The Effect of Atmospheric Carbon Dioxide Concentration on the Growth and Chlorophyll Fluorescence Characteristics of Hazelnut Leaves under Cadmium Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Determination of Experimental Parameters
2.4. Data Analysis
3. Results
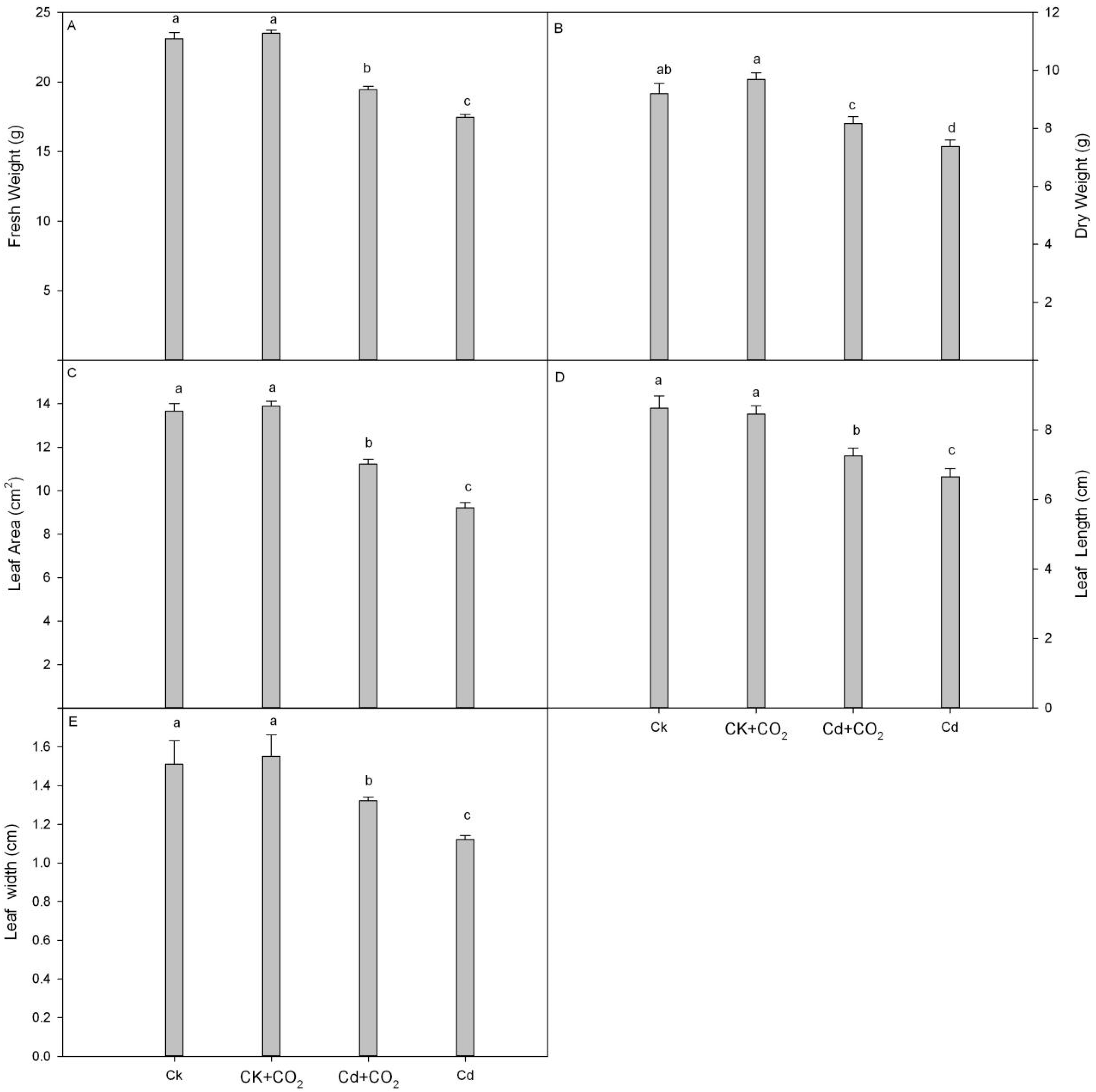
3.1. Growth Characteristics
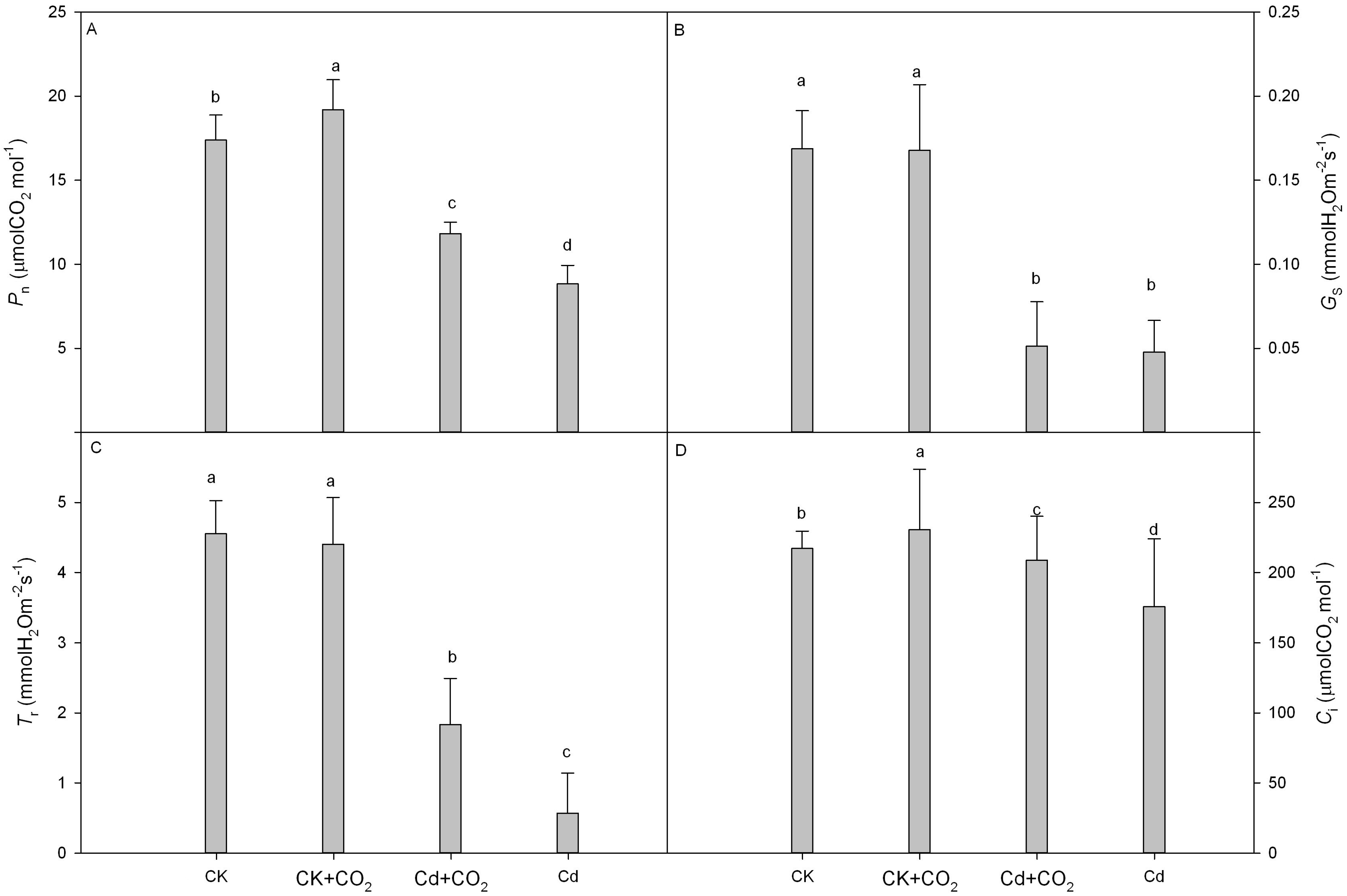
3.2. Photosynthetic Gas Exchange Parameters
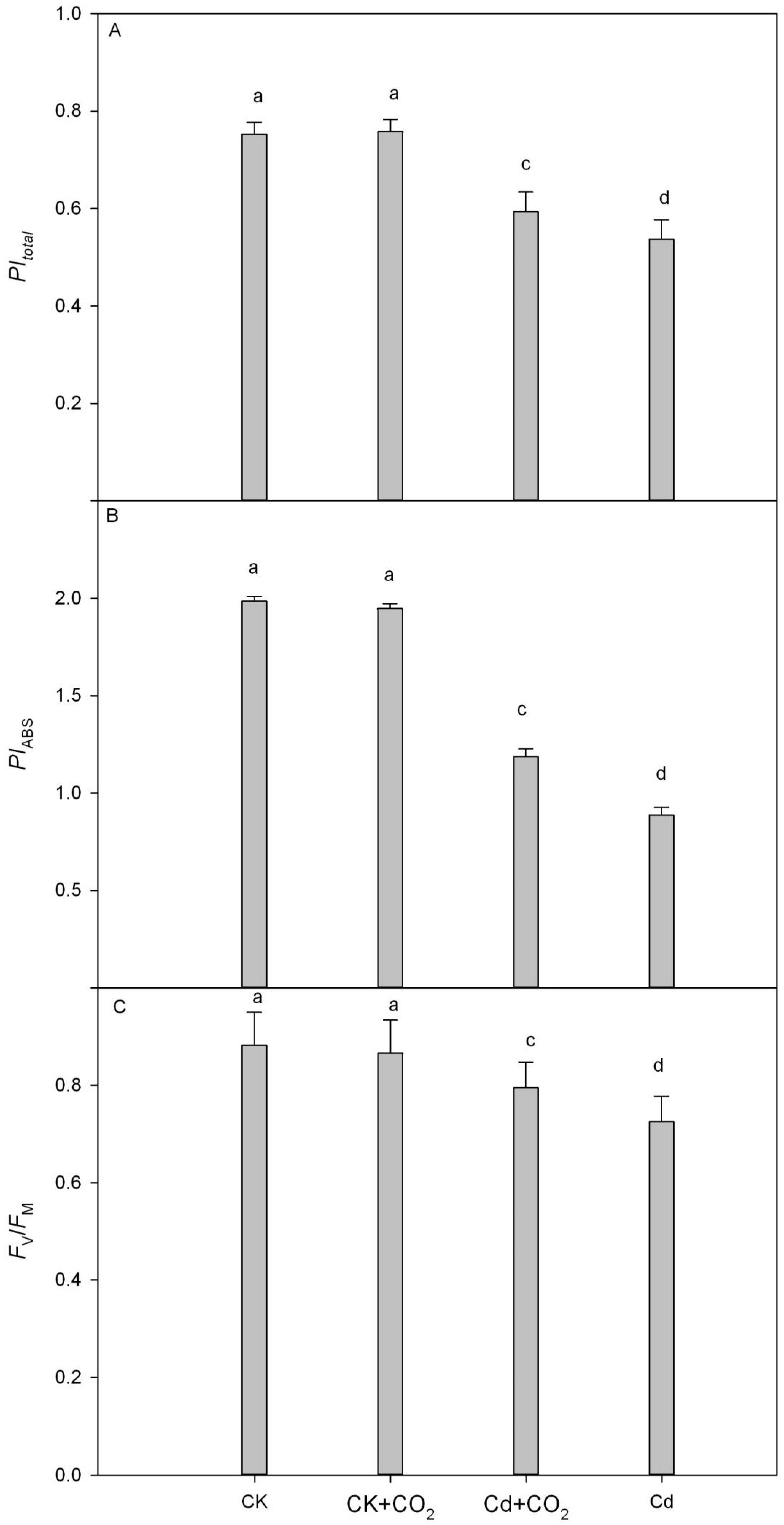
3.3. Chlorophyll Fluorescence Characteristics
3.4. PSII Photochemical Efficiency
3.5. Standardized O-P Curve and Relative Variable Fluorescence VJ, VK, and VL
3.6. Parameters of Energy Distribution per Unit of Reaction Center
4. Discussion
4.1. The Growth and Development of Hazelnut under Cd Stress Conditions
4.2. Effects of High CO2 Concentration on Gas Exchange Parameters under Cd Stress
4.3. Effects of High CO2 Concentration on PSII Photochemical Efficiency under Cd Stress Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Woodward, F.I.; Kelly, C.K. The influence of CO2 concentration on stomatal density. New Phytol. 2006, 131, 311–327. [Google Scholar] [CrossRef]
- Melillo, J.M.; Steudler, P.A.; Aber, J.D. Soil warming and carbon-cycle feedbacks to the climate system. Science 2002, 298, 2173–2176. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.K.; Huggett, B.A.; Tofte, A.N. Cutting xylem under tension or supersaturated with gas can generate PLC and the appearance of rapid recovery from embolism. Plant. Cell Environ. 2013, 36, 1938–1949. [Google Scholar] [CrossRef] [PubMed]
- Suslov, M.A. Dynamics of intercellular water transfer in the roots of intact Zea mays L. plants under elevated concentrations of atmospheric CO2. Plant Physiol. Biochem. 2020, 151, 516–525. [Google Scholar] [CrossRef]
