef] [PubMed]
- Wegner, L.H. Root pressure and beyond: Energetically uphill water transport into xylem vessels? J. Exp. Bot. 2014, 65, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Trifilò, P.; Kiorapostolou, N.; Petruzzellis, F.; Vitti, S.; Petit, G.; Gullo, M.A.L.; Nardini, A.; Casolo, V. Hydraulic recovery from xylem embolism in excised branches of twelve woody species: Relationships with parenchyma cells and non-structural carbohydrates. Plant Physiol. Biochem. 2019, 139, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; (Institute for Systematic Botany and Ecology, Ulm University, Ulm, Germany). Unpublished work. 2015.
- Secchi, F.; Pagliarani, C.; Cavalletto, S.; Petruzzellis, F.; Tonel, G.; Savi, T.; Tromba, G.; Obertino, M.M.; Lovisolo, C.; Nardini, A.; et al. Chemical inhibition of xylem cellular activity impedes the removal of drought-induced embolisms in poplar stems—New insights from micro-CT analysis. New Phytol. 2021, 229, 820–830. [Google Scholar] [CrossRef]
- Brodersen, C.R.; Knipfer, T.; McElrone, A.J. In vivo visualization of the final stages of xylem vessel refilling in grapevine (Vitis vinifera) stems. New Phytol. 2018, 217, 117–126. [Google Scholar] [CrossRef]
- Ryu, J.; Hwang, B.G.; Lee, S.J. In vivo dynamic analysis of water refilling in embolized xylem vessels of intact Zea mays leaves. Ann. Bot. 2016, 118, 1033–1042. [Google Scholar] [CrossRef]
- Choat, B.; Nolf, M.; Lopez, R.; Peters, J.M.R.; Carins-Murphy, M.R.; Creek, D.; Brodribb, T.J. Non-invasive imaging shows no evidence of embolism repair after drought in tree species of two genera. Tree Physiol. 2019, 39, 113–121. [Google Scholar] [CrossRef]
- Hawes, J.E.; Peres, C.A.; Riley, L.B.; Hess, L.L. Landscape-scale variation in structure and biomass of Amazonian seasonally flooded and unflooded forests. For. Ecol. Manag. 2012, 281, 163–176. [Google Scholar] [CrossRef]
- Allen, S.T.; Keim, R.F.; Dean, T.J. Contrasting effects of flooding on tree growth and stand density determine aboveground production, in baldcypress forests. For. Ecol. Manag. 2019, 432, 345–355. [Google Scholar] [CrossRef]
- Araújo, F.D.C.; Tng, D.Y.P.; Apgaua, D.M.G.; Morel, J.D.; Pereira, D.G.S.; Santos, P.F.; Santos, R.M.d.; Collins, B. Flooding regime drives tree community structure in Neotropical dry forests. J. Veg. Sci. 2019, 30, 1195–1205. [Google Scholar] [CrossRef]
- Jackson, M.B.; Ishizawa, K.; Ito, O. Evolution and mechanisms of plant tolerance to flooding stress. Ann. Bot. 2009, 103, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.; Tezara, W.; Rengifo, E.; Flores, S. Changes with seasonal flooding in sap flow of the tropical flood-tolerant tree species, Campsiandra laurifolia. Trees 2008, 22, 551–558. [Google Scholar] [CrossRef]
- Li, M.; López, R.; Venturas, M.; Pita, P.; Gordaliza, G.G.; Gil, L.; Rodríguez-Calcerrada, J. Greater resistance to flooding of seedlings of Ulmus laevis than Ulmus minor is related to the maintenance of a more positive carbon balance. Trees 2015, 29, 835–848. [Google Scholar] [CrossRef]
- Tan, X.; Zwiazek, J.J. Stable expression of aquaporins and hypoxia-responsive genes in adventitious roots are linked to maintaining hydraulic conductance in tobacco (Nicotiana tabacum) exposed to root hypoxia. PLoS ONE 2019, 14, e0212059. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Sheng, J.; Jin, S.; Zhou, F.; Hu, Z.; Diao, Y. Transcriptome, physiological and biochemical analysis of Triarrhena sacchariflora in response to flooding stress. BMC Genetics 2019, 20, 88. [Google Scholar] [CrossRef]
- Ferner, E.; Rennenberg, H.; Kreuzwieser, J. Effect of flooding on C metabolism of flood-tolerant (Quercus robur) and non-tolerant (Fagus sylvatica) tree species. Tree Physiol. 2012, 32, 135–145. [Google Scholar] [CrossRef]
- Habibi, F.; Liu, T.; Shahid, M.A.; Schaffer, B.; Sarkhosh, A. Physiological, biochemical, and molecular responses of fruit trees to root zone hypoxia. Environ. Exp. Bot. 2023, 206, 105179. [Google Scholar] [CrossRef]
- Kwak, J.M.; Mori, I.C.; Pei, Z.-M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef]
- Bashar, K.; Tareq, M.; Amin, M.; Honi, U.; Tahjib-Ul-Arif, M.; Sadat, M.; Hossen, Q. Phytohormone-mediated stomatal response, escape and quiescence strategies in plants under flooding stress. Agronomy 2019, 9, 43. [Google Scholar] [CrossRef]
- Nicolás, E.; Torrecillas, A.; Dell’Amico, J.; Alarcón, J.J. The effect of short-term flooding on the sap flow, gas exchange and hydraulic conductivity of young apricot trees. Trees 2005, 19, 51–57. [Google Scholar] [CrossRef]
- Ashraf, M.A. Waterlogging stress in plants: A review. Afr. J. Agric. Res. 2012, 7, 1976–1981. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X. Can early wilting of old leaves account for much of the ABA accumulation in flooded pea plants? J. Exp. Bot. 1994, 45, 1335–1342. [Google Scholar] [CrossRef]
- Domingo, R.; Pérez-Pastor, A.; Ruiz-Sánchez, M.C. Physiological responses of apricot plants grafted on two different rootstocks to flooding conditions. J. Plant Physiol. 2002, 159, 725–732. [Google Scholar] [CrossRef]
- Krauss, K.W.; Young, P.J.; Chambers, J.L.; Doyle, T.W.; Twilley, R.R. Sap flow characteristics of neotropical mangroves in flooded and drained soils. Tree Physiol. 2007, 27, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Steppe, K.; Cochard, H.; Lacointe, A.; Améglio, T. Could rapid diameter changes be facilitated by a variable hydraulic conductance? Plant Cell Environ. 2012, 35, 150–157. [Google Scholar] [CrossRef]
- Rodríguez-Gamir, J.; Ancillo, G.; González-Mas, M.C.; Primo-Millo, E.; Iglesias, D.J.; Forner-Giner, M.A. Root signalling and modulation of stomatal closure in flooded citrus seedlings. Plant Physiol. Biochem. 2011, 49, 636–645. [Google Scholar] [CrossRef]
- Bhusal, N.; Kim, H.S.; Han, S.-G.; Yoon, T.-M. Photosynthetic traits and plant–water relations of two apple cultivars grown as bi-leader trees under long-term waterlogging conditions. Environ. Exp. Bot. 2020, 176, 104111. [Google Scholar] [CrossRef]
