Flavonoid Metabolome-Based Active Ingredient Mining and Callus Induction in Catalpa bungei C. A. Mey
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Reagents
2.2. Sterilization of Leaves of Catalpa bungei C. A. Mey
2.3. Callus Induction in Leaves of Catalpa bungei C. A. Mey
2.4. Establishment of Flavonoids Widely Target Metabolomics from Catalpa bungei C. A. Mey
2.5. Elicitor Selection for Flavonoids Accumucation in Catalpa bungei C. A. Mey Leaf Callus
2.6. Determination of Total Flavonoid Content of Catalpa bungei C. A. Mey Callus
2.7. Determination of Diosmetin-7-O-rutinoside (Diosmin) Content of Catalpa bungei C. A. Mey Callus
2.8. Statistical Analysis
3. Results
3.1. Effect of Sterilization Treatments on the In Vitro Culture of Young Leaves of Catalpa bungei C. A. Mey
3.2. Effect of Culture Medium on the Induction of Leaf Callus and Flavonoids Content
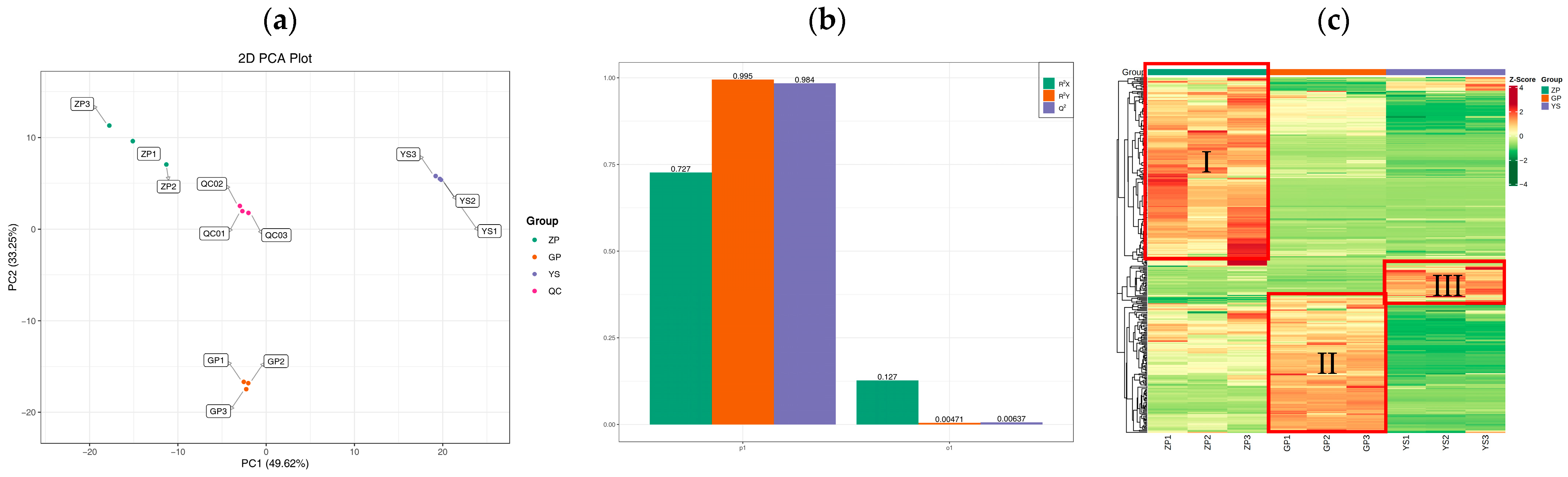
3.3. Quality Control of Extensively Targeted Metabolomic Samples of Catalpa bungei C. A. Mey Flavonoids
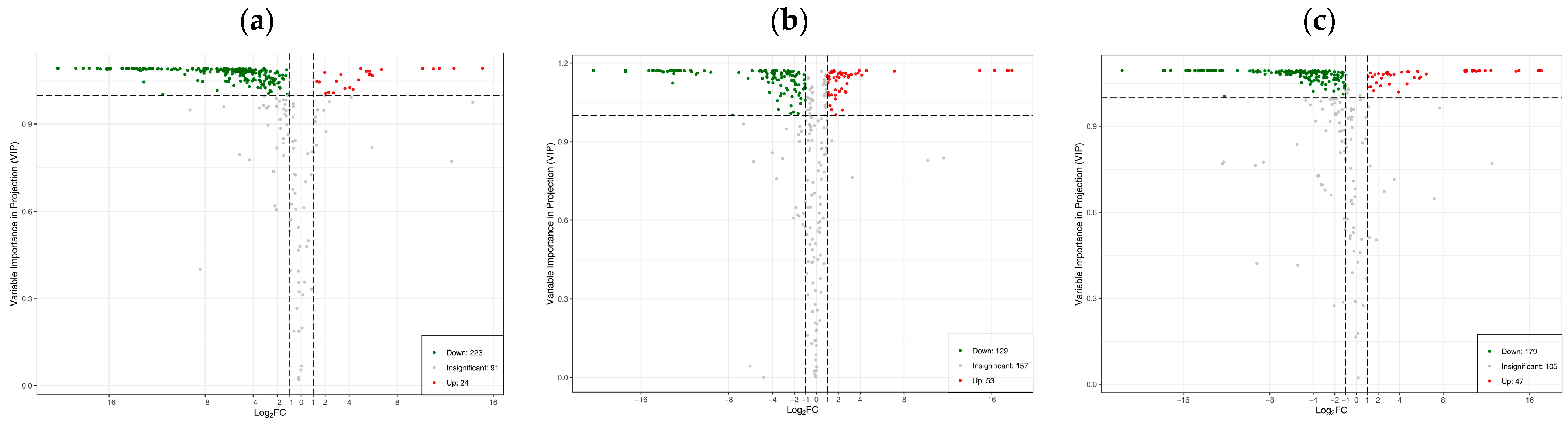
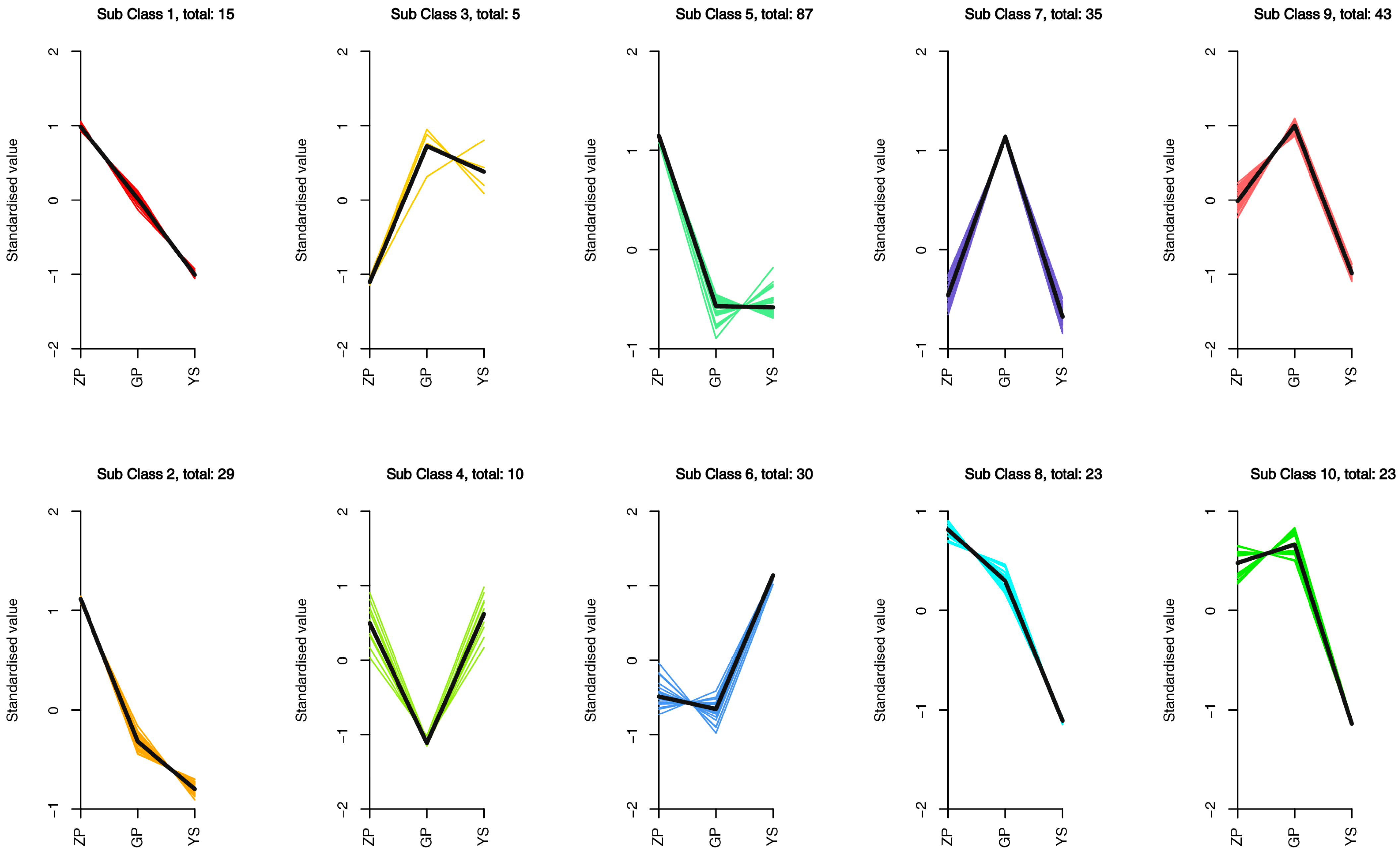
3.4. Differences in Flavonoid Metabolome of Different Materials of Catalpa bungei C. A. Mey
3.5. Effect of Elicitors on the Induction of Callus in Catalpa bungei C. A. Mey
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, H.; Hu, G.; Dong, J.; Wei, Q.; Shao, H.; Lei, M. Antioxidative Activities and Active Compounds of Extracts from Catalpa Plant Leaves. Sci. World J. 2014, 857982. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Bai, M.-M.; Tian, J.-M.; Pescitelli, G.; Lvsic, T.; Huang, X.-H.; Lee, H.; Son, Y.N.; Kim, J.H.; Kim, Y.H.; et al. Chemical components from the seeds of Catalpa bungei and their inhibitions of soluble epoxide hydrolase, cholinesterase and nuclear factor kappa B activities. RSC Adv. 2016, 6, 40706–40716. [Google Scholar] [CrossRef]
- Zhuo, Z.-Q. Study on Growth Law of Catalpa bungei and Accumulation Law of the Total Flavone in Catalpa bungei Leaf Blade. Master’s Thesis, Northwest A&F University, Xianyang, China, 2014. [Google Scholar]
- Xu, H. Studies on Chemical Constituents and Biological Activities of the catalpa bungei C.A. Mey. Leaves and Seeds. Doctor’s Thesis, Northwest A&F University, Xianyang, China, 2015. [Google Scholar]
