Physiological, Photosynthetic and Stomatal Ultrastructural Responses of Quercus acutissima Seedlings to Drought Stress and Rewatering
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Drought Treatment
2.2. Measurement of Physiological Indexes
2.2.1. Photosynthetic Parameters
2.2.2. Antioxidant Enzyme Activity, MDA, PRO Measurement
2.2.3. Determination of Soluble Sugar and Protein Contents
2.2.4. Determination of Relative Water Content and Electrical Conductivity
2.2.5. Ultrastructural Changes in Q. acutissima Leaves
2.3. Statistical Analysis
3. Results
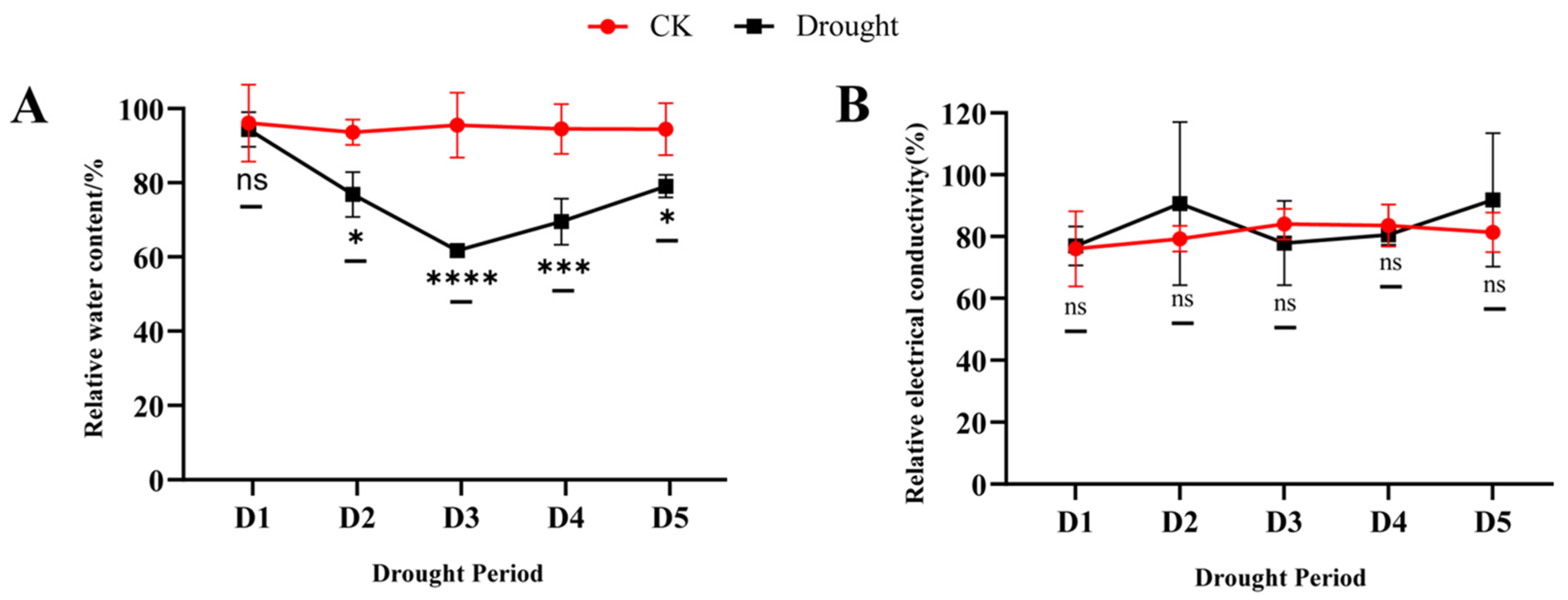
3.1. Effect of Drought Stress and Rewatering on Leaf Morphology, Relative Water Content and Relative Electrical Conductivity
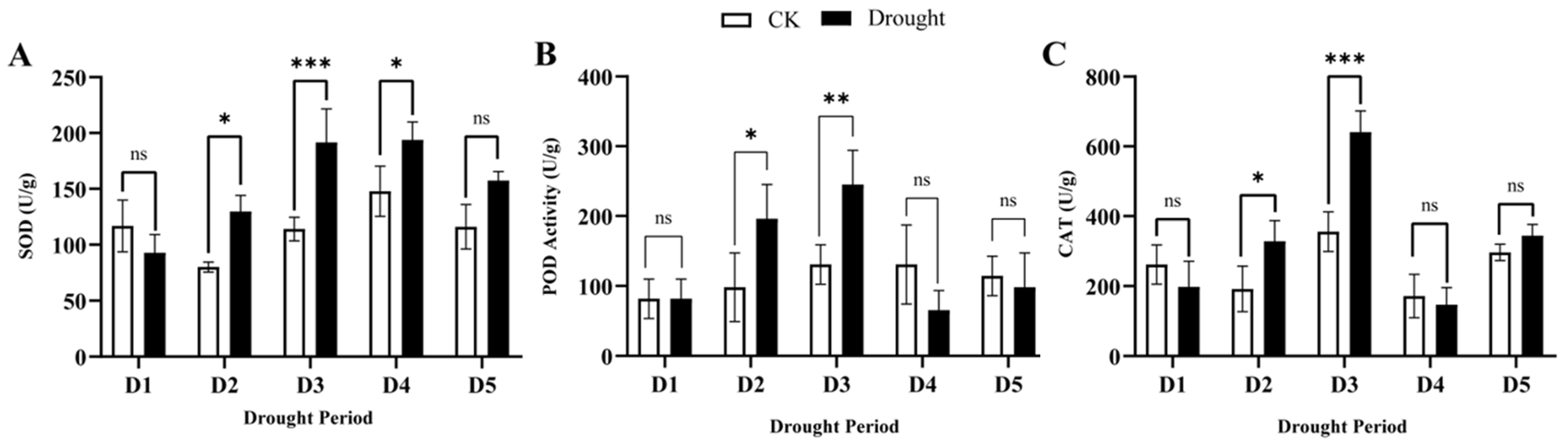
3.2. Effect of Drought Stress and Rewatering on Antioxidant Enzyme Activities in Q. acutissima Leaves
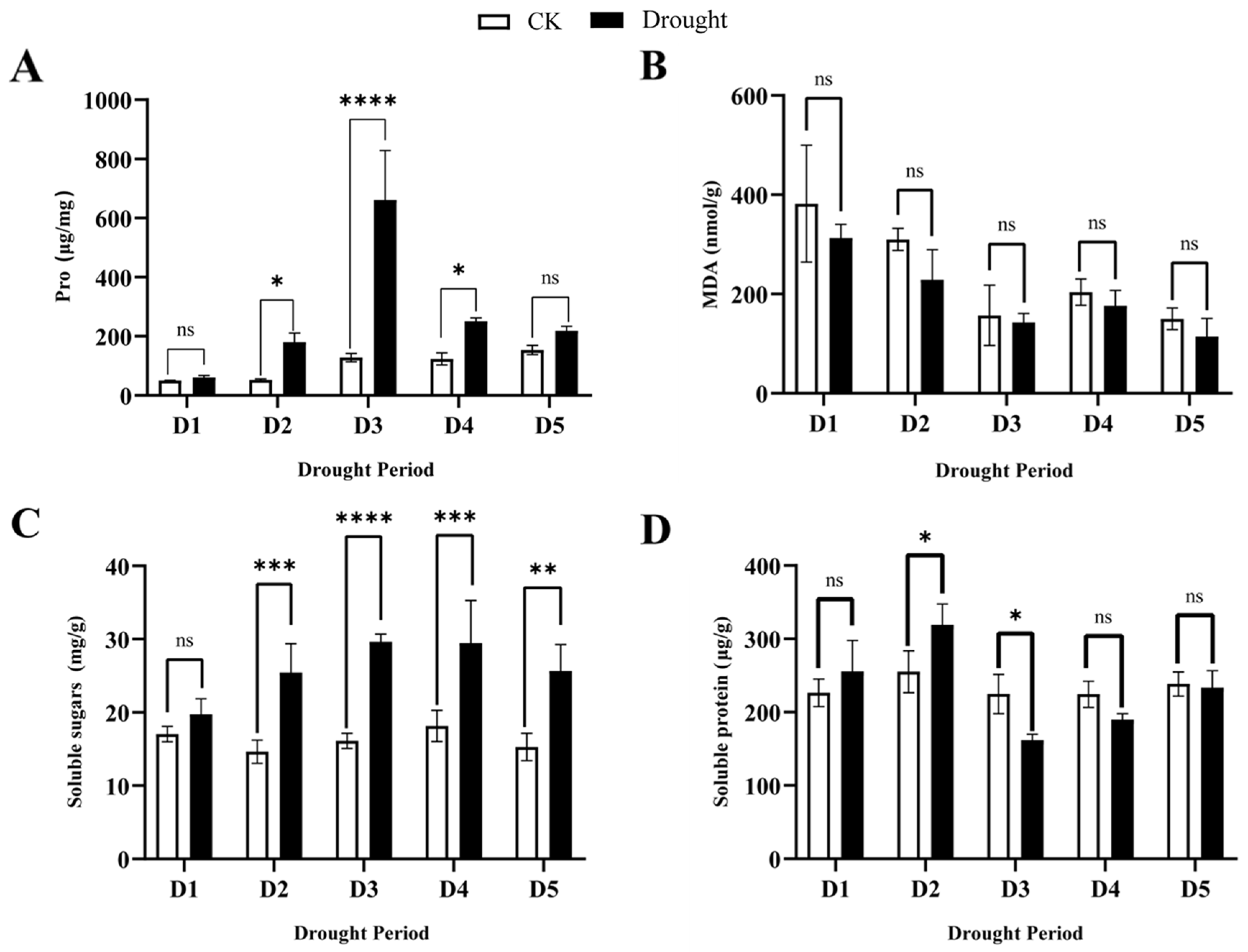
3.3. Effect of Drought Stress and Rewatering on MDA, Proline, Soluble Sugar and Soluble Protein in Q. acutissima Leaves
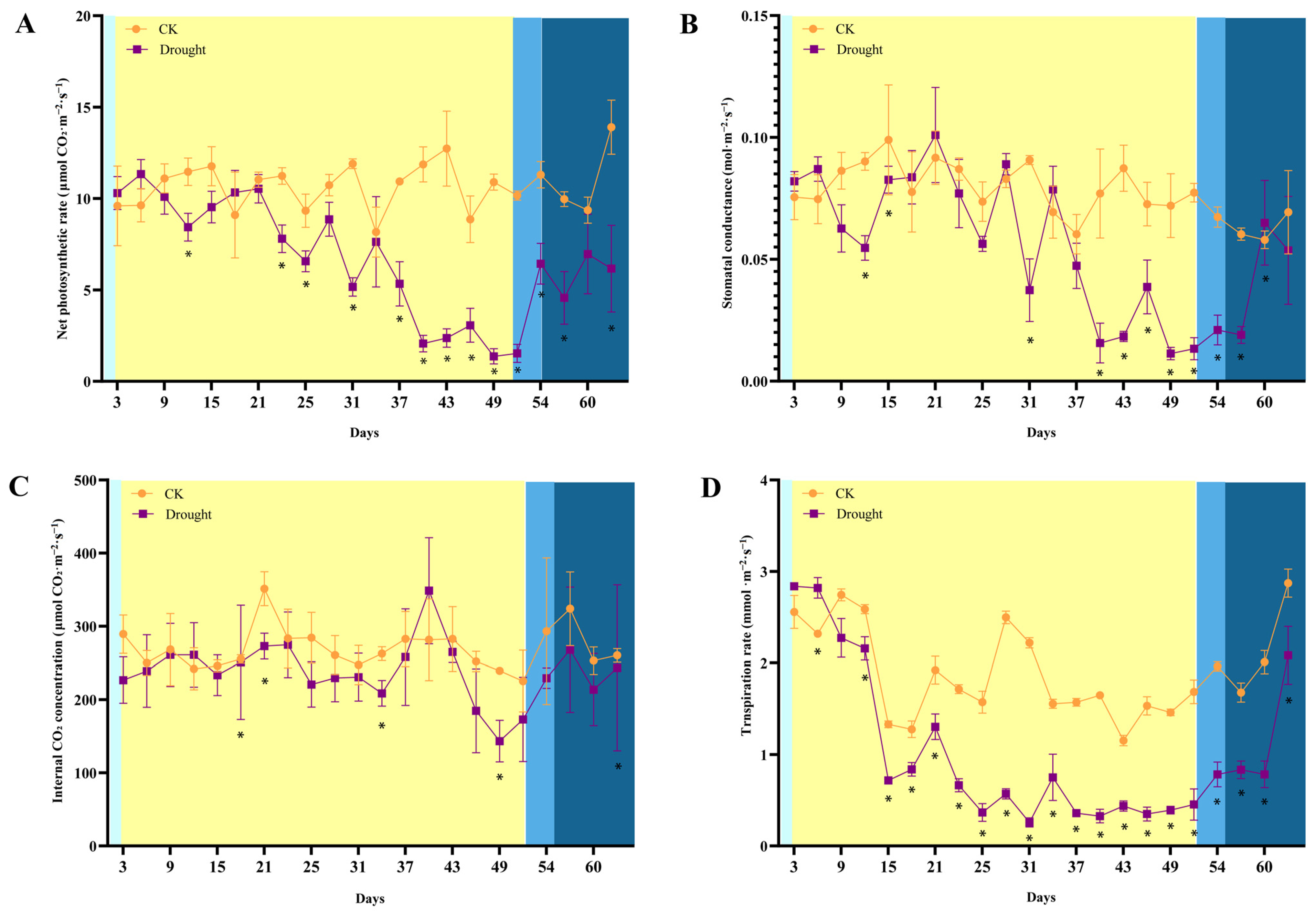
3.4. Effect of Drought Stress and Rewatering on Photosynthesis in Q. acutissima Leaves
3.5. Effect of Drought Stress and Rewatering on Stomatal Ultrastructure in Q. acutissima Seedling Leaves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.L.; Yin, C.B.; Lu, Q.; Wu, B.; Que, X.E. Economic analysis of desertification causes and irrational human activities. Chin. J. Agric. Resour. Reg. Plan. 2016, 37, 123–129. [Google Scholar] [CrossRef]
- Xu, L. Effects of drought on agriculture in China and countermeasures. Guangdong Agric. Sci. 2011, 38, 201–203. [Google Scholar] [CrossRef]
- Gong, Z.Z.; Xiong, L.M.; Shi, H.Z.; Yang, S.H.; Herrera-Estrella, L.R.; Xu, G.H.; Chao, D.Y.; Li, J.R.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.B.; Lu, J. The Advance on Foreign Biomass Mode. Chin. Agric. Sci. Bull. 2012, 28, 6–11. [Google Scholar] [CrossRef]
