Nematode Community Characteristics Indicate Soil Restoration under Different Revegetation Approaches in the Semiarid Area of the Chinese Loess Plateau
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Soil Sampling
2.3. Soil Physicochemical Analysis
2.4. Nematode Community Analysis
2.5. Data Analysis
3. Results
3.1. Soil Physicochemical Properties
3.2. Composition and Structure of Soil Nematode Communities
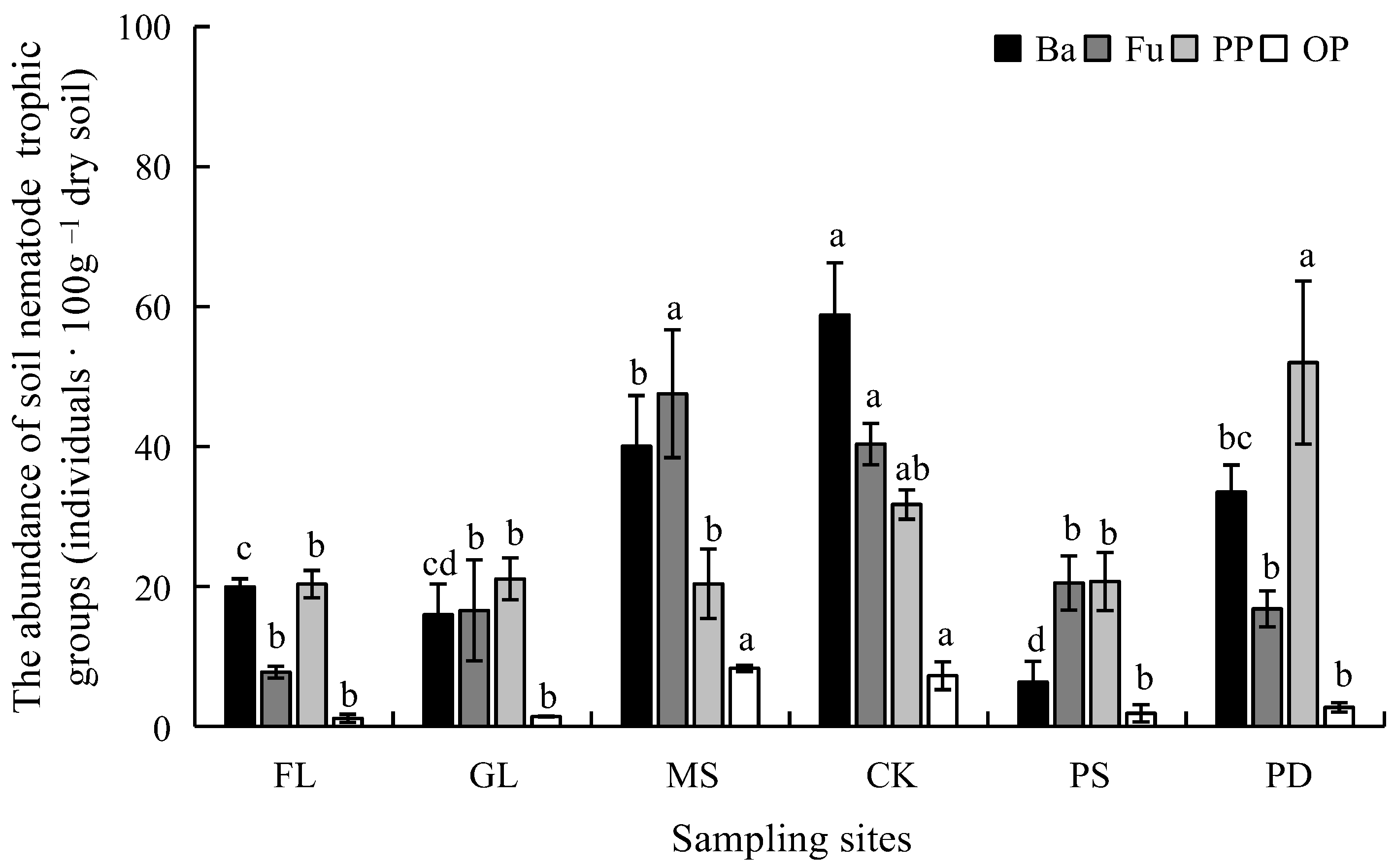
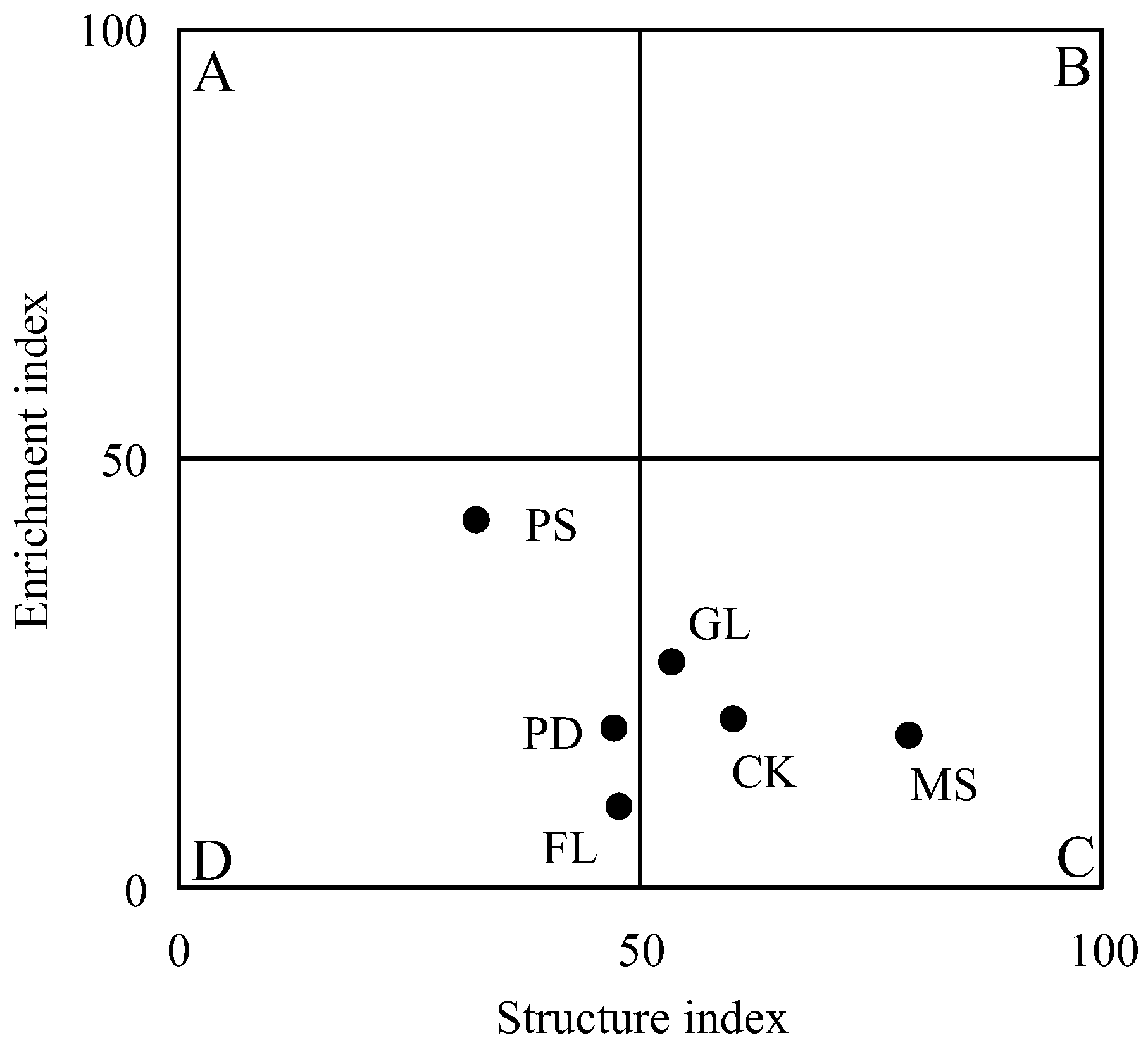
3.3. Ecological Indices of Soil Nematode Communities
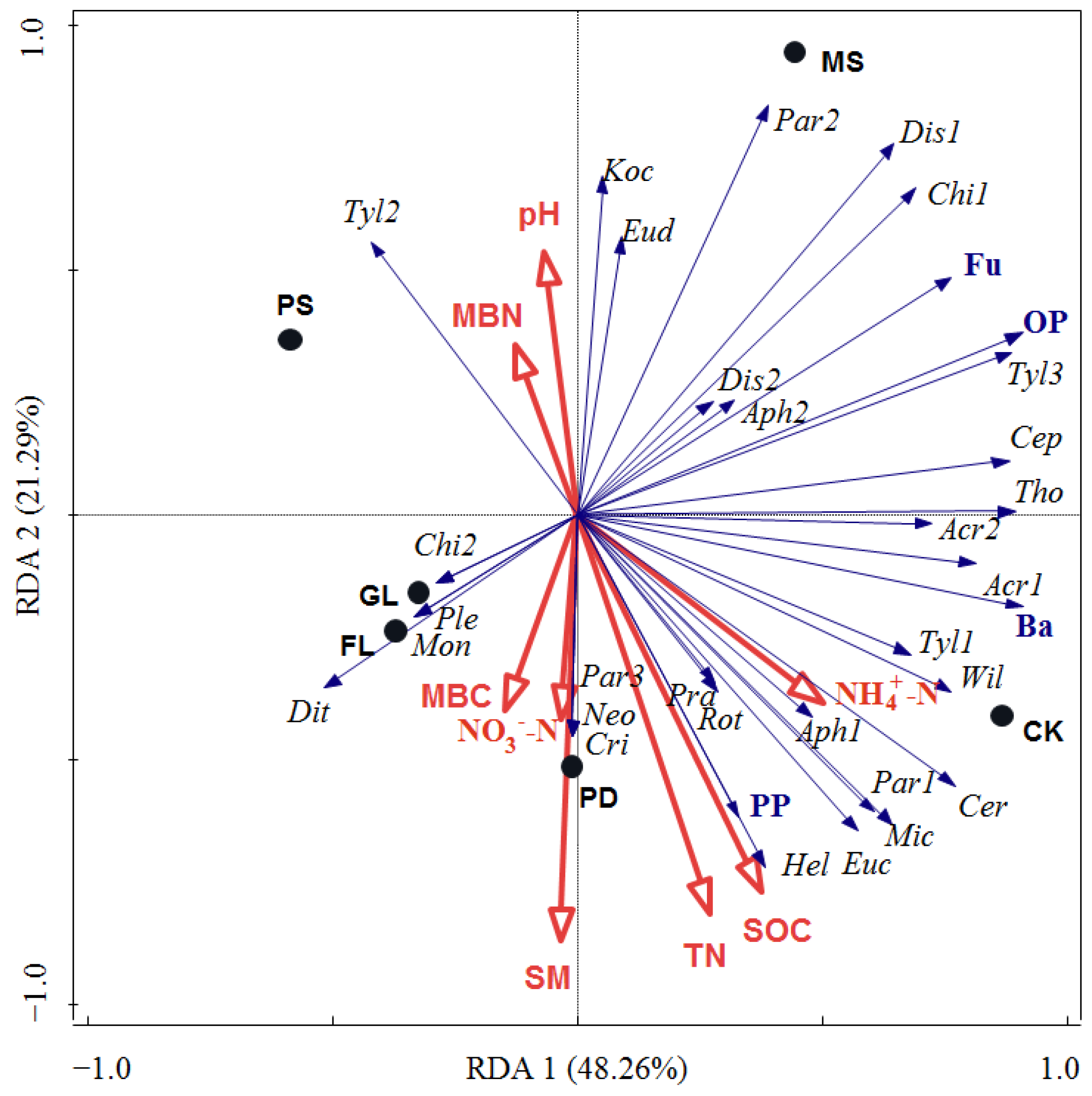
3.4. Relationships between Soil Nematode Communities and Physicochemical Properties
4. Discussion
4.1. Effects of Revegetation Approaches on Soil Physicochemical Properties
4.2. Effects of Revegetation Approaches on Soil Nematode Community Characteristics
4.3. Driving Forces of Soil Nematode Communities under Different Revegetation Approaches
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, P.; House, J.I.; Bustamante, M.; Sobocká, J.; Harper, R.; Pan, G.; West, P.C.; Clark, J.M.; Adhya, T.; Rumpel, C.; et al. Global change pressures on soils from land use and management. Glob. Chang. Biol. 2016, 22, 1008–1028. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, X.; Hou, L.; Wei, X.; Zhang, Y.; Shao, M.; Zhao, X. Variable response of inorganic carbon and consistent increase of organic carbon as a consequence of afforestation in areas with semiarid soils. Land Degrad. Dev. 2019, 30, 1345–1356. [Google Scholar] [CrossRef]
- Bullock, J.M.; Aronson, J.; Newton, A.C.; Pywell, R.F.; Rey-Benayas, J.M. Restoration of ecosystem services and biodiversity: Conflicts and opportunities. Trends Ecol. Evol. 2011, 26, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A. Measurements of the soil microbial community for estimating the success of restoration. Eur. J. Soil Sci. 2003, 54, 801–808. [Google Scholar] [CrossRef]
- Khorchani, M.; Nadal-Romero, E.; Lasanta, T.; Tague, C. Carbon sequestration and water yield tradeoffs following restoration of abandoned agricultural lands in Mediterranean mountains. Environ. Res. 2022, 207, 112203. [Google Scholar] [CrossRef]
- Azeria, E.T.; Santala, K.; McIntosh, A.C.S.; Aubin, I. Plant traits as indicators of recovery of reclaimed wellsites in forested areas: Slow but directional succession trajectory. For. Ecol. Manag. 2020, 468, 118180. [Google Scholar] [CrossRef]
- Bai, X.; Zhao, W.; Wang, J.; Ferreira, C.S.S. Reducing plant community variability and improving resilience for sustainable restoration of temperate grassland. Environ. Res 2022, 207, 112149. [Google Scholar] [CrossRef]
- Fu, D.; Wu, X.; Qiu, Q.; Duan, C.; Jones, D.L. Seasonal variations in soil microbial communities under different land restoration types in a subtropical mountains region, Southwest China. Appl. Soil Ecol. 2020, 153, 103634. [Google Scholar] [CrossRef]
- Kardol, P.; De Deyn, G.B.; Laliberté, E.; Mariotte, P.; Hawkes, C.V. Biotic plant–soil feedbacks across temporal scales. J. Ecol. 2013, 101, 309–315. [Google Scholar] [CrossRef]