- Gu, R.-H.; Hong, L.-Y.; Long, C.-L. The ways of producing secondary metabolites via plant cell culture. Plant Physiol. J. 2013, 49, 869–881. [Google Scholar]
- Zhong, J.J. Biochemical engineering of the production of plant-specific secondary metabolites by cell suspension cultures. Adv. Biochem. Eng./Biotechnol. 2001, 72, 1. [Google Scholar] [PubMed]
- Meng, L.; Liu, Y.; Wang, X.; Li, J.; He, G.; Xue, D.; Xing, L.; Li, S. Comparison study on rapid reproductive capacity of four cultivars of Catalpa bungei in tissue culture. J. Cent. South Univ. For. Technol. 2020, 40, 82–88. [Google Scholar]
- Liu, W.; Wang, C.; Shen, X.; Liang, H.; Wang, Y.; He, H.; Zhang, D.; Chen, F. Comparative transcriptome analysis highlights the hormone effects on somatic embryogenesis in Catalpa bungei. Plant Reprod. 2018, 11, 12. [Google Scholar] [CrossRef]
- Gao, H.; Chen, F.; Wang, Y.; Liang, H. Establishment of embryogenic cell suspension culture and plant regeneration of Catalpa bungei. Genom. Appl. Biol. 2018, 37, 895–899. [Google Scholar]
- Smith, C.J. Accumulation of phytoalexins: Defence mechanism and stimulus response system. New Phytol. 1996, 132, 1–45. [Google Scholar] [CrossRef]
- Yang, J.; Wang, S.; Huang, Z.; Guo, P. The complete chloroplast genome sequence of Catalpa bungei (Bignoniaceae): A high-quality timber species from China. Mitochondrial DNA Part B 2020, 5, 3854–3855. [Google Scholar] [CrossRef]
- Sawada, Y.; Akiyama, K.; Sakata, A.; Kuwahara, A.; Otsuki, H.; Sakurai, T.; Saito, K.; Hirai, M.Y. Widely Targeted Metabolomics Based on Large-Scale MS/MS Data for Elucidating Metabolite Accumulation Patterns in Plants. Plant Cell Physiol. 2009, 50, 37–47. [Google Scholar] [CrossRef]
- Zhou, J.; Hou, D.; Zou, W.; Wang, J.; Luo, R.; Wang, M.; Yu, H. Comparison of Widely Targeted Metabolomics and Untargeted Metabolomics of Wild Ophiocordyceps sinensis. Molecules 2022, 27, 3645. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.; Zhang, X.; Huang, L.; Yuan, X.; Xue, C.; Chen, X. Widely targeted metabolomics analysis characterizes the phenolic compounds profiles in mung bean sprouts under sucrose treatment. Food Chem. 2022, 395, 133601. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wu, F.; Lu, A.; Tan, D.; Zhang, Q.; He, Y.; Qin, L. Widely Targeted Metabolomics Analysis of Dendrobium officinale at Different Altitudes. Chem. Biodiver. 2023, 20, e202201082. [Google Scholar] [CrossRef] [PubMed]