- Geßler, A.; Keitel, C.; Kreuzwieser, J.; Matyssek, R.; Seiler, W.; Rennenberg, H. Potential risks for European beech (Fagus sylvatica L.) in a changing climate. Trees 2006, 21, 1–11. [Google Scholar] [CrossRef]
- Ohlemuller, R.; Gritti, E.S.; Sykes, M.T.; Thomas, C.D. Quantifying components of risk for European woody species under climate change. Glob. Chang. Biol. 2006, 12, 1788–1799. [Google Scholar] [CrossRef]
- Zimmermann, N.E.; Bolliger, J.; Gehrig-Fasel, J.; Guisan, A.; Kienast, F.; Lischke, H.; Rickebusch, S.; Wohlgemuth, T. Wo wachsen die Bäume in 100 Jahren? Forum. Für. Wissen 2006, 2006, 63–71. [Google Scholar]
- Yang, C.; Dang, K.L.; Liu, J.J. Study on water conservation efficiency of Quercus aculeatus plantation. J. Northwest For. Univ. 1997, 12, 16–20. [Google Scholar]
- You, L.X.; Li, Y.; Yin, Z.F.; Fang, Y.M. A Study of Sawtooth Oak in China: Provenance Test, Afforestation and Forest Management. World For. Res. 2017, 30, 75–79. [Google Scholar] [CrossRef]
- Hao, P.L.; Zhang, J.P.; Han, X.Y.; Dong, Y.F. Effect on transpiration among Quercus acutissima clones under drought stress. J. For. Eng. 2012, 26, 62–65. [Google Scholar]
- Dong, Y.F.; Jiang, Y.Z.; Wang, H.T.; Kong, L.G.; Ge, Z.Q. Study on resistance of Quercus acutissima clones to different soil drought conditions. J. Shandong For. Sci. Technol. 2010, 40, 28–31 + 42. [Google Scholar]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought Stress in Plants: An Overview. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. [Google Scholar] [CrossRef]
- Wu, J.S.; Zhang, J.H.; Li, R.J.; Li, Y.Y.; Gao, H.J.; Zhao, Q.J. The Plant’s physiological mechanism and adaptability to drought stress. J. Shanxi Agric. Univ. 2017, 37, 452–456. [Google Scholar] [CrossRef]
- Tian, X.R.; Lei, Y.B. Physiological responses of wheat seedlings to drought and UV-B radiation. Effect of exogenous sodium nitroprusside application. Russ. J. Plant Physiol. 2007, 54, 676–682. [Google Scholar] [CrossRef]