- Gérard, B.; Alaoui-Sossé, B.; Badot, P.-M. Flooding effects on starch partitioning during early growth of two oak species. Trees 2009, 23, 373–380. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Li, J.; Li, Z.; Li, H.; Mao, J.; Zhai, F.; Liu, J.; Sun, Z. Gender-specific responses of Salix viminalis roots in morphology and physiology subjected to flooding. Flora 2023, 303, 152296. [Google Scholar] [CrossRef]
- Bogarín, M.R.A.; Reis, L.K.; Laura, V.A.; Pott, A.; Szabo, J.K.; Garcia, L.C. Morphological and phenological strategies for flooding tolerance in Cerrado and Pantanal trees: Implications for restoration under new legislation. Restor. Ecol. 2022, 31, e13660. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, F.; Meng, Y.; Chandrasekaran, U.; Luo, X.; Yang, W.; Shu, K. Plant waterlogging/flooding stress responses: From seed germination to maturation. Plant Physiol. Biochem. 2020, 148, 228–236. [Google Scholar] [CrossRef]
- He, B.; Lai, T.; Fan, H.; Wang, W.; Zheng, H. Comparison of flooding-tolerance in four mangrove species in a diurnal tidal zone in the Beibu Gulf. Estuar. Coast. Shelf Sci. 2007, 74, 254–262. [Google Scholar] [CrossRef]
- Parolin, P. Senna reticulata, a pioneer tree from Amazonian várzea floodplains. Bot. Rev. 2001, 67, 239–254. [Google Scholar] [CrossRef]
- Kim, Y.; Shahzad, R.; Lee, I.-J. Regulation of flood stress in plants. In Plant Life Under Changing Environment; Academic Press: Cambridge, MA, USA, 2020; pp. 157–173. [Google Scholar]
- Oliveira, A.S.d.; Ferreira, C.S.; Graciano-Ribeiro, D.; Franco, A.C. Anatomical and morphological modifications in response to flooding by six Cerrado tree species. Acta Bot. Bras. 2015, 29, 478–488. [Google Scholar] [CrossRef]
- Steffens, B.; Rasmussen, A. The physiology of adventitious roots. Plant Physiol. 2016, 170, 603–617. [Google Scholar] [CrossRef]
- Calvo-Polanco, M.; Señorans, J.; Zwiazek, J.J. Role of adventitious roots in water relations of tamarack (Larix laricina) seedlings exposed to flooding. BMC Plant Biol. 2012, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Xu, H.; Khan, S.; Equiza, M.A.; Lee, S.H.; Vaziriyeganeh, M.; Zwiazek, J.J. Plant water transport and aquaporins in oxygen-deprived environments. J. Plant Physiol. 2018, 227, 20–30. [Google Scholar] [CrossRef]
- Vasellati, V.; Oesterheld, M.; Medan, D.; Loreti, J. Effects of flooding and drought on the anatomy of Paspalum dilatatum. Ann. Bot. 2001, 88, 355–360. [Google Scholar] [CrossRef]
- Yamauchi, T.; Noshita, K.; Tsutsumi, N. Climate-smart crops: Key root anatomical traits that confer flooding tolerance. Breed. Sci. 2021, 71, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Parvin, D.; Rashid, P.; Karmoker, J. Anatomical responses of jute (Corchorus capsularis L. cv. D-154) to waterlogging. Dhaka Univ. J. Biol. Sci. 2018, 27, 213–219. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, Z.; Tong, R.; Hu, X.; Du, K. Anatomy and ultrastructure adaptations to soil flooding of two full-sib poplar clones differing in flood-tolerance. Flora 2017, 233, 90–98. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Sun, J.; Yu, X.; Wang, H.; Jia, G.; Zhao, Y.; Tu, Z.; Deng, W.; Jia, J.; Chen, J. Effects of forest structure on hydrological processes in China. J. Hydrol. 2018, 561, 187–199. [Google Scholar] [CrossRef]
- Parente, J.; Pereira, M.G.; Amraoui, M.; Fischer, E.M. Extreme weather conditions: The role of an heat wave on wildfires in Portugal. In Advances in Forest Fire Research 2018; Coimbra University Press: Coimbra, Portugal, 2018; pp. 1200–1204. [Google Scholar]
- Bradford, J.B.; Bell, D.M. A window of opportunity for climate-change adaptation: Easing tree mortality by reducing forest basal area. Front. Ecol. Environ. 2016, 15, 11–17. [Google Scholar] [CrossRef]
- Peñuelas, J.; Ogaya, R.; Boada, M.; Jump, A.S. Migration, invasion and decline: Changes in recruitment and forest structure in a warming-linked shift of European beech forest in Catalonia (NE Spain). Ecography 2007, 30, 829–837. [Google Scholar] [CrossRef]
- Stoddard, M.T.; Huffman, D.W.; Fulé, P.Z.; Crouse, J.E.; Sánchez Meador, A.J. Forest structure and regeneration responses 15 years after wildfire in a ponderosa pine and mixed-conifer ecotone, Arizona, USA. Fire Ecol. 2018, 14, 12. [Google Scholar] [CrossRef]
- Reilly, S.; Clark, M.L.; Bentley, L.P.; Matley, C.; Piazza, E.; Oliveras Menor, I. The Potential of Multispectral Imagery and 3D Point Clouds from Unoccupied Aerial Systems (UAS) for Monitoring Forest Structure and the Impacts of Wildfire in Mediterranean-Climate Forests. Remote Sens. 2021, 13, 3810. [Google Scholar] [CrossRef]
- Pareek, A.; Sopory, S.K.; Bohnert, H. Abiotic Stress Adaptation in Plants; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Rehschuh, R.; Ruehr, N.K. Unrevealing water and carbon relations during and after heat and hot drought stress in Pinus sylvestris. Tree Phisiol. 2022, 42, 1532–1548. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, R.; Xu, X.; Fowler, J.C.; Miller, T.E.X.; Dong, T. Effect of summer warming on growth, photosynthesis and water status in female and male Populus cathayana: Implications for sex-specific drought and heat tolerances. Tree Physiol. 2020, 40, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xuan, A.; Bu, C.; Liu, X.; Zhang, D. Identification of a transcriptional regulatory module that reduces leaf temperature in poplar under heat stress. Tree Physiol. 2020, 40, 1108–1125. [Google Scholar] [CrossRef]