- Frouz, J.; Toyota, A.; Mudrák, O.; Jílková, V.; Filipová, A.; Cajthaml, T. Effects of soil substrate quality, microbial diversity and community composition on the plant community during primary succession. Soil Biol. Biochem. 2016, 99, 75–84. [Google Scholar] [CrossRef]
- Korthals, G.W.; Smilauer, P.; Van Dijk, C.; Van Der Putten, W.H. Linking above- and below-ground biodiversity: Abundance and trophic complexity in soil as a response to experimental plant communities on abandoned arable land. Funct. Ecol. 2001, 15, 506–514. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, Y.; An, S. How C:N:P stoichiometry in soils and plants responds to succession in Robinia pseudoacacia forests on the Loess Plateau, China. For. Ecol. Manag. 2020, 475, 118394. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, P.; Wang, Y.; Li, Q.; Zhang, S.; Zhang, Z.; Bezemer, T.M.; Liang, W. Community composition, diversity and metabolic footprints of soil nematodes in differently-aged temperate forests. Soil Biol. Biochem. 2015, 80, 118–126. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- van der Putten, W.H.; Bradford, M.A.; Pernilla Brinkman, E.; van de Voorde, T.F.J.; Veen, G.F. Where, when and how plant–soil feedback matters in a changing world. Funct. Ecol. 2016, 30, 1109–1121. [Google Scholar] [CrossRef]
- Bongers, T.; Ferris, H. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 1999, 14, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Yeates, G.W. Nematodes as soil indicators: Functional and biodiversity aspects. Biol. Fertil. Soils 2003, 37, 199–210. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera-an outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar] [PubMed]
- Bardgett, R.D.; Cook, R.; Yeates, G.W.; Denton, C.S. The influence of nematodes on below-ground processes in grassland ecosystems. Plant Soil 1999, 212, 23–33. [Google Scholar] [CrossRef]
- Ekschmitt, K.; Bakonyi, G.; Bongers, M.; Bongers, T.; Boström, S.; Dogan, H.; Harrison, A.; Nagy, P.; O’Donnell, A.G.; Papatheodorou, E.M.; et al. Nematode community structure as indicator of soil functioning in European grassland soils. Eur. J. Soil Biol. 2001, 37, 263–268. [Google Scholar] [CrossRef]
- Ritz, K.; Trudgill, D.L. Utility of nematode community analysis as an integrated measure of the functional state of soils: Perspectives and challenges. Plant Soil 1999, 212, 1–11. [Google Scholar] [CrossRef]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Urzelai, A.; Hernández, A.J.; Pastor, J. Biotic indices based on soil nematode communities for assessing soil quality in terrestrial ecosystems. Sci. Total Environ. 2000, 247, 253–261. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.V.C.d.L.; Hirschfeld, M.N.C.; Cares, J.E.; Esteves, A.M. Land use, soil properties and climate variables influence the nematode communities in the Caatinga dry forest. Appl. Soil Ecol. 2020, 150, 103474. [Google Scholar] [CrossRef]
