Invasive Pest and Invasive Host: Where Might Spotted-Wing Drosophila (Drosophila suzukii) and American Black Cherry (Prunus serotina) Cross Paths in Europe?
Abstract
1. Introduction
2. Materials and Methods
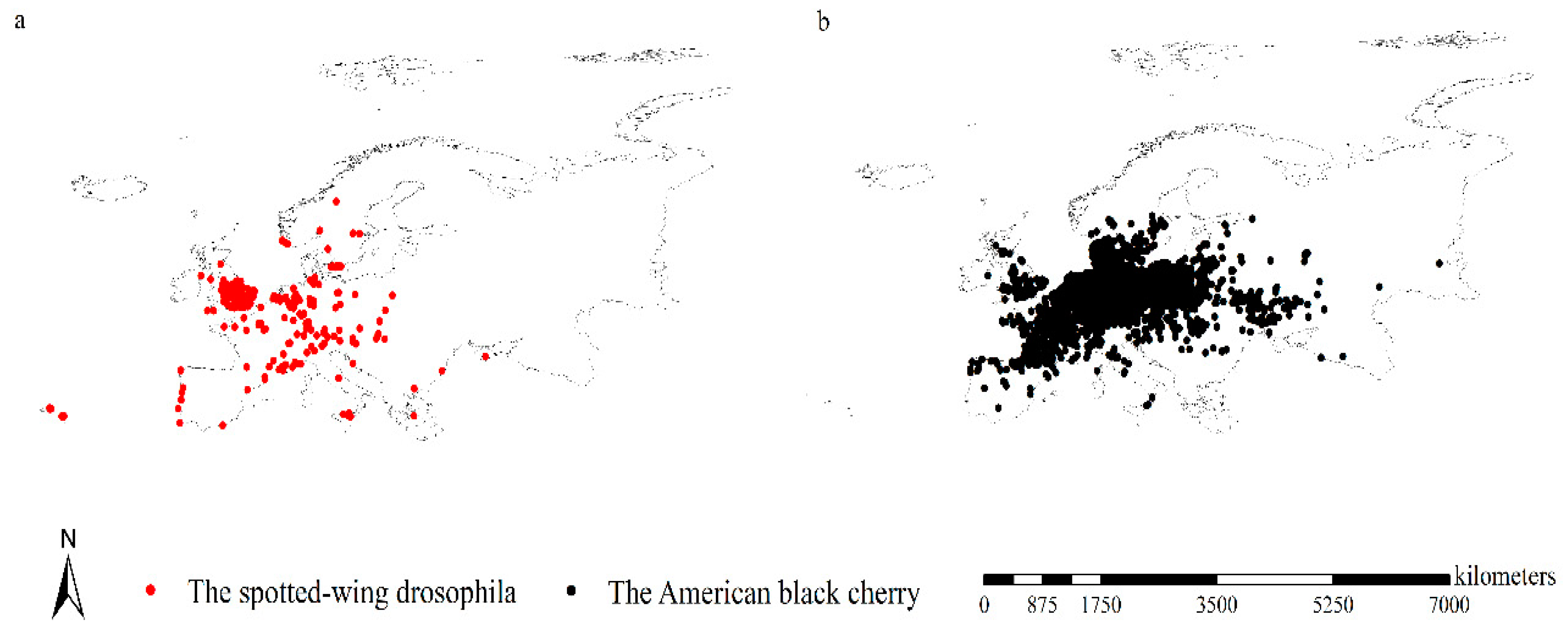
2.1. Occurrence Records
2.2. Predictors in the Species Distribution Models (SDMs)
2.3. Model Construction
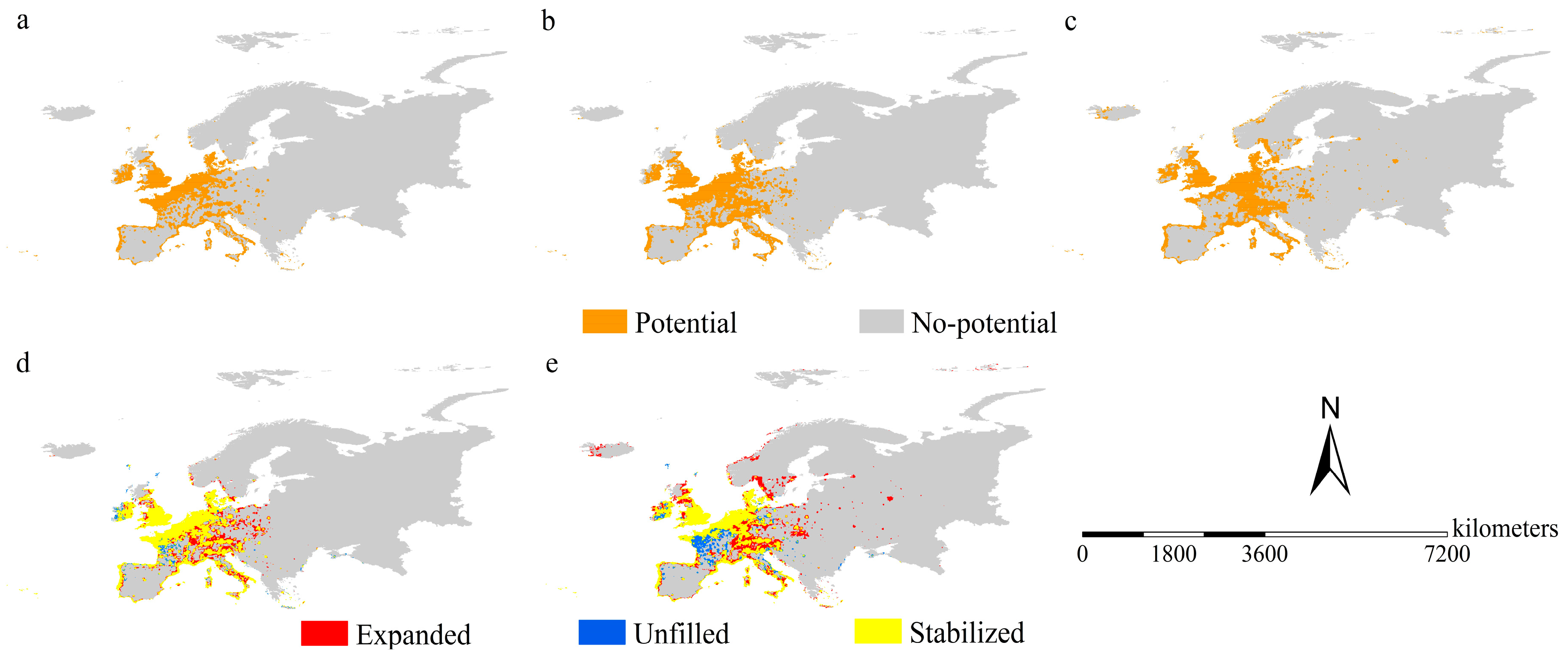
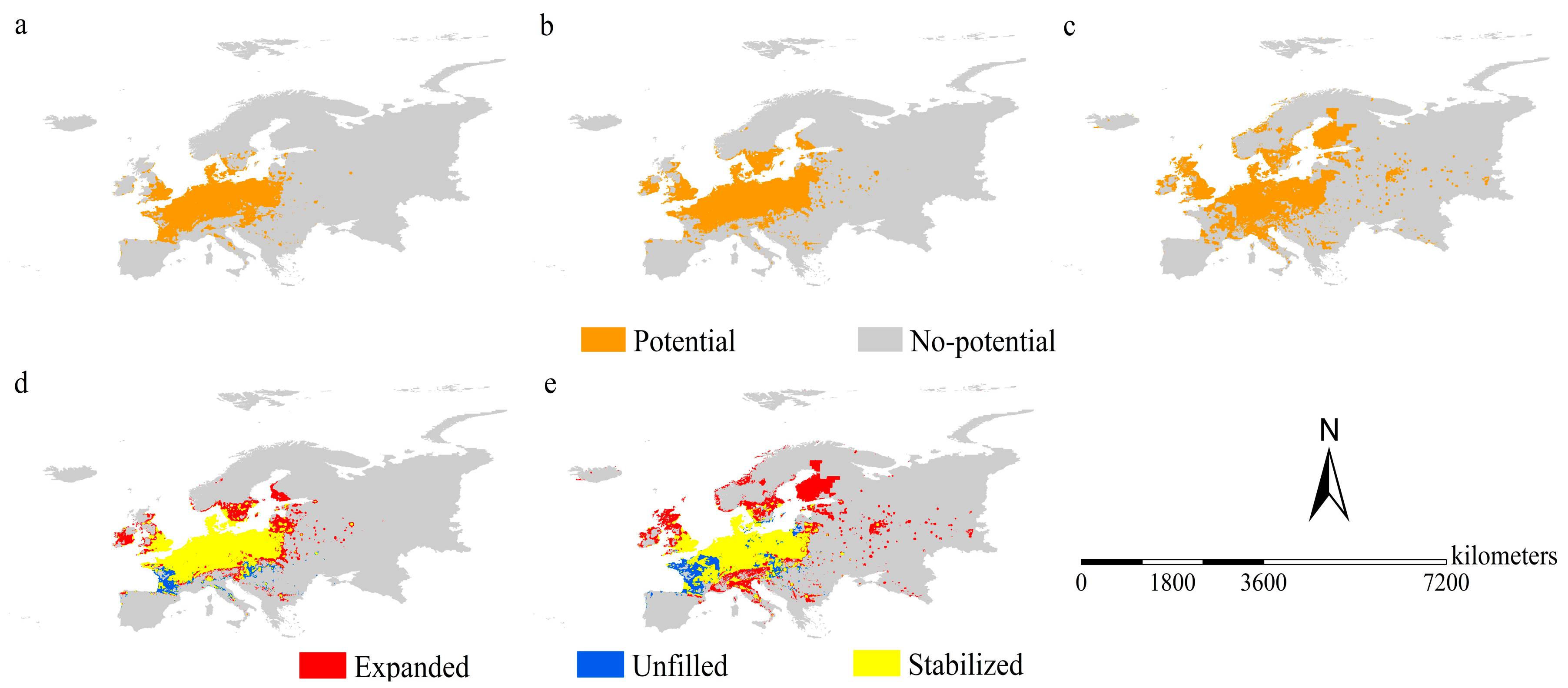
2.4. Range Shifts of the Two Species
3. Results
3.1. Model Reliability
3.2. Relative Importance of Predictors
3.3. Range Shifts of Spotted-Wing Drosophila
3.4. Range Shifts of American Black Cherry
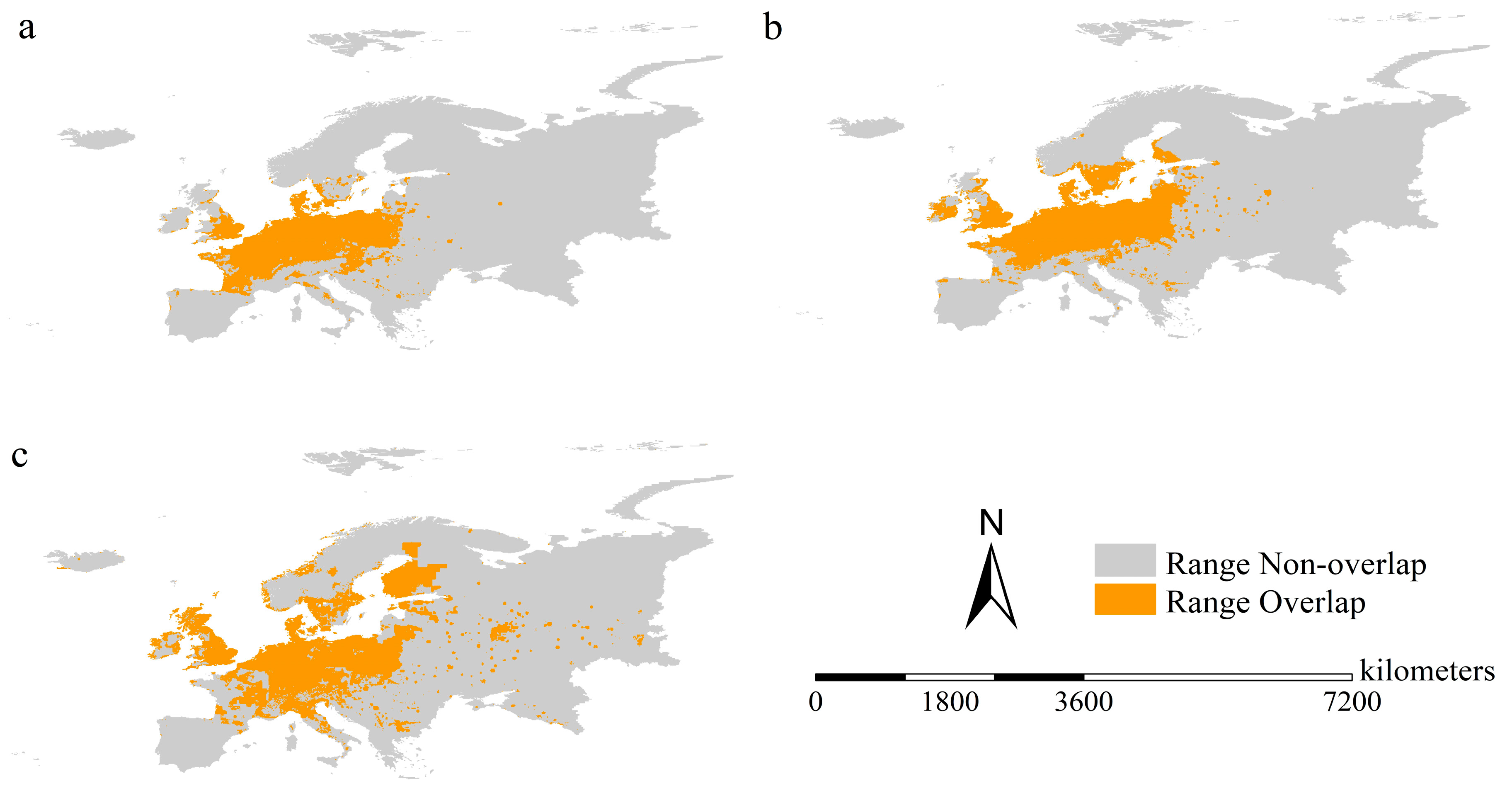
3.5. Potential Range Overlap between the Pest and Its Host
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Integr. Pest Manag. 2011, 2, G1–G7. [Google Scholar] [CrossRef]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.