Physiological and Endophytic Fungi Changes in Grafting Seedlings of Qi-Nan Clones (Aquilaria sinensis)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Graft Healing Observation
2.3. Malondialdehyde (MDA) Determination
2.4. Phytohormonal Estimation
2.5. Endogenous Fungal Properties
2.5.1. DNA Extraction and PCR Amplification
2.5.2. Library Construction and Sequencing
2.5.3. Processing of Sequencing Data
2.6. Data Preprocessing and Statistical Analysis
3. Results
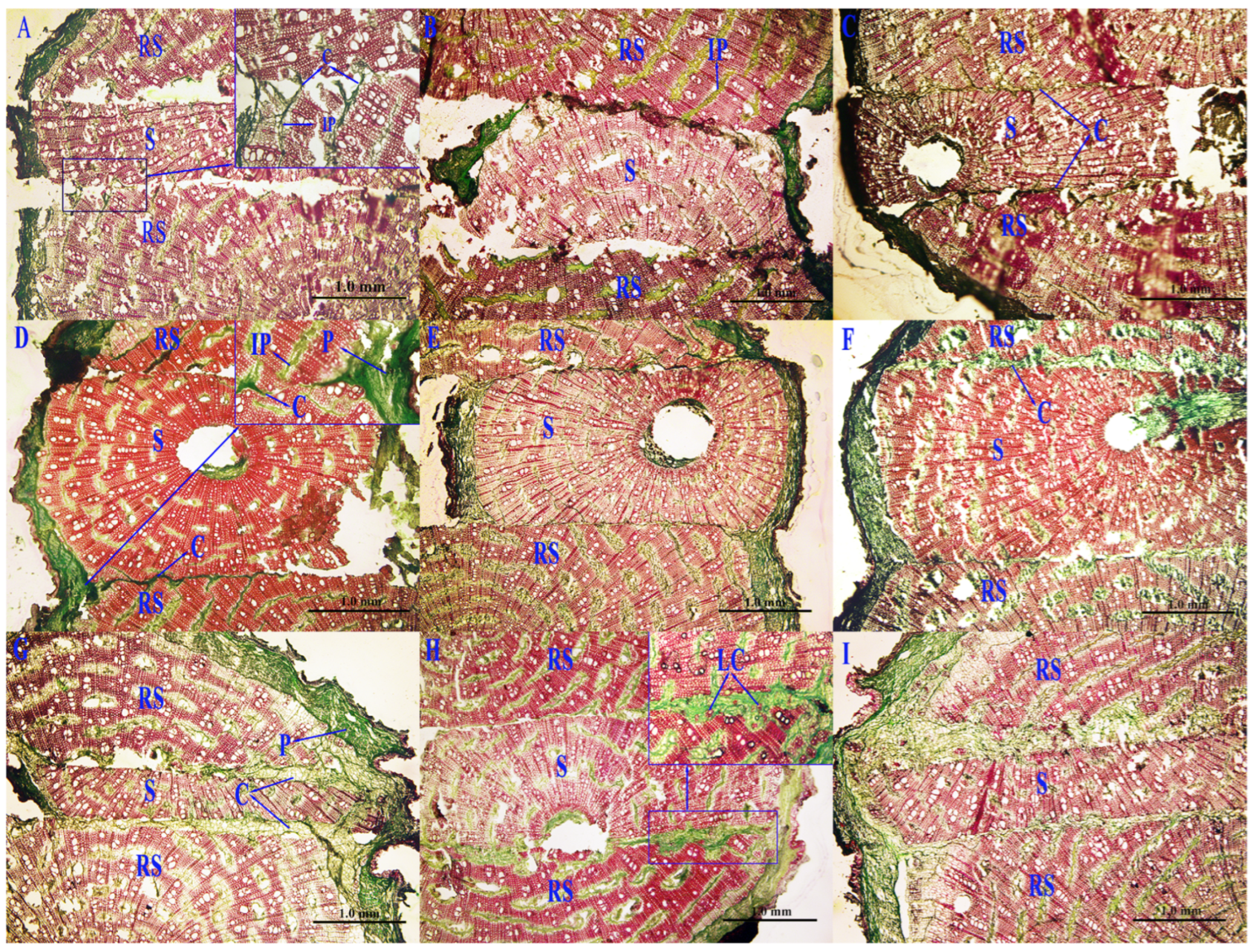
3.1. Graft Healing Situation
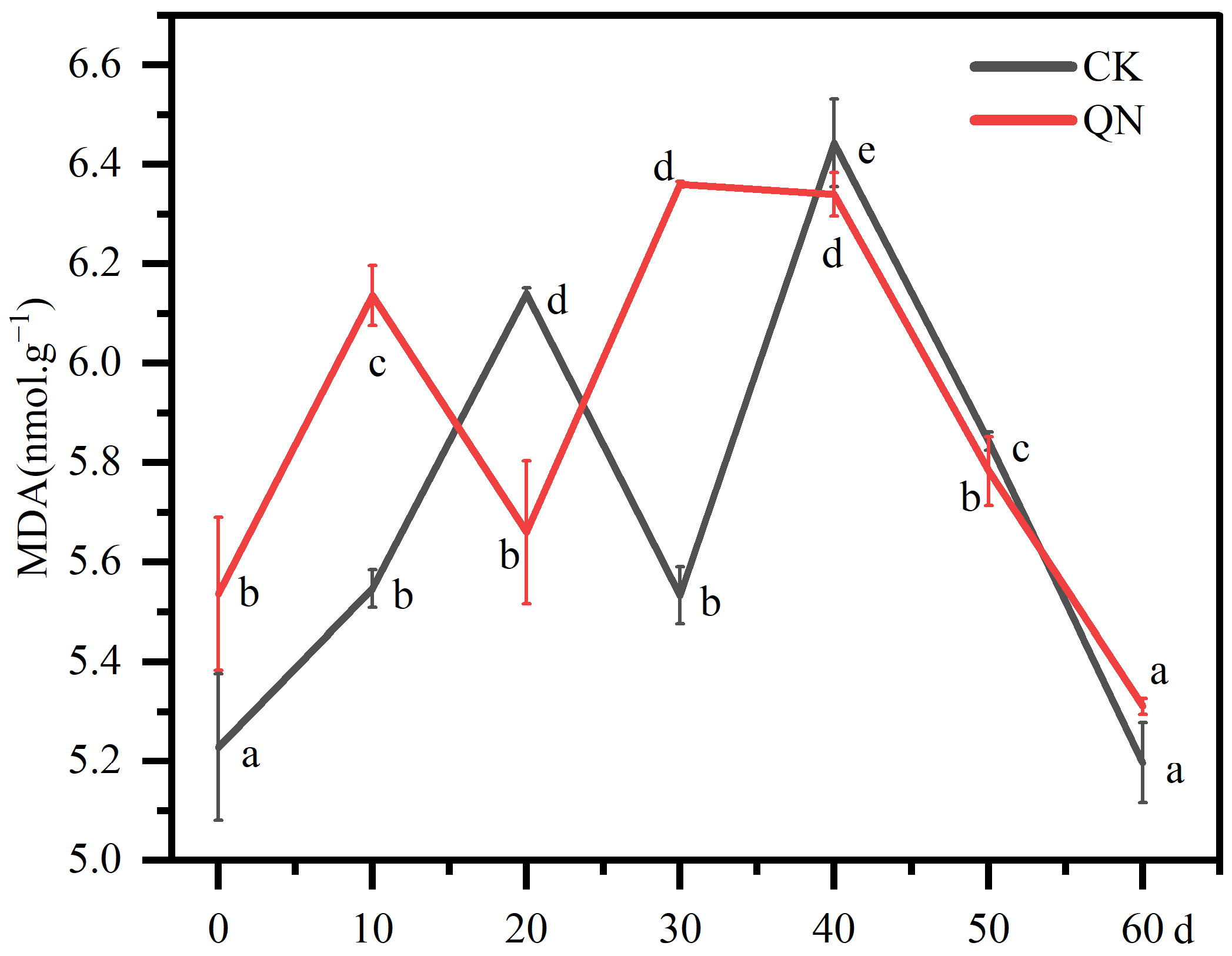
3.2. Malondialdehyde (MDA) Changes in Graft Healing
3.3. Physiological Index Changes
3.4. Endophytic Fungi Changes
3.4.1. Characterization of Sequencing Data
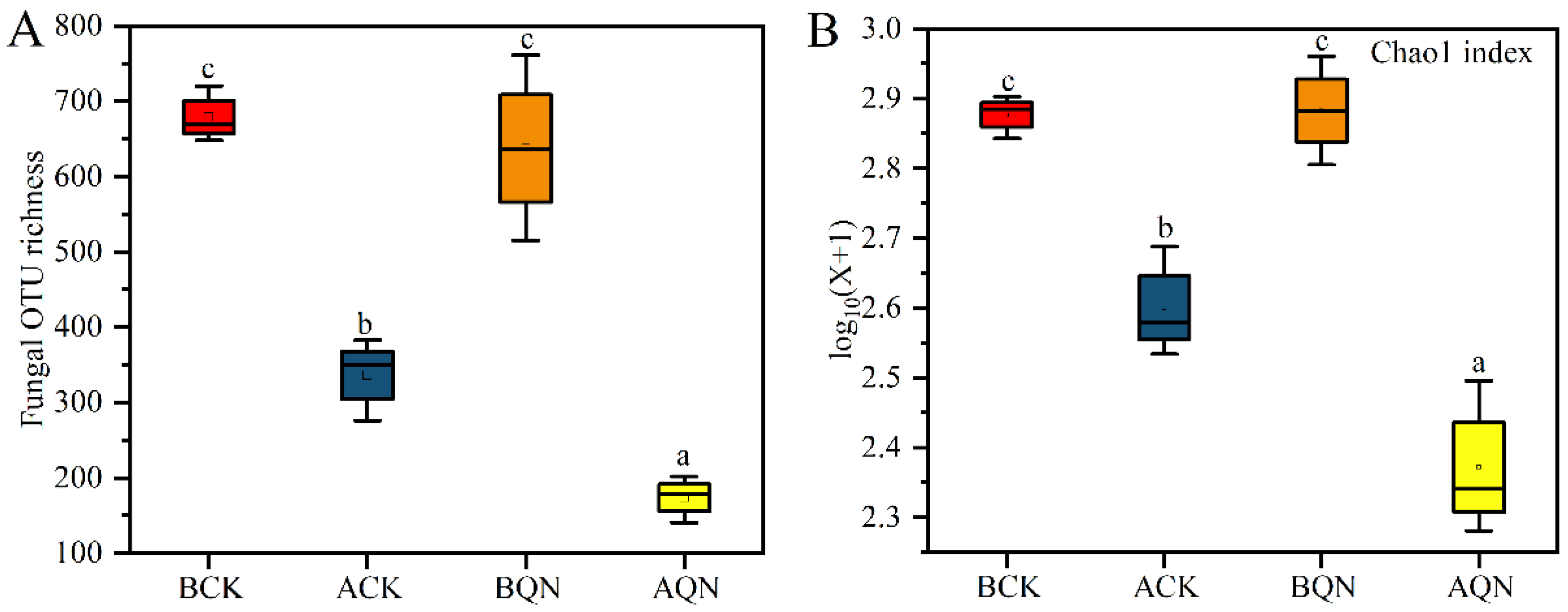
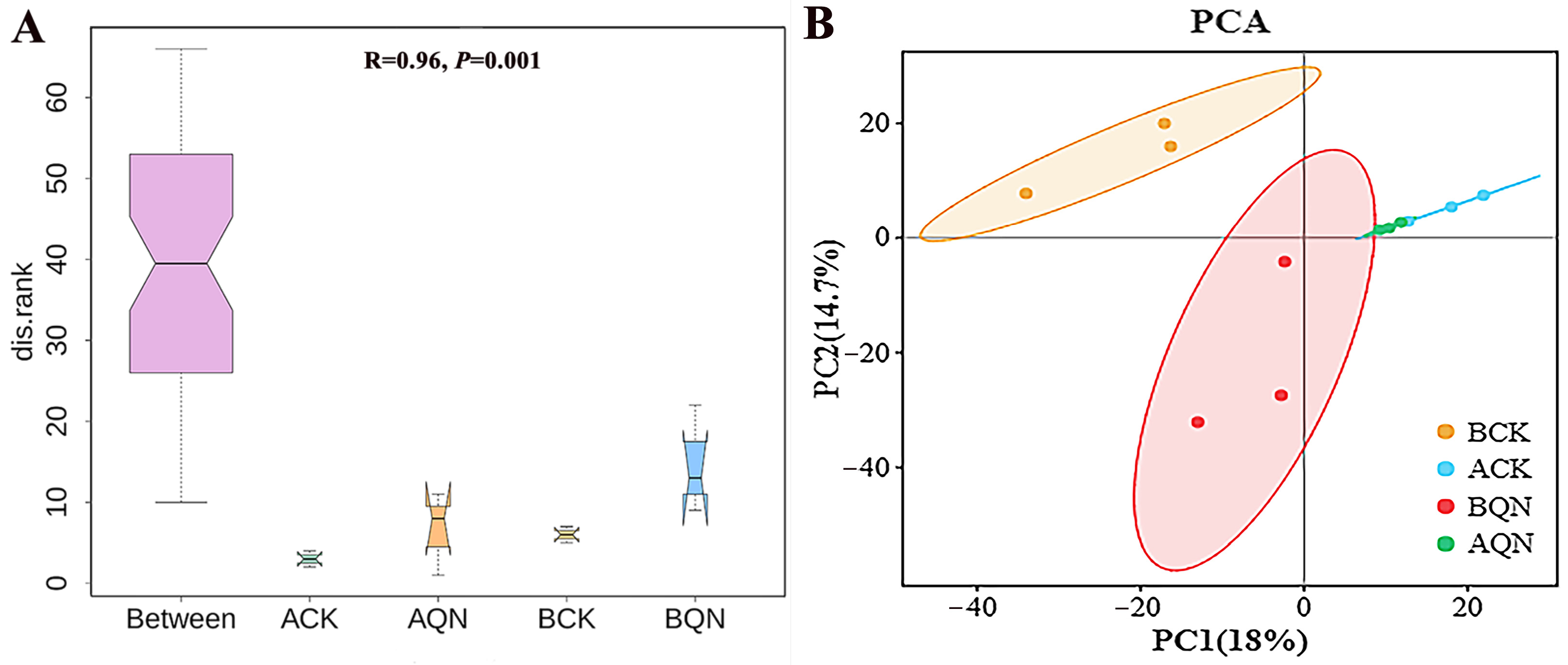
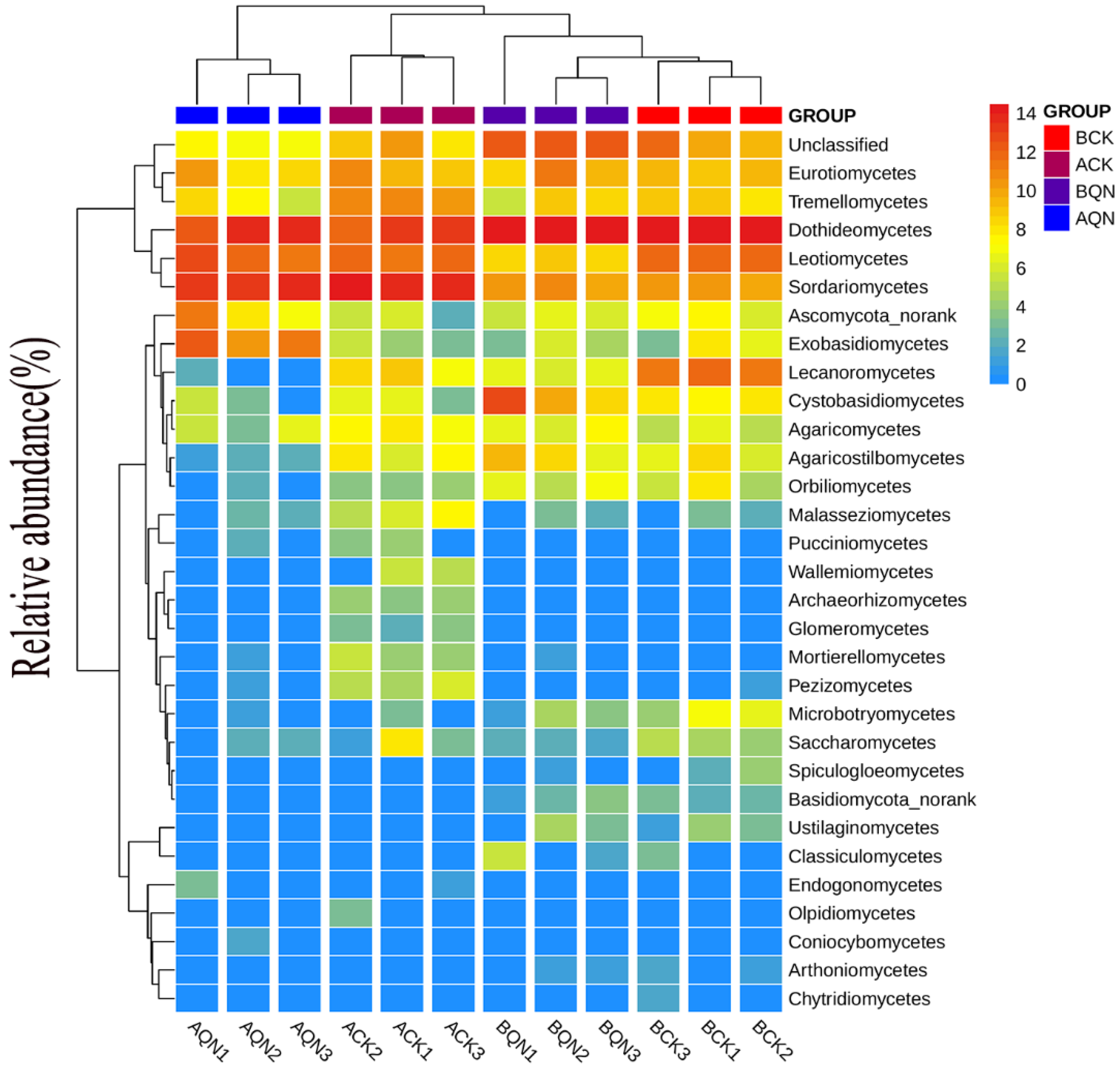
3.4.2. Endophytic Fungi Community Structure
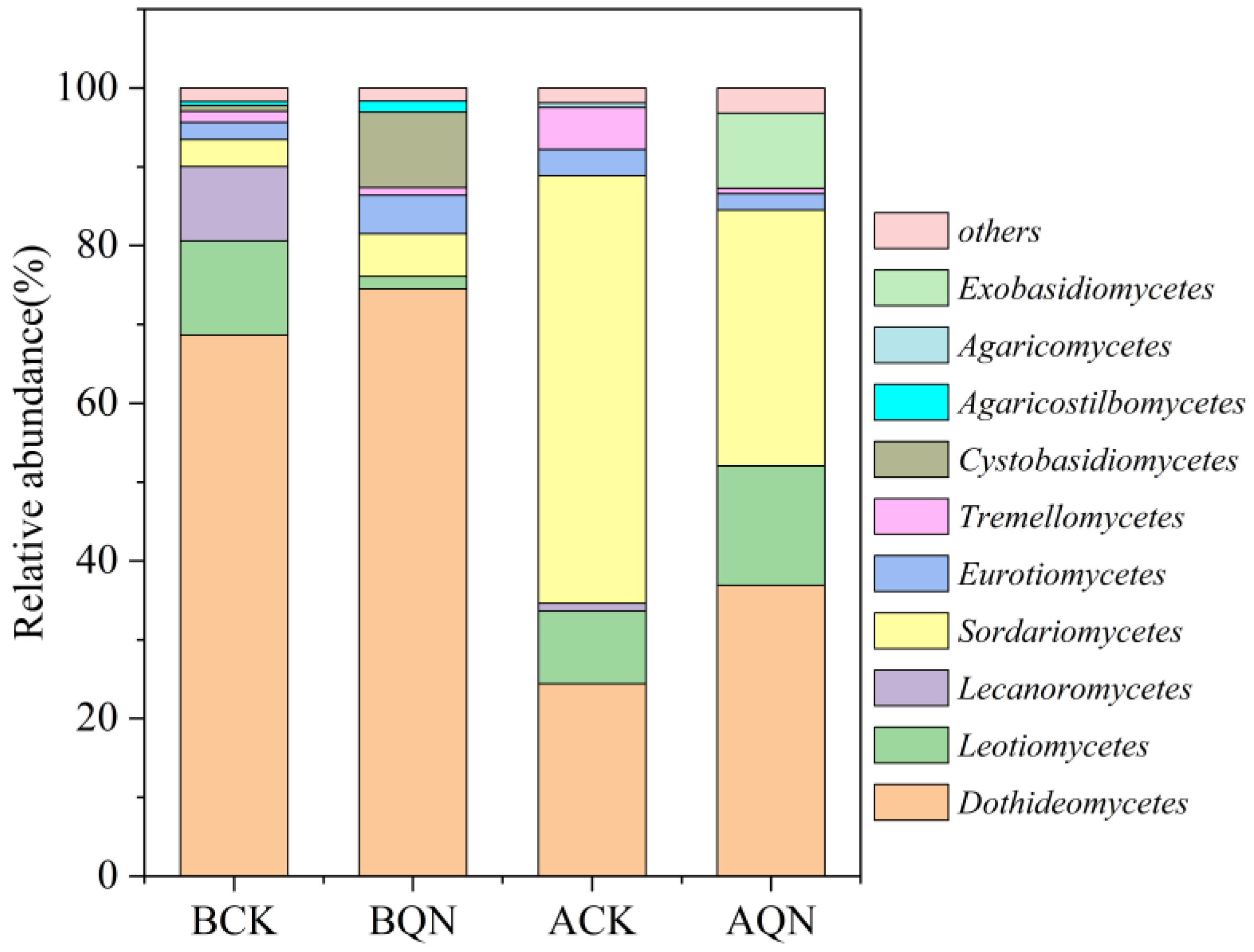
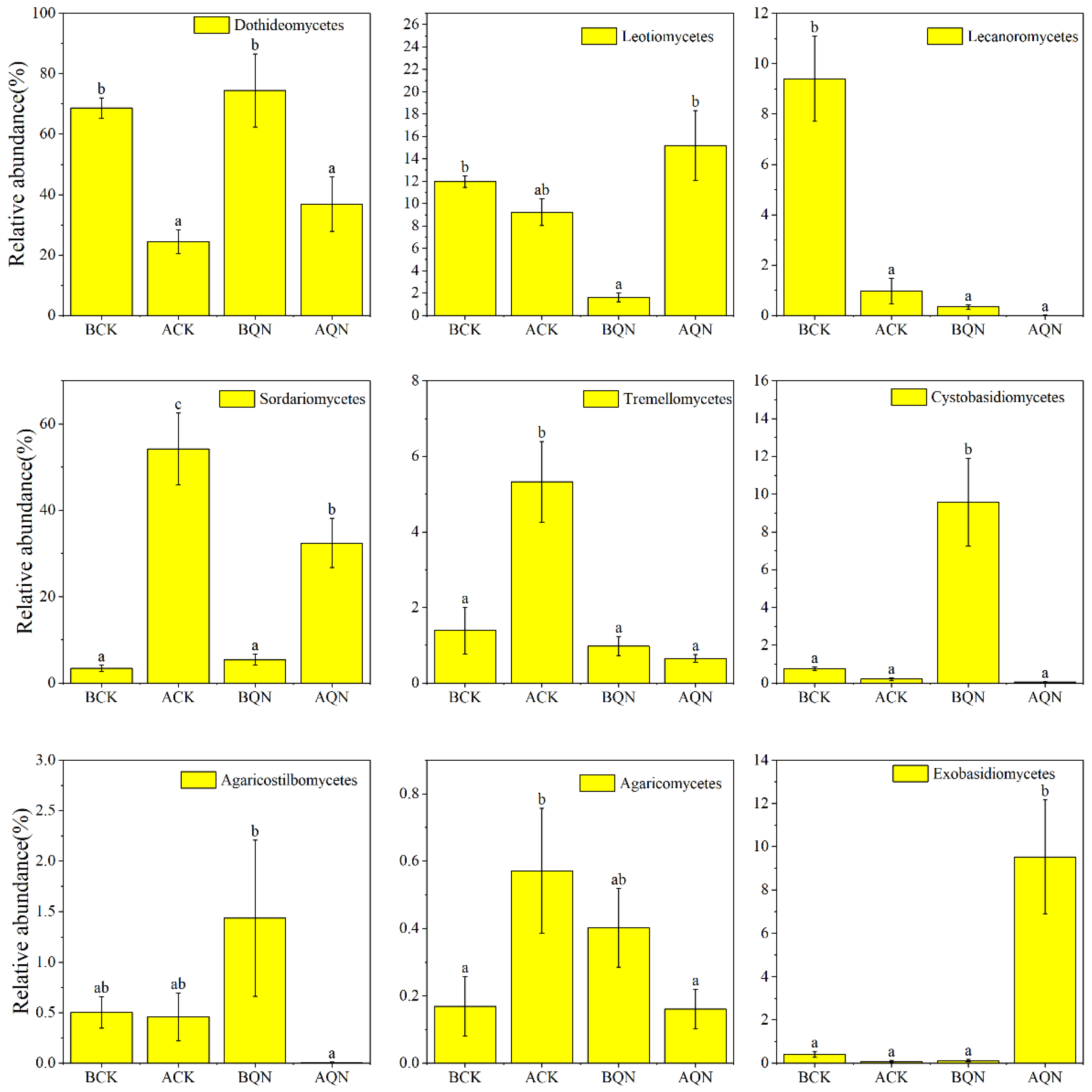
3.4.3. Community Composition of Endophytic Fungi before and after Grafting between Ordinary A. sinensis and Qi-Nan
3.4.4. Co-Occurrence Network of the Endophytic Fungal Communities
3.5. Functional Prediction of Endophytic Fungi from Ordinary A. sinensis and Qi-Nan
4. Discussion
4.1. Grafting Interface Healing
4.2. Physiological Index Changes of Grafting Seedlings
4.3. Endophytic Fungi of Grafting Seedlings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azren, P.D.; Lee, S.Y.; Emang, D.; Mohamed, R. History and Perspectives of Induction Technology for Agarwood Production from Cultivated Aquilaria in Asia: A Review. J. For. Res. 2019, 30, 1–11. [Google Scholar] [CrossRef]
- Chen, X.Y.; Sui, C.; Liu, Y.Y.; Yang, Y.; Liu, P.W.; Zhang, Z.; Wei, J.H. Agarwood Formation Induced by Fermentation Liquid of Lasiodiplodia Theobromae, the Dominating Fungus in Wounded Wood of Aquilaria sinensis. Curr. Microbiol. 2017, 74, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Han, X.M.; Sun, Y.; Chen, H.J.; Yang, Y.; Liu, Y.; Meng, H.; Gao, Z.; Xu, Y.; Zhang, Z.; et al. Overview of Sesquiterpenes and Chromones of Agarwood Originating from Four Main Species of the Genus Aquilaria. RSC Adv. 2019, 9, 4113–4130. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Q.; Liu, W.Z.; Li, J.; Yu, L.; Lin, L. Dynamic Analysis of Gene Expression and Determination of Chemicals in Agarwood in Aquilaria sinensis. J. For. Res. 2020, 31, 1833–1841. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Jiang, C.; Zhou, J.H.; Zhao, Y.Y.; Huang, L.Q. Volatile Organic Compound and Endogenous Phytohormone Characteristics during Callus Browning in Aquilaria sinensis. Ind. Crops Prod. 2021, 168, 113605. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Y.Y.; Feng, J.; Chen, D.L.; Yang, Y.; Liu, P.; Yu, Z.; Wei, J. Remarkable Phytochemical Characteristics of Chi-Nan Agarwood Induced from New-Found Chi-Nan Germplasm of Aquilaria sinensis Compared with Ordinary Agarwood. Int. J. Anal. Chem. 2021, 2021, 5593730. [Google Scholar] [CrossRef]
- Hou, W.C.; Liu, P.W.; Liu, Y.Y.; Kang, Y.; Yang, Y.; Zhang, Y.; Gao, Z.; Yu, M.; Feng, J.; Lv, F.; et al. Chi-Nan Agarwood Germplasms Constitute a New Chemotype of Aquilaria sinensis (Lour.) Spreng. Ind. Crops Prod. 2022, 187, 115494. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, P.W.; Lv, F.F.; Zhang, Y.X.; Yang, Y.; Wei, J.H. Genetic Relationship and Source Species Identification of 58 Qi-Nan Germplasms of Aquilaria Species in China That Easily Form Agarwood. PLoS ONE 2022, 17, e0270167. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.F.; Cui, Z.Y.; Xu, D.P. Morphological, Physiological, Biochemical and Molecular Analyses Reveal Wounding-Induced Agarwood Formation Mechanism in Two Types of Aquilaria sinensis (Lour.) Spreng. Ind. Crops Prod. 2022, 178, 114603. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, T.T.; Zhang, Y.G.; Wang, Q.; Li, G.Y. Characterization of the Incense Ingredients of Cultivated Grafting Kynam by TG-FTIR and HS-GC-MS. Fitoterapia 2020, 142, 104493. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.Q.; Wang, H.; Mei, W.L.; Dai, H.F. Natural Products in Agarwood and Aquilaria Plants: Chemistry, Biological Activities and Biosynthesis. Nat. Prod. Rep. 2021, 38, 528–565. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Cui, Z.Y.; Liu, X.J.; Hong, Z.; Zhang, P.; Xu, D.P. Comparative Morphological, Anatomical and Physiological Analyses Explain the Difference of Wounding-Induced Agarwood Formation between Ordinary Agarwood Nongrafted Plants and Five Grafted Qi-Nan Clones (Aquilaria sinensis). Forests 2022, 13, 1618. [Google Scholar] [CrossRef]
- Yang, L.; Yang, J.L.; Dong, W.H.; Wang, Y.L.; Zeng, J.; Yuan, J.Z.; Wang, H.; Mei, W.L.; Dai, H.F. The Characteristic Fragrant Sesquiterpenes and 2-(2-Phenylethyl)Chromones in Wild and Cultivated “Qi-Nan” Agarwood. Mol. Basel Switz. 2021, 26, 436. [Google Scholar] [CrossRef] [PubMed]