- Nikam, T.D.; Savant, R.S. Multiple shoot regeneration and alkaloid cerpegin accumulation in callus culture of Ceropegia juncea Roxb. Physiol. Mol. Biol. Plants 2009, 15, 71–77. [Google Scholar] [CrossRef][Green Version]
- Legha, M.R.; Prasad, K.V.; Singh, S.K.; Kaur, C.; Arora, A.; Kumar, S. Induction of carotenoid pigments in callus cultures of Calendula officinalis L. in response to nitrogen and sucrose levels. In Vitro Cell. Dev. Biol. Plant 2012, 48, 99–106. [Google Scholar] [CrossRef]
- Jain, D.; Bansal, M.K.; Dalvi, R.; Upganlawar, A.; Somani, R. Protective effect of diosmin against diabetic neuropathy in experimental rats. J. Integr. Med. 2014, 12, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Silambarasan, T.; Raja, B. Diosmin, a bioflavonoid reverses alterations in blood pressure, nitric oxide, lipid peroxides and antioxidant status in DOCA-salt induced hypertensive rats. Eur. J. Pharmacol. 2012, 679, 81–89. [Google Scholar] [CrossRef]
- Queenthy, S.S.; John, B. Diosmin exhibits anti-hyperlipidemic effects in isoproterenol induced myocardial infarcted rats. Eur. J. Pharmacol. 2013, 718, 213–218. [Google Scholar] [CrossRef]
- Garner, R.C.; Gamer, J.V.; Gregory, S.; Whattam, M.; Calam, A.; Eong, D. Comparison of the absorption of micronizedt4c-diosmin tablets after oral administration to healthy volunteers by accelerator mass spectrometry and liquid scintillation counting. J. Pharm. Sci. 2002, 91, 32–40. [Google Scholar] [CrossRef]
- Ener, B.K. Dorsal pedal veuous oximetry for assessing treatment of chronic venous in sufficiency:effects of Daflon 500 mg. Vasc Surg. 2000, 34, 43–49. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Kwasniewicz, E.; Deregowska, A.; Wnuk, M. Diosmin-induced senescence, apoptosis and autophagy in breast cancer cells of different p53 status and ERK activity. Toxicol. Lett. 2017, 265, 117–130. [Google Scholar] [CrossRef]