- Ruehr, N.K.; Gast, A.; Weber, C.; Daub, B.; Arneth, A. Water availability as dominant control of heat stress responses in two contrasting tree species. Tree Physiol. 2016, 36, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Schönbeck, L.C.; Schuler, P.; Lehmann, M.M.; Mas, E.; Mekarni, L.; Pivovaroff, A.L.; Turberg, P.; Grossiord, C. Increasing temperature and vapour pressure deficit lead to hydraulic damages in the absence of soil drought. Plant Cell Environ. 2022, 45, 3275–3289. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.E.; Tjoelker, M.G.; Varhammar, A.; Medlyn, B.E.; Reich, P.B.; Leigh, A.; Pfautsch, S.; Blackman, C.J.; Lopez, R.; Aspinwall, M.J.; et al. Trees tolerate an extreme heatwave via sustained transpirational cooling and increased leaf thermal tolerance. Glob. Chang. Biol. 2018, 24, 2390–2402. [Google Scholar] [CrossRef]
- Bar, A.; Michaletz, S.T.; Mayr, S. Fire effects on tree physiology. New Phytol. 2019, 223, 1728–1741. [Google Scholar] [CrossRef] [PubMed]
- Michaletz, S.T. Xylem dysfunction in fires: Towards a hydraulic theory of plant responses to multiple disturbance stressors. New Phytol. 2018, 217, 1391–1393. [Google Scholar] [CrossRef]
- Partelli-Feltrin, R.; Smith, A.M.S.; Adams, H.D.; Thompson, R.A.; Kolden, C.A.; Yedinak, K.M.; Johnson, D.M. Death from hunger or thirst? Phloem death, rather than xylem hydraulic failure, as a driver of fire-induced conifer mortality. New Phytol. 2022, 237, 1154–1163. [Google Scholar] [CrossRef]
- Gricar, J.; Hafner, P.; Lavric, M.; Ferlan, M.; Ogrinc, N.; Krajnc, B.; Eler, K.; Vodnik, D. Post-fire effects on development of leaves and secondary vascular tissues in Quercus pubescens. Tree Physiol. 2020, 40, 796–809. [Google Scholar] [CrossRef]
- Bauweraerts, I.; Ameye, M.; Wertin, T.M.; McGuire, M.A.; Teskey, R.O.; Steppe, K. Water availability is the decisive factor for the growth of two tree species in the occurrence of consecutive heat waves. Agr. For. Meteorol. 2014, 189–190, 19–29. [Google Scholar] [CrossRef]
- Filewod, B.; Thomas, S.C. Impacts of a spring heat wave on canopy processes in a northern hardwood forest. Glob. Chang. Biol. 2014, 20, 360–371. [Google Scholar] [CrossRef]
- Teskey, R.; Wertin, T.; Bauweraerts, I.; Ameye, M.; McGuire, M.A.; Steppe, K. Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 2015, 38, 1699–1712. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, L.; Wittemann, M.; Mujawamariya, M.; Manishimwe, A.; Zibera, E.; Ntirugulirwa, B.; Ract, C.; Manzi, O.J.L.; Andersson, M.X.; Spetea, C.; et al. Handling the heat—Photosynthetic thermal stress in tropical trees. New Phytol. 2022, 233, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Leigh, A.; Sevanto, S.; Ball, M.C.; Close, J.D.; Ellsworth, D.S.; Knight, C.A.; Nicotra, A.B.; Vogel, S. Do thick leaves avoid thermal damage in critically low wind speeds? New Phytol. 2012, 194, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Mackay, D.S.; Savoy, P.R.; Grossiord, C.; Tai, X.; Pleban, J.R.; Wang, D.R.; McDowell, N.G.; Adams, H.D.; Sperry, J.S. Conifers depend on established roots during drought: Results from a coupled model of carbon allocation and hydraulics. New Phytol. 2020, 225, 679–692. [Google Scholar] [CrossRef]
- Thomas, D.S.; Montagu, K.D.; Conroy, J.P. Changes in wood density of Eucalyptus camaldulensis due to temperature—The physiological link between water viscosity and wood anatomy. For. Ecol. Manag. 2004, 193, 157–165. [Google Scholar] [CrossRef]
- Mundo, I.A.; González, C.V.; Stoffel, M.; Ballesteros-Cánovas, J.A.; Villalba, R. Fire damage to cambium affects localized xylem anatomy and hydraulics: The case of Nothofagus pumilio in Patagonia. Am. J. Bot. 2019, 106, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. The cold range limit of trees. Trends Ecol. Evol. 2021, 36, 979–989. [Google Scholar] [CrossRef]
- Reich, P.B.; Sendall, K.M.; Stefanski, A.; Wei, X.; Rich, R.L.; Montgomery, R.A. Boreal and temperate trees show strong acclimation of respiration to warming. Nature 2016, 531, 633–636. [Google Scholar] [CrossRef]
- Carroll, C.J.W.; Martin, P.H.; Knapp, A.K.; Ocheltree, T.W. Temperature induced shifts in leaf water relations and growth efficiency indicate climate change may limit aspen growth in the Colorado Rockies. Environ. Exp. Bot. 2019, 159, 132–137. [Google Scholar] [CrossRef]
- Lafon, C.W. Forest disturbance by ice storms in Quercus forests of the southern Appalachian Mountains, USA. Ecoscience 2006, 13, 30–43. [Google Scholar] [CrossRef]
- Rubio-Cuadrado, A.; Gomez, C.; Rodriguez-Calcerrada, J.; Perea, R.; Gordaliza, G.G.; Julio Camarero, J.; Montes, F.; Gil, L. Differential response of oak and beech to late frost damage: An integrated analysis from organ to forest. Agr. For. Meteorol. 2021, 297, 108243. [Google Scholar] [CrossRef]
- Girardin, M.P.; Guo, J.; Gervais, D.; Metsaranta, J.; Campbell, E.M.; Arsenault, A.; Isaac-Renton, M.; Hogg, E.H. Cold-season freeze frequency is a pervasive driver of subcontinental forest growth. Proc. Natl. Acad. Sci. USA 2022, 119, e2117464119. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Zhu, J.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef]
- Vicente, E.; Didion-Gency, M.; Morcillo, L.; Morin, X.; Vilagrosa, A.; Grossiord, C. Aridity and cold temperatures drive divergent adjustments of European beech xylem anatomy, hydraulics and leaf physiological traits. Tree Physiol. 2022, 42, 1720–1735. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, S.; Arzac, A.; Cooper, D.J.; Jin, Y.; Yuan, D.; Zhu, Y.; Zhang, X.; Li, Z.; Zhang, Y.; et al. Different response of earlywood vessel features of Fraxinus mandshurica to rapid warming in warm-dry and cold-wet areas. Agric. For. Meteorol. 2021, 307, 108523. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Knipfer, T.; Fricke, W.; McElrone, A.J. Aquaporins and root water uptake. In Plant Aquaporins; Signaling and Communication in Plants; Springer: Berlin/Heidelberg, Germany, 2017; pp. 133–153. [Google Scholar]
- Maruta, E.; Yazaki, K.; Ogasa, M.Y.; Taneda, H. Pit aspiration causes an apparent loss of xylem hydraulic conductivity in a subalpine fir (Abies mariesii Mast.) overwintering at the alpine timberline. Tree Physiol. 2021, 42, 1228–1238. [Google Scholar] [CrossRef]
