Desiccation Sensitivity Characteristics and Low-Temperature Storage of Recalcitrant Quercus variabilis Seed
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Desiccation Sensitivity of Q. variabilis Seeds (Embryonic Axis)
2.2.1. Determination of Moisture Content
2.2.2. Preparation of Seeds (Embryonic Axes) with Different Moisture Contents
2.2.3. Germination Experiments of Seeds (Embryonic Axes) with Different Moisture Contents
2.3. Thermograms of Seeds (Embryonic Axes)
2.4. Effect of Desiccation on the Activity of Antioxidant Enzymes in Seed Cells
2.5. Low-Temperature Storage of Seeds (Embryonic Axes)
2.5.1. Low-Temperature Storage of Seeds
2.5.2. Germination of Seeds at Low-Temperature Storage
2.5.3. Selection of Embryonic Axes of Different Sizes
2.5.4. Cryopreservation of Embryonic Axes
- Load procedure: the pre-chilled upload solution was added to the 2 mL cryogenic vials, and treated for 20–30 min in an ice bath. Loading solution formula: 2 M glycerol + 0.4 M sucrose, dissolved in WPM medium and adjusted to a pH of 5.8.
- Vitrification procedure: the loading solution was sucked up with a pipette. The vitrification solution PVS2 was pre-chilled on ice, and then added to the samples, and the samples were incubated in an ice bath for 15 min, 30 min, 45 min, or 60 min. PVS2 solution formula: 30% glycerol + 15% ethylene glycol + 15% dimethyl sulfoxide + 0.4 mol/L sucrose, dissolved in WPM medium and adjusted to a pH of 5.8.
- Cryopreservation: fresh PVS2 solution was replaced to the cryogenic vials, and quickly immersed in LN2 for 24 h.
- Thawing procedure: cryogenic vials were removed from the LN2, and quickly thawed at 40 °C for 3–5 min.
- Unloading procedure: the PVS2 solution was sucked up with a pipette, the unloading solution was added, and the samples were incubated at 25 °C for 20–30 min. The unloading solution was changed every 10 min. After incubation, the unloading solution was poured away, and the samples were shaken gently to remove as much unloading solution as possible. WPM medium was added after the unloading was finished, to remove any remaining tissue from the solution. Unloading solution formula: 1.2 M sucrose, dissolved in WPM medium and adjusted to a pH of 5.8.