- Buddhan, R.; Manoharan, S. Diosmin reduces cell viability of A431 skin cancer cells through apoptotic induction. J. Cancer Res. Ther. 2017, 13, 471–476. [Google Scholar]
- Hu, S.; Ma, Y.; Jiang, H.; Feng, D.; Yu, W.; Dai, D.; Mei, L. Production of paeoniflorin and albiflorin by callus tissue culture of Paeonia lactiflora Pall. Chin. J. Chem. Eng. 2015, 23, 451–455. [Google Scholar] [CrossRef]
- Edahiro, J.I.; Nakamura, M.; Seki, M.; Furusaki, S. Enhanced accumulation of anthocyanin in cultured strawberry cells by repetitive feeding of l-Phenylalanine into the medium. J. Biosci. Bioeng. 2005, 99, 43–47. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Z.; Zhang, Y. Induction and in vitro alkaloid yield of calluses and protocorm-like bodies (PLBs) from Pinellia ternate. In Vitro Cell. Dev. Biol. Plant 2010, 46, 239–245. [Google Scholar] [CrossRef]
- Wang, Y.D.; Wu, J.C.; Yuan, Y.J. Salicylic acid-induced taxol production and is opentenyl pyrophosphate biosynthesis in suspension cultures of Taxus chinensis var. mairei. Cell Biol. Int. 2013, 31, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Abbasi, B.H.; Ali, G.S. Elicitation of antioxidant secondary metabolites with jasmonates and gibberellic acid in cell suspension cultures of Artemisia absinthium L. Plant Cell Tissue Organ Cult. 2015, 120, 1099–1106. [Google Scholar] [CrossRef]
- Bhanbhani, S.; Karwasara, V.S.; Dixit, V.K.; Banerjee, S. Enhanced production of vasicine in Adhatoda vasica (L.) Nees. cellculture by elicitation. Acta Physiol. Plant. 2012, 34, 1571. [Google Scholar] [CrossRef]
- Ali, A.M.A.; El-Nour, M.E.M.; Yagi, S.M. Total phenolic and flavonoid contents and antioxidant activity of ginger (Zingiber officinale Rosc.) rhizome, callus and callus treated with some elicitors. J. Genet. Eng. Biotechnol. 2018, 16, 677–682. [Google Scholar] [CrossRef]
- Sardar, T.; Maqbool, M.; Ishtiaq, M.; Mazhar, M.W.; El-Sheikh, M.A.; Casini, R.; Mahmoud, E.A.; Elansary, H.O. Synergistic influence of yeast extract and calcium oxide nanoparticles on the synthesis of bioactive antioxidants and metabolites in swertia chirata in vitro callus cultures. Molecules 2023, 28, 4607. [Google Scholar] [CrossRef]