- Sperry, J.S.; Sullivan, J.E. Xylem embolism in response to freeze-thaw cycles and water stress in ring-porous, diffuse-porous, and conifer species. Plant Physiol. 1992, 100, 605–613. [Google Scholar] [CrossRef]
- Niu, C.; Meinzer, F.C.; Hao, G.; Watling, J. Divergence in strategies for coping with winter embolism among co-occurring temperate tree species: The role of positive xylem pressure, wood type and tree stature. Funct. Ecol. 2017, 31, 1550–1560. [Google Scholar] [CrossRef]
- Zanne, A.E.; Tank, D.C.; Cornwell, W.K.; Eastman, J.M.; Smith, S.A.; FitzJohn, R.G.; McGlinn, D.J.; O’Meara, B.C.; Moles, A.T.; Reich, P.B.; et al. Three keys to the radiation of angiosperms into freezing environments. Nature 2014, 506, 89–92. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, F.; Tyree, M.T. Seasonality of cavitation and frost fatigue in Acer mono Maxim. Plant Cell Environ. 2018, 41, 1278–1286. [Google Scholar] [CrossRef]
- Lintunen, A.; Lindfors, L.; Kolari, P.; Juurola, E.; Nikinmaa, E.; Holtta, T. Bursts of CO2 released during freezing offer a new perspective on avoidance of winter embolism in trees. Ann. Bot. 2014, 114, 1711–1718. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S. Functional and ecological xylem anatomy. In Perspectives in Plant Ecology, Evolution and Systematics; Elsevier: Amsterdam, The Netherlands, 2001; Volume 4, pp. 97–115. [Google Scholar]
- Mayr, S.; Sperry, J.S. Freeze-thaw-induced embolism in Pinus contorta: Centrifuge experiments validate the ‘thaw-expansion hypothesis’ but conflict with ultrasonic emission data. New Phytol. 2010, 185, 1016–1024. [Google Scholar] [CrossRef]
- Pittermann, J.; Sperry, J.S. Analysis of freeze-thaw embolism in conifers. The interaction between cavitation pressure and tracheid size. Plant Physiol. 2006, 140, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Lintunen, A.; Salmon, Y.; Hölttä, T.; Suhonen, H. Inspection of gas bubbles in frozen Betula pendula xylem withmicro-CT: Conduit size, water status and bark permeability affect bubble characteristics. Physiol. Plant 2022, 174, e13749. [Google Scholar] [CrossRef]
- Charra-Vaskou, K.; Lintunen, A.; Améglio, T.; Badel, E.; Cochard, H.; Mayr, S.; Salmon, Y.; Suhonen, H.; van Rooij, M.; Charrier, G. Xylem embolism and bubble formation during freezing suggest complex dynamics of pressure-tension in Betula pendula stems. J. Exp. Bot. 2022, erad275. [Google Scholar]
- Lens, F.; Tixier, A.; Cochard, H.; Sperry, J.S.; Jansen, S.; Herbette, S. Embolism resistance as a key mechanism to understand adaptive plant strategies. Curr. Opin. Plant Biol. 2013, 16, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Ogasa, M.Y.; Taneda, H.; Ooeda, H.; Ohtsuka, A.; Maruta, E. Repair of severe winter xylem embolism supports summer water transport and carbon gain in flagged crowns of the subalpine conifer Abies veitchii. Tree Physiol. 2019, 39, 1725–1735. [Google Scholar] [CrossRef]
- Sperry, J.S.; Nichols, K.L.; Sullivan, J.E.; Eastlack, S.E. Xylem embolism in ring-porous, diffuse-porous, and coniferous trees of northern Utah and interior Alaska. Ecology 1994, 75, 1736–1752. [Google Scholar] [CrossRef]
- Nardini, A.; Lo Gullo, M.A.; Salleo, S. Refilling embolized xylem conduits: Is it a matter of phloem unloading? Plant Sci. 2011, 180, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Bowling, D.R.; Logan, B.A.; Hufkens, K.; Aubrecht, D.M.; Richardson, A.D.; Burns, S.P.; Anderegg, W.R.; Blanken, P.D.; Eiriksson, D.P. Limitations to winter and spring photosynthesis of a Rocky Mountain subalpine forest. Agric. For. Meteorol. 2018, 252, 241–255. [Google Scholar] [CrossRef]
- Morris, H.; Plavcova, L.; Gorai, M.; Klepsch, M.M.; Kotowska, M.; Jochen Schenk, H.; Jansen, S. Vessel-associated cells in angiosperm xylem: Highly specialized living cells at the symplast-apoplast boundary. Am. J. Bot. 2018, 105, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Willick, I.R.; Kasuga, J.; Livingston III, D.P. Responses of the plant cell wall to sub-zero temperatures: A brief update. Plant Cell Physiol. 2021, 62, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Pearce, R. Plant freezing and damage. Ann. Bot. 2001, 87, 417–424. [Google Scholar] [CrossRef]
- Arias, N.S.; Scholz, F.G.; Goldstein, G.; Bucci, S.J. The cost of avoiding freezing in stems: Trade-off between xylem resistance to cavitation and supercooling capacity in woody plants. Tree Physiol. 2017, 37, 1251–1262. [Google Scholar] [CrossRef]
- Mayr, S.; Ameglio, T. Freezing stress in tree xylem. Prog. Bot. 2016, 77, 381–414. [Google Scholar] [CrossRef]
- Feng, F.; Ding, F.; Tyree, M.T. Investigations concerning cavitation and frost fatigue in clonal 84K poplar using high-resolution cavitron measurements. Plant Physiol. 2015, 168, 144–155. [Google Scholar] [CrossRef]
- Mayr, S.; Schmid, P.; Beikircher, B.; Feng, F.; Badel, E. Die hard: Timberline conifers survive annual winter embolism. New Phytol. 2020, 226, 13–20. [Google Scholar] [CrossRef]
- Caselles, V.; Casadesus, A.; Munne-Bosch, S. A dual role for abscisic acid integrating the cold stress response at the whole-plant level in Iris pseudacorus L. growing in a natural wetland. Front. Plant Sci. 2021, 12, 722525. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, Y.; Han, X.; Wang, H.; Pan, J.; Yu, D. Jasmonate regulates leaf senescence and tolerance to cold stress: Crosstalk with other phytohormones. J. Exp. Bot. 2017, 68, 1361–1369. [Google Scholar] [CrossRef]
- Ployet, R.; Soler, M.; Carocha, V.; Ladouce, N.; Alves, A.; Rodrigues, J.C.; Harvengt, L.; Marque, C.; Teulieres, C.; Grima-Pettenati, J.; et al. Long cold exposure induces transcriptional and biochemical remodelling of xylem secondary cell wall in Eucalyptus. Tree Physiol. 2018, 38, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Nabais, C.; Vieira, J.; Rossi, S.; Campelo, F. Plastic response of tracheids in Pinus pinaster in a water-limited environment: Adjusting lumen size instead of wall thickness. PLoS ONE 2015, 10, e0136305. [Google Scholar] [CrossRef]
