Tree Species Affect Beetle Diversity on the Common Deciduous Dead Wood in Lithuanian Unmanaged Forests
Abstract
1. Introduction
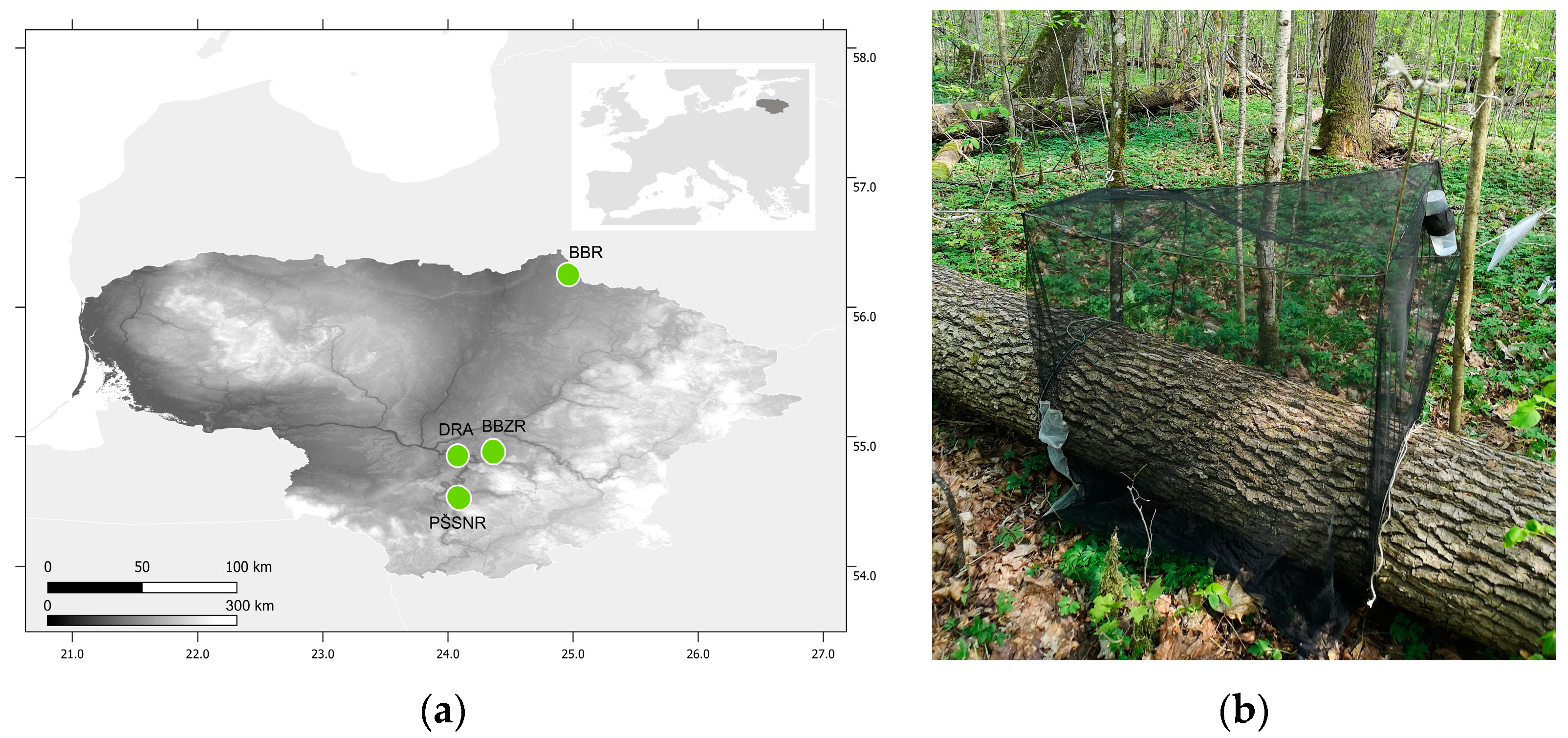
2. Materials and Methods
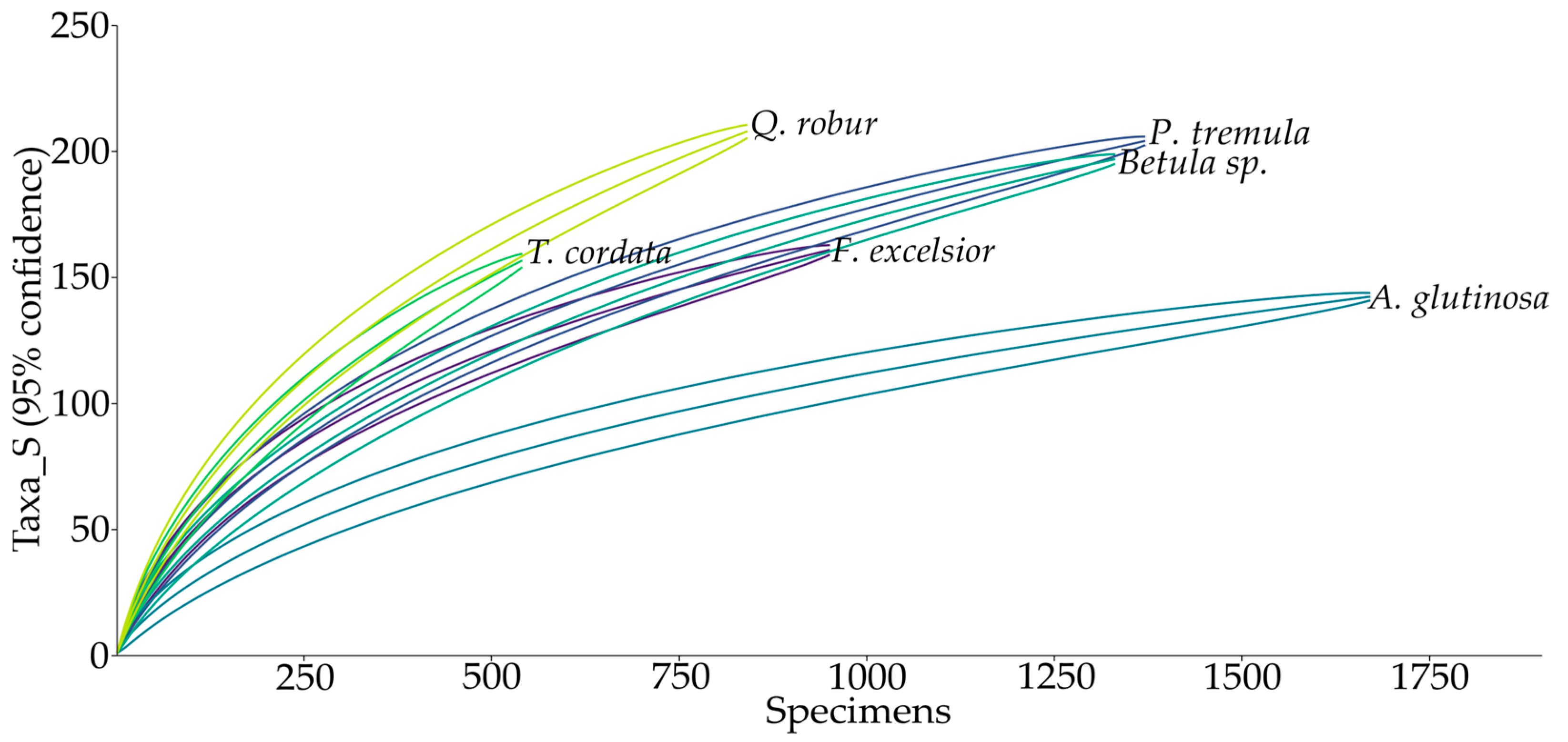
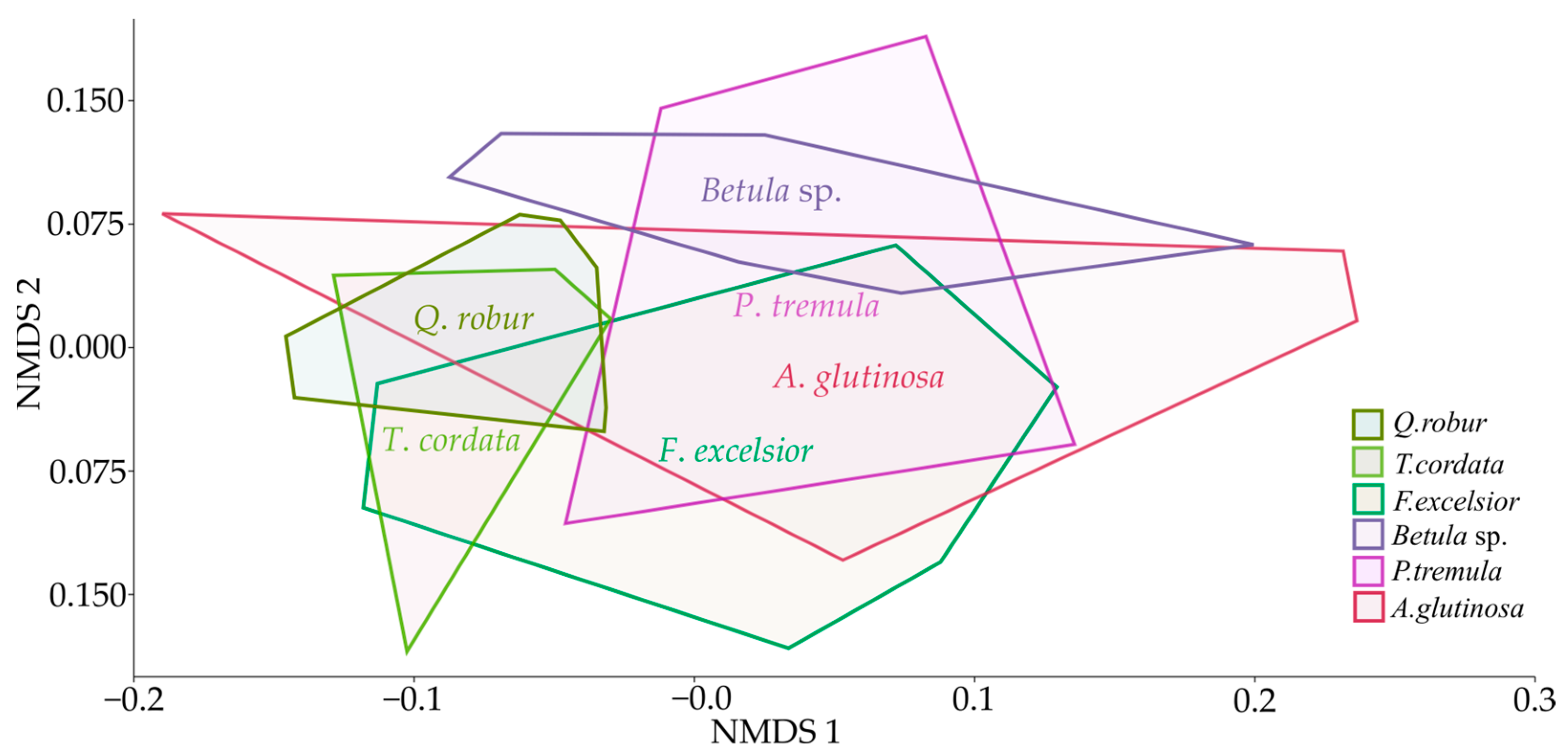
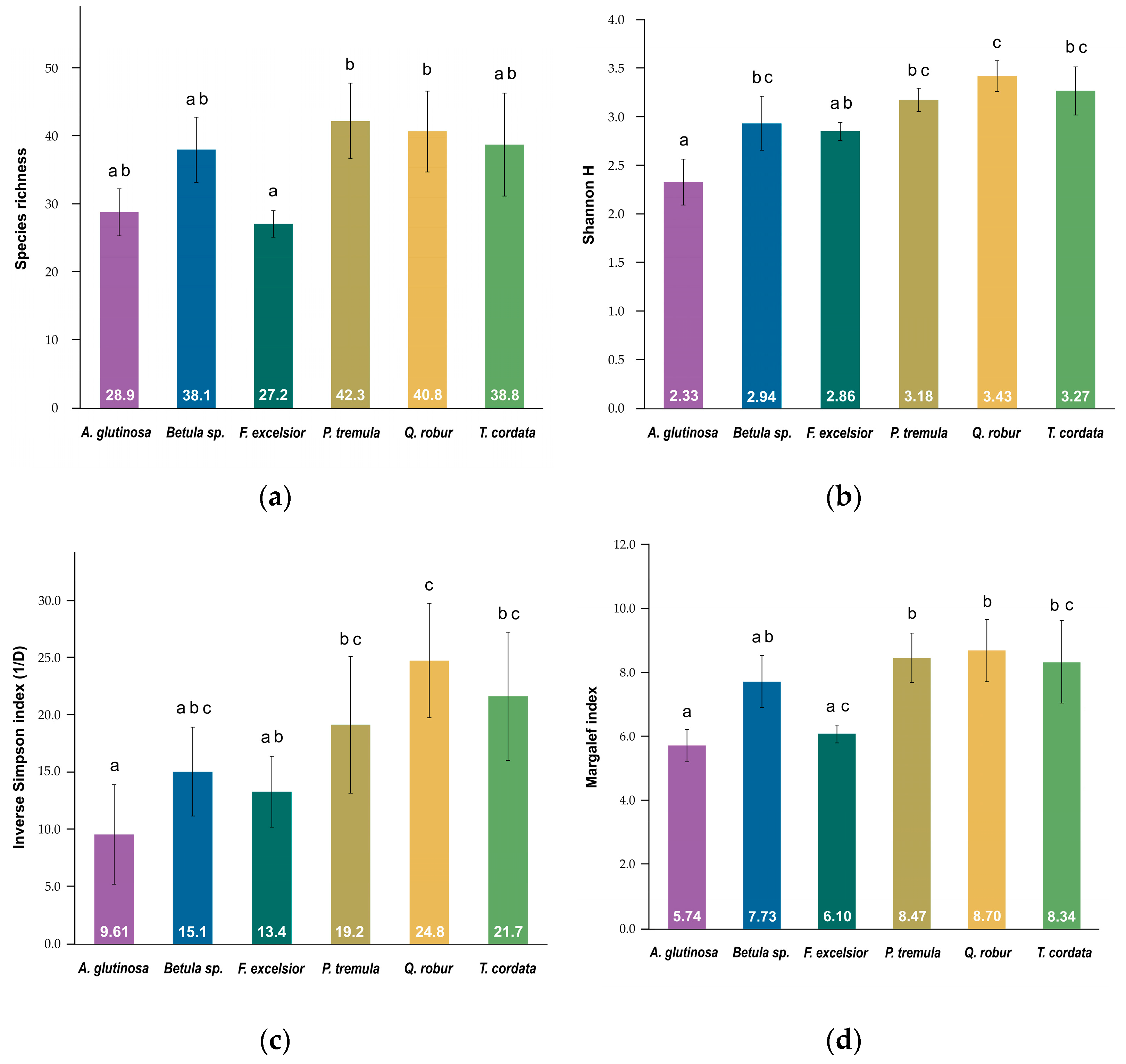
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.; Skutsch, M.; Paneque-Gálvez, J.; Ghilardi, A. Remote sensing of forest degradation: A review. Environ. Res. Lett. 2020, 15, 103001. [Google Scholar] [CrossRef]
- Barlow, J.; Lennox, G.D.; Ferreira, J.; Berenguer, E.; Lees, A.C.; Nally, R.M.; Thomson, J.R.; Ferraz, S.F.D.B.; Louzada, J.; Oliveira, V.H.F.; et al. Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature 2016, 535, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Thorn, S.; Seibold, S.; Leverkus, A.B.; Michler, T.; Müller, J.; Noss, R.F.; Stork, N.; Vogel, S.; Lindenmayer, D.B. The living dead: Acknowledging life after tree death to stop forest degradation. Front. Ecol. Environ. 2020, 18, 505–512. [Google Scholar] [CrossRef]
- Pastorelli, R.; De Meo, I.; Lagomarsino, A. The necrobiome of deadwood: The life after death. Ecologies 2022, 4, 20–38. [Google Scholar] [CrossRef]
- Puletti, N.; Canullo, R.; Mattioli, W.; Gawryś, R.; Corona, P.; Czerepko, J. A dataset of forest volume deadwood estimates for Europe. Ann. For. Sci. 2019, 76, 68. [Google Scholar] [CrossRef]
- Merganičová, K.; Merganič, J.; Svoboda, M.; Bače, R.; Šebeň, V. Deadwood in forest ecosystems. In Forest Ecosystems—More than Just Trees; Blanco, J.A., Lo, Y.H., Eds.; InTech: London, UK, 2012; pp. 81–108. [Google Scholar] [CrossRef]
- Speight, M.C.D. Saproxylic Invertebrates and Their Conservation; Nature and Environment Series; Council of Europe: Strasbourg, France, 1989; pp. 1–81. [Google Scholar]
- Stokland, J.N.; Siitonen, J.; Jonsson, B.G. Biodiversity in Dead Wood; Cambridge University Press: New York, NY, USA, 2012; pp. 1–509. [Google Scholar]