- Ayub, M.; Ashraf, M.Y.; Kausar, A.; Saleem, S.; Anwar, S.; Altay, V.; Ozturk, M. Growth and physio-biochemical responses of maize (Zea mays L.) to drought and heat stresses. Plant Biosyst. 2021, 155, 535–542. [Google Scholar] [CrossRef]
- Smith, S.D.; Huxman, T.E.; Zitzer, S.F.; Charlet, T.N.; Housman, D.C.; Coleman, J.S.; Fenstermaker, L.K.; Seemann, J.R.; Nowak, R.S. Elevated CO2 increases productivity and invasive species success in an arid ecosystem. Nature 2000, 408, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 5–86. [Google Scholar] [CrossRef]
- Bressana, R.; Bohnertb, H.; Zhu, J.-K. Abiotic Stress Tolerance: From Gene Discovery in Model Organisms to Crop Improvement. Mol. Plant 2009, 2, 1–2. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Feng, Y.L.; Cao, K.F. Photosynthesis and photoinhibition after night chilling in seedlings of two tropical tree species grown under three irradiances. Photosynthetica 2005, 43, 567–574. [Google Scholar] [CrossRef]
- Sperry, J.S.; Venturas, M.D.; Anderegg, W.R.L.; Mencuccini, M.; Mackay, D.S.; Wang, Y.J.; Love, D.M. Predicting stomatal responses to the environment from the optimization of photosynthetic gain and hydraulic cost. Plant Cell Environ. 2016, 40, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Zarrouk, O.; Francisco, R.; Costa, J.M.; Santos, T.; Regalado, A.P.; Rodrigues, M.L.; Lopes, C.M. Grapevine under deficit irrigation: Hints from physiological and molecular data. Ann. Bot. 2010, 105, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, H.Y.; Liu, X.W.; Xia, B.; Sun, S.W.; Sun, Y.; He, M. Effects of different light intensities on morphogenesis and ultrastructure of Gibasis pellucida leaf. Acta Prataculturae Sin. 2019, 28, 175–185. [Google Scholar] [CrossRef]
- Li, L.; Li, D.N.; Yang, Y.Y.; Pan, X.Y.; Wan, Y.Q.; Zhang, G.Y.; Xi, J.J.; Wang, Y.F.; Yang, P.Z. Analysis of Metabolomics and Screening of Stomatal Regulating Substances in Alfalfa Leaves under Drought Stress. Acta Agrestia Sin. 2023, 31, 2671–2683. [Google Scholar]