- Savage, J.A.; Kiecker, T.; McMann, N.; Park, D.; Rothendler, M.; Mosher, K. Leaf out time correlates with wood anatomy across large geographic scales and within local communities. New Phytol. 2022, 235, 953–964. [Google Scholar] [CrossRef]
- Davis, S.D.; Sperry, J.S.; Hacke, U.G. The relationship between xylem conduit diameter and cavitation caused by freezing. Am. J. Bot. 1999, 86, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Medek, D.E.; Stuart, S.A.; Pasquet-Kok, J.; Egerton, J.J.; Salari, H.; Sack, L.; Ball, M.C. Xylem traits mediate a trade-off between resistance to freeze–thaw-induced embolism and photosynthetic capacity in overwintering evergreens. New Phytol. 2011, 191, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, L.; Wan, X. Frost fatigue and its spring recovery of xylem conduits in ring-porous, diffuse-porous, and coniferous species in situ. Plant Physiol. Biochem. 2020, 146, 177–186. [Google Scholar] [CrossRef]
- Tedla, B.; Dang, Q.-L.; Inoue, S. Freeze-thaw events delay spring budburst and leaf expansion while longer photoperiods have opposite effect under different [CO2] in white birch: Advance it under elevated but delay it under ambient [CO2]. Environ. Exp. Bot. 2020, 173, 103982. [Google Scholar] [CrossRef]
- Clough, B.J.; Curzon, M.T.; Domke, G.M.; Russell, M.B.; Woodall, C.W. Climate-driven trends in stem wood density of tree species in the eastern United States: Ecological impact and implications for national forest carbon assessments. Glob. Ecol. Biogeogr. 2017, 26, 1153–1164. [Google Scholar] [CrossRef]
- Cochard, H.; Tyree, M.T. Xylem dysfunction in Quercus: Vessel sizes, tyloses, cavitation and seasonal changes in embolism. Tree Physiol. 1990, 6, 393–407. [Google Scholar] [CrossRef]
- Jansen, S.; Schuldt, B.; Choat, B. Current controversies and challenges in applying plant hydraulic techniques: International Workshop on Plant Hydraulic Techniques, Ulm University, Germany, September 2014. New Phytol 2015, 205, 961–964. [Google Scholar] [CrossRef]
- Ahmed, S.; Sarker, S.K.; Friess, D.A.; Kamruzzam, M.; Jacobs, M.; Islam, M.A.; Alam, M.A.; Suvo, M.J.; Sani, M.N.H.; Dey, T.; et al. Salinity reduces site quality and mangrove forest functions. From monitoring to understanding. Sci. Total Environ. 2022, 853, 158662. [Google Scholar] [CrossRef] [PubMed]
- Perri, S.; Detto, M.; Porporato, A.; Molini, A. Salinity-induced limits to mangrove canopy height. Glob. Ecol. Biogeogr. 2023, 32, 1561–1574. [Google Scholar] [CrossRef]
- Qie, Y.; Jiang, L.; Lv, G.; Yang, X.; Wang, H.; Teng, D. Response of plant leaf functional traits to soil aridity and salinity in temperate desert ecosystem. Ecol. Environ. Sci. 2018, 27, 2000–2010. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Guo, R.; Yang, Z.; Li, F.; Yan, C.; Zhong, X.; Liu, Q.; Xia, X.; Li, H.; Zhao, L. Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum) to salt and alkali stress. BMC Plant Biol. 2015, 15, 170. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Physiological Ecology; Springer: Berlin/Heidelberg, Germany, 2008; Volume 2. [Google Scholar]
- Liao, Q.; Gu, S.; Kang, S.; Du, T.; Tong, L.; Wood, J.D.; Ding, R. Mild water and salt stress improve water use efficiency by decreasing stomatal conductance via osmotic adjustment in field maize. Sci. Total Environ. 2022, 805, 150364. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Fatehi, F.; Coventry, S.; Rengasamy, P.; McDonald, G.K. Additive effects of Na+ and Cl– ions on barley growth under salinity stress. J. Exp. Bot. 2011, 62, 2189–2203. [Google Scholar] [CrossRef]
- Vysotskaya, L.; Hedley, P.E.; Sharipova, G.; Veselov, D.; Kudoyarova, G.; Morris, J.; Jones, H.G. Effect of salinity on water relations of wild barley plants differing in salt tolerance. AoB Plants 2010, 2010, plq006. [Google Scholar] [CrossRef]
- Acosta-Motos, J.; Ortuño, M.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.; Hernandez, J. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Navarro, A.; Bañon, S.; Olmos, E.; Sánchez-Blanco, M.d.J. Effects of sodium chloride on water potential components, hydraulic conductivity, gas exchange and leaf ultrastructure of Arbutus unedo plants. Plant Sci. 2007, 172, 473–480. [Google Scholar] [CrossRef]
- Baraldi, R.; Przybysz, A.; Facini, O.; Pierdonà, L.; Carriero, G.; Bertazza, G.; Neri, L. Impact of drought and salinity on sweetgum tree (Liquidambar styraciflua L.): Understanding tree ecophysiological responses in the urban context. Forests 2019, 10, 1032. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Wang, W.; Pivovaroff, A.L.; Li, W.; Zhang, P.; Ward, N.D.; Myers-Pigg, A.; Adams, H.D.; Leff, R.; et al. Seawater exposure causes hydraulic damage in dying Sitka-spruce trees. Plant Physiol. 2021, 187, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Ewers, F.W.; Lopez-Portillo, J.; Angeles, G.; Fisher, J.B. Hydraulic conductivity and embolism in the mangrove tree Laguncularia racemosa. Tree Physiol. 2004, 24, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, N.; Wang, H.; Ren, J.; Yao, Y. Hydraulic characteristics and carbon metabolism of Lycium chinense Miller and Tamarix chinensis Lour.under saline-alkali stress. Plant Sci. J. 2017, 35, 865–873. [Google Scholar] [CrossRef]
- Feng, S.; Ren, L.; Sun, H.; Qiao, K.; Liu, S.; Zhou, A. Morphological and physiological responses of two willow species from different habitats to salt stress. Sci. Rep. 2020, 10, 18228. [Google Scholar] [CrossRef]
- Regni, L.; Del Pino, A.M.; Mousavi, S.; Palmerini, C.A.; Baldoni, L.; Mariotti, R.; Mairech, H.; Gardi, T.; D’Amato, R.; Proietti, P. Behavior of four olive cultivars during salt stress. Front. Plant Sci. 2019, 10, 867. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Beritognolo, I.; Muleo, R.; Piazzai, M.; Sabatti, M.; Mugnozza, G.S.; Kuzminsky, E. Leaf morphological plasticity and stomatal conductance in three Populus alba L. genotypes subjected to salt stress. Environ. Exp. Bot. 2009, 66, 381–388. [Google Scholar] [CrossRef]