- Chen, H.; Chen, F. Effect of yeast elicitor on the secondary metabolism of Ti-transformed Salvia miltiorrhiza cell suspension cultures. Plant Cell Rep. 2000, 19, 710–717. [Google Scholar] [CrossRef]




| Group | Sterilization Methods | Contamination Rate (%) | Browning Rate (%) | Survival Rate (%) |
|---|---|---|---|---|
| 1 | 75% alcohol 15 s + 0.1% mercuric chloride 2 min | 17.78 ± 4.01 a | 10 ± 3.85 c | 72.22 ± 1.11 a |
| 2 | 75% alcohol 15 s + 0.1% mercuric chloride 4 min | 8.89 ± 2.93 b | 11.11 ± 2.94 c | 80 ± 5.09 a |
| 3 | 75% alcohol 30 s + 0.1% mercuric chloride 2 min | 4.44 ± 4.44 b | 33.34 ± 3.33 b | 62.22 ± 6.76 ab |
| 4 | 75% alcohol 30 s + 0.1% mercuric chloride 4 min | 0 b | 16.67 ± 10.72 bc | 83.33 ± 10.72 a |
| 5 | 75% alcohol 30 s + 0.1% mercuric chloride 6 min | 0 b | 34.44 ± 5.88 b | 65.56 ± 5.88 a |
| 6 | 75% alcohol 30 s + 0.1% mercuric chloride 8 min | 0 b | 58.89 ± 7.78 a | 41.11 ± 7.78 b |
| Group | Hormone Concentration (mg·L−1) | Basic Medium | Induction Rate (%) | Total Flavonoid Content (mg·g−1) | |
|---|---|---|---|---|---|
| 6-BA | NAA | ||||
| A | 0.5 | 0.5 | MS | 52.78 ± 2.78 Bb | 66.50 ± 0.55 e |
| B | 1.0 | 1.5 | MS | 61.11 ± 2.78 ABab | 77.67 ± 1.34 d |
| C | 2.0 | 1.0 | MS | 66.67 ± 4.82 ABa | 77.29 ± 2.46 d |
| D | 0.5 | 1.5 | N6 | 0 Cc | 0 g |
| E | 1.0 | 1.0 | N6 | 2.78 ± 2.78 Cc | 0 g |
| F | 2.0 | 0.5 | N6 | 8.33 ± 4.81 Cc | 46.21 ± 0.41 f |
| G | 0.5 | 1.0 | DKW | 63.89 ± 2.78 ABa | 108.71 ± 1.04 a |
| H | 1.0 | 0.5 | DKW | 69.45 ± 2.78 Aa | 86.55 ± 1.37 c |
| I | 2.0 | 1.5 | DKW | 66.67 ± 4.81 ABa | 98.34 ± 1.15 b |
| K1 | 1.1667 | 1.3056 | 1.8056 | ||
| K2 | 1.3343 | 1.3334 | 0.1111 | ||
| K3 | 1.4167 | 1.2778 | 2.0001 | ||
| R1 | 0.2500 | 0.0556 | 1.8889 | ||
| K4 | 175.21 | 199.26 | 221.46 | ||
| K5 | 164.22 | 186.00 | 46.21 | ||
| K6 | 221.84 | 176.01 | 293.60 | ||
| R2 | 57.62 | 23.25 | 247.39 | ||
| Source | Type III Sum of Squares | Degree of Freedom | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Basic medium | 21,590.012 | 2 | 10,795.006 | 285.417 | 0.000 |
| 6-BA | 324.074 | 2 | 162.037 | 4.284 | 0.028 |
| NAA | 15.438 | 2 | 7.719 | 0.204 | 0.817 |
| Error | 756.438 | 20 | 37.822 |