- Kosiba, A.M.; Schaberg, P.G.; Rayback, S.A.; Hawley, G.J. The surprising recovery of red spruce growth shows links to decreased acid deposition and elevated temperature. Sci. Total Environ. 2018, 637, 1480–1491. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Govindjee. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising CO2: Mechanisms and environmental interactions. Plant. Cell. Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Murray, D.R. Plant responses to carbon dioxide. Am. J. Bot. 1995, 82, 690–697. [Google Scholar] [CrossRef]
- Taylor, B.N.; Strand, A.E.; Cooper, E.R.; Beidler, R.E.; Katilyn, V.; Schonholz, M. Root length, biomass, tissue chemistry and mycorrhizal colonization following 14 years of CO2 enrichment and 6 years of N fertilization in a warm temperate forest. Tree Physiol. 2014, 34, 955–965. [Google Scholar] [CrossRef]
- Hassan, W.; Bano, R.; Bashir, S.; Aslam, Z. Cadmium toxicity and soil biological index under potato (Solanum tuberosum L.) cultivation. Soil Res. 2016, 54, 460–468. [Google Scholar] [CrossRef]
- Pazzaglia, P.T.; Weiner, J.; Liu, F. Effects of CO2 elevation and irrigation regimes on leaf gas exchange plant water relations, and water use efficiency of two tomato cultivars. Agric. Water Manag. 2019, 169, 26–33. [Google Scholar] [CrossRef]
- Wu, D.X.; Wang, G.X. Interaction of CO2, enrichment and drought on growth, water use, and yield of broad bean (Vicia faba). Environ. Experi. Bot. 2000, 43, 13–139. [Google Scholar] [CrossRef]
- Zavalloni, C.; Vicca, S.; Buscher, M.; de la Providencia, I.E.; de Boulois, H.D.; Declerck, S.; Nijs, I.; Ceulemans, R. Exposure to warming and CO2 enrichment promotes greater above-ground biomass, nitrogen, phosphorus and arbuscular mycorrhizal colonization in newly established grasslands. Plant Soil 2012, 359, 121–136. [Google Scholar] [CrossRef]