- Kapazoglou, A.; Tani, E.; Avramidou, E.V.; Abraham, E.M.; Gerakari, M.; Megariti, S.; Doupis, G.; Doulis, A.G. Epigenetic Changes and Transcriptional Reprogramming Upon Woody Plant Grafting for Crop Sustainability in a Changing Environment. Front. Plant Sci. 2020, 11, 613004. [Google Scholar] [CrossRef] [PubMed]
- Gautier, A.T.; Chambaud, C.; Brocard, L.; Ollat, N.; Gambetta, G.A.; Delrot, S.; Cookson, S.J. Merging Genotypes: Graft Union Formation and Scion-Rootstock Interactions. J. Exp. Bot. 2019, 70, 747–755. [Google Scholar] [CrossRef]
- Melnyk, C.W.; Schuster, C.; Leyser, O.; Meyerowitz, E.M. A Developmental Framework for Graft Formation and Vascular Reconnection in Arabidopsis Thaliana. Curr. Biol. 2015, 25, 1306–1318. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Zheng, B.S. Molecular Responses during Plant Grafting and Its Regulation by Auxins, Cytokinins, and Gibberellins. Biomolecules 2019, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Kapazoglou, A.; Ganopoulos, I.; Tani, E.; Tsaftaris, A. Epigenetics, Epigenomics and Crop Improvement. In Advances in Botanical Research; Kuntz, M., Ed.; Transgenic Plants; Academic Press: Cambridge, MA, USA, 2018; Volume 86, pp. 287–324. [Google Scholar]
- Wang, W.Q.; Allan, A.; Yin, X.R. Small RNAs With a Big Impact on Horticultural Traits. Crit. Rev. Plant Sci. 2020, 39, 30–43. [Google Scholar] [CrossRef]
- Gaut, B.S.; Miller, A.J.; Seymour, D.K. Living with Two Genomes: Grafting and Its Implications for Plant Genome-to-Genome Interactions, Phenotypic Variation, and Evolution. Annu. Rev. Genet. 2019, 53, 195–215. [Google Scholar] [CrossRef]
- Nanda, A.K.; Melnyk, C.W. The Role of Plant Hormones during Grafting. J. Plant Res. 2018, 131, 49–58. [Google Scholar] [CrossRef]
- Thomas, H.R.; Frank, M.H. Connecting the Pieces: Uncovering the Molecular Basis for Long-Distance Communication through Plant Grafting. New Phytol. 2019, 223, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, B.S.; Babalola, O.O.; Glick, B.R. Plant Growth-Promoting Root-Colonizing Bacterial Endophytes. Rhizosphere 2021, 20, 100433. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G. Influence of Fungal Endophytes on Plant Physiology Is More Pronounced under Stress than Well-Watered Conditions: A Meta-Analysis. Planta 2018, 248, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.L.