-S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D. Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Bellamy, D.E.; Sisterson, M.S.; Walse, S.S. Quantifying host potentials: Indexing postharvest fresh fruits for spotted wing drosophila, Drosophila suzukii. PLoS ONE 2013, 8, e61227. [Google Scholar] [CrossRef]
- Burrack, H.J.; Fernandez, G.E.; Spivey, T.; Kraus, D.A. Variation in selection and utilization of host crops in the field and laboratory by Drosophila suzukii Matsumura (Diptera: Drosophilidae), an invasive frugivore. Pest Manag. Sci. 2013, 69, 1173–1180. [Google Scholar] [CrossRef]
- Tochen, S.; Dalton, D.T.; Wiman, N.G.; Hamm, C.; Shearer, P.W.; Walton, V.M. Temperature-related development and population parameters for Drosophila suzukii (Diptera: Drosophilidae) on cherry and blueberry. Environ. Entomol. 2014, 43, 501–510. [Google Scholar] [CrossRef]
- Cini, A.; Ioriatti, C.; Anfora, G. A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull. Insectology 2012, 65, 149–160. [Google Scholar]
- Cini, A.; Anfora, G.; Escudero-Colomar, L.; Grassi, A.; Santosuosso, U.; Seljak, G.; Papini, A. Tracking the invasion of the alien fruit pest Drosophila suzukii in Europe. J. Pest Sci. 2014, 87, 559–566. [Google Scholar] [CrossRef]
- Ioriatti, C.; Anfora, G.; Grassi, A.; Puppato, S.; Rossi Stacconi, M.V. Current status of the Drosophila suzukii control in Europe. Acta Hortic. 2020, 1277, 387–396. [Google Scholar] [CrossRef]
- Nagy, A.; Szalárdi, T.; Gombos, D.; Szanyi, S. Distribution of the spotted-wing drosophila (Drosophila suzukii) in the north-eastern part of the Carpathian lowlands. EPPO Bull. 2020, 50, 197–200. [Google Scholar] [CrossRef]
- Lee, J.C.; Bruck, D.J.; Dreves, A.J.; Ioriatti, C.; Vogt, H.; Baufeld, P. In focus: Spotted wing drosophila, Drosophila suzukii, across perspectives. Pest Manag. Sci. 2011, 67, 1349–1351. [Google Scholar] [CrossRef]
- De Ros, G.; Anfora, G.; Grassi, A.; Ioriatti, C. The potential economic impact of Drosophila suzukii on small fruits production in Trentino (Italy). IOBC-WPRS Bull. 2013, 91, 317–321. [Google Scholar] [CrossRef]
- De Ros, G.; Conci, S.; Pantezzi, T.; Savini, G. The economic impact of invasive pest Drosophila suzukii on berry production in the Province of Trento, Italy. J. Berry Res. 2015, 5, 89–96. [Google Scholar] [CrossRef]
- Starfinger, U. Introduction and naturalization of Prunus serotina in central Europe. In Plant Invasions: Studies from North America and Europe; Brock, J.H., Wade, M., Pyšek, P., Green, D., Eds.; Backhuys: Leiden, The Netherlands, 1997; Volume 161. [Google Scholar]
- Lull, H.W. A Forest Atlas of the Northeast; USDA Forest Service, Northeastern Forest Experiment Station: Upper Darby, PA, USA, 1968.