- Tóth, S.Z.; Schansker, G.; Kissimon, J.; Kovã, C.L.; Garab, G.; Strasser, R.J. Biophysical studies of photosystem II-related recovery processes after a heat pulse in barley seedlings (Hordeum vulgare L.). J. Plant. Physiol. 2005, 162, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.W.; Ren, L.K.; Fan, J.W.; Li, Q.; Wang, K.J.; Guo, M.M.; Wang, L.; Li, J.; Zhang, G.X.; Yang, Z.Y.; et al. Salt response of photosynthetic electron transport system in wheat cultivars with contrasting tolerance. Plant. Soil Environ. 2016, 62, 515–521. [Google Scholar] [CrossRef]
- Jiang, H.X.; Chen, L.S.; Zheng, J.G.; Zheng, J.G.; Han, S.; Tang, N.; Smith, B.R. Aluminum-induced effects on Photosystem II photochemistry in Citrus leaves assessed by the chlorophyll a fluorescence transient. Tree Physiol. 2008, 28, 1863–1871. [Google Scholar] [CrossRef]
- Farquhar, G.D.; O’Leary, M.H.; Berry, J.A. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust. J. Plant Physiol. 1982, 9, 121–137. [Google Scholar] [CrossRef]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Sobia, A.; Hafiz, N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Pedroso, D.D.; Barbosa, M.V.; Dos Santos, J.V.; Pinto, F.A.; Siqueira, J.O.; Carneiro, M.A.C. Arbuscular mycorrhizal fungi favor the initial growth of Acacia mangium, Sorghum bicolor, and Urochloa brizantha in soil contaminated with Zn, Cu, Pb, and Cd. Bull. Environ. Contam. Toxicol. 2018, 101, 386–391. [Google Scholar] [CrossRef]
- Xu, H.; Li, L.; Li, Q.H.; Zhou, L.; Zhu, X.J.; Chen, F.J.; Wang, Y.H.; Fang, W.P. Effects of elevated atmospheric CO2 concentration and temperature on photosynthesis system and quality components in tea plant. J. Nanjing Agric. Univ. 2016, 39, 550–556. [Google Scholar]