- Guan, P.; Zhang, X.; Yu, J.; Ma, N.; Liang, W. Variation of soil nematode community composition with increasing sand-fixation year of Caragana microphylla: Bioindication for desertification restoration. Ecol. Eng. 2015, 81, 93–101. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, Q.Z.; Wang, Y.Z.; Sun, H.Y.; Bai, C.Q.; Lewis, E.E. Different changes of soil nematode communities in replant and continuous-planting peach orchards and their indicative value for peach replant problem. Helminthologia 2015, 52, 261–269. [Google Scholar] [CrossRef]
- Coffey, V.; Otfinowski, R. Legacies of afforestation on soil nematode community composition, structure, and diversity in a northern Canadian prairie. Plant Soil 2019, 435, 437–447. [Google Scholar] [CrossRef]
- Vink, S.N.; Bienkowski, D.; Roberts, D.M.; Daniell, T.J.; Neilson, R. Impact of land use and management practices on soil nematode communities of Machair, a low-input calcareous ecosystem of conservation importance. Sci. Total Environ. 2020, 738, 140164. [Google Scholar] [CrossRef]
- Huo, N.; Zhao, S.; Huang, J.; Geng, D.; Wang, N.; Yang, P. Seasonal Stabilities of Soil Nematode Communities and Their Relationships with Environmental Factors in Different Temperate Forest Types on the Chinese Loess Plateau. Forests 2021, 12, 246. [Google Scholar] [CrossRef]
- Kardol, P.; De Long, J. How anthropogenic shifts in plant community composition alter soil food webs [version 1; peer review: 2 approved]. F1000Research 2018, 7, 4. [Google Scholar] [CrossRef]
- Deng, L.; Shangguan, Z.-p.; Sweeney, S. “Grain for Green” driven land use change and carbon sequestration on the Loess Plateau, China. Sci. Rep. 2014, 4, 7039. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dou, Y.; Huang, Y.; An, S. Links between Soil Fungal Diversity and Plant and Soil Properties on the Loess Plateau. Front. Microbiol. 2017, 8, 2198. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Tan, Q.; Liu, G.; Xu, M. Impacts of species mixture on soil nitrogen stocks in the Loess Plateau of China. For. Ecol. Manag. 2021, 491, 119145. [Google Scholar] [CrossRef]
- Li, G.; Huang, J.; Guo, H.; Du, S. Projecting species loss and turnover under climate change for 111 Chinese tree species. For. Ecol. Manag. 2020, 477, 118488. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, W.; Martinez-Murillo, J.F.; Pereira, P. Loess Plateau: From degradation to restoration. Sci. Total Environ. 2020, 738, 140206. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Xue, S.; Wang, G. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biol. Biochem. 2016, 97, 40–49. [Google Scholar] [CrossRef]
- Guo, Y.; Hou, L.; Zhang, Z.; Zhang, J.; Cheng, J.; Wei, G.; Lin, Y. Soil microbial diversity during 30 years of grassland restoration on the Loess Plateau, China: Tight linkages with plant diversity. Land Degrad. Dev. 2019, 30, 1172–1182. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Huang, J.; Wen, Z.; Du, S. Afforestation and climatic niche dynamics of black locust (Robinia pseudoacacia). For. Ecol. Manag. 2018, 407, 184–190. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Liu, J.; Liu, T.; Cheng, J.; Wei, G.; Lin, Y. Temporal and spatial succession and dynamics of soil fungal communities in restored grassland on the Loess Plateau in China. Land Degrad. Dev. 2019, 30, 1273–1287. [Google Scholar] [CrossRef]