- Terwei, A.; Zerbe, S.; Zeileis, A.; Annighöfer, P.; Kawaletz, H.; Mölder, I.; Ammer, C. Which are the factors controlling tree seedling establishment in North Italian floodplain forests invaded by non-native tree species? For. Ecol. Manag. 2013, 304, 192–203. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Drivers of invasive tree and shrub natural regeneration in temperate forests. Biol. Invasions 2018, 20, 2363–2379. [Google Scholar] [CrossRef]
- Vegini, E.; Lastrucci, L.; Lazzaro, L.; Cardarelli, E.; Martignoni, M. Impact of Prunus serotina Ehrh. invasion on heathland vegetation: A case of study in North-Western Italy. Biologia 2020, 75, 327–336. [Google Scholar] [CrossRef]
- Closset-Kopp, D.; Chabrerie, O.; Valentin, B.; Delachapelle, H.; Decocq, G. When Oskar meets Alice: Does a lack of trade-off in r/K-strategies make Prunus serotina a successful invader of European forests? For. Ecol. Manag. 2007, 247, 120–130. [Google Scholar] [CrossRef]
- Vanhellemont, M.; Wauters, L.; Baeten, L.; Bijlsma, R.-J.; De Frenne, P.; Hermy, M.; Verheyen, K. Prunus serotina unleashed: Invader dominance after 70 years of forest development. Biol. Invasions 2010, 12, 1113–1124. [Google Scholar] [CrossRef]
- Kawaletz, H.; Mölder, I.; Zerbe, S.; Annighöfer, P.; Terwei, A.; Ammer, C. Exotic tree seedlings are much more competitive than natives but show underyielding when growing together. J. Plant Ecol. 2013, 6, 305–315. [Google Scholar] [CrossRef][Green Version]
- Halarewicz, A.; Żołnierz, L. Changes in the understorey of mixed coniferous forest plant communities dominated by the American black cherry (Prunus serotina Ehrh.). For. Ecol. Manag. 2014, 313, 91–97. [Google Scholar] [CrossRef]
- Godefroid, S.; Phartyal, S.S.; Weyembergh, G.; Koedam, N. Ecological factors controlling the abundance of non-native invasive black cherry (Prunus serotina) in deciduous forest understory in Belgium. For. Ecol. Manag. 2005, 210, 91–105. [Google Scholar] [CrossRef]
- Aerts, R.; Ewald, M.; Nicolas, M.; Piat, J.; Skowronek, S.; Lenoir, J.; Hattab, T.; Garzón-López, C.X.; Feilhauer, H.; Schmidtlein, S. Invasion by the alien tree Prunus serotina alters ecosystem functions in a temperate deciduous forest. Front. Plant Sci. 2017, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Nentwig, W.; Bacher, S.; Kumschick, S.; Pyšek, P.; Vilà, M. More than “100 worst” alien species in Europe. Biol. Invasions 2018, 20, 1611–1621. [Google Scholar] [CrossRef]
- Gutierrez, A.P.; Ponti, L.; Dalton, D.T. Analysis of the invasiveness of spotted wing Drosophila (Drosophila suzukii) in North America, Europe, and the Mediterranean Basin. Biol. Invasions 2016, 18, 3647–3663. [Google Scholar] [CrossRef]
- Ørsted, I.V.; Ørsted, M. Species distribution models of the Spotted Wing Drosophila (Drosophila suzukii, Diptera: Drosophilidae) in its native and invasive range reveal an ecological niche shift. J. Appl. Ecol. 2019, 56, 423–435. [Google Scholar] [CrossRef]
- Reyes, J.A.; Lira-Noriega, A. Current and future global potential distribution of the fruit fly Drosophila suzukii (Diptera: Drosophilidae). Can. Entomol. 2020, 152, 587–599. [Google Scholar] [CrossRef]
- Macedo, F.L.; Ragonezi, C.; Reis, F.; de Freitas, J.G.; Lopes, D.H.; Aguiar, A.M.F.; Cravo, D.; de Carvalho, M.A.A.P. Prediction of the Potential Distribution of Drosophila suzukii on Madeira Island Using the Maximum Entropy Modeling. Agriculture 2023, 13, 1764. [Google Scholar] [CrossRef]
- Dos Santos, L.A.; Mendes, M.F.; Krüger, A.P.; Blauth, M.L.; Gottschalk, M.S.; Garcia, F.R. Global potential distribution of Drosophila suzukii (Diptera, Drosophilidae). PLoS ONE 2017, 12, e0174318. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Peterson, A.T. Mapping the global distribution of invasive pest Drosophila suzukii and parasitoid Leptopilina japonica: Implications for biological control. PeerJ 2023, 11, e15222. [Google Scholar] [CrossRef]
- Verheyen, K.; Vanhellemont, M.; Stock, T.; Hermy, M. Predicting patterns of invasion by black cherry (Prunus serotina Ehrh.) in Flanders (Belgium) and its impact on the forest understorey community. Divers. Distrib. 2007, 13, 487–497. [Google Scholar] [CrossRef]
- Vanhellemont, M.; Verheyen, K.; De Keersmaeker, L.; Vandekerkhove, K.; Hermy, M. Does Prunus serotina act as an aggressive invader in areas with a low propagule pressure? Biol. Invasions 2009, 11, 1451–1462. [Google Scholar] [CrossRef]
- Gentili, R.; Gilardelli, S.; Ghiani, A.; Ciappetta, S.; Citterio, S. Distribution Range of Four Invasive Alien Species in Italy: Ambrosia artemisiifolia L., Reynoutria japonica Houtt., Prunus serotina Ehrh., Senecio inaequidens DC. 2014. Available online: https://hdl.handle.net/10281/57139 (accessed on 1 October 2023).