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A.N.; Yadav, N.; Dhaliwal, H.S.; Saxena, A.K. Endophytic Microbes: Biodiversity, Plant Growth-Promoting Mechanisms and Potential Applications for Agricultural Sustainability. Antonie Van Leeuwenhoek 2020, 113, 1075–1107. [Google Scholar] [CrossRef] [PubMed]
- Mochahari, D.; Kharnaior, S.; Sen, S.; Thomas, S. Isolation of Endophytic Fungi from Juvenile Aquilaria malaccensis and Their Antimicrobial Properties. J. Trop. For. Sci. 2020, 32, 97–104. [Google Scholar] [CrossRef]
- Liu, J.; Li, T.X.; Chen, T.; Gao, J.Q.; Zhang, X.; Jiang, C.; Yang, J.; Zhou, J.; Wang, T.; Chi, X.; et al. Integrating Multiple Omics Identifies Phaeoacremonium Rubrigenum Acting as Aquilaria sinensis Marker Fungus to Promote Agarwood Sesquiterpene Accumulation by Inducing Plant Host Phosphorylation. Microbiol. Spectr. 2022, 10, e0272221. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.W.; Zhang, X.L.; Yang, Y.; Sui, C.; Xu, Y.H.; Wei, J.H. Interxylary Phloem and Xylem Rays Are the Structural Foundation of Agarwood Resin Formation in the Stems of Aquilaria sinensis. Trees 2019, 33, 533–542. [Google Scholar] [CrossRef]
- Farsaraei, S.; Mehdizadeh, L.; Moghaddam, M. Seed Priming with Putrescine Alleviated Salinity Stress During Germination and Seedling Growth of Medicinal Pumpkin. J. Soil Sci. Plant Nutr. 2021, 21, 1782–1792. [Google Scholar] [CrossRef]
- Almeida Trapp, M.; De Souza, G.D.; Rodrigues-Filho, E.; Boland, W.; Mithöfer, A. Validated Method for Phytohormone Quantification in Plants. Front. Plant Sci. 2014, 5, 417. [Google Scholar] [CrossRef]
- Yao, H.; Sun, X.; He, C.; Maitra, P.; Li, X.-C.; Guo, L.-D. Phyllosphere Epiphytic and Endophytic Fungal Community and Network Structures Differ in a Tropical Mangrove Ecosystem. Microbiome 2019, 7, 57. [Google Scholar] [CrossRef]
- Duong, L.; Jeewon, R.; Lumyong, S. DGGE Coupled with Ribosomal DNA Gene Phylogenies Reveal Uncharacterized Fungal Phylotypes. Fungal Divers. 2006, 23, 121–138. [Google Scholar]