- Zeng, Q.; An, S.; Liu, Y. Soil bacterial community response to vegetation succession after fencing in the grassland of China. Sci. Total Environ. 2017, 609, 2–10. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, X.; Zhang, J.; Cao, L.; Xu, X.; Zhang, K.; Chen, Y. Spatio-temporal variation of reference evapotranspiration and aridity index in the Loess Plateau Region of China, during 1961–2012. Quat. Int. 2014, 349, 196–206. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, C.; Wang, Y.; Zhu, Y.; Wei, X.; Shao, M. Traditional dry soil layer index method overestimates soil desiccation severity following conversion of cropland into forest and grassland on China’s Loess Plateau. Agric. Ecosyst. Environ. 2020, 291, 106794. [Google Scholar] [CrossRef]
- Wen, M.; Cheng, D.; Song, J.; Zhang, G.; Lai, W.; Jiang, W. Impacts of climate change on aridity index and its spatiotemporal variation in the Loess Plateau of China, from 1961 to 2014. Environ. Earth Sci. 2018, 77, 137. [Google Scholar] [CrossRef]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Wu, J.; O’Donnell, A.G.; He, Z.L.; Syers, J.K. Fumigation-extraction method for the measurement of soil microbial biomass-S. Soil Biol. Biochem. 1994, 26, 117–125. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Liu, M.; Chen, X.; Qin, J.; Wang, D.; Griffiths, B.; Hu, F. A sequential extraction procedure reveals that water management affects soil nematode communities in paddy fields. Appl. Soil Ecol. 2008, 40, 250–259. [Google Scholar] [CrossRef]
- Bongers, A.M.T. De Nematoden Van Nederland; Pirota Schoorl, Bibliotheek uitgave KNNV, nr. 46: Utrecht, The Netherlands, 1988; 408p. [Google Scholar]
- Ahmad, W.; Jairajpuri, M.S. Mononchida: The Predaceous Nematodes (Nematology Monographs and Perspectives 7); Brill Academic Publishers: Leiden, The Netherlands, 2010. [Google Scholar]
- Geisen, S.; Snoek, L.B.; ten Hooven, F.C.; Duyts, H.; Kostenko, O.; Bloem, J.; Martens, H.; Quist, C.W.; Helder, J.A.; van der Putten, W.H. Integrating quantitative morphological and qualitative molecular methods to analyse soil nematode community responses to plant range expansion. Methods Ecol. Evol. 2018, 9, 1366–1378. [Google Scholar] [CrossRef]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1949; Volume 60, p. 144. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1967, 15, 177. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Margalef, R. La teoria de la informacion en ecologia. Mem. Real Acad. Cienc. Artes Barc. 1957, 32, 373–449. [Google Scholar]
- Heip, C. Biota and abiotic environment in the Westerschelde estuary. Hydrobiol. Bull. 1988, 22, 31–34. [Google Scholar] [CrossRef]
- Bongers, T.; van der Meulen, H.; Korthals, G. Inverse relationship between the nematode maturity index and plant parasite index under enriched nutrient conditions. Appl. Soil Ecol. 1997, 6, 195–199. [Google Scholar] [CrossRef]
- Wasilewska, L. The effect of age of meadows on succession and diversity in soil nematode communities. Pedobiologia 1994, 38, 1–11. [Google Scholar]