- Germination: the method followed that described in Section 2.2.3 for embryonic axis germination. Three repetitions were conducted, with 20 seeds per set.
2.5.5. Thermograms of Embryonic Axis before and after Cryopreservation
2.6. Statistical Analysis
3. Results
3.1. Desiccation Sensitivity of Q. variabilis Seeds
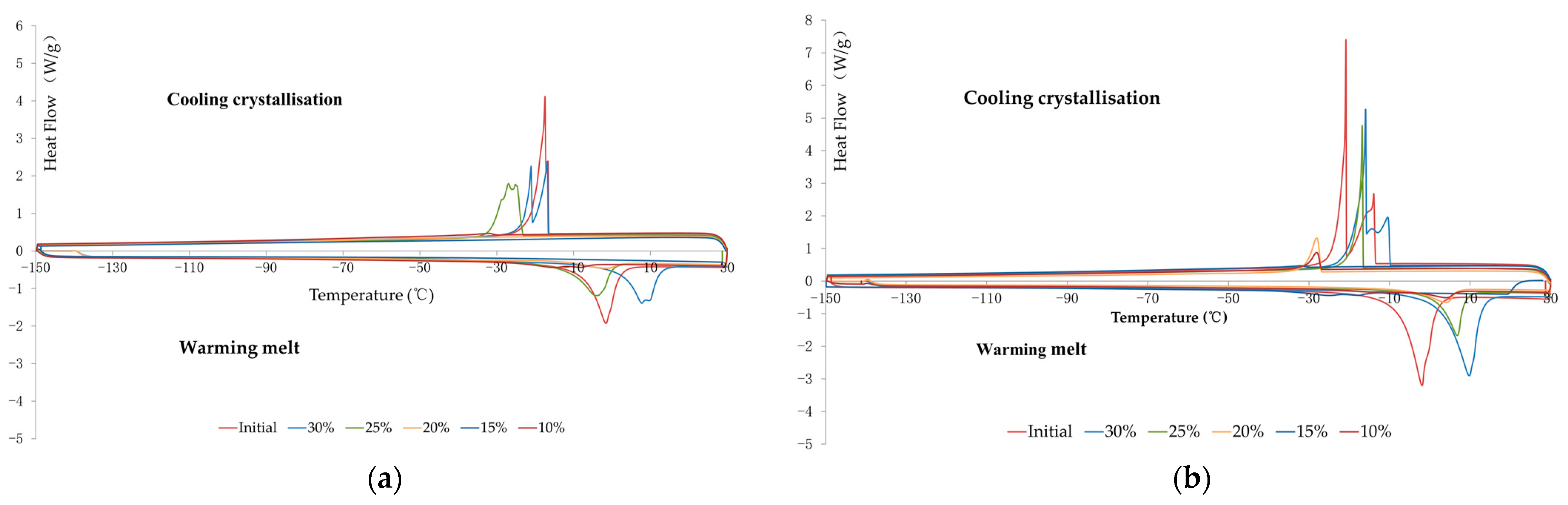
3.2. Analysis of Thermograms and Determination of Critical Moisture Content
3.3. Effects of Desiccation on the Antioxidant Enzyme Activity in Seeds
3.4. Research on Low-Temperature Storage of Q. variabilis Seeds (Embryonic Axes)
3.4.1. Low-Temperature Storage of Seeds
3.4.2. Cryopreservation of Q. variabilis Embryonic Axes
Germination of Different Volumes of Embryonic Axes
The Effect of PVS2 Treatment Time on the Survival Percentage of the Q. variabilis Embryonic Axis following Cryopreservation
3.4.3. Effect of Cryopreservation on Thermograms
4. Discussion
4.1. Desiccation Sensitivity and Critical Moisture Content
4.2. Low-Temperature Preservation Technology
5. Conclusions
- (1)
- The exploration of precise seed drying techniques that allow seeds to be dried to the desired or critical moisture content in order to lessen the influence of variations in individual moisture content on the outcomes of the experiment.
- (2)
- The refinement of cryopreservation techniques and materials. Before the current method can be used to preserve the seeds of Q. variabilis, in particular, research into various parts of the Q. variabilis seed and various pretreatment techniques should be conducted to improve the viability of seeds (or embryonic axes) after cryopreservation. The relevant research should be extended to the entire Quercus genus.
- (3)