- Amato, K.R.; Yeoman, C.J.; Kent, A.; Righini, N.; Carbonero, F.; Estrada, A.; Gaskins, H.R.; Stumpf, R.M.; Yildirim, S.; Torralba, M.; et al. Habitat Degradation Impacts Black Howler Monkey (Alouatta pigra) Gastrointestinal Microbiomes. ISME J. 2013, 7, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An Effective Distance Metric for Microbial Community Comparison. ISME J. 2011, 5, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Bantis, F.; Koukounaras, A.; Siomos, A.S.; Dangitsis, C. Impact of Scion and Rootstock Seedling Quality Selection on the Vigor of Watermelon–Interspecific Squash Grafted Seedlings. Agriculture 2020, 10, 326. [Google Scholar] [CrossRef]
- Baron, D.; Esteves Amaro, A.C.; Pina, A.; Ferreira, G. An Overview of Grafting Re-Establishment in Woody Fruit Species. Sci. Hortic. 2019, 243, 84–91. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and Oxidative Burst: Roots in Plant Development. Plant Divers. 2019, 42, 33–43. [Google Scholar] [CrossRef]
- Mi, Y.F.; Ma, X.W.; Chen, S.C. Resistant Evaluation of Kiwifruit Rootstocks to Root Zone Hypoxia Stress. Am. J. Plant Sci. 2013, 4, 945–954. [Google Scholar] [CrossRef]
- Reckova, S.; Tuma, J.; Dobrev, P.; Vankova, R. Influence of Copper on Hormone Content and Selected Morphological, Physiological and Biochemical Parameters of Hydroponically Grown Zea mays Plants. Plant Growth Regul. 2019, 89, 191–201. [Google Scholar] [CrossRef]
- Husain, T.; Fatima, A.; Suhel, M.; Singh, S.; Sharma, A.; Prasad, S.M.; Singh, V.P. A Brief Appraisal of Ethylene Signaling under Abiotic Stress in Plants. Plant Signal. Behav. 2020, 15, 1782051. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.W.; Wang, J.; Li, S.H.; Kakan, X.; Zhou, Y.; Miao, Y.; Wang, F.; Qin, H.; Huang, R. Ascorbic Acid Integrates the Antagonistic Modulation of Ethylene and Abscisic Acid in the Accumulation of Reactive Oxygen Species. Plant Physiol. 2019, 179, 1861–1875. [Google Scholar] [CrossRef] [PubMed]
- Bücker-Neto, L.; Paiva, A.; Machado, R.; Arenhart, R.; Margis-Pinheiro, M. Interactions between Plant Hormones and Heavy Metals Responses. Genet. Mol. Biol. 2017, 40, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, V. Responses of Wild Type and Abscisic Acid Mutants of Arabidopsis Thaliana to Cadmium. J. Plant Physiol. 2002, 159, 1323–1327. [Google Scholar] [CrossRef]
- Grant, M.; Lamb, C. Systemic Immunity. Curr. Opin. Plant Biol. 2006, 9, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.G.; Liu, H.B.; Li, X.H.; Xiao, J.H.; Wang, S.P. Rice OsPAD4 Functions Differently from Arabidopsis AtPAD4 in Host-Pathogen Interactions. Plant J. 2014, 78, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, H.Y.; Ruan, W.Y.; Deng, M.J.; Wang, F.; Peng, J.; Luo, J.; Chen, Z.; Yi, K. ABNORMAL INFLORESCENCE MERISTEM1 Functions in Salicylic Acid Biosynthesis to Maintain Proper Reactive Oxygen Species Levels for Root Meristem Activity in Rice. Plant Cell 2017, 29, 560–574. [Google Scholar] [CrossRef]