- Li, Q.; Liu, B.; Wu, Y.; Zou, Z. Interactive effects of drought stresses and elevated CO2 concentration on photochemistry efficiency of cucumber seedlings. J. Int. Plant. Biol. 2008, 50, 1307–1317. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, Y.H.; Liu, T.; He, Y.H. Leaf defense system of Robinia pseudoacacia L. seedlings exposed to 3 years of elevated atmospheric CO2 and Cd-contaminated soils. Sci. Total Environ. 2017, 605, 48–57. [Google Scholar] [CrossRef]
- Li, Q.M.; Liu, B.B.; Zou, Z.R. Effects of doubled CO2 concentration on photosynthetic characteristics of cucumber seedlings under drought stresses. Sci. Agric. Sin. 2011, 44, 963–971. [Google Scholar]
- Curtis, P.S.; Wang, X.Z. A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 1998, 113, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.S.; Li, G.; Gao, H.Y.; Zhang, L.T.; Yang, C.; Liu, P.; Meng, Q.W. Characterization of photosynthetic performance during senescence in stay-green and quick-Leaf-senescence Zea mays L. inbred lines. PLoS ONE 2012, 7, e42936. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Khanboluki, G.; Hosseini, H.M.; Holford, P.; Zadeh, B.M.; Milham, P.J. Effect of elevated atmospheric CO2 concentration on growth and physiology of wheat and sorghum under cadmium stress. Commun. Soil. Sci. Plant Ana. 2018, 49, 2867–2882. [Google Scholar] [CrossRef]
- Guo, B.H.; Dai, S.X.; Wang, R.G.; Guo, J.K.; Ding, Y.A.; Xu, Y.M. Combined effects of elevated CO2 and Cd-contaminated soil on the growth, gas exchange, antioxidant defense, and Cd accumulation of poplars and willows. Environ. Exper Bot. 2015, 115, 1–10. [Google Scholar] [CrossRef]