- Carvalho, M.H.C.D. Drought stress and reactive oxygen species. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Du, C.Y.; Duan, Z.Y.; Pan, Y.H.; Lei, B.K.; Hu, W.L.; Fu, B.; Chen, A.Q.; Chen, S.H.; Yang, Y.X.; Jin, G.M. Effect of drought stress on growth and activities of antioxidant enzymes of maize seedling. Agric. Res. Arid. Areas. 2015, 33, 124–129. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Pan, K.; Li, W.; Zhang, L.; Shen, X.; Liu, L.; Deng, M. Responses of the antioxidant defense system to drought stress in the leaves of Fargesia denudata seedlings, the staple food of the giant panda. Russ. J. Plant Physiol. 2014, 61, 374–383. [Google Scholar] [CrossRef]
- Arndt, S.K.; Irawan, A.; Sanders, G.J. Apoplastic water fraction and rehydration techniques introduce significant errors in measurements of relative water content and osmotic potential in plant leaves. Physiol. Plant. 2015, 155, 355–368. [Google Scholar] [CrossRef]
- Zhang, M.J.; Han, B.; Zhu, Q.; Cui, C.C.; Xian, Y.; Yang, K.Q.; Tong, B.Q. Effects of Osmotic Treatment on the Biological Properties of Quercus acutissima and Quercus variabilis’ Seeds. Genom. Appl. Biol. 2023, Accepted. [Google Scholar]
- Farrell, C.; Szota, C.; Arndt, S.K. Does the turgor loss point characterize drought response in dryland plants? Plant Cell Environ. 2017, 40, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.D.; Chen, Y.J.; Ye, Q.; He, P.C.; Liu, H.; Li, R.H.; Fu, P.L.; Jiang, G.F.; Cao, K.F. Leaf turgor loss point is correlated with drought tolerance and leaf carbon economics traits. Tree Physiol. 2018, 38, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Kaproth, M.A.; Fredericksen, B.W.; Antonio González-Rodríguez, A.; Hipp, A.L.; Cavender-Bares, J. Drought response strategies are coupled with leaf habit in 35 evergreen and deciduous oak (Quercus) species across a climatic gradient in the Americas. New Phytol. 2023, 239, 888–904. [Google Scholar] [CrossRef] [PubMed]