- The development of a reliable recovery culture system after cryopreservation, in addition to the need to optimize variables like medium formulation and disinfection techniques suitable for the growth of explants after cryopreservation.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roberts, E.H. Predicting the storage life of seeds. Seed Sci. Technol. 1973, 1, 499–514. [Google Scholar]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. An intermediate category of seed storage behaviour? J. Exp. Bot. 1991, 42, 653–657. [Google Scholar] [CrossRef]
- Yuan, M.; Zhu, M.W.; Hou, J.; Zhu, Y.Y. Changes of water content in Robinia pseudoacacia seeds during imbibition by a low nuclear magnetic resonance. J. Nanjing For. Univ. 2022, 46, 135–142. [Google Scholar]
- Kenzo, T.; Ichie, T.; Ninomiya, I.; Koike, T. Photosynthetic activity in seed wings of Dipterocarpaceae in a masting year: Does wing photosynthesis contribute to reproduction? Photosynthetic 2003, 41, 551–557. [Google Scholar] [CrossRef]
- King, M.W.; Roberts, E.H. The Storage of Recalcitrant Seeds: Achievements and Possible Approaches; IBPGR: Rome, Italy, 1979; 99p. [Google Scholar]
- Al, Z.O.M.; Normah, M.N. Critical moisture content for successful cryopreservation of embryonic axes of Fortunella polyandra determined by differential scanning calorimetry (DSC). Acta Physiol. Plant. 2014, 37, 1727. [Google Scholar]
- Han, B. Analysis on Recalcitrant Characteristics and Preservation of Castanea Mollissima Bl. Seed. Ph. D. Thesis, Beijing Forestry University, Beijing, China, 2020. [Google Scholar]
- Engelmann, F. Cryopreservation of embryos: An overview. Methods Mol. Biol. 2011, 86, 155–184. [Google Scholar]
- Araldi, C.G.; Coelho, C.M.M.; Gaziola, S.A.; Azeyedo, R.A. Storage elicits a fast antioxidant enzyme activity in Araucaria angustifolia embryos. Acta Physiol. Plant. 2016, 38, 201. [Google Scholar] [CrossRef]
- Tian, X.M.; Li, H.L.; He, Y.; Hong, Q.M.; Hu, W.B.; Li, Q. Research progress on recalcitrant seeds storage of tropical crops. Chin. J. Trop. Agric. 2014, 34, 52–58. [Google Scholar]
- Zheng, Y.S.; Chen, L.G.; Li, Q.R.; Lin, Z.B.; Wu, Z.X. Study of cryopreservation on Castanea Mollissima seeds. Sci. Silvae Sin. 2002, 389, 146–149. [Google Scholar]