- Segura, S.; Guzmán-Díaz, F.; López-Upton, J.; Mathuriau, C.; López-Medina, J. Distribution of Prunus serotina Ehrh. in North America and its invasion in Europe. J. Geosci. Environ. Prot. 2018, 6, 111–124. [Google Scholar] [CrossRef]
- Hamby, K.A.; E Bellamy, D.; Chiu, J.C.; Lee, J.C.; Walton, V.M.; Wiman, N.G.; York, R.M.; Biondi, A. Biotic and abiotic factors impacting development, behavior, phenology, and reproductive biology of Drosophila suzukii. J. Pest Sci. 2016, 89, 605–619. [Google Scholar] [CrossRef]
- Ørsted, M.; Lye, J.; Umina, P.A.; Maino, J.L. Global analysis of the seasonal abundance of the invasive pest Drosophila suzukii reveal temperature extremes determine population activity potential. Pest Manag. Sci. 2021, 77, 4555–4563. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.; Jung, J.; Kleinhenz, B.; Racca, P. Estimating temperature effects on Drosophila suzukii life cycle parameters. Agric. For. Entomol. 2021, 23, 361–377. [Google Scholar] [CrossRef]
- Mosher, E.S.; Silander, J.A.; Latimer, A.M. The role of land-use history in major invasions by woody plant species in the northeastern North American landscape. Biol. Invasions 2009, 11, 2317–2328. [Google Scholar] [CrossRef]
- Thomas, S.M.; Moloney, K.A. Combining the effects of surrounding land-use and propagule pressure to predict the distribution of an invasive plant. Biol. Invasions 2015, 17, 477–495. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Hu, X.; Feng, J. Land-use change drives present and future distributions of Fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Sci. Total Environ. 2020, 706, 135872. [Google Scholar] [CrossRef]
- Sirami, C.; Caplat, P.; Popy, S.; Clamens, A.; Arlettaz, R.; Jiguet, F.; Brotons, L.; Martin, J.L. Impacts of global change on species distributions: Obstacles and solutions to integrate climate and land use. Glob. Ecol. Biogeogr. 2017, 26, 385–394. [Google Scholar] [CrossRef]
- Gong, X.; Chen, Y.; Wang, T.; Jiang, X.; Hu, X.; Feng, J. Double-edged effects of climate change on plant invasions: Ecological niche modeling global distributions of two invasive alien plants. Sci. Total Environ. 2020, 740, 139933. [Google Scholar] [CrossRef]
- Reeve, J.D.; Cronin, J.T. The effect of plant-and parasitoid-induced egg mortality on the interspecific distribution of an oligophagous herbivore. Oecologia 1994, 100, 89–93. [Google Scholar] [CrossRef]
- Cai, C.; Xiao, J.; Wan, J.; Ren, Z.; van Kleunen, M.; Li, J. Implications of climate change for environmental niche overlap between five Cuscuta pest species and their two main Leguminosae host crop species. Weed Sci. 2022, 70, 543–552. [Google Scholar] [CrossRef]
- Castro-Sosa, R.; Castillo-Peralta, M.d.R.; Monterroso-Rivas, A.I.; Gomez-Díaz, J.D.; Flores-González, E.; Rebollar-Alviter, Á. Potential distribution of Drosophila suzukii (Diptera: Drosophilidae) in relation to alternate hosts in Mexico. Fla. Entomol. 2017, 100, 787–794. [Google Scholar] [CrossRef]
- Robinet, C. Mathematical Modelling of Invasion Processes in Ecology: The Pine Processionary Moth as a Case Study. Ph.D. Thesis, EHESS, Paris, France, 2006. [Google Scholar]
- Battisti, A.; Stastny, M.; Netherer, S.; Robinet, C.; Schopf, A.; Roques, A.; Larsson, S. Expansion of geographic range in the pine processionary moth caused by increased winter temperatures. Ecol. Appl. 2005, 15, 2084–2096. [Google Scholar] [CrossRef]
- Poyet, M.; Eslin, P.; Heraude, M.; Le Roux, V.; Prevost, G.; Gibert, P.; Chabrerie, O. Invasive host for invasive pest: When the A siatic cherry fly (Drosophila suzukii) meets the A merican black cherry (Prunus serotina) in Europe. Agric. For. Entomol. 2014, 16, 251–259. [Google Scholar] [CrossRef]