- Álvarez, S.; Sanchez-Blanco, M. Long-term effect of salinity on plant quality, water relations, photosynthetic parameters and ion distribution in Callistemon citrinus. Plant Biol. 2014, 16, 757–764. [Google Scholar] [CrossRef]
- Cao, B.; Li, N.; Xu, K. Crosstalk of phenylpropanoid biosynthesis with hormone signaling in Chinese cabbage is key to counteracting salt stress. Environ. Exp. Bot. 2020, 179, 104209. [Google Scholar] [CrossRef]
- Gómez-Bellot, M.J.; Alvarez, S.; Castillo, M.; Bañón, S.; Ortuño, M.F.; Sánchez-Blanco, M.J. Water relations, nutrient content and developmental responses of Euonymus plants irrigated with water of different degrees of salinity and quality. J. Plant Res. 2013, 126, 567–576. [Google Scholar] [CrossRef]
- Franco, J.; Bañón, S.; Vicente, M.; Miralles, J.; Martínez-Sánchez, J. Root development in horticultural plants grown under abiotic stress conditions–a review. J. Hortic. Sci. Biotechnol. 2011, 86, 543–556. [Google Scholar] [CrossRef]
- Croser, C.; Renault, S.; Franklin, J.; Zwiazek, J. The effect of salinity on the emergence and seedling growth of Picea mariana, Picea glauca, and Pinus banksiana. Environ. Pollut. 2001, 115, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Stiller, V. Soil salinity and drought alter wood density and vulnerability to xylem cavitation of baldcypress (Taxodium distichum (L.) Rich.) seedlings. Environ. Exp. Bot. 2009, 67, 164–171. [Google Scholar] [CrossRef]
- Escalante-Perez, M.; Lautner, S.; Nehls, U.; Selle, A.; Teuber, M.; Schnitzler, J.P.; Teichmann, T.; Fayyaz, P.; Hartung, W.; Polle, A.; et al. Salt stress affects xylem differentiation of grey poplar (Populus x canescens). Planta 2009, 229, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Eckert, C.; Sharmin, S.; Kogel, A.; Yu, D.; Kins, L.; Strijkstra, G.-J.; Polle, A. What makes the wood? Exploring the molecular mechanisms of xylem acclimation in hardwoods to an ever-changing environment. Forests 2019, 10, 358. [Google Scholar] [CrossRef]
- Junghans, U.; Polle, A.; Düchting, P.; Weiler, E.; Kuhlman, B.; Gruber, F.; Teichmann, T. Adaptation to high salinity in poplar involves changes in xylem anatomy and auxin physiology. Plant Cell Environ. 2006, 29, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Aloni, R. The induction of vascular tissues by auxin. In Plant Hormones; Springer: Berlin/Heidelberg, Germany, 2010; pp. 485–518. [Google Scholar]
- Hori, C.; Yu, X.; Mortimer, J.C.; Sano, R.; Matsumoto, T.; Kikuchi, J.; Demura, T.; Ohtani, M. Impact of abiotic stress on the regulation of cell wall biosynthesis in Populus trichocarpa. Plant Biotechnol. 2020, 37, 273–283. [Google Scholar] [CrossRef]
- Janz, D.; Lautner, S.; Wildhagen, H.; Behnke, K.; Schnitzler, J.P.; Rennenberg, H.; Fromm, J.; Polle, A. Salt stress induces the formation of a novel type of ‘pressure wood’in two Populus species. New Phytol. 2012, 194, 129–141. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Long, S.; Zhao, C. Maintenance of cell wall integrity under high salinity. Int. J. Mol. Sci. 2021, 22, 3260. [Google Scholar] [CrossRef]
- Kankia, H.; Abdulhamid, Y. Determination of accumulated heavy metals in benthic invertebrates found in Ajiwa Dam, Katsina State, Northern Nigeria. Arch. Appl. Sci. Res. 2014, 6, 80–87. [Google Scholar]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Trammell, T.L.E.; Schneid, B.P.; Carreiro, M.M. Forest soils adjacent to urban interstates: Soil physical and chemical properties, heavy metals, disturbance legacies, and relationships with woody vegetation. Urban. Ecosyst. 2011, 14, 525–552. [Google Scholar] [CrossRef]
- Barker, A.V.; Pilbeam, D.J. Handbook of Plant Nutrition; CRC press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology. Experientia Supplementum; Springer: Berlin/Heidelberg, Germany, 2012; pp. 133–164. [Google Scholar] [CrossRef]
- Ke, W.; Wang, W.; Yang, Y. The characteristic of some micrometal elements accumulation in elsholtizia spledens population at tongllushan copper mine area of Daye in Hubei province. Environ. Sci. Technol. 2000, S1, 33–35. [Google Scholar] [CrossRef]
- Ghori, N.-H.; Ghori, T.; Hayat, M.; Imadi, S.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Zhang, J.; Shu, W. Mechanisms of heavy metal cadmium tolerance in plants. J. Plant Physiol. Mol. Biol. 2006, 32, 1–8. [Google Scholar]
- Houda, Z.; Bejaoui, Z.; Albouchi, A.; Gupta, D.K.; Corpas, F.J. Comparative study of plant growth of two poplar tree species irrigated with treated wastewater, with particular reference to accumulation of heavy metals (Cd, Pb, As, and Ni). Environ. Monit. Assess. 2016, 188, 99. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Luo, Y.; Xing, X.; Christie, P. EDTA-enhanced phytoremediation of heavy metal contaminated soil with Indian mustard and associated potential leaching risk. Agric. Ecosyst. Environ. 2004, 102, 307–318. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Yang, L.; Jin, L.; Song, Y.; Jiang, S.; Qin, L. Transport pathways of cadmium (Cd) and its regulatory mechanisms in plant. Acta Ecol. Sin. 2015, 35, 7921–7929. [Google Scholar]
- Álvarez-Fernández, A.; Díaz-Benito, P.; Abadía, A.; López-Millán, A.-F.; Abadía, J. Metal species involved in long distance metal transport in plants. Front. Plant Sci. 2014, 5, 105. [Google Scholar] [CrossRef]
- Page, V.; Feller, U. Heavy metals in crop plants: Transport and redistribution processes on the whole plant level. Agronomy 2015, 5, 447–463. [Google Scholar] [CrossRef]
- Li, S.; (Department of Environmental Science and Ecology, School of Environmental Science and Engineering, Shaanxi University of Science and Technology, Xi’an, China); Wang, X.; (Department of Environmental Science and Ecology, School of Environmental Science and Engineering, Shaanxi University of Science and Technology, Xi’an, China). Unpublished work. 2022.