- Duan, Y.; Liu, Y.W.; Wang, Z.Y.; Wang, C.X. A study of Nicotiana tabacum seeds using differential scanning calorimetry. In Abstracts of the Thirteenth National Conference on Chemical Thermodynamics and Thermal Analysis of the Chinese Chemical Society; Henan Normal University: Xinxiang, China, 2006. [Google Scholar]
- Wen, B. Cytological and physiological changes related to cryotolerance in recalcitrant Livistona chinensis embryos during seed development. Protoplasma 2011, 248, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.Q.; Zhang, C.X.; Zhang, C.S.; Li, H.; Zhang, W.H. Cryopreservation of embryogenic tissues in Quercus variabilis. J. Northwest. For. Univ. 2012, 27, 88–92. [Google Scholar]
- Spitz, D.R.; Oberley, L.W. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 1989, 179, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xu, Q.; Ma, J.C.; Yuan, B.; Li, W.Q. Cryopreservation of protocorm-like body-derived shoot tips of Calanthe davidii by droplet-vitrification. Cryo. Lett. 2014, 35, 129–137. [Google Scholar]
- Xia, K.; Daws, M.I.; Hay, F.R.; Zhou, Z.K.; Pritchard, H.W. A comparative study of desiccation responses of seeds of Asian evergreen oaks, Quercus subgenus Cyclobalanopsis and Quercus subgenus Quercus. S. Afr. J. Bot. 2012, 78, 47–54. [Google Scholar] [CrossRef]
- Wen, B. Rambling stubborn seed. Life World 2019, 11, 22–33. [Google Scholar]
- Fu, J.R.; Xia, Q.H.; Tan, L.F. Effects of desiccation on exised embryonic axes of three recalcitrant seeds and studies on cryoproservation. Seed Sci. Technol. 1993, 21, 85–95. [Google Scholar]
- Han, B.; Li, W.Q.; Guo, S.J.; Lu, L.; Xie, X.M. Cryopreservation and critical moisture content of embryo axis of Castanea mollissima based on differential scanning calorimetry. Sci. Silvae Sin. 2020, 56, 21–27. [Google Scholar]
- Li, D.X.; Qian, J.L.; Xu, H.H.; Zhang, G.W.; Ren, J.J.; Wang, Q.; Xu, Y.; Wang, L.B.; Yu, H.Y.; Li, Y.C. Expression analysis of genes in response to desiccation of Quercus variabilis seeds. J. Northeast For. Univ. 2022, 50, 1–8. [Google Scholar]