- Ferris, H.; Bongers, T.; de Goede, R.G.M. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- ter Braak, C.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software of Ordination, version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Wang, Z.; Guo, S.; Sun, Q.; Li, N.; Jiang, J.; Wang, R.; Zhang, Y.; Liu, Q.; Wu, D.; Li, R.; et al. Soil organic carbon sequestration potential of artificial and natural vegetation in the hilly regions of Loess Plateau. Ecol. Eng. 2015, 82, 547–554. [Google Scholar] [CrossRef]
- Fang, X.; Xue, Z.; Li, B.; An, S. Soil organic carbon distribution in relation to land use and its storage in a small watershed of the Loess Plateau, China. Catena 2012, 88, 6–13. [Google Scholar] [CrossRef]
- Fu, B.J.; Liu, S.L.; Ma, K.M.; Zhu, Y.G. Relationships between soil characteristics, topography and plant diversity in a heterogeneous deciduous broad-leaved forest near Beijing, China. Plant Soil 2004, 261, 47–54. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, G.; Hai, X.; Li, J.; Shangguan, Z.; Peng, C.; Deng, L. Long-term forest succession improves plant diversity and soil quality but not significantly increase soil microbial diversity: Evidence from the Loess Plateau. Ecol. Eng. 2020, 142, 105631. [Google Scholar] [CrossRef]
- Jia, G.-M.; Liu, B.-R.; Wang, G.; Zhang, B. The microbial biomass and activity in soil with shrub (Caragana korshinskii K.) plantation in the semi-arid loess plateau in China. Eur. J. Soil Biol. 2010, 46, 6–10. [Google Scholar] [CrossRef]
- She, D.; Xia, Y.; Shao, M.; Peng, S.; Yu, S. Transpiration and canopy conductance of Caragana korshinskii trees in response to soil moisture in sand land of China. Agrofor. Syst. 2013, 87, 667–678. [Google Scholar] [CrossRef]
- Cao, C.; Jiang, D.; Teng, X.; Jiang, Y.; Liang, W.; Cui, Z. Soil chemical and microbiological properties along a chronosequence of Caragana microphylla Lam. plantations in the Horqin sandy land of Northeast China. Appl. Soil Ecol. 2008, 40, 78–85. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Raaijmakers, C.E.; van Ruijven, J.; Berendse, F.; van der Putten, W.H. Plant species identity and diversity effects on different trophic levels of nematodes in the soil food web. Oikos 2004, 106, 576–586. [Google Scholar] [CrossRef]
- Ferris, H.; Matute, M.M. Structural and functional succession in the nematode fauna of a soil food web. Appl. Soil Ecol. 2003, 23, 93–110. [Google Scholar] [CrossRef]
- Yeates, G.W.; Hawke, M.F.; Rijkse, W.C. Changes in soil fauna and soil conditions under Pinus radiata agroforestry regimes during a 25-year tree rotation. Biol. Fertil. Soils 2000, 31, 391–406. [Google Scholar] [CrossRef]
- Khan, A.; Shaukat, S.S. Plant nematodes associated with almond seedlings in Kalat District, Balochistan. Pak. J. Zool. 2016, 48, 1579–1580. [Google Scholar]
- Baxter, C.; Rowan, J.S.; Mckenzie, B.M.; Neilson, R. Understanding soil erosion impacts in temperate agroecosystems: Bridging the gap between geomorphology and soil ecology using nematodes as a model organism. Biogeosciences 2013, 10, 7133–7145. [Google Scholar] [CrossRef]