- Kunert, N.; Tomaskova, I. Leaf turgor loss point at full hydration for 41 native and introduced tree and shrub species from Central Europe. J. Plant Ecol. 2020, 13, 754–756. [Google Scholar] [CrossRef]
- Zhang, H.L.; Qi, J.C.; Wang, X.J. Effects of Water Stress on Epicuticular Wax Content and Main Physiological Parameters of Barley. J. Triticeae Crops 2012, 32, 280–283. [Google Scholar] [CrossRef]
- Pei, B.; Zhang, G.C.; Zhang, S.Y.; Wu, Q.; Xu, Z.Q.; Xu, P. Effects of soil drought stress on photosynthetic characteristics and antioxidant enzyme activities in Hippophae rhamnoides Linn. seedings. Acta Ecol. Sin. 2013, 33, 1386–1396. [Google Scholar] [CrossRef]
- Guo, Z.F.; Gong, D.Z.; Hao, W.P.; Mei, X.R.; Li, Y.Q.; Liu, B.H. Study on Compensation of Maize Varieties under Drought Stress and Re-watering in Different Growth Stages. J. Maize Sci. 2011, 19, 84–88. [Google Scholar] [CrossRef]
- Du, J.X.; Hou, X.Y.; Liu, J.R. A study on physiological response to drought and re-watering treatments in Kentucky bluegrass. Acta Prataculturae Sin. 2010, 19, 31–38. [Google Scholar] [CrossRef]
- Rankenberg, T.; Geldhof, B.; Veen, H.V.; Holsteens, K.; Poel, B.V.D.; Sasidharan, R. Age-Dependent Abiotic Stress Resilience in Plants. Trends Plant Sci. 2021, 26, 692–705. [Google Scholar] [CrossRef]
- Du, M.F. Molecular Mechanisms of Drought-Resistant Germplasm in Response to Drought Stress in Masson Pine (Pinus massoniana). Ph.D. Thesis, Guizhou University, Guizhou, China, 2018. [Google Scholar]
- Liu, X.; Luo, G.J. Effects of water stress on growth and physiological characteristics in Quercus Acutissima. J. North. Agric. 2019, 47, 11–14. [Google Scholar]
- Ji, Z.B.; Wang, J.X.; Li, J.W.; Xue, S.; Zhang, M.L. Dynamic Changes of Soluble Sugar in the Seedlings of Robinia pseudoacacia under Drought Stress and Rewatering in Different Seasons. Acta Bot. Boreal. 2009, 29, 1358–1363. [Google Scholar]