| Source | Type III Sum of Squares | Degree of Freedom | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Basic medium | 32,371.802 | 2 | 16,185.901 | 101.729 | 0.000 |
| 6-BA | 1872.123 | 2 | 936.061 | 5.883 | 0.010 |
| NAA | 271.954 | 2 | 135.977 | 0.855 | 0.440 |
| Error | 3182.152 | 20 | 159.108 |
| Metabolite | ZP vs. YS | GP vs. YS | |||||
|---|---|---|---|---|---|---|---|
| CAS | VIP | p-Value | Log2FC | VIP | p-Value | Log2FC | |
| Diosmetin-7-O-rutinoside (diosmin) | 520-27-4 | 1.05 | 0.04 | 1.29 | 1.07 | 0.02 | 2.31 |
| Tricin-7-O-(2″-feruloyl)glucoside | -- | 1.07 | 0.01 | 5.95 | 1.10 | 0.01 | 16.81 |
| Kaempferol-3-O-(2″-galloyl)glucoside | 76343-90-3 | 1.01 | 0.14 | 2.71 | 1.09 | 0.11 | 14.75 |
| Quercetin-3-O-(2‴-O-feruloyl) sophoroside | -- | 1.09 | 0.00 | 6.70 | 1.10 | 0.00 | 16.92 |
| Chrysoeriol-7-O-(6″-sinapoyl)glucoside | -- | 1.07 | 0.03 | 5.69 | 1.09 | 0.03 | 17.06 |
| Kaempferol-3-O-neohesperidoside-7-O-glucoside | -- | 1.03 | 0.02 | 4.05 | 1.09 | 0.02 | 15.01 |
| Quercetin-3-O-(2‴-O-caffeoyl)sophoroside | -- | 1.07 | 0.00 | 3.30 | 1.09 | 0.01 | 4.74 |
| Quercetin-3-O-(6″-O-Caffeoyl)sophoroside-7-O-rhamnoside | -- | 1.05 | 0.00 | 2.94 | 1.09 | 0.00 | 5.65 |
| Group | Hormone Concentration (mg·L−1) | Basic Medium | SA (μmol·L−1) | YE (mg·L−1) | Induction Rate (%) | |
|---|---|---|---|---|---|---|
| 6-BA | NAA | |||||
| J | 2.0 | 1.0 | DKW | 0 | 0 | 71.11 ± 2.22 d |
| J-SA1 | 2.0 | 1.0 | DKW | 10 | -- | 100.00 ± 0.00 a |
| J-SA2 | 2.0 | 1.0 | DKW | 50 | -- | 52.22 ± 1.11 e |
| J-SA3 | 2.0 | 1.0 | DKW | 100 | -- | 52.22 ± 2.94 e |
| J-YE1 | 2.0 | 1.0 | DKW | -- | 50 | 44.44 ± 1.11 f |
| J-YE2 | 2.0 | 1.0 | DKW | -- | 100 | 77.78 ± 1.11 c |
| J-YE3 | 2.0 | 1.0 | DKW | -- | 200 | 36.67 ± 1.93 g |
| K | 2.0 | 0.5 | DKW | 0 | 0 | 65.56 ± 1.11 d |
| K-SA1 | 2.0 | 0.5 | DKW | 10 | -- | 92.22 ± 2.94 b |
| K-SA2 | 2.0 | 0.5 | DKW | 50 | -- | 76.67 ± 1.93 c |
| K-SA3 | 2.0 | 0.5 | DKW | 100 | -- | 50.00 ± 1.92 e |
| K-YE1 | 2.0 | 0.5 | DKW | -- | 50 | 67.78 ± 1.11 d |
| K-YE2 | 2.0 | 0.5 | DKW | -- | 100 | 34.44 ± 2.94 g |
| K-YE3 | 2.0 | 0.5 | DKW | -- | 200 | 37.78 ± 1.11 g |