- Feng, J.; Shen, Y.B.; Shi, F.H. Study on desiccation sensitivity of Ginkgo biloba seeds. J. Nanjing For. Univ. 2019, 43, 193–200. [Google Scholar]
- Wei, S.G. Study on the Biological Characteristics of Recalcitrant Seeds and Mechanisms of Desiccation Sensitivity of Taxillus chinensis (DC.) Danser. Ph. D. Thesis, Peking Union Medical College Hospital, Beijing, China, 2017. [Google Scholar]
- Luo, T.T. A Preliminary Study on Seed Preservation Technology Based on Post- Ripening Physiological Characteristics of Coptis chinensis Franch. Seeds. Master’s Thesis, Chengdu University of TCM, Chengdu, China, 2017. [Google Scholar]
- Li, L.; Sun, X.T.; Zhang, G.H.; Long, G.Q.; Meng, G.Z.; Yang, S.C.; Chen, W.J. Effect of drying rates on the desiccation sensitivity and antioxidant enzyme activities of recalcitrant Panax notoginseng seeds. Seed 2014, 33, 1–5. [Google Scholar]
- Xue, P.; Wen, B. Effects of drying rates on the desiccation tolerance of Citrus maxima ‘Feizhouyou’ seeds. Plant Divers. Resour. 2015, 37, 293–300. [Google Scholar]
- Zhang, M.J.; Han, B.; Zhu, Q.; Cui, C.C.; Xian, Y.; Yang, K.Q.; Tong, B.Q. Effects of osmotic treatment on the biological properties of Quercus acutissima and Quercus variabilis’ seeds. Genom. Appl. Bio. 2023, 1–17. Available online: https://kns.cnki.net/kcms2/detail/45.1369.Q.20230629.1741.004.html (accessed on 5 September 2023).
- Pammenter, N.W.; Beriak, P.; Walters, C. The Effect of Drying Rate on Recalcitrant Seeds: Lethal Water Contents, Causes of Damage, and Quantification of Recalcitrance; CABI Publishing: Wallingford, UK, 2000; pp. 215–221. [Google Scholar]
- Yan, X.F.; Du, Q.; Wang, J.L.; Ren, Y.F. Efects of dehydrating treatments on seeds germination of Ligustrum obtusifolium. Seed 2009, 28, 93–96. [Google Scholar]
- Pammenter, N.W.; Vertucci, C.W.; Berjak, P. Homeohydrous (recalcitrant) seeds: Dehydration, the state of water and 501 viability characteristics in Landolphia kirkii. Plant Physiol. 1991, 96, 1093–1098. [Google Scholar] [CrossRef]