- Xiong, S.F.; Wu, L.W.; Chen, Y.C.; Gao, M.; Jiang, X.G.; Li, Q.Y.; Huang, S.H.; Wang, Y.D. Response of leaf of Quercus fabri seedlings from different provenances to drought stress and drought resistance evaluation. Chin. J. Ecol. 2020, 39, 3924–3933. [Google Scholar] [CrossRef]
- He, B.R. Effects of Drought Stress on Physiological Characteristics and Anatomical Structure of Quercus mongolica. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2019. [Google Scholar]
- Liu, M.Y.; Chen, P.Y. Effects of stomatal and nonstomatal factors on wheat photosynthesis under water stress. Plant Physiol. Commun. 1990, 4, 24–27. [Google Scholar] [CrossRef]
- Yao, Q.Q.; Xie, G.S. The Photosynthetic Stomatal and Nonstomatal Limitation under Drought Stress. Chin. J. Trop. Agric. 2005, 25, 84–89. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dwivedi, S.; Bhagavatula, L.; Datta, S. Integration of light and ABA signaling pathways to combat drought stress in plants. Plant Cell Rep. 2023, 42, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Bi, M.H.; Nie, Z.F.; Jiang, H.; Liu, X.D.; Fang, X.W.; Brodribb, T.J. Evolution of stomatal closure to optimize water-use efficiency in response to dehydration in ferns and seed plants. New Phytol. 2021, 230, 2001–2010. [Google Scholar] [CrossRef]
- Gallé, A.; Haldimann, P.; Feller, U. Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytol. 2007, 174, 799–810. [Google Scholar] [CrossRef]
- Grzesiak, M.T.; Grzesiak, S.; Skoczowski, A. Changes of leaf water potential and gas exchange during and after drought in triticale and maize genotypes differing in drought tolerance. Photosynthetica 2006, 44, 561–568. [Google Scholar] [CrossRef]
- Waseem, M.; Nie, Z.F.; Yao, G.Q.; Hasan, M.; Xiang, Y.; Fang, X.W. Dew absorption by leaf trichomes in Caragana korshinskii: An alternative water acquisition strategy for withstanding drought in arid environments. Physiol. Plant. 2021, 172, 528–539. [Google Scholar] [CrossRef]