- Kumar, S.; Shah, S.H.; Vimala, Y.; Jatav, H.S.; Ahmad, P.; Chen, Y.; Siddique, K.H.M. Abscisic acid: Metabolism, transport, crosstalk with other plant growth regulators, and its role in heavy metal stress mitigation. Front. Plant Sci. 2022, 13, 972856. [Google Scholar] [CrossRef] [PubMed]
- Sridharamurthy, M.; Kovach, A.; Zhao, Y.; Zhu, J.-K.; Xu, H.E.; Swaminathan, K.; Melcher, K. H2O2 inhibits ABA-signaling protein phosphatase HAB1. PLoS ONE 2014, 9, e113643. [Google Scholar] [CrossRef] [PubMed]
- Jentschke, G.; Godbold, D. Metal toxicity and ectomycorrhizas. Physiol. Plant 2000, 109, 107–116. [Google Scholar] [CrossRef]
- de Silva, N.D.; Cholewa, E.; Ryser, P. Effects of combined drought and heavy metal stresses on xylem structure and hydraulic conductivity in red maple (Acer rubrum L.). J. Exp. Bot. 2012, 63, 5957–5966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tan, X.; Zhou, Y.; Liu, N. Effects of a heavy metal (cadmium) on the responses of subtropical coastal tree species to drought stress. Environ. Sci. Pollut. Res. 2022, 30, 12682–12694. [Google Scholar] [CrossRef]
- Przedpelska-Wasowicz, E.M.; Wierzbicka, M. Gating of aquaporins by heavy metals in Allium cepa L. epidermal cells. Protoplasma 2011, 248, 663–671. [Google Scholar] [CrossRef]
- Rucińska-Sobkowiak, R.; Nowaczyk, G.; Krzesłowska, M.; Rabęda, I.; Jurga, S. Water status and water diffusion transport in lupine roots exposed to lead. Environ. Exp. Bot. 2013, 87, 100–109. [Google Scholar] [CrossRef]
- Tanentzap, F.M.; Ryser, P. Decreased resistance to embolism in red maple (Acer rubrum L.) saplings within a heavy metal contaminated region. Environ. Exp. Bot. 2015, 109, 40–44. [Google Scholar] [CrossRef]
- Staňová, A.; Ďurišová, E.; Banásová, V.; Gurinová, E.; Nadubinská, M.; Kenderešová, L.; Ovečka, M.; Čiamporová, M. Root system morphology and primary root anatomy in natural non-metallicolous and metallicolous populations of three Arabidopsis species differing in heavy metal tolerance. Biologia 2012, 67, 505–516. [Google Scholar] [CrossRef]
- Gomes, M.P.; Nogueira, M.d.O.G.; Castro, E.M.d.; Soares, Â.M. Ecophysiological and anatomical changes due to uptake and accumulation of heavy metal in Brachiaria decumbens. Sci. Agric. 2011, 68, 566–573. [Google Scholar] [CrossRef]
- Li, J.; Yu, J.; Du, D.; Liu, J.; Lu, H.; Yan, C. Analysis of anatomical changes and cadmium distribution in Aegiceras corniculatum (L.) Blanco roots under cadmium stress. Mar. Pollut. Bull. 2019, 149, 110536. [Google Scholar] [CrossRef] [PubMed]
- Bazihizina, N.; Taiti, C.; Marti, L.; Rodrigo-Moreno, A.; Spinelli, F.; Giordano, C.; Caparrotta, S.; Gori, M.; Azzarello, E.; Mancuso, S. Zn2+-induced changes at the root level account for the increased tolerance of acclimated tobacco plants. J. Exp. Bot. 2014, 65, 4931–4942. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.A.; Vassileva, V.; Petrov, P.; Popova, L.P. Cadmium-induced structural disturbances in Pisum sativum leaves are alleviated by nitric oxide. Turkish J. Bot. 2013, 37, 698–707. [Google Scholar] [CrossRef]
- Sagardoy, R.; Vázquez, S.; Florez-Sarasa, I.; Albacete, A.; Ribas-Carbó, M.; Flexas, J.; Abadía, J.; Morales, F. Stomatal and mesophyll conductances to CO2 are the main limitations to photosynthesis in sugar beet (Beta vulgaris) plants grown with excess zinc. New Phytol. 2010, 187, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Bora, M.S.; Sarma, K.P. Anatomical and ultrastructural alterations in Ceratopteris pteridoides under cadmium stress: A mechanism of cadmium tolerance. Ecotoxicol. Environ. Saf. 2021, 218, 112285. [Google Scholar] [CrossRef]
- Vaculik, M.; Konlechner, C.; Langer, I.; Adlassnig, W.; Puschenreiter, M.; Lux, A.; Hauser, M.T. Root anatomy and element distribution vary between two Salix caprea isolates with different Cd accumulation capacities. Environ. Pollut. 2012, 163, 117–126. [Google Scholar] [CrossRef]
- Górska, M.; Roszyk, E. Wood structure of Scots pine (L.) growing on flotation tailings. Folia For. Pol. 2019, 61, 112–122. [Google Scholar] [CrossRef]
- Mrak, T.; Grašič, B.; Prislan, P.; Gričar, J.; Laznik, Ž.; Voglar, G.E. Soil contamination with potentially toxic elements and root herbivory: Effects on root surface area and stem secondary xylem of young common beech (Fagus sylvatica L.). Acta Physiol. Plant 2022, 45, 23. [Google Scholar] [CrossRef]
- Li, S.; (Department of Environmental Science and Ecology, School of Environmental Science and Engineering, Shaanxi University of Science and Technology, Xi’an, China); Sun, X.; (Department of Environmental Science and Ecology, School of Environmental Science and Engineering, Shaanxi University of Science and Technology, Xi’an, China); Zhu, X.; (Department of Environmental Science and Ecology, School of Environmental Science and Engineering, Shaanxi University of Science and Technology, Xi’an, China). Unpublished work. 2021.
- Rucińska-Sobkowiak, R. Water relations in plants subjected to heavy metal stresses. Acta Physiol. Plant 2016, 38, 257. [Google Scholar] [CrossRef]
- Solanki, R.; Dhankhar, R. Biochemical changes and adaptive strategies of plants under heavy metal stress. Biologia 2011, 66, 195–204. [Google Scholar] [CrossRef]
- Jia, H.; Wang, X.; Wei, T.; Wang, M.; Liu, X.; Hua, L.; Ren, X.; Guo, J.; Li, J. Exogenous salicylic acid regulates cell wall polysaccharides synthesis and pectin methylation to reduce Cd accumulation of tomato. Ecotoxicol. Environ. Saf. 2021, 207, 111550. [Google Scholar] [CrossRef] [PubMed]
- Krzeslowska, M.; Rabeda, I.; Basinska, A.; Lewandowski, M.; Mellerowicz, E.J.; Napieralska, A.; Samardakiewicz, S.; Wozny, A. Pectinous cell wall thickenings formation—A common defense strategy of plants to cope with Pb. Environ. Pollut. 2016, 214, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, K.; Zheng, W.; Zhang, H.; Cao, X.; Lan, Y.; Yang, C.; Li, C. Characterization of early transcriptional responses to cadmium in the root and leaf of Cd-resistant Salix matsudana Koidz. BMC Genomics 2015, 16, 705. [Google Scholar] [CrossRef]
- da Silva Cunha, L.F.; de Oliveira, V.P.; do Nascimento, A.W.S.; da Silva, B.R.S.; Batista, B.L.; Alsahli, A.A.; Lobato, A.K.d.S. Leaf application of 24-epibrassinolide mitigates cadmium toxicity in young Eucalyptus urophylla plants by modulating leaf anatomy and gas exchange. Physiol. Plantarum 2021, 173, 67–87. [Google Scholar] [CrossRef]
- Hrkic-Ilic, Z.; Borisev, M.; Zoric, L.; Arsenov, D.; Lukovic, J. Assessment of differences in anatomical and hydraulic properties of the root and xylem of three willow (Salix L.) clones during phytostabilization after exposure to elevated cadmium. Arch. Biol. Sci. 2022, 74, 169–180. [Google Scholar] [CrossRef]