- Köhler, F. Totholzkäfer in Naturwaldzellen des Nördlichen Rheinlandes. Vergleichende Studien zur Totholzkäferfauna Deutschlands und deutschen Naturwaldforschung; LÖBF/ Landesamt für Agrarordnung Nordrhein-Westfalen: Recklinghausen, Germany, 2000; pp. 1–351. [Google Scholar]
- Franc, N. Standing or downed dead trees—Does it matter for saproxylic beetles in temperate oak-rich forest? Can. J. For. Res. 2007, 37, 2494–2507. [Google Scholar] [CrossRef]
- Macagno, A.L.M.; Hardersen, S.; Nardi, G.; Lo Giudice, G.; Mason, F. Measuring saproxylic beetle diversity in small and medium diameter dead wood: The “grab-and-go” method. Eur. J. Entomol. 2015, 112, 510–519. [Google Scholar] [CrossRef]
- Muñoz-López, N.Z.; Andrés-Hernández, A.R.; Carrillo-Ruiz, H.; Rivas-Arancibia, S.P. Coleoptera associated with decaying wood in a tropical deciduous Forest. Neotrop. Entomol. 2016, 45, 341–350. [Google Scholar] [CrossRef]
- Procházka, J.; Schlaghamerský, J. Does dead wood volume affect saproxylic beetles in montane beech-fir forests of central Europe? J. Insect Conserv. 2019, 23, 157–173. [Google Scholar] [CrossRef]
- Jonsell, M. Saproxylic beetle species in logging residues: Which are they and which residues do they use? Norw. J. Entomol. 2008, 55, 109–122. [Google Scholar]
- Jonsell, M.; Nittérus, K.; Stighäll, K. Saproxylic beetles in natural and man-made deciduous high stumps retained for conservation. Biol. Conserv. 2004, 118, 163–173. [Google Scholar] [CrossRef]
- Seibold, S.; Brandl, R.; Buse, J.; Hothorn, T.; Schmidl, J.; Thorn, S.; Müller, J. Association of extinction risk of saproxylic beetles with ecological degradation of forests in Europe: Beetle extinction and forest degradation. Conserv. Biol. 2015, 29, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Bouget, C.; Larrieu, L.; Nusillard, B.; Parmain, G. In search of the best local habitat drivers for saproxylic beetle diversity in temperate deciduous forests. Biodivers. Conserv. 2013, 22, 2111–2130. [Google Scholar] [CrossRef]