- Beriak, P.; Vertucci, C.W.; Pammenter, N.W. Effects of developmental status and dehydration rate on characteristics of water and desiccation-sensitivity in recalcitrant seeds of Camellia sinensis. Seed Sci. Res. 1993, 3, 155–166. [Google Scholar] [CrossRef]
- Pence, V.C. Cryostorage of embryo axes of several large-seeded temperate tree species. Cryobiology 1990, 27, 212–218. [Google Scholar] [CrossRef]
- Li, Y.L.; Qu, S.C.; Luan, Y.T.; Gao, Z.H. Establishment of cryopreservation detoxification system of apple shoot-tips. Mol. Plant Breed. 2019, 17, 2982–2995. [Google Scholar]
- He, M.G.; Wang, R.X.; Song, X.Q.; Song, S.Q.; Zhang, R.L. Study on Cryopreservation of Dendrobium chrysanthum (Orchidaceae) Seeds. Acta Bot. Yunnan. 2010, 32, 334–338. [Google Scholar]

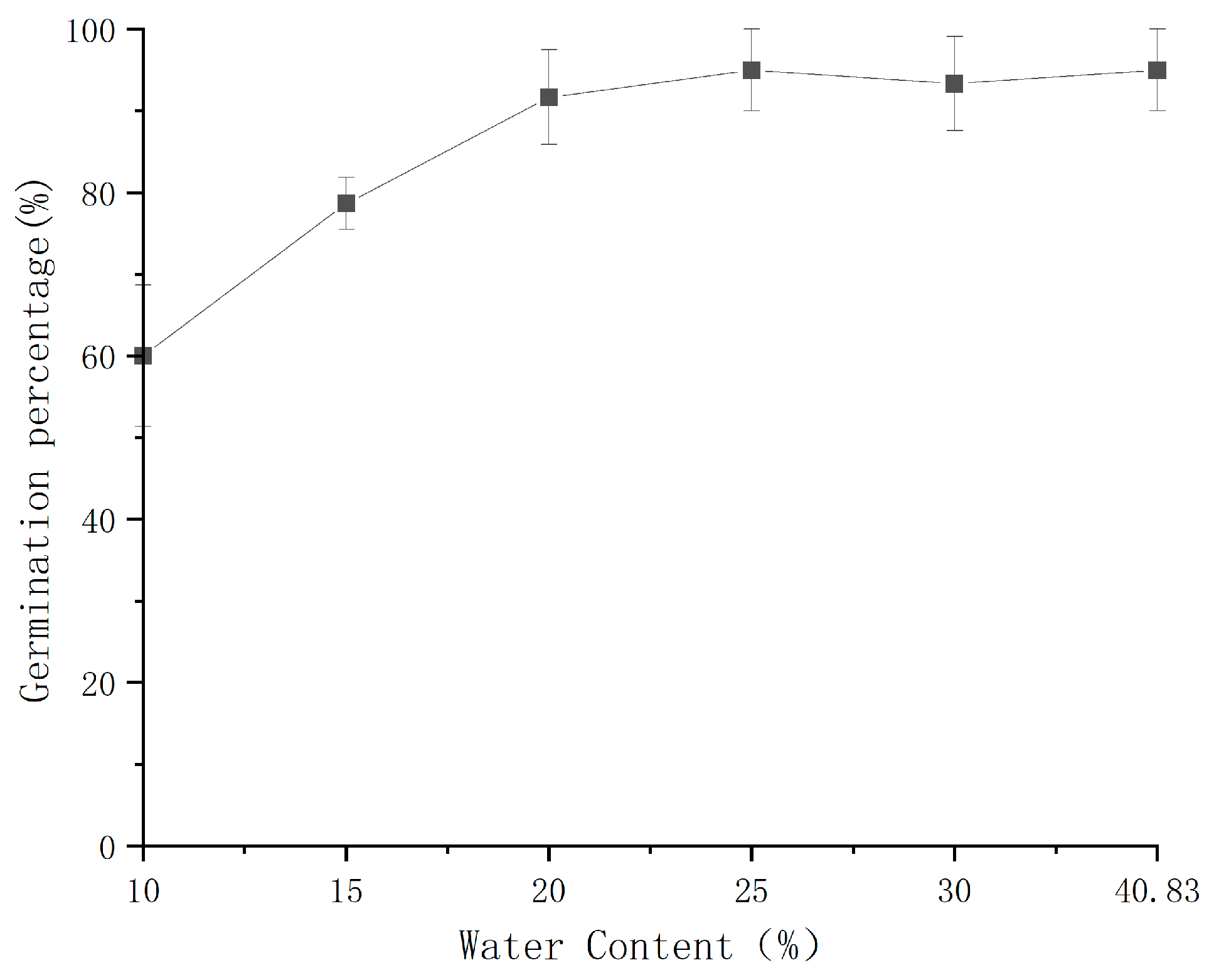


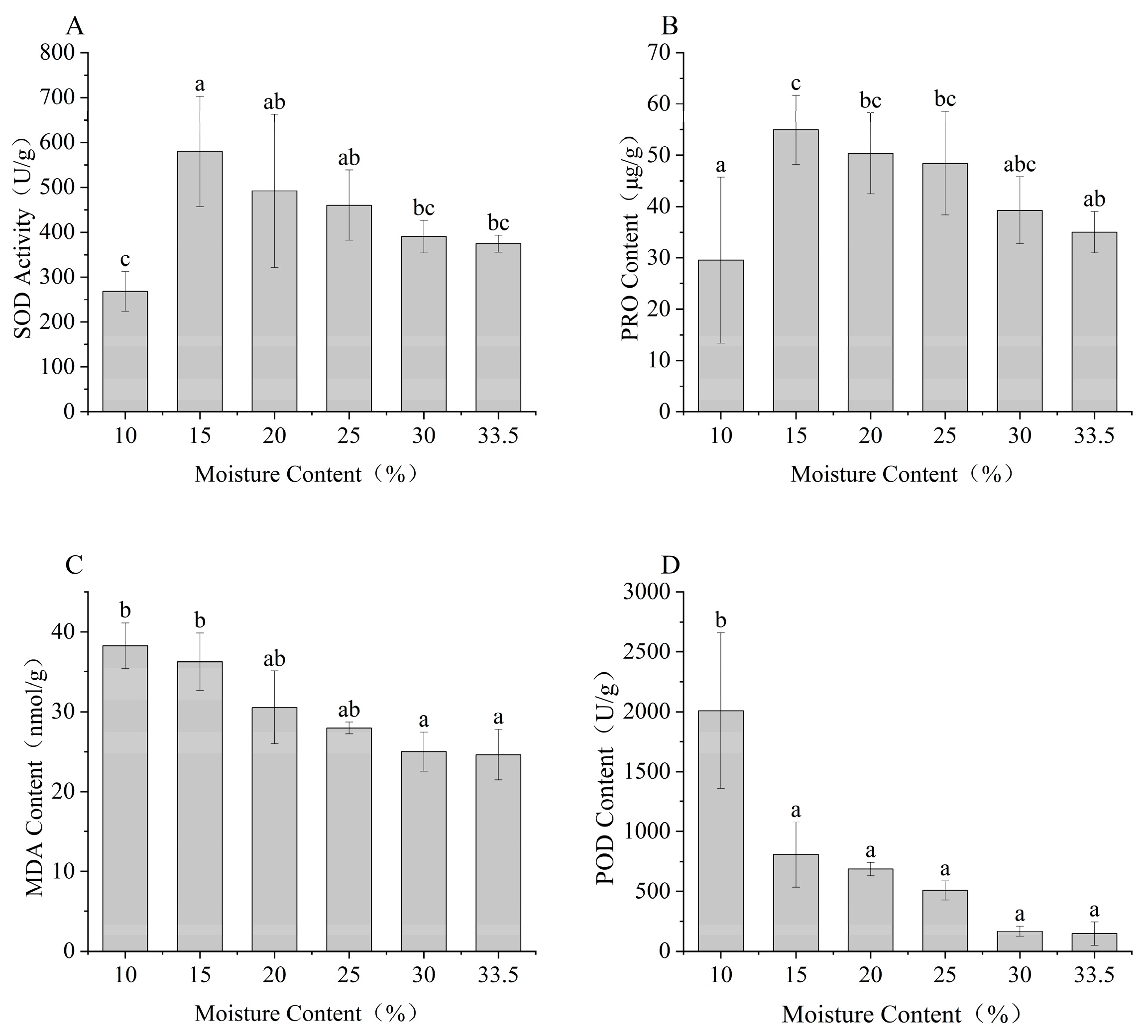
| Moisture Content (%) | Germination Percentage (%) | Germinability | Mean Germination Time (d) | Root Length (mm) | Number of Rotten Seeds |
|---|---|---|---|---|---|
| Initial | 95.60 ± 0.02 c | 2.21 ± 0.08 b | 8.50 ± 0.79 a | 7.78 ± 0.99 a | 0.66 ± 0.33 a |
| 30 | 93.90 ± 0.00 c | 3.47 ± 0.32 c | 7.30 ± 0.79 a | 7.82 ± 1.35 a | 0.66 ± 0.33 a |
| 25 | 77.80 ± 0.04 c | 2.05 ± 0.16 b | 11.13 ± 0.53 a | 7.02 ± 1.47 a | 1.66 ± 0.33 ab |
| 20 | 39.80 ± 0.09 b | 0.63 ± 0.05 a | 11.34 ± 2.28 a | 6.58 ± 1.24 a | 2.33 ± 0.33 b |
| 15 | 13.50 ± 0.08 a | 0.21 ± 0.10 a | 18.63 ± 1.96 b | 3.37 ± 0.83 a | 4.33 ± 0.66 c |
| 10 | 6.30 ± 0.03 a | 0.29 ± 0.25 a | 9.75 ± 4.25 a | 2.33 ± 0.44 a | 7.33 ± 0.33 d |
| Seed Site | Moisture Content (%) | Cooling Crystallization | Warming Melt | ||||
|---|---|---|---|---|---|---|---|
| Onset Temperature (°C) | Peak Temperature (°C) | Enthalpy (Jg-1dw) | Onset Temperature (°C) | Peak Temperature (°C) | Enthalpy (Jg-1dw) | ||
| embryonic axis | 10 | −39.53 ± 6.55 a | −43.88 ± 2.67 a | 70.33 ± 6.88 a | −103.79 ± 44.89 a | −61.35 ± 44.25 a | 29.17 ± 28.20 a |
| 15 | −34.01 ± 5.84 a | −43.25 ± 3.06 a | 76.04 ± 6.44 a | −80.25 ± 39.46 ab | −41.86 ± 33.64 a | 42.22 ± 16.40 a | |