- Stefanovska, T.; Skwiercz, A.; Zouhar, M.; Pidlisnyuk, V.; Zhukov, O. Plant-feeding nematodes associated with Miscanthus × giganteus and their use as potential indicators of the plantations’ state. Int. J. Environ. Sci. Technol. 2021, 18, 57–72. [Google Scholar] [CrossRef]
- Verschoor, B.C.; Pronk, T.E.; De Goede, R.G.M.; Brussaard, L. Could plant-feeding nematodes affect the competition between grass species during succession in grasslands under restoration management? J. Ecol. 2002, 90, 753–761. [Google Scholar] [CrossRef]
- Brinkman, E.P.; Duyts, H.; Karssen, G.; van der Stoel, C.D.; van der Putten, W.H. Plant-feeding nematodes in coastal sand dunes: Occurrence, host specificity and effects on plant growth. Plant Soil 2015, 397, 17–30. [Google Scholar] [CrossRef]
- Cesarz, S.; Reich, P.B.; Scheu, S.; Ruess, L.; Schaefer, M.; Eisenhauer, N. Nematode functional guilds, not trophic groups, reflect shifts in soil food webs and processes in response to interacting global change factors. Pedobiologia 2015, 58, 23–32. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Wu, Y.; Zhang, L.; Cheng, J.; Wei, G.; Lin, Y. Natural revegetation of a semiarid habitat alters taxonomic and functional diversity of soil microbial communities. Sci. Total Environ. 2018, 635, 598–606. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Song, Z.; Qu, D.; Fang, L.; Deng, L. Natural succession on abandoned cropland effectively decreases the soil erodibility and improves the fungal diversity. Ecol. Appl. 2017, 27, 2142–2154. [Google Scholar] [CrossRef] [PubMed]
- Kardol, P.; Newton, J.S.; Bezemer, T.M.; Maraun, M.; van der Putten, W.H. Contrasting diversity patterns of soil mites and nematodes in secondary succession. Acta Oecol. 2009, 35, 603–609. [Google Scholar] [CrossRef]
- Neilson, R.; Caul, S.; Fraser, F.C.; King, D.; Mitchell, S.M.; Roberts, D.M.; Giles, M.E. Microbial community size is a potential predictor of nematode functional group in limed grasslands. Appl. Soil Ecol. 2020, 156, 103702. [Google Scholar] [CrossRef]
- Ciobanu, M.; Popovici, I.; Zhao, J.; Stoica, I.-A. Patterns of relative magnitudes of soil energy channels and their relationships with environmental factors in different ecosystems in Romania. Sci. Rep. 2015, 5, 17606. [Google Scholar] [CrossRef]
- Ferris, H.; Sánchez-Moreno, S.; Brennan, E.B. Structure, functions and interguild relationships of the soil nematode assemblage in organic vegetable production. Appl. Soil Ecol. 2012, 61, 16–25. [Google Scholar] [CrossRef]
- Schöning, C.; Wurst, S. Positive effects of root-knot nematodes (Meloidogyne incognita) on nitrogen availability do not outweigh their negative effects on fitness in Nicotiana attenuata. Plant Soil 2016, 400, 381–390. [Google Scholar] [CrossRef]
- Wardle, D.A.; Williamson, W.M.; Yeates, G.W.; Bonner, K.I. Trickle-down effects of aboveground trophic cascades on the soil food web. Oikos 2005, 111, 348–358. [Google Scholar] [CrossRef]
- Taylor, R.A.J. Taylor’s Power Law: Order and Pattern in Nature; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Abd-Elgawad, M.M.M. Optimizing Sampling and Extraction Methods for Plant-Parasitic and Entomopathogenic Nematodes. Plants 2021, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