- Vogel, S.; Bussler, H.; Finnberg, S.; Müller, J.; Stengel, E.; Thorn, S. Diversity and conservation of saproxylic beetles in 42 European tree species: An experimental approach using early successional stages of branches. Insect Conserv. Divers. 2021, 14, 132–143. [Google Scholar] [CrossRef]
- Zuo, J.; Berg, M.P.; Van Hal, J.; Van Logtestijn, R.S.P.; Goudzwaard, L.; Hefting, M.M.; Poorter, L.; Sterck, F.J.; Cornelissen, J.H.C. Fauna community convergence during decomposition of deadwood across tree species and forests. Ecosystems 2021, 24, 926–938. [Google Scholar] [CrossRef]
- Wu, J.; Yu, X.D.; Zhou, H.Z. The saproxylic beetle assemblage associated with different host trees in southwest China. Insect Sci. 2008, 15, 251–261. [Google Scholar] [CrossRef]
- Thunes, K.H.; Søli, G.E.E.; Thuróczy, C.; Fjellberg, A.; Olberg, S.; Roth, S.; Coulianos, C.C.; Disney, R.H.L.; Starý, J.; Vierbergen, G.; et al. The arthropod fauna of oak (Quercus spp., Fagaceae) canopies in Norway. Diversity 2021, 13, 332. [Google Scholar] [CrossRef]
- Milberg, P.; Bergman, K.O.; Johansson, H.; Jansson, N. Low host-tree preferences among saproxylic beetles: A comparison of four deciduous species. Insect Conserv. Divers. 2014, 7, 508–522. [Google Scholar] [CrossRef]
- Sverdrup-Thygeson, A.; Ims, R.A. The effect of forest clearcutting in Norway on the community of saproxylic beetles on aspen. Biol. Conserv. 2002, 106, 347–357. [Google Scholar] [CrossRef]
- Kouki, J.; Arnold, K.; Martikainen, P. Long-term persistence of aspen—A key host for many threatened species—Is endangered in old-growth conservation areas in Finland. J. Nat. Conserv. 2004, 12, 41–52. [Google Scholar] [CrossRef]
- Lindhe, A.; Lindelöw, Å. Cut high stumps of spruce, birch, aspen and oak as breeding substrates for saproxylic beetles. For. Ecol. Manag. 2004, 203, 1–20. [Google Scholar] [CrossRef]
- Jonsell, M.; Weslien, J.; Ehnström, B. Substrate Requirements of Red-Listed Saproxylic Invertebrates in Sweden. Biodivers. Conserv. 1998, 7, 749–764. [Google Scholar] [CrossRef]