- Pan, Z.L.; Guo, W.; Wang, T.; Li, Y.P.; Yang, S.J. Research progress on foliar water uptake. Plant Physiol. J. 2021, 57, 19–32. [Google Scholar] [CrossRef]
- Liu, W.Y.; Ma, W.Z.; Yang, L.P. Advances in ecological studies on epiphytes in forest canopies. Chin. J. Plant Ecol. 2006, 30, 522–533. [Google Scholar] [CrossRef][Green Version]
- Fernández, V.; Sancho-Knapik, D.; Guzmán, P.; Peguero-Pina, J.J.; Gil, L.; Karabourniotis, G.; Khayet, M.; Fasseas, C.; Heredia-Guerrero, J.A.; Heredia, A.; et al. Wettability, Polarity, and Water Absorption of Holm oak Leaves: Effect of Leaf Side and Age. Plant Physiol. 2014, 166, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Schwerbrock, R.; Leuschner, C. Air humidity as key determinant of morphogenesis and productivity of the rare temperate woodland fern Polystichum braunii. Plant Biol. 2016, 18, 649–657. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Guo, H.; Yan, L.-P.; Gao, L.; Zhai, S.; Xu, Y. Physiological, Photosynthetic and Stomatal Ultrastructural Responses of Quercus acutissima Seedlings to Drought Stress and Rewatering. Forests 2024, 15, 71. https://doi.org/10.3390/f15010071
Liu D, Guo H, Yan L-P, Gao L, Zhai S, Xu Y. Physiological, Photosynthetic and Stomatal Ultrastructural Responses of Quercus acutissima Seedlings to Drought Stress and Rewatering. Forests. 2024; 15(1):71. https://doi.org/10.3390/f15010071
Chicago/Turabian StyleLiu, Dan, Haili Guo, Li-Ping Yan, Lei Gao, Shasha Zhai, and Yan Xu. 2024. "Physiological, Photosynthetic and Stomatal Ultrastructural Responses of Quercus acutissima Seedlings to Drought Stress and Rewatering" Forests 15, no. 1: 71. https://doi.org/10.3390/f15010071
APA StyleLiu, D., Guo, H., Yan, L.-P., Gao, L., Zhai, S., & Xu, Y. (2024). Physiological, Photosynthetic and Stomatal Ultrastructural Responses of Quercus acutissima Seedlings to Drought Stress and Rewatering. Forests, 15(1), 71. https://doi.org/10.3390/f15010071