- Yang, L.; Li, B.S.; Zheng, X.Y.; Li, J.G.; Yang, M.; Dong, X.; He, G.; An, C.; Deng, X.W. Salicylic Acid Biosynthesis is Enhanced and Contributes to Increased Biotrophic Pathogen Resistance in Arabidopsis hybrids. Nat. Commun. 2015, 6, 7309. [Google Scholar] [CrossRef]
- Zhou, X.T.; Jia, L.J.; Wang, H.J.; Zhao, P.; Wang, W.Y.; Liu, N.; Song, S.-W.; Wu, Y.; Su, L.; Zhang, J.; et al. The Potato Transcription Factor StbZIP61 Regulates Dynamic Biosynthesis of Salicylic Acid in Defense against Phytophthora infestans Infection. Plant J. 2018, 95, 1055–1068. [Google Scholar] [CrossRef]
- Bali, S.G.; Kaur, P.; Kohli, S.K.; Ohri, P.; Thukral, A.K.; Bhardwaj, R.; Wijaya, L.; Alyemeni; Ahmad, P. Jasmonic Acid Induced Changes in Physio-Biochemical Attributes and Ascorbate-Glutathione Pathway in Lycopersicon esculentum under Lead Stress at Different Growth Stages. Sci. Total Environ. 2018, 645, 1344–1360. [Google Scholar] [CrossRef]
- Hanaka, A.; Lechowski, L.; Mroczek-Zdyrska, M.; Strubińska, J. Oxidative Enzymes Activity during Abiotic and Biotic Stresses in Zea Mays Leaves and Roots Exposed to Cu, Methyl Jasmonate and Trigonotylus caelestialium. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2018, 24, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Du, T.Y.; Dao, C.J.; Mapook, A.; Stephenson, S.L.; Elgorban, A.M.; Al-Rejaie, S.; Suwannarach, N.; Karunarathna, S.C.; Tibpromma, S. Diversity and Biosynthetic Activities of Agarwood Associated Fungi. Diversity 2022, 14, 211. [Google Scholar] [CrossRef]
- Nagajothi, M.S.; Parthiban, K.T.; Kanna, S.U.; Karthiba, L.; Saravanakumar, D. Fungal Microbes Associated with Agarwood Formation. Am. J. Plant Sci. 2016, 7, 1445–1452. [Google Scholar] [CrossRef]
- Cui, J.; Guo, S.; Fu, S.; Xiao, P.; Wang, M. Effects of Inoculating Fungi on Agilawood Formation in Aquilaria sinensis. Chin. Sci. Bull. 2013, 58, 3280–3287. [Google Scholar] [CrossRef]
- Siburian, R.H.; Siregar, U.J.; Siregar, I.Z.; Santoso, E. Identification of Morphological Characters of Aquilaria microcarpa in the Interaction with Fusarium solani. Int. J. Sci. Basic Appl. Res. 2015, 20, 119–128. [Google Scholar]