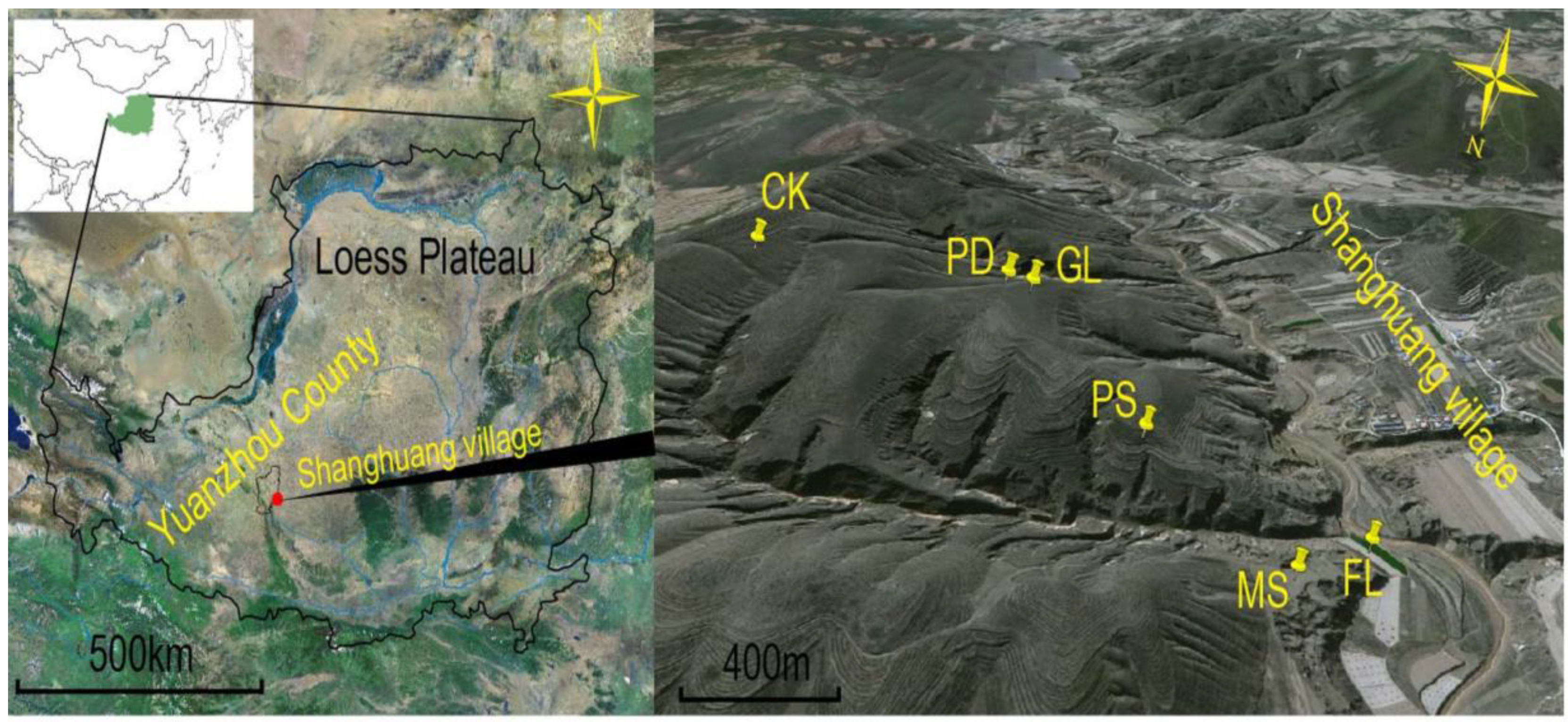
| Sampling Sites | Dominant Plant Species | Planting Time | Longitude | Latitude | Altitude (m) |
|---|---|---|---|---|---|
| FL | Corn (Zea mays) | / | 106°27′56″ | 36°0′42″ | 1583 |
| GL | T. mongolicus; S. bungeana; H. altaicus | 2004 | 106°28′31″ | 36°0′25″ | 1681 |
| MS | M. sativa | 2005 | 106°28′00″ | 36°0′46″ | 1593 |
| CK | C. korshinskii | 2005 | 106°29′00″ | 36°0′26″ | 1652 |
| PS | P. sibirica | 2002 | 106°28′06″ | 36°0′38″ | 1651 |
| PD | P. davidiana | 2004 | 106°28′31″ | 36°0′24″ | 1670 |
| Sampling Sites | pH | SM (%) | SOC (g/kg) | TN (g/kg) | NH4+-N (mg/kg) | NO3−-N (mg/kg) | MBC (mg/kg) | MBN (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| FL | 8.15 ± 0.03 a | 13.66 ± 0.28 ab | 6.98 ± 0.15 b | 0.78 ± 0.02 ab | 0.75 ± 0.10 b | 2.92 ± 0.06 a | 628.61 ± 27.94 ab | 91.03 ± 3.68 ab |
| GL | 7.88 ± 0.12 b | 16.82 ± 0.50 a | 13.00 ± 0.59 a | 1.18 ± 0.17 ab | 1.72 ± 0.10 a | 0.23 ± 0.14 c | 739.57 ± 37.27 a | 39.97 ± 6.12 b |
| MS | 8.14 ± 0.02 a | 10.87 ± 0.18 b | 5.65 ± 0.31 c | 0.60 ± 0.03 b | 0.98 ± 0.09 ab | 0.19 ± 0.08 c | 428.42 ± 75.38 bc | 110.94 ± 14.15 a |
| CK | 7.98 ± 0.01 ab | 15.37 ± 0.16 ab | 13.74 ± 0.03 a | 1.18 ± 0.20 ab | 1.77 ± 0.35 a | 1.23 ± 0.40 b | 506.16 ± 11.46 b | 55.42 ± 17.40 ab |
| PS | 8.08 ± 0.02 ab | 12.66 ± 0.07 ab | 5.35 ± 0.29 c | 0.56 ± 0.04 b | 0.80 ± 0.08 ab | 0.11 ± 0.02 c | 342.40 ± 91.60 c | 100.18 ± 32.45 ab |
| PD | 8.00 ± 0.03 ab | 17.56 ± 2.31 a | 12.83 ± 0.27 a | 1.38 ± 0.04 a | 1.05 ± 0.24 ab | 0.51 ± 0.35 bc | 434.81 ± 34.66 bc | 118.94 ± 16.22 a |
| Genus | Trophic Group | c-p Value | FL | GL | MS | CK | PS | PD |
|---|---|---|---|---|---|---|---|---|
| Acrobeles | Ba | 2 | 16.84 | 10.26 | 17.81 | 13.72 | 6.00 | 20.89 |
| Acrobeloides | Ba | 2 | 2.11 | 4.27 | 2.83 | 1.81 | 1.00 | 2.67 |
| Cephalobus | Ba | 2 | 4.21 | 3.42 | 4.05 | 6.14 | 2.00 | 0.89 |
| Cervidellus | Ba | 2 | 12.63 | 9.40 | 4.45 | 13.72 | 2.00 | 7.56 |
| Chiloplacus | Ba | 2 | 1.05 | 0.00 | 5.26 | 2.17 | 2.00 | 0.00 |
| Chiloplectus | Ba | 2 | 0.00 | 0.85 | 0.00 | 0.00 | 0.00 | 0.00 |
| Eucephalobus | Ba | 2 | 3.16 | 0.85 | 0.00 | 3.25 | 0.00 | 0.44 |
| Plectus | Ba | 2 | 1.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Wilsoema | Ba | 2 | 0.00 | 0.00 | 0.00 | 1.44 | 0.00 | 0.00 |
| Aphelenchoides | Fu | 2 | 1.05 | 0.00 | 0.00 | 2.53 | 2.00 | 0.44 |
| Aphelenchus | Fu | 2 | 5.26 | 17.09 | 9.31 | 11.19 | 35.00 | 8.89 |
| Tylencholaimus | Fu | 4 | 9.47 | 12.82 | 31.58 | 15.88 | 5.00 | 6.67 |
| Ditylenchus | PP | 2 | 23.16 | 17.09 | 7.29 | 5.05 | 17.00 | 12.00 |
| Neopsilenchus | PP | 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.44 |
| Paratylenchus | PP | 2 | 0.00 | 1.71 | 0.00 | 5.78 | 1.00 | 1.78 |
| Tylenchus | PP | 2 | 0.00 | 0.00 | 0.40 | 0.00 | 5.00 | 0.00 |
| Criconema | PP | 3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.44 |
| Helicotylenchus | PP | 3 | 2.11 | 4.27 | 0.40 | 2.89 | 2.00 | 1.33 |