- Warren, M.S.; Key, R.S. Woodlands: Past, present and potential for insects. In The Conservation of Insects and Their Habitats, Proceedings of the 15th Symposium of the Royal Entomological Society of London, at the Department of Physics Lecture Theatre, Imperial College, London, UK, 14–15 Septempber 1989; Collins, N.M., Thomas, J.A., Eds.; Academic Press: London, UK, 1991; pp. 155–211. [Google Scholar]
- Kärvemo, S.; Schroeder, M.; Ranius, T. Beetle diversity in dead wood is lower in non-native than native tree species, especially those more distantly related to native species. J. Appl. Ecol. 2023, 60, 170–180. [Google Scholar] [CrossRef]
- Losos, J.B. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol. Lett. 2008, 11, 995–1003. [Google Scholar] [CrossRef]
- Pyron, R.A.; Costa, G.C.; Patten, M.A.; Burbrink, F.T. Phylogenetic niche conservatism and the evolutionary basis of ecological speciation: Niche conservatism and speciation. Biol. Rev. 2015, 90, 1248–1262. [Google Scholar] [CrossRef] [PubMed]
- Crisp, M.D.; Cook, L.G. Phylogenetic niche conservatism: What are the underlying evolutionary and ecological causes? New Phytol. 2012, 196, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Berg, M.P.; Klein, R.; Nusselder, J.; Neurink, G.; Decker, O.; Hefting, M.M.; Sass-Klaassen, U.; Logtestijn, R.S.P.; Goudzwaard, L.; et al. Faunal community consequence of interspecific bark trait dissimilarity in early-stage decomposing logs. Funct. Ecol. 2016, 30, 1957–1966. [Google Scholar] [CrossRef]
- Kraus, D.; Bütler, R.; Krumm, F.; Lachat, T.; Larrieu, L.; Mergner, U.; Paillet, Y.; Rydkvist, T.; Schuck, A.; Winter, S. Catalogue of Tree Microhabitats—Reference Field List, Integrate + Technical Paper; European Forest Institute: Freiburg, Germany, 2016; p. 16. [Google Scholar]
- Ulyshen, M.D.; Šobotník, J. An introduction to the diversity, ecology, and conservation of saproxylic insects. In Saproxylic Insects; Ulyshen, M.D., Ed.; Zoological Monographs; Springer International Publishing: Cham, Switzerland, 2018; Volume 1, pp. 1–47. [Google Scholar] [CrossRef]