- Li, T.X.; Qiu, Z.D.; Yih Lee, S.; Li, X.; Gao, J.Q.; Jiang, C.; Huang, L.; Liu, J. Biodiversity and Application Prospects of Fungal Endophytes in the Agarwood-Producing Genera, Aquilaria and Gyrinops (Thymelaeaceae): A Review. Arab. J. Chem. 2023, 16, 104435. [Google Scholar] [CrossRef]
- Sen, S.; Dehingia, M.; Talukdar, N.C.; Khan, M. Chemometric Analysis Reveals Links in the Formation of Fragrant Bio-Molecules during Agarwood (Aquilaria malaccensis) and Fungal Interactions. Sci. Rep. 2017, 7, 44406. [Google Scholar] [CrossRef]
- Xu, Y.H.; Zhang, Z.; Wang, M.X.; Wei, J.H.; Chen, H.J.; Gao, Z.; Sui, C.; Luo, H.; Zhang, X.; Yang, Y.; et al. Identification of Genes Related to Agarwood Formation: Transcriptome Analysis of Healthy and Wounded Tissues of Aquilaria sinensis. BMC Genomics 2013, 14, 227. [Google Scholar] [CrossRef]
- Chhipa, H.; Kaushik, N. Fungal and Bacterial Diversity Isolated from Aquilaria malaccensis Tree and Soil, Induces Agarospirol Formation within 3 Months after Artificial Infection. Front. Microbiol. 2017, 8, 1286. [Google Scholar] [CrossRef]
- Premalatha, K.; Kalra, A. Molecular Phylogenetic Identification of Endophytic Fungi Isolated from Resinous and Healthy Wood of Aquilaria malaccensis, a Red Listed and Highly Exploited Medicinal Tree. Fungal Ecol. 2013, 6, 205–211. [Google Scholar] [CrossRef]
- Rasool, S.; Mohamed, R. Understanding Agarwood Formation and Its Challenges. In Agarwood: Science Behind the Fragrance; Mohamed, R., Ed.; Springer: Berlin, Germany, 2016; pp. 39–56. [Google Scholar] [CrossRef]
- Rastogi, G.; Sani, R.K. Molecular Techniques to Assess Microbial Community Structure, Function, and Dynamics in the Environment. In Microbes and Microbial Technology; Iqbal, A., Farah, A., John, P., Eds.; Springer: New York, NY, USA, 2011; pp. 29–57. [Google Scholar]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cai, C.H.; Dong, W.H.; Guo, Z.K.; Wang, H.; Mei, W.L.; Dai, H.F. 2-(2-Phenylethyl)Chromone Derivatives from Chinese Agarwood Induced by Artificial Holing. Fitoterapia 2014, 98, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.N.; Dong, W.H.; Huang, S.Z.; Li, W.; Kong, F.D.; Wang, H.; Wang, J.; Mei, W.-L.; Dai, H.-F. Three New Sesquiterpenoids from Agarwood of Aquilaria crassna. Fitoterapia 2016, 114, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Hussain, M.; Jiang, Z.B.; Wang, Z.H.; Gao, J.; Ye, F.; Mao, R.; Li, H. Aquilaria Species (Thymelaeaceae) Distribution, Volatile and Non-Volatile Phytochemicals, Pharmacological Uses, Agarwood Grading System, and Induction Methods. Molecules 2021, 26, 7708. [Google Scholar] [CrossRef]