| Group | Hormone Concentration (mg·L−1) | Basic Medium | SA (μmol·L−1) | YE (mg·L−1) | Total Flavonoid Content (mg.g−1) | Diosmetin-7-O-rutinoside (Diosmin) Content (mg.g−1) | |
|---|---|---|---|---|---|---|---|
| 6-BA | NAA | ||||||
| J | 2.0 | 1.0 | DKW | 0 | 0 | 119.064 ± 1.062 i | 6.090 ± 0.903 bcd |
| J-SA1 | 2.0 | 1.0 | DKW | 10 | -- | 133.760 ± 1.116 g | 4.380 ± 0.006 de |
| J-SA2 | 2.0 | 1.0 | DKW | 50 | -- | 127.732 ± 3.01 gh | 4.440 ± 0.592 de |
| J-SA3 | 2.0 | 1.0 | DKW | 100 | -- | 143.308 ± 0.754 de | 5.505 ± 0.551 cde |
| J-YE1 | 2.0 | 1.0 | DKW | -- | 50 | 146.256 ± 1.284 d | 6.660 ± 0.195 bc |
| J-YE2 | 2.0 | 1.0 | DKW | -- | 100 | 168.652 ± 0.781 a | 7.590 ± 0.561 ab |
| J-YE3 | 2.0 | 1.0 | DKW | -- | 200 | 150.084 ± 1.010 c | 8.595 ± 0.602 a |
| K | 2.0 | 0.5 | DKW | 0 | 0 | 101.376 ± 1.00 j | 7.275 ± 0.283 abc |
| K-SA1 | 2.0 | 0.5 | DKW | 10 | -- | 140.272 ± 2.017 e | 6.840 ± 0.588 bc |
| K-SA2 | 2.0 | 0.5 | DKW | 50 | -- | 137.060 ± 2.026 f | 4.890 ± 0.208 de |
| K-SA3 | 2.0 | 0.5 | DKW | 100 | -- | 139.832 ± 1.260 f | 4.200 ± 0.563 e |
| K-YE1 | 2.0 | 0.5 | DKW | -- | 50 | 131.252 ± 1.010 g | 4.860 ± 0.928 de |
| K-YE2 | 2.0 | 0.5 | DKW | -- | 100 | 161.392 ± 0.746 b | 5.850 ± 0.190 cde |
| K-YE3 | 2.0 | 0.5 | DKW | -- | 200 | 126.632 ± 2.082 h | 6.945 ± 0.053 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Wang, X.; Zeng, Y.; Hou, J.; Liu, Z. Flavonoid Metabolome-Based Active Ingredient Mining and Callus Induction in Catalpa bungei C. A. Mey. Forests 2023, 14, 1814. https://doi.org/10.3390/f14091814
Zeng X, Wang X, Zeng Y, Hou J, Liu Z. Flavonoid Metabolome-Based Active Ingredient Mining and Callus Induction in Catalpa bungei C. A. Mey. Forests. 2023; 14(9):1814. https://doi.org/10.3390/f14091814
Chicago/Turabian StyleZeng, Xiaofeng, Xiao Wang, Yanling Zeng, Jinbo Hou, and Zhiming Liu. 2023. "Flavonoid Metabolome-Based Active Ingredient Mining and Callus Induction in Catalpa bungei C. A. Mey" Forests 14, no. 9: 1814. https://doi.org/10.3390/f14091814
APA StyleZeng, X., Wang, X., Zeng, Y., Hou, J., & Liu, Z. (2023). Flavonoid Metabolome-Based Active Ingredient Mining and Callus Induction in Catalpa bungei C. A. Mey. Forests, 14(9), 1814. https://doi.org/10.3390/f14091814