- Wu, K.R.; Li, J.X.; Luo, J.P.; Liu, Y.K.; Song, Y.C.; Liu, N.L.; Rafiq, M.T.; Li, T.Q. Effects of elevated CO2 and endophytic bacterium on photosynthetic characteristics and cadmium accumulation in Sedum alfredii. Sci. Total Environ. 2018, 643, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Zhang, X.L.; Li, X.; Ding, J.N.; Zhu, W.X.; Qi, F.; Zhang, T.; Tian, Y.; Sun, G.Y. Effects of NaCl and Na2CO3 stresses on the growth and photosynthesis characteristics of Morus alba seedlings. Chin. J. Appl. Ecol. 2012, 23, 625–631. [Google Scholar]
- Tang, L.; Hamid, Y.; Gurajala, H.K.; He, Z.L.; Yang, X.E. Effects of CO2 application and endophytic bacterial inoculation on morphological properties, photosynthetic characteristics and cadmium uptake of two ecotypes of Sedum alfredii Hance. Environ. Sci. Poll. Res. 2019, 26, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.N.; Zhong, H.X.; Li, J.B.; Xing, J.H.; Xu, N.; Zou, H.F. Water use efficiency and photosynthesis of Calamagrostis angustifolia leaves under drought stress through CO2 concentration increase. J. Plant Interact. 2022, 17, 60–74. [Google Scholar] [CrossRef]
- Shuang, H.; Ning, T.; Jiang, H.X.; Yang, L.T.; Li, Y.; Chen, L.S. CO2 assimilation, photosystem II photochemistry, carbohydrate metabolism and antioxidant system of citrus leaves in response to boron stress. Plant Sci. 2009, 176, 143–153. [Google Scholar]
- Shi, Y.; Liu, Y.Q.; Li, H.Y.; Pei, H.P.; Ju, X.H. Phytochelatins formation kinetics and Cd-induced growth inhibition in Lolium perenne L. at elevated CO2 level under Cd stress. Environ. Sci. Poll. Res. 2021, 28, 35751–35763. [Google Scholar] [CrossRef]
- Cohen, I.; Halpern, M.; Yermiyahu, U.; Bar-Tal, A.; Gendler, T.; Rachmilevitch, S. CO2 and nitrogen interaction alters root anatomy, morphology, nitrogen partitioning and photosynthetic acclimation of tomato plants. Planta 2019, 250, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Habeeb, T.H.; Abdel-Mawgoud, M.; Yehia, R.S.; Khalil, A.M.A.; Saleh, A.; Abdelgawad, H. Interactive impact of arbuscular mycorrhizal fungi and elevated CO2 on growth and functional food value of Thymus vulgare. J. Fung. 2020, 6, 168. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Li, Y.; Lu, H.; Wang, S. Combined effects of elevated temperature and CO2 concentration on Cd and Zn accumulation dynamics in Triticum aestivum L. J. Environ. Sci. 2016, 47, 109–119. [Google Scholar] [CrossRef]
- Balabanova, D.A.; Paunov, M.; Goltsev, V.; Cuypers, A.; Vangronsveld, J.; Vassilev, A. Photosynthetic performance of the imidazolinone resistant sunflower exposed to single and combined treatment by the herbicide imazamox and an amino acid extract. Front. Plant Sci. 2016, 7, 1559. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Cai, Y.; Ni, P.; Liu, B.; Tang, X. The Effect of Atmospheric Carbon Dioxide Concentration on the Growth and Chlorophyll Fluorescence Characteristics of Hazelnut Leaves under Cadmium Stress. Forests 2023, 14, 1791. https://doi.org/10.3390/f14091791
Liu X, Cai Y, Ni P, Liu B, Tang X. The Effect of Atmospheric Carbon Dioxide Concentration on the Growth and Chlorophyll Fluorescence Characteristics of Hazelnut Leaves under Cadmium Stress. Forests. 2023; 14(9):1791. https://doi.org/10.3390/f14091791
Chicago/Turabian StyleLiu, Xiaojia, Yan Cai, Peiyan Ni, Binghan Liu, and Xuedong Tang. 2023. "The Effect of Atmospheric Carbon Dioxide Concentration on the Growth and Chlorophyll Fluorescence Characteristics of Hazelnut Leaves under Cadmium Stress" Forests 14, no. 9: 1791. https://doi.org/10.3390/f14091791
APA StyleLiu, X., Cai, Y., Ni, P., Liu, B., & Tang, X. (2023). The Effect of Atmospheric Carbon Dioxide Concentration on the Growth and Chlorophyll Fluorescence Characteristics of Hazelnut Leaves under Cadmium Stress. Forests, 14(9), 1791. https://doi.org/10.3390/f14091791