- Shivanand, P.; Arbie, N.; Krishnamoorthy, S.; Ahmad, N. Agarwood—The Fragrant Molecules of a Wounded Tree. Molecules 2022, 27, 3386. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C. The Endophytic Continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef]
- Xia, A.N.; Liu, J.; Kang, D.C.; Zhang, H.G.; Zhang, R.H.; Liu, Y.G. Assessment of Endophytic Bacterial Diversity in Rose by High-Throughput Sequencing Analysis. PLoS ONE 2020, 15, e0230924. [Google Scholar] [CrossRef]



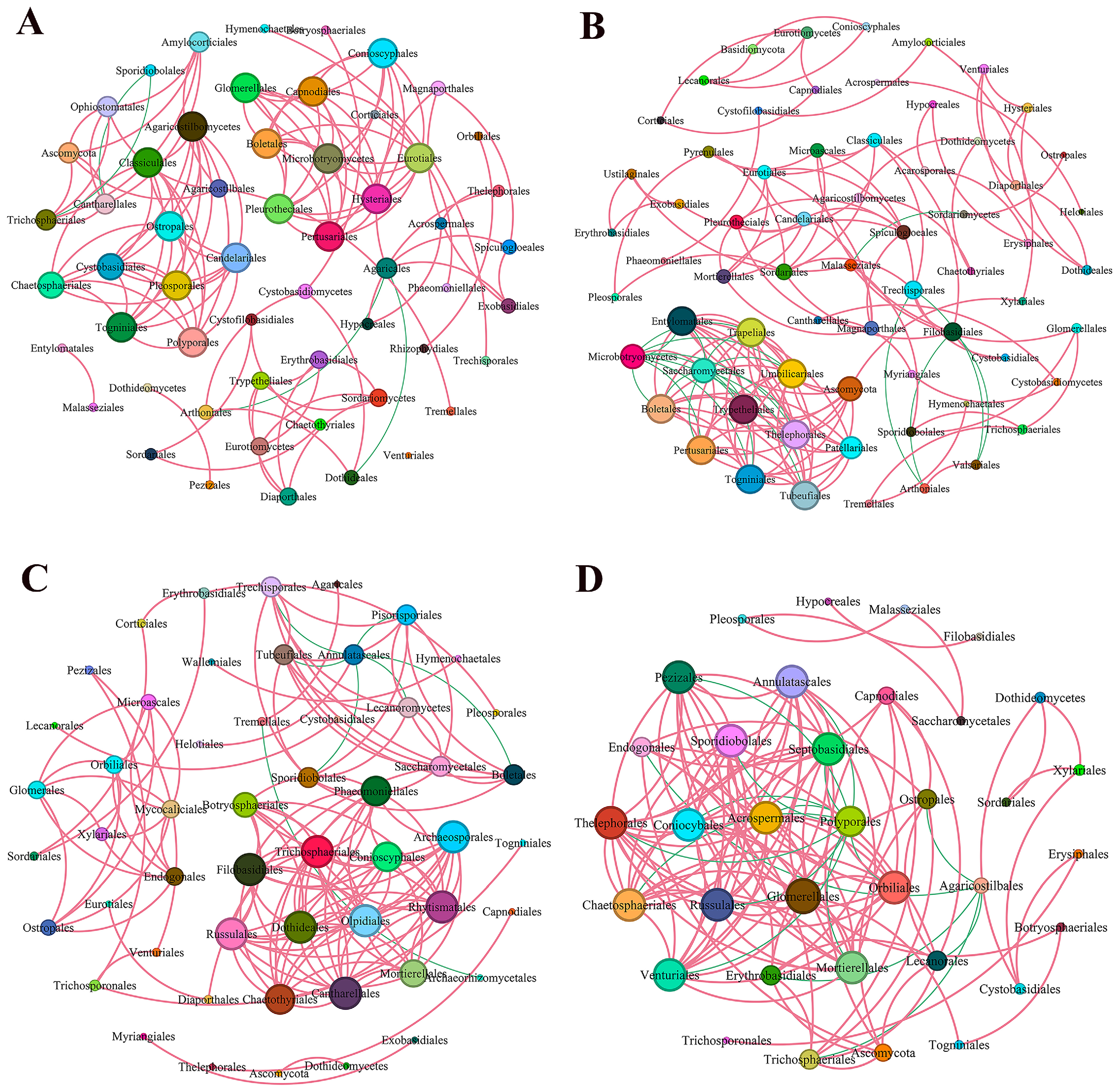
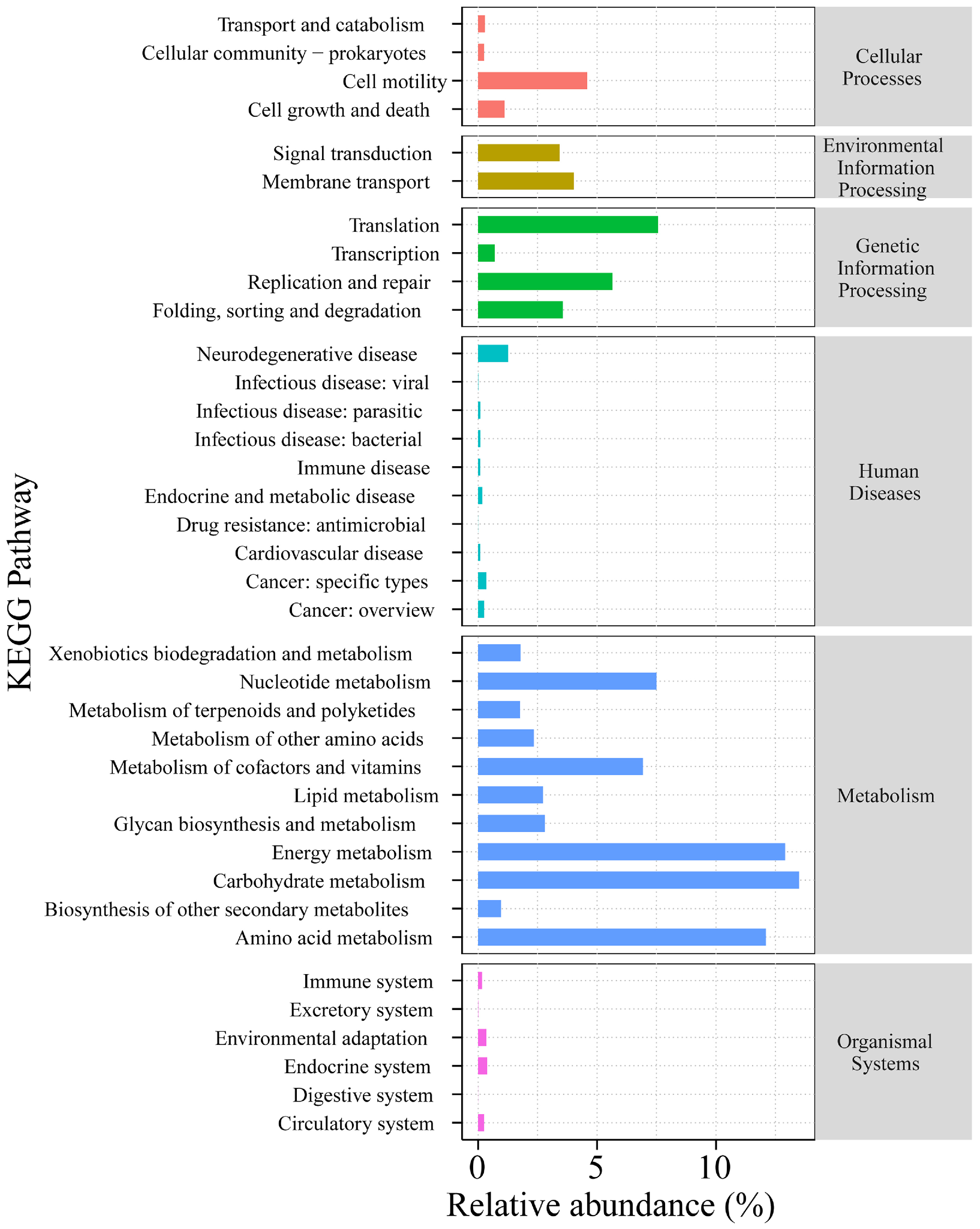
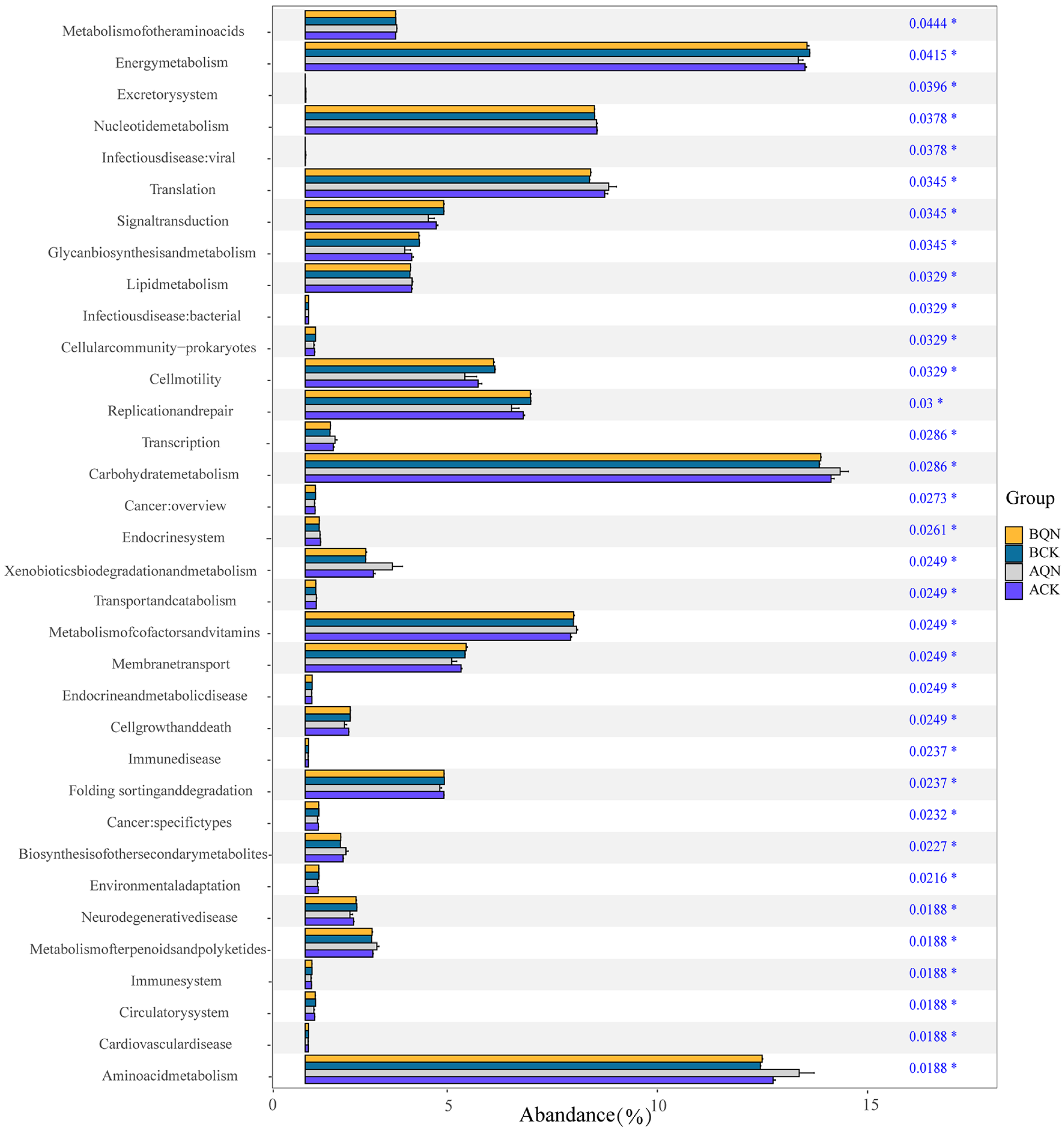
| Topological Properties | BCK | BQN | ACK | AQN |
|---|---|---|---|---|
| Nodes | 56 | 65 | 52 | 35 |
| Edges | 120 | 152 | 138 | 130 |
| Positive (red) | 96.67% | 84.87% | 94.93% | 86.92% |
| Negative (green) | 3.33% | 15.13% | 5.07% | 13.08% |
| Average clustering coefficient | 0.91 | 0.774 | 0.853 | 0.899 |
| Modularity | 0.809 | 0.947 | 0.648 | 0.529 |
| Average path length | 1.295 | 2.276 | 1.293 | 1.263 |
| Network diameter | 4 | 8 | 5 | 4 |
| Graph density | 0.078 | 0.073 | 0.104 | 0.218 |
| Average degree | 4.286 | 4.677 | 5.308 | 7.429 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Li, X.; Zhang, Q.; Hu, H.; Hong, Z.; Liu, X.; Cui, Z.; Xu, D. Physiological and Endophytic Fungi Changes in Grafting Seedlings of Qi-Nan Clones (Aquilaria sinensis). Forests 2024, 15, 106. https://doi.org/10.3390/f15010106
Fang X, Li X, Zhang Q, Hu H, Hong Z, Liu X, Cui Z, Xu D. Physiological and Endophytic Fungi Changes in Grafting Seedlings of Qi-Nan Clones (Aquilaria sinensis). Forests. 2024; 15(1):106. https://doi.org/10.3390/f15010106
Chicago/Turabian StyleFang, Xiaoying, Xiaofei Li, Qilei Zhang, Houzhen Hu, Zhou Hong, Xiaojin Liu, Zhiyi Cui, and Daping Xu. 2024. "Physiological and Endophytic Fungi Changes in Grafting Seedlings of Qi-Nan Clones (Aquilaria sinensis)" Forests 15, no. 1: 106. https://doi.org/10.3390/f15010106
APA StyleFang, X., Li, X., Zhang, Q., Hu, H., Hong, Z., Liu, X., Cui, Z., & Xu, D. (2024). Physiological and Endophytic Fungi Changes in Grafting Seedlings of Qi-Nan Clones (Aquilaria sinensis). Forests, 15(1), 106. https://doi.org/10.3390/f15010106