- Turcotte, R.M.; Larcenaire, C.; Long, R.; Martin, D.K.; Barringer, L. The spotted wing Drosophila, Drosophila suzukii (Diptera: Drosophilidae): A new pest of concern for black cherry, Prunus serotina, on the High Allegheny Plateau in Pennsylvania. Entomol. News 2018, 127, 390–399. [Google Scholar] [CrossRef]
- Nie, P.; Feng, J. Niche and Range Shifts of Aedes aegypti and Ae. albopictus Suggest That the Latecomer Shows a Greater Invasiveness. Insects 2023, 14, 810. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Zhang, M.-Z.; Xu, Z.; Han, Y.; Guo, W. Evaluation of CMIP6 models toward dynamical downscaling over 14 CORDEX domains. Clim. Dyn. 2022, 1–15. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Rujing, Y.; Xiang, G.; Xiaokang, H.; Yawen, H.; Jianmeng, F. Global cultivation of wheat crops induces considerable shifts in the range and niche of species relative to their wild progenitors. Environ. Res. Commun. 2021, 3, 115012. [Google Scholar] [CrossRef]
- Cao, R.; Feng, J. Future Range Shifts Suggest That the Six-Spined Spruce Bark Beetle Might Pose a Greater Threat to Norway Spruce in Europe than the Eight-Spined Spruce Bark Beetle. Forests 2023, 14, 2048. [Google Scholar] [CrossRef]
- Wan, J.Z.; Wang, C.J.; Tan, J.F.; Yu, F.H. Climatic niche divergence and habitat suitability of eight alien invasive weeds in China under climate change. Ecol. Evol. 2017, 7, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Aidoo, O.F.; Souza, P.G.C.; da Silva, R.S.; Santana, P.A., Jr.; Picanço, M.C.; Kyerematen, R.; Sètamou, M.; Ekesi, S.; Borgemeister, C. Climate-induced range shifts of invasive species (Diaphorina citri Kuwayama). Pest Manag. Sci. 2022, 78, 2534–2549. [Google Scholar] [CrossRef] [PubMed]
- Merow, C.; Bois, S.T.; Allen, J.M.; Xie, Y.; Silander, J.A., Jr. Climate change both facilitates and inhibits invasive plant ranges in New England. PNAS 2017, 114, E3276–E3284. [Google Scholar] [CrossRef]
- Colautti, R.I.; Barrett, S.C. Rapid adaptation to climate facilitates range expansion of an invasive plant. Science 2013, 342, 364–366. [Google Scholar] [CrossRef]
- Hong, S.H.; Lee, Y.H.; Lee, G.; Lee, D.-H.; Adhikari, P. Predicting impacts of climate change on northward range expansion of invasive weeds in South Korea. Plants 2021, 10, 1604. [Google Scholar] [CrossRef]
- Artaev, O. Prediction of Current and Future Suitable Habitats for Three Invasive Freshwater Fish Species in Europe. Water 2023, 15, 2091. [Google Scholar] [CrossRef]
- Denlinger, D.L.; Lee, R.E. Low Temperature Biology of Insects; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Osland, M.J.; Feher, L.C. Winter climate change and the poleward range expansion of a tropical invasive tree (Brazilian pepper—Schinus terebinthifolius). Glob. Chang. Biol. 2020, 26, 607–615. [Google Scholar] [CrossRef]
- Osland, M.J.; Chivoiu, B.; Feher, L.C.; Dale, L.L.; Lieurance, D.; Daniel, W.M.; Spencer, J.E. Plant migration due to winter climate change: Range expansion of tropical invasive plants in response to warming winters. Biol. Invasions 2023, 25, 2813–2830. [Google Scholar] [CrossRef]
- Sebert-Cuvillier, E.; Paccaut, F.; Chabrerie, O.; Endels, P.; Goubet, O.; Decocq, G. Local population dynamics of an invasive tree species with a complex life-history cycle: A stochastic matrix model. Ecol. Model. 2007, 201, 127–143. [Google Scholar] [CrossRef]
- Stephens, A.; Asplen, M.; Hutchison, W.D.; Venette, R. Cold hardiness of winter-acclimated Drosophila suzukii (Diptera: Drosophilidae) adults. Environ. Entomol. 2015, 44, 1619–1626. [Google Scholar] [CrossRef]
- Shearer, P.W.; West, J.D.; Walton, V.M.; Brown, P.H.; Svetec, N.; Chiu, J.C. Seasonal cues induce phenotypic plasticity of Drosophila suzukii to enhance winter survival. BMC Ecol. 2016, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Oliver, T.H.; Morecroft, M.D. Interactions between climate change and land use change on biodiversity: Attribution problems, risks, and opportunities. Wiley Interdiscip. Rev. Clim. Chang. 2014, 5, 317–335. [Google Scholar] [CrossRef]