| 20 | −32.40 ± 2.51 a | −42.55 ± 3.37 a | 94.22 ± 7.73 a | −18.22 ± 0.93 b | −9.39 ± 0.47 a | 77.73 ± 8.89 a | |
| 25 | −31.92 ± 4.02 b | −34.94 ± 5.71 ab | 125.20 ± 14.39 a | −14.69 ± 2.41 b | −6.38 ± 1.77 a | 59.54 ± 10.98 a | |
| 30 | −26.29 ± 0.94 b | −28.07 ± 2.24 b | 138.96 ± 3.72 ab | −12.55 ± 2.06 b | −4.96 ± 1.28 a | 71.88 ± 3.72 a | |
| Initial | −18.01 ± 1.73 c | −16.77 ± 1.64 c | 346.80 ± 13.73 b | −6.03 ± 0.24 b | −0.86 ± 0.19 a | 137.53 ± 6.90 b | |
| cotyledon | 10 | no peak | no peak | no peak | no peak | no peak | no peak |
| 15 | no peak | no peak | no peak | no peak | no peak | no peak | |
| 20 | −26.73 ± 2.80 a | −28.66 ± 4.06 a | 74.35 ± 3.94 a | −13.38 ± 1.93 a | −6.19 ± 0.98 a | 43.63 ± 4.43 a | |
| 25 | −21.98 ± 1.23 ab | −24.19 ± 2.81 ab | 106.67 ± 12.61 a | −9.71 ± 0.43 b | −3.88 ± 0.45 b | 55.41 ± 13.84 a | |
| 30 | −20.82 ± 1.68 b | −20.70 ± 1.90 ab | 136.67 ± 10.60 a | −9.43 ± 0.68 b | −3.78 ± 0.55 b | 55.41 ± 13.89 a | |
| Initial | −18.71 ± 1.05 b | −18.13 ± 1.12 b | 179.89 ± 40.62 a | −7.00 ± 0.51 b | −2.45 ± 0.44 b | 76.46 ± 5.44 a | |
| Moisture Content (%) | Embryonic Axis Enthalpy (Jg-1dw) | Cotyledon Enthalpy (Jg-1dw) | Average Enthalpy of Cotyledons and Embryonic Axes (Jg-1dw) | ||||
|---|---|---|---|---|---|---|---|
| Cooling Crystallization | Warming Melt | Average Enthalpy (Jg-1dw) | Cooling Crystallization | Warming Melt | Average Enthalpy (Jg-1dw) | ||
| 10 | 70.33 | 29.17 | 49.75 | - | - | - | - |
| 15 | 76.04 | 42.22 | 59.13 | - | - | - | - |
| 20 | 94.22 | 77.73 | 85.98 | 74.25 | 43.63 | 78.99 | 84.24 |
| 25 | 125.20 | 59.54 | 92.37 | 106.67 | 55.41 | 91.04 | 115.94 |
| 30 | 138.96 | 71.88 | 105.42 | 136.67 | 55.41 | 96.04 | 137.82 |
| Initial | 346.80 | 137.53 | 242.17 | 179.89 | 76.46 | 128.18 | 263.35 |
| Temperature (°C) | Moisture Content (%) | |||||
|---|---|---|---|---|---|---|
| Initial | 30% | 25% | 20% | 15% | 10% | |
| 4 °C | 94.13 ± 5.86 a | 59.66 ± 11.49 a | 46.60 ± 6.41 b | 26.63 ± 6.21 bc | 6.33 ± 0.36 c | 5.00 ± 2.88 d |