- Bani, A.; Pioli, S.; Ventura, M.; Panzacchi, P.; Borruso, L.; Tognetti, R.; Tonon, G.; Brusetti, L. The role of microbial community in the decomposition of leaf litter and deadwood. Appl. Soil Ecol. 2018, 126, 75–84. [Google Scholar] [CrossRef]
- Lekoveckaitė, A.; Podėnienė, V.; Ferenca, R. Beetles (Coleoptera) in deciduous dead wood tree species trunks in Lithuania. Biodivers. Data J. 2023, 11, e106132. [Google Scholar] [CrossRef]
- Lithuanian State Forest Service. M-GIS Geoinformacija Apie Miškus [M-GIS Geoinformation about Forests]. Available online: https://kadastras.amvmt.lt/vartai/ (accessed on 23 July 2023).
- Varnagirytė-Kabašinskienė, I.; Lukminė, D.; Mizaras, S.; Beniušienė, L.; Armolaitis, K. Lithuanian forest biomass resources: Legal, economic and ecological aspects of their use and potential. Energy Sustain. Soc. 2019, 9, 41. [Google Scholar] [CrossRef]
- Palviainen, M.; Laiho, R.; Mäkinen, H.; Finér, L. Do decomposing Scots Pine, Norway Spruce, and Silver Birch stems retain nitrogen? Can. J. For. Res. 2008, 38, 3047–3055. [Google Scholar] [CrossRef]
- Renvall, P. Community structure and dynamics of wood-rotting Basidiomycetes on decomposing conifer trunks in Northern Finland. Karstenia 1995, 35, 1–51. [Google Scholar] [CrossRef]
- Halme, P.; Vartija, N.; Salmela, J.; Penttinen, J.; Norros, V. High within- and between-trunk variation in the nematoceran (Diptera) community and its physical environment in decaying aspen trunks. Insect Conserv. Divers. 2013, 6, 502–512. [Google Scholar] [CrossRef]
- Käfer Europas. Die Käfer Europas. Ein Bestimmungswerk Im Internet. Herausgegeben von Arved Lompe, Nienburg/Weser. Begründet Im September 2002. Available online: http://Coleonet.de/Coleo/Index.Htm (accessed on 19 August 2023).
- Mike’s Insect Keys. Keys for the Identification of British Insects. Available online: https://Sites.Google.Com/Site/Mikesinsectkeys/ (accessed on 19 August 2023).