- Hanspach, J.; Schweiger, O.; Kühn, I.; Plattner, M.; Pearman, P.B.; Zimmermann, N.E.; Settele, J. Host plant availability potentially limits butterfly distributions under cold environmental conditions. Ecography 2014, 37, 301–308. [Google Scholar] [CrossRef]
- Silva, D.P.; Varela, S.; Nemesio, A.; De Marco, P., Jr. Adding biotic interactions into paleodistribution models: A host-cleptoparasite complex of Neotropical orchid bees. PLoS ONE 2015, 10, e0129890. [Google Scholar] [CrossRef]
- Poyet, M.; Le Roux, V.; Gibert, P.; Meirland, A.; Prevost, G.; Eslin, P.; Chabrerie, O. The wide potential trophic niche of the Asiatic fruit fly Drosophila suzukii: The key of its invasion success in temperate Europe? PLoS ONE 2015, 10, e0142785. [Google Scholar] [CrossRef]
| Spotted-Wing Drosophila | American Black Cherry | ||||
|---|---|---|---|---|---|
| Category | Predictors | Importance Values | Category | Predictors | Importance Values |
| Climate predictors | MTCQ | 0.534 | Climate predictors | MTCM | 0.441 |
| Land-use predictors | URBAN | 0.179 | Climate predictors | MTWS | 0.108 |
| Climate predictors | MTWM | 0.094 | Land-use predictors | PRIMFL | 0.052 |
| Climate predictors | TAR | 0.092 | Land-use predictors | URBAN | 0.031 |
| Host availability | HAVA | 0.036 | Climate predictors | PWS | 0.030 |
| Climate predictors | PDM | 0.035 | Land-use predictors | SECDNL | 0.024 |
| Climate predictors | ISO | 0.031 | Land-use predictors | PRIMN | 0.019 |
| Climate predictors | PWS | 0.027 | Land-use predictors | PASTER | 0.018 |
| Climate predictors | PS | 0.023 | Topographical predictors | SLOP | 0.015 |
| Climate predictors | PCQ | 0.022 | Topographical predictors | ELEV | 0.013 |
| Land-use predictors | SECDF | 0.019 | Land-use predictors | CROPL | 0.012 |
| Land-use predictors | PRIMFL | 0.017 | Climate predictors | MTWETS | 0.011 |
| Land-use predictors | CROPL | 0.015 | Climate predictors | PS | 0.007 |
| Climate predictors | MTWETS | 0.008 | Land-use predictors | RANG | 0.007 |
| Land-use predictors | PASTR | 0.007 | Climate predictors | PDS | 0.007 |
| Topographical predictors | ELEV | 0.007 | Land-use predictors | SECDFL | 0.006 |
| Land-use predictors | SECDNL | 0.006 | Climate predictors | MDR | 0.004 |
| Land-use predictors | PRIMN | 0.006 | Topographical predictors | ASP | 0.003 |
| Land-use predictors | RANG | 0.006 | |||
| Topographical predictors | ASP | 0.005 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Wu, C.; Nie, P.; Feng, J.; Hu, X. Invasive Pest and Invasive Host: Where Might Spotted-Wing Drosophila (Drosophila suzukii) and American Black Cherry (Prunus serotina) Cross Paths in Europe? Forests 2024, 15, 206. https://doi.org/10.3390/f15010206
Zhou Y, Wu C, Nie P, Feng J, Hu X. Invasive Pest and Invasive Host: Where Might Spotted-Wing Drosophila (Drosophila suzukii) and American Black Cherry (Prunus serotina) Cross Paths in Europe? Forests. 2024; 15(1):206. https://doi.org/10.3390/f15010206
Chicago/Turabian StyleZhou, Yefu, Chunhong Wu, Peixiao Nie, Jianmeng Feng, and Xiaokang Hu. 2024. "Invasive Pest and Invasive Host: Where Might Spotted-Wing Drosophila (Drosophila suzukii) and American Black Cherry (Prunus serotina) Cross Paths in Europe?" Forests 15, no. 1: 206. https://doi.org/10.3390/f15010206
APA StyleZhou, Y., Wu, C., Nie, P., Feng, J., & Hu, X. (2024). Invasive Pest and Invasive Host: Where Might Spotted-Wing Drosophila (Drosophila suzukii) and American Black Cherry (Prunus serotina) Cross Paths in Europe? Forests, 15(1), 206. https://doi.org/10.3390/f15010206