- Sruthi, P.; Puthur, J.T. Characterization of physiochemical and anatomical features associated with enhanced phytostabilization of copper in Bruguiera cylindrica (L.) Blume. Int. J. Phytorem 2019, 21, 1423–1441. [Google Scholar] [CrossRef] [PubMed]
- Marques, D.M.; Veroneze Júnior, V.; da Silva, A.B.; Mantovani, J.R.; Magalhães, P.C.; de Souza, T.C. Copper toxicity on photosynthetic responses and root morphology of Hymenaea courbaril L.(Caesalpinioideae). Water Air Soil. Pollut. 2018, 229, 138. [Google Scholar] [CrossRef]
- Ribeiro, A.T.; de Oliveira, V.P.; de Oliveira Barros Junior, U.; da Silva, B.R.S.; Batista, B.L.; da Silva Lobato, A.K. 24-Epibrassinolide mitigates nickel toxicity in young Eucalyptus urophylla ST Blake plants: Nutritional, physiological, biochemical, anatomical and morphological responses. Ann. For. Sci. 2020, 77, 5. [Google Scholar] [CrossRef]
- Iqbal, T.; Shah, S.K.; Ullah, F.; Mehmood, S.; Zeb, M.A. Analysis of deformable distortion in the architecture of leaf xylary vessel elements of Carthamus oxycantha caused by heavy metals stress using image registration. Microsc. Res. Tech. 2020, 83, 843–849. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Marchin, R.M.; Backes, D.; Ossola, A.; Leishman, M.R.; Tjoelker, M.G.; Ellsworth, D.S. Extreme heat increases stomatal conductance and drought-induced mortality risk in vulnerable plant species. Glob. Chang. Biol. 2022, 28, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Rivero, R.M.; Martínez, V.; Gómez-Cadenas, A.; Arbona, V. Tolerance of citrus plants to the combination of high temperatures and drought is associated to the increase in transpiration modulated by a reduction in abscisic acid levels. BMC Plant Biol. 2016, 16, 105. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A.; Inupakutika, M.A.; Mittler, R. ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J. Exp. Bot. 2016, 67, 5381–5390. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Zhou, M.; Shabala, S. How does stomatal density and residual transpiration contribute to osmotic stress tolerance? Plants 2023, 12, 494. [Google Scholar] [CrossRef] [PubMed]
- Alabdallah, N.M.; Alluqmani, S.M. The synthesis of polysaccharide crude nanoparticles extracts from taif rose petals and its effect on eggplant seedlings under drought and salt stress. J. King Saud. Univ. Sci. 2022, 34, 102055. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, Y.; Yan, Z.; Chen, J.; Eziz, A.; Li, K.; Han, W. Resorptions of 10 mineral elements in leaves of desert shrubs and their contrasting responses to aridity. J. Plant Ecol. 2019, 12, 358–366. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Richards, J. Low leaf N and P resorption contributes to nutrient limitation in two desert shrubs. Plant Ecol. 2006, 183, 305–314. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Chen, J.; Zhang, S.; Xu, J.; Han, X.; Feng, Y.; Chen, Y.; Zhang, X.; Dong, G.; et al. Xylem development, cadmium bioconcentration, and antioxidant defense in Populus × euramericana stems under combined conditions of nitrogen and cadmium. Environ. Exp. Bot. 2019, 164, 1–9. [Google Scholar] [CrossRef]
- Sruthi, P.; Puthur, J.T. Cadmium stress alleviation potential of Bruguiera cylindrica (L.) Blume enhances in combination with NaCl. Biorem J. 2021, 26, 89–112. [Google Scholar] [CrossRef]
- Hao, L.; Chen, L.; Zhu, P.; Zhang, J.; Zhang, D.; Xiao, J.; Xu, Z.; Zhang, L.; Liu, Y.; Li, H.; et al. Sex-specific responses of Populus deltoides to interaction of cadmium and salinity in root systems. Ecotoxicol. Environ. Saf. 2020, 195, 110437. [Google Scholar] [CrossRef]
- Lu, Y.W.; Miao, X.L.; Song, Q.Y.; Peng, S.M.; Duan, B.L. Morphological and ecophysiological plasticity in dioecious plant Populus tomentosa under drought and alkaline stresses. Photosynthetica 2018, 56, 1353–1364. [Google Scholar] [CrossRef]
- Kilpeläinen, A.; Gerendiain, A.Z.; Luostarinen, K.; Peltola, H.; Kellomäki, S. Elevated temperature and CO2 concentration effects on xylem anatomy of Scots pine. Tree Physiol. 2007, 27, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Austen, K.; MacLean, J.; Balanzategui, D.; Hölker, F. Microplastic inclusion in birch tree roots. Sci. Total Environ. 2022, 808, 152085. [Google Scholar] [CrossRef]
- Murazzi, M.E.; Cherubini, P.; Brunner, I.; Kägi, R.; Saurer, M.; Ballikaya, P.; Hagedorn, F.; Al Sid Cheikh, M.; Onandia, G.; Gessler, A. Can forest trees take up and transport nanoplastics? iForest 2022, 15, 128–132. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Sun, S.; Scheckel, K.G.; Malysheva, A.; McKenna, B.A.; Menzies, N.W.; Zhao, F.-J.; Kopittke, P.M. Characterizing the uptake, accumulation and toxicity of silver sulfide nanoparticles in plants. Environ. Sci. Nano 2017, 4, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chang, X.; Zhang, J.; Wang, Y.; Zhong, R.; Wang, L.; Wei, J.; Wang, Y. Uptake and distribution of microplastics of different particle sizes in maize (Zea mays) seedling roots. Chemosphere 2023, 313, 137491. [Google Scholar] [CrossRef] [PubMed]



| Heavy Metal | Species | Morphological and Anatomical Response | References |
|---|---|---|---|
| Cd | E. urophylla | Leaf Ds↓ | [277] |
| S. viminalis | Root Dc↓ | [278] | |
| S. alba | Root Dc↑ | [278] | |
| Cr | A. rubrum | Xylem area↓ | [256] |
| Cu | B. cylindrica | Number and size of vessels↓ | [279] |
| Hymenaea courbaril | Ds↓ | [280] | |
| Ni | E. urophylla | Dc and Ds↓ | [281] |
| Carthamus oxycatha | Dc↓ | [282] | |
| A. rubrum. | Xylem area ↓ | [256] | |
| Pb | Lupinus luteus | Cell wall thickness ↑ and vacuole volume↑ | [259] |
| Carthamus oxycatha | Dc↓ | [282] | |
| Zn | Populus alba | Wavy and thinner cell walls Number of vascular bundles per unit area in leaves↓ | |
| Multiple heavy metals | Pisum sylvestris | Tracheid size↓ | [269] |
| F. sylvatica | Growth ring width↓, radial Dc ↓, vessel density↑, vessel grouping index↑ | [270] | |
| S. caprea | Root Dc↓ | [268] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Lu, S.; Wang, J.; Chen, Z.; Zhang, Y.; Duan, J.; Liu, P.; Wang, X.; Guo, J. Responses of Physiological, Morphological and Anatomical Traits to Abiotic Stress in Woody Plants. Forests 2023, 14, 1784. https://doi.org/10.3390/f14091784
Li S, Lu S, Wang J, Chen Z, Zhang Y, Duan J, Liu P, Wang X, Guo J. Responses of Physiological, Morphological and Anatomical Traits to Abiotic Stress in Woody Plants. Forests. 2023; 14(9):1784. https://doi.org/10.3390/f14091784
Chicago/Turabian StyleLi, Shan, Sen Lu, Jing Wang, Zhicheng Chen, Ya Zhang, Jie Duan, Peng Liu, Xueyan Wang, and Junkang Guo. 2023. "Responses of Physiological, Morphological and Anatomical Traits to Abiotic Stress in Woody Plants" Forests 14, no. 9: 1784. https://doi.org/10.3390/f14091784