- De Jong, Y.; Verbeek, M.; Michelsen, V.; Bjørn, P.D.P.; Los, W.; Steeman, F.; Bailly, N.; Basire, C.; Chylarecki, P.; Stloukal, E.; et al. Fauna Europaea—All European animal species on the web. Biodivers. Data J. 2014, 2, e4034. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for education and data analysis. Paleontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-4. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 23 August 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 23 August 2023).
- Dormann, C.F.; Gruber, B.; Fründ, J. Introducing the bipartite package: Analysing ecological networks. Interaction 2008, 8, 8–11. [Google Scholar]
- Cálix, M.; Alexander, K.N.A.; Nieto, A.; Dodelin, B.; Soldati, F.; Telnov, D.; Vazquez-Albalate, X.; Aleksandrowicz, O.; Audisio, P.; Istrate, P.; et al. European Red List of Saproxylic Beetles; IUCN: Brussels, Belgium, 2018; pp. 1–24. Available online: https://portals.iucn.org/library/node/47296 (accessed on 20 August 2023).
- Durka, W.; Michalski, S.G. Daphne: A dated phylogeny of a large European flora for phylogenetically informed ecological analyses: Ecological Archives E093-214. Ecology 2012, 93, 2297. [Google Scholar] [CrossRef]
- Gossner, M.M.; Wende, B.; Levick, S.; Schall, P.; Floren, A.; Linsenmair, K.E.; Steffan-Dewenter, I.; Schulze, E.D.; Weisser, W.W. Deadwood enrichment in European forests—Which tree species should be used to promote saproxylic beetle diversity? Biol. Conserv. 2016, 201, 92–102. [Google Scholar] [CrossRef]
- Goßner, M.M.; Chao, A.; Bailey, R.I.; Prinzing, A. Native fauna on exotic trees: Phylogenetic conservatism and geographic contingency in two lineages of phytophages on two lineages of trees. Am. Nat. 2009, 173, 599–614. [Google Scholar] [CrossRef]
- Baber, K.; Otto, P.; Kahl, T.; Gossner, M.M.; Wirth, C.; Gminder, A.; Bässler, C. Disentangling the effects of forest-stand type and dead-wood origin of the early successional stage on the diversity of wood-inhabiting fungi. For. Ecol. Manag. 2016, 377, 161–169. [Google Scholar] [CrossRef]
- Sawoniewicz, M. Beetles (Coleoptera) occurring in decaying birch (Betula spp.) Wood in the Kampinos National Park. For. Res. Pap. 2013, 74, 71–85. [Google Scholar] [CrossRef]
- Maňák, V.; Schlaghamerský, J. The saproxylic beetles of Dlúhý Hrúd, an old-growth remnant on the lower Dyje river (Czechia). In Saproxylic Beetles—Their Role and Diversity in European Woodland and Tree Habitats; Buse, J., Alexander, K.N.A., Ranius, T., Assmann, T., Eds.; Pensoft Publishers: Moscow, Russia, 2009; pp. 49–76. [Google Scholar]
- Byk, A.; Mokrzycki, T.; Perliński, S.; Rutkiewicz, A. Saproxylic Beetles—In the Monitoring of Anthropogenic Transformations of Białowieża Primeval Forest; Szujecki, A., Ed.; Warsaw Agricultural University Press: Warsaw, Poland, 2006; pp. 325–397. [Google Scholar]
- Toivanen, T.; Kotiaho, J.S. The Preferences of saproxylic beetle species for different dead wood types created in forest restoration treatments. Can. J. For. Res. 2010, 40, 445–464. [Google Scholar] [CrossRef]
- Horák, J. Response of saproxylic beetles to tree species composition in a secondary urban forest area. Urban For. Urban Green. 2011, 10, 213–222. [Google Scholar] [CrossRef]
- Papis, M.; Mokrzycki, T. Saproxylic beetles (Coleoptera) of the strictly protected area Bukowa Góra in the Roztoczański National Park. For. Res. Pap. 2015, 76, 229–239. [Google Scholar] [CrossRef][Green Version]
- Cucujus cinnaberinus. Available online: https://doi.org/10.2305/IUCN.UK.2010-1.RLTS.T5935A11921415.en (accessed on 20 August 2023). [CrossRef]
- Ferenca, R. Purpurinis plokščiavabalis. Cucujus cinnaberinus (Scopoli, 1763). In Red Data Book of Lithuania. Animals, Plants, Fungi; Rašomavičius, V., Ed.; Lututė: Vilnius, Lithuania, 2021; p. 131. [Google Scholar]
- Vrezec, A.; Ambrožič, Š.; Kobler, A.; Kapla, A.; De Groot, M. Cucujus cinnaberinus (Scopoli, 1763) at its terra typica in Slovenia: Historical overview, distribution patterns and habitat selection. Nat. Conserv. 2017, 19, 219–229. [Google Scholar] [CrossRef]
- Lygis, V.; Bakys, R.; Gustiene, A.; Burokiene, D.; Matelis, A.; Vasaitis, R. Forest self-regeneration following clear-felling of dieback-affected Fraxinus Excelsior: Focus on ash. Eur. J. Forest Res. 2014, 133, 501–510. [Google Scholar] [CrossRef]
- National Forestry Accounting [Valstybinė Miškų Apskaita]. Available online: https://amvmt.lrv.lt/lt/atviri-duomenys-1/misku-statistikos-leidiniai/valstybine-misku-apskaita (accessed on 25 July 2023).