| −20 °C | 0 | 0 | 0 | 0 | 0 | 0 |
| −40 °C | 0 | 0 | 0 | 0 | 0 | 0 |
| −80 °C | 0 | 0 | 0 | 0 | 0 | 0 |
| Embryonic Axis Size | Germination Percentage (%) | Contamination Percentage (%) |
|---|---|---|
| Large (2.5–3 g) | 88.78 ± 2.11 a | 53.33 ± 7.26 a |
| Medium (1.5–2 g) | 88.89 ± 1.11 a | 48.33 ± 3.33 a |
| Small (0.5–1 g) | 92.22 ± 2.22 a | 1.78 ± 1.66 b |
| Moisture Content (%) | Initial Germination Percentage (%) | PVS2 Processing Time (min) | |||
|---|---|---|---|---|---|
| 15 | 30 | 45 | 60 | ||
| 10 | 60.00 ± 5.00 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 15.00 ± 0.05 a |
| 15 | 78.60 ± 1.84 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| 20 | 91.67 ± 3.33 c | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| 25 | 95.00 ± 2.89 c | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| 30 | 93.33 ± 3.33 c | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| Initial | 95.00 ± 2.89 c | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.-J.; Wang, Y.-Z.; Xian, Y.; Cui, C.-C.; Xie, X.-M.; Tong, B.-Q.; Han, B. Desiccation Sensitivity Characteristics and Low-Temperature Storage of Recalcitrant Quercus variabilis Seed. Forests 2023, 14, 1837. https://doi.org/10.3390/f14091837
Zhang M-J, Wang Y-Z, Xian Y, Cui C-C, Xie X-M, Tong B-Q, Han B. Desiccation Sensitivity Characteristics and Low-Temperature Storage of Recalcitrant Quercus variabilis Seed. Forests. 2023; 14(9):1837. https://doi.org/10.3390/f14091837
Chicago/Turabian StyleZhang, Ming-Jia, Yong-Zheng Wang, Yang Xian, Cheng-Cheng Cui, Xiao-Man Xie, Bo-Qiang Tong, and Biao Han. 2023. "Desiccation Sensitivity Characteristics and Low-Temperature Storage of Recalcitrant Quercus variabilis Seed" Forests 14, no. 9: 1837. https://doi.org/10.3390/f14091837
APA StyleZhang, M.-J., Wang, Y.-Z., Xian, Y., Cui, C.-C., Xie, X.-M., Tong, B.-Q., & Han, B. (2023). Desiccation Sensitivity Characteristics and Low-Temperature Storage of Recalcitrant Quercus variabilis Seed. Forests, 14(9), 1837. https://doi.org/10.3390/f14091837