| Pratylenchus | PP | 3 | 11.50 | 7.69 | 6.48 | 4.69 | 8.00 | 23.56 |
| Rotylenchus | PP | 3 | 2.11 | 7.69 | 2.43 | 3.97 | 8.00 | 8.89 |
| Tylenchorhynchus | PP | 3 | 2.11 | 0.00 | 0.40 | 0.72 | 0.00 | 0.44 |
| Eudorylaimus | Om | 4 | 0.00 | 0.85 | 0.40 | 0.00 | 0.00 | 0.00 |
| Microdorylaimus | Om | 4 | 0.00 | 0.00 | 0.00 | 0.36 | 0.00 | 0.44 |
| Kochinema | Om | 4 | 0.00 | 0.00 | 0.81 | 0.00 | 1.00 | 0.44 |
| Thonus | Om | 4 | 1.05 | 0.85 | 1.62 | 3.97 | 2.00 | 0.89 |
| Paraxonchium | Om | 5 | 0.00 | 0.00 | 0.40 | 0.00 | 0.00 | 0.00 |
| Mononchus | Pr | 4 | 1.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Discolaimium | Pr | 5 | 0.00 | 0.00 | 3.64 | 0.72 | 1.00 | 0.00 |
| Discolaimus | Pr | 5 | 0.00 | 0.00 | 0.40 | 0.00 | 0.00 | 0.44 |
| Nygolaimus | Pr | 5 | 0.00 | 0.85 | 0.00 | 0.00 | 0.00 | 0.00 |
| Paravulvus | Pr | 5 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.44 |
| Total genera number | 17 | 16 | 19 | 20 | 17 | 21 | ||
| Total nematode abundance | 49.18 ± 2.74 b | 55.00 ± 9.21 b | 116.26 ± 17.67 a | 138.10 ± 11.60 a | 49.43 ± 6.26 b | 105.00 ± 11.43 a | ||
| Ecological Index | FL | GL | MS | CK | PS | PD |
|---|---|---|---|---|---|---|
| H’ | 2.07 ± 0.12 b | 2.15 ± 0.10 b | 2.13 ± 0.19 b | 2.48 ± 0.13 a | 1.94 ± 0.22 b | 2.14 ± 0.07 b |
| J’ | 0.88 ± 0.02 a | 0.89 ± 0.02 a | 0.83 ± 0.05 a | 0.88 ± 0.02 a | 0.82 ± 0.01 a | 0.83 ± 0.07 a |
| λ | 0.15 ± 0.02 ab | 0.14 ± 0.01 ab | 0.16 ± 0.03 ab | 0.11 ± 0.02 b | 0.21 ± 0.07 a | 0.15 ± 0.01 ab |
| SR | 2.49 ± 0.32 a | 2.58 ± 0.34 a | 2.62 ± 0.41 a | 3.25 ± 0.32 a | 2.56 ± 0.44 a | 2.67 ± 0.31 a |
| TD | 2.74 ± 0.04 ab | 2.84 ± 012 ab | 3.11 ± 0.14 a | 3.08 ± 0.02 a | 2.50 ± 0.22 b | 2.59 ± 0.11 ab |
| NCR | 0.72 ± 0.05 a | 0.51 ± 0.21 ab | 0.46 ± 0.01 b | 0.59 ± 0.05 ab | 0.21 ± 0.10 c | 0.67 ± 0.05 ab |
| WI | 1.38 ± 0.09 b | 1.54 ± 0.15 b | 4.67 ± 0.82 a | 3.13 ± 0.18 ab | 1.50 ± 0.46 b | 1.12 ± 0.29 b |
| MI | 1.41 ± 0.08 c | 1.54 ± 0.05 bc | 2.47 ± 0.56 a | 1.96 ± 0.13 b | 1.30 ± 0.38 c | 1.23 ± 0.42 c |
| PPI | 2.44 ± 0.02 b | 2.53 ± 0.13 ab | 2.52 ± 0.13 ab | 2.54 ± 0.15 ab | 2.43 ± 0.08 b | 2.71 ± 0.02 a |
| PPI/MI | 1.72 ± 0.05 ab | 1.67 ± 0.11 ab | 1.03 ± 0.07 b | 1.30 ± 0.30 b | 2.14 ± 0.51 a | 2.30 ± 0.27 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Huang, T.; Chen, J.; Li, G.; Wang, Z.; Huo, N. Nematode Community Characteristics Indicate Soil Restoration under Different Revegetation Approaches in the Semiarid Area of the Chinese Loess Plateau. Forests 2023, 14, 1886. https://doi.org/10.3390/f14091886
Huang J, Huang T, Chen J, Li G, Wang Z, Huo N. Nematode Community Characteristics Indicate Soil Restoration under Different Revegetation Approaches in the Semiarid Area of the Chinese Loess Plateau. Forests. 2023; 14(9):1886. https://doi.org/10.3390/f14091886
Chicago/Turabian StyleHuang, Jinghua, Tianyuan Huang, Jing Chen, Guoqing Li, Zijun Wang, and Na Huo. 2023. "Nematode Community Characteristics Indicate Soil Restoration under Different Revegetation Approaches in the Semiarid Area of the Chinese Loess Plateau" Forests 14, no. 9: 1886. https://doi.org/10.3390/f14091886