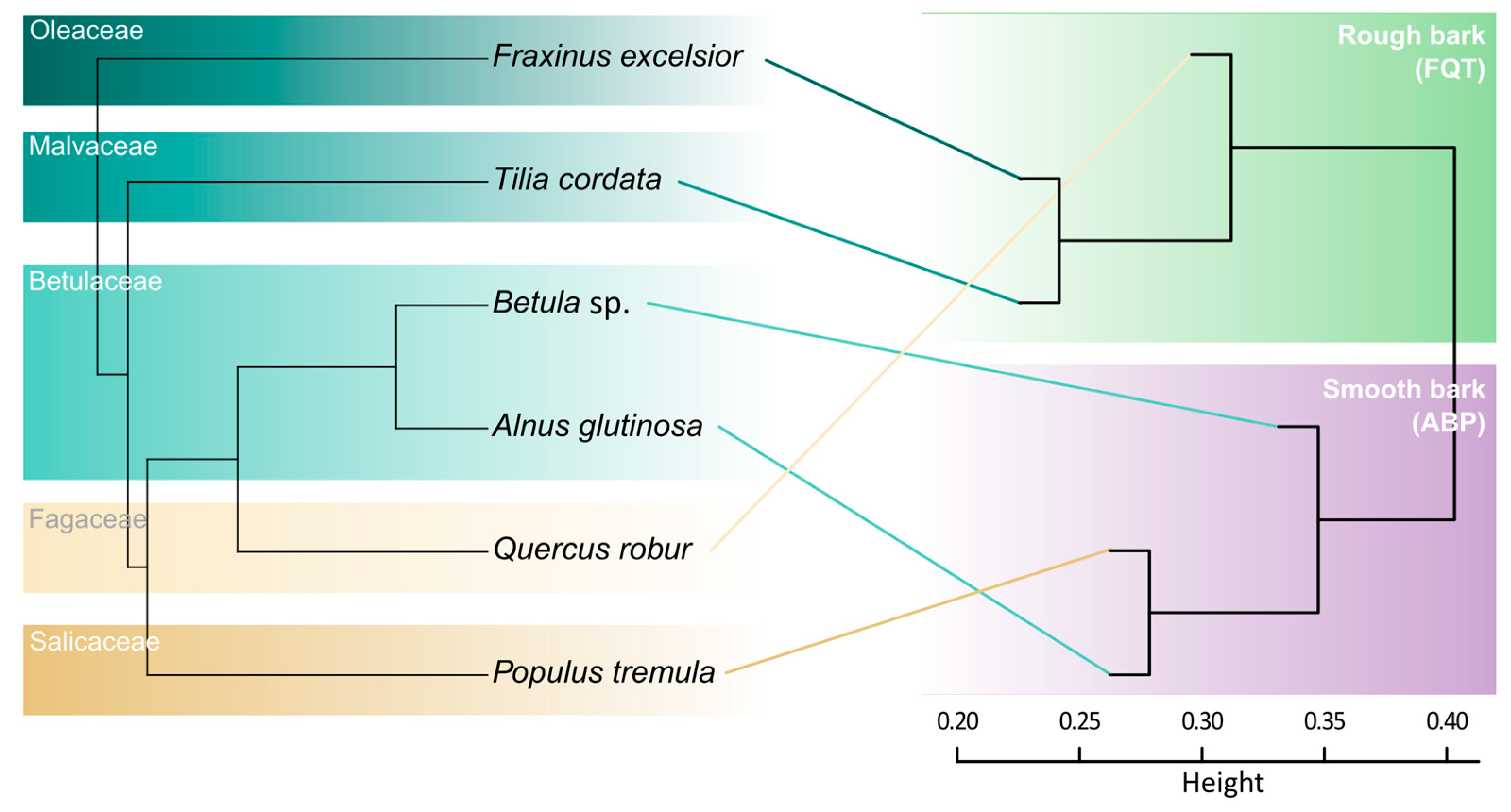
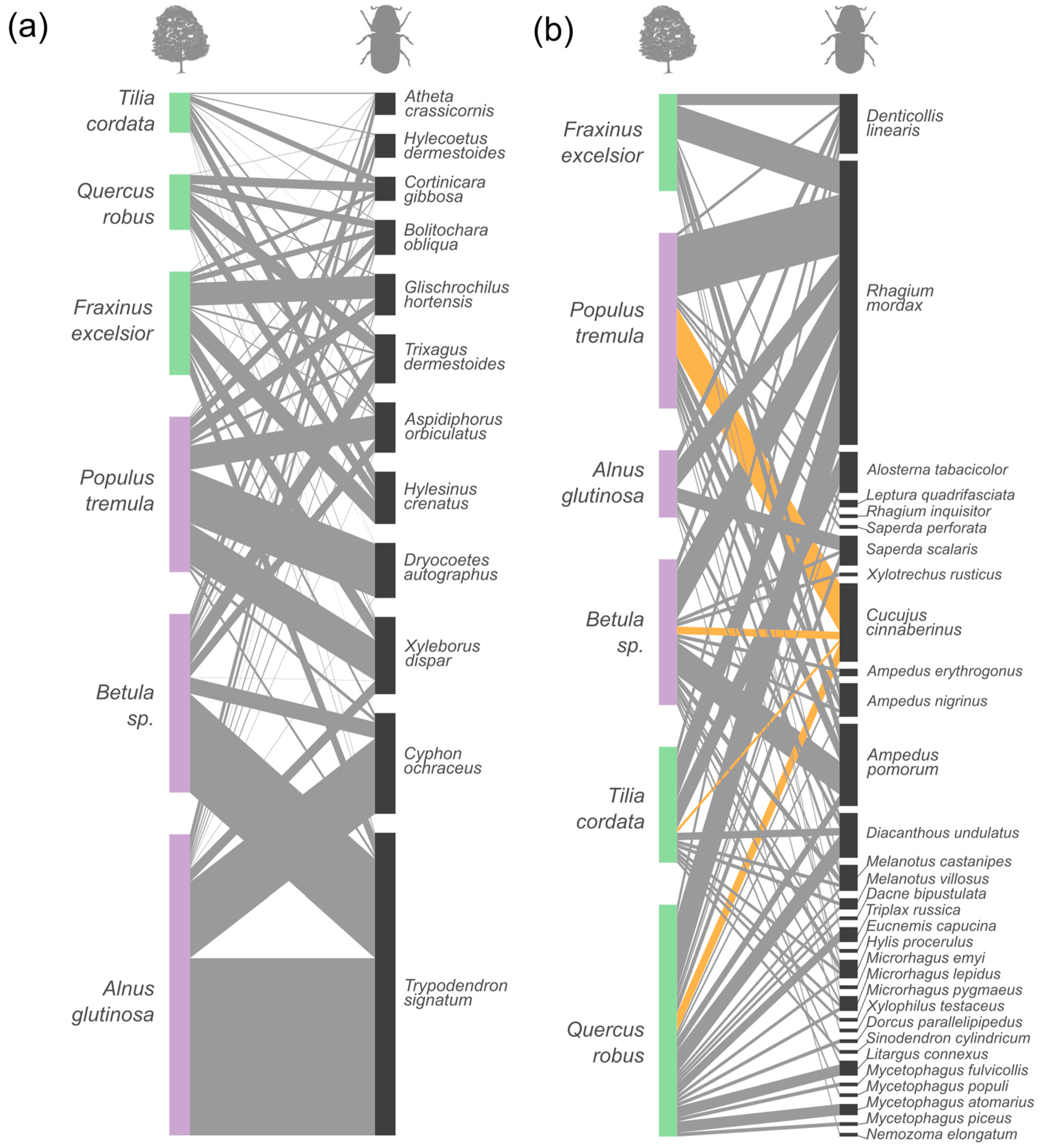
| Tree | n | MTD (cm) | SD (cm) | Locality | Year |
|---|---|---|---|---|---|
| Alnus glutinosa | 9 | 24.0 | 2.1 | BBR, BBZR | 2018–2019, 2021 |
| Betula sp. | 9 | 26.0 | 4.2 | BBZR, DRA, PŠSNR | 2021 |
| Fraxinus excelsior | 12 | 27.2 | 3.0 | BBR, BBZR | 2018–2019 |
| Populus tremula | 9 | 27.8 | 4.1 | BBZR, DRA | 2018–2019, 2021 |
| Quercus robur | 9 | 36.8 | 8.6 | BBZR, PŠSNR | 2020–2021 |
| Tilia cordata | 6 | 32.5 | 8.6 | BBZR, PŠSNR | 2020 |
| Tree_species | Beetle_ind. | Taxa_S | Shannon_H’ | (1/D) | Margalef |
|---|---|---|---|---|---|
| Alnus glutinosa | 1686 | 143 | 2.70 | 4.57 | 19.11 |
| Betula sp. | 1347 | 198 | 3.73 | 9.78 | 27.34 |
| Fraxinus excelsior | 965 | 162 | 4.14 | 26.52 | 23.43 |
| Populus tremula | 1383 | 205 | 4.08 | 19.40 | 28.21 |
| Quercus robur | 859 | 210 | 4.71 | 51.84 | 30.94 |
| Tilia cordata | 556 | 159 | 4.42 | 35.10 | 25.00 |
| Mean (±SD) | 1133 (±412.39) | 180 (±28.22) | 3.96 (±0.70) | 24.54 (±17.34) | 25.67 (±4.14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lekoveckaitė, A.; Jimenez, M.F.T.; Trakimas, G.; Ferenca, R.; Podėnienė, V. Tree Species Affect Beetle Diversity on the Common Deciduous Dead Wood in Lithuanian Unmanaged Forests. Forests 2023, 14, 1836. https://doi.org/10.3390/f14091836
Lekoveckaitė A, Jimenez MFT, Trakimas G, Ferenca R, Podėnienė V. Tree Species Affect Beetle Diversity on the Common Deciduous Dead Wood in Lithuanian Unmanaged Forests. Forests. 2023; 14(9):1836. https://doi.org/10.3390/f14091836
Chicago/Turabian StyleLekoveckaitė, Aistė, Maria Fernanda Torres Jimenez, Giedrius Trakimas, Romas Ferenca, and Virginija Podėnienė. 2023. "Tree Species Affect Beetle Diversity on the Common Deciduous Dead Wood in Lithuanian Unmanaged Forests" Forests 14, no. 9: 1836. https://doi.org/10.3390/f14091836
APA StyleLekoveckaitė, A., Jimenez, M. F. T., Trakimas, G., Ferenca, R., & Podėnienė, V. (2023). Tree Species Affect Beetle Diversity on the Common Deciduous Dead Wood in Lithuanian Unmanaged Forests. Forests, 14(9), 1836. https://doi.org/10.3390/f14091836






