Aboveground Biomass Component Plasticity and Allocation Variations of Bamboo (Pleioblastus amarus) of Different Regions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Determining Component Biomass and Morphological Indicators
2.3. Determining Soil Property Indicators
2.4. Data Analysis
3. Result
3.1. Culm Morphology within the Three Regions
3.2. Crown Morphology within the Three Regions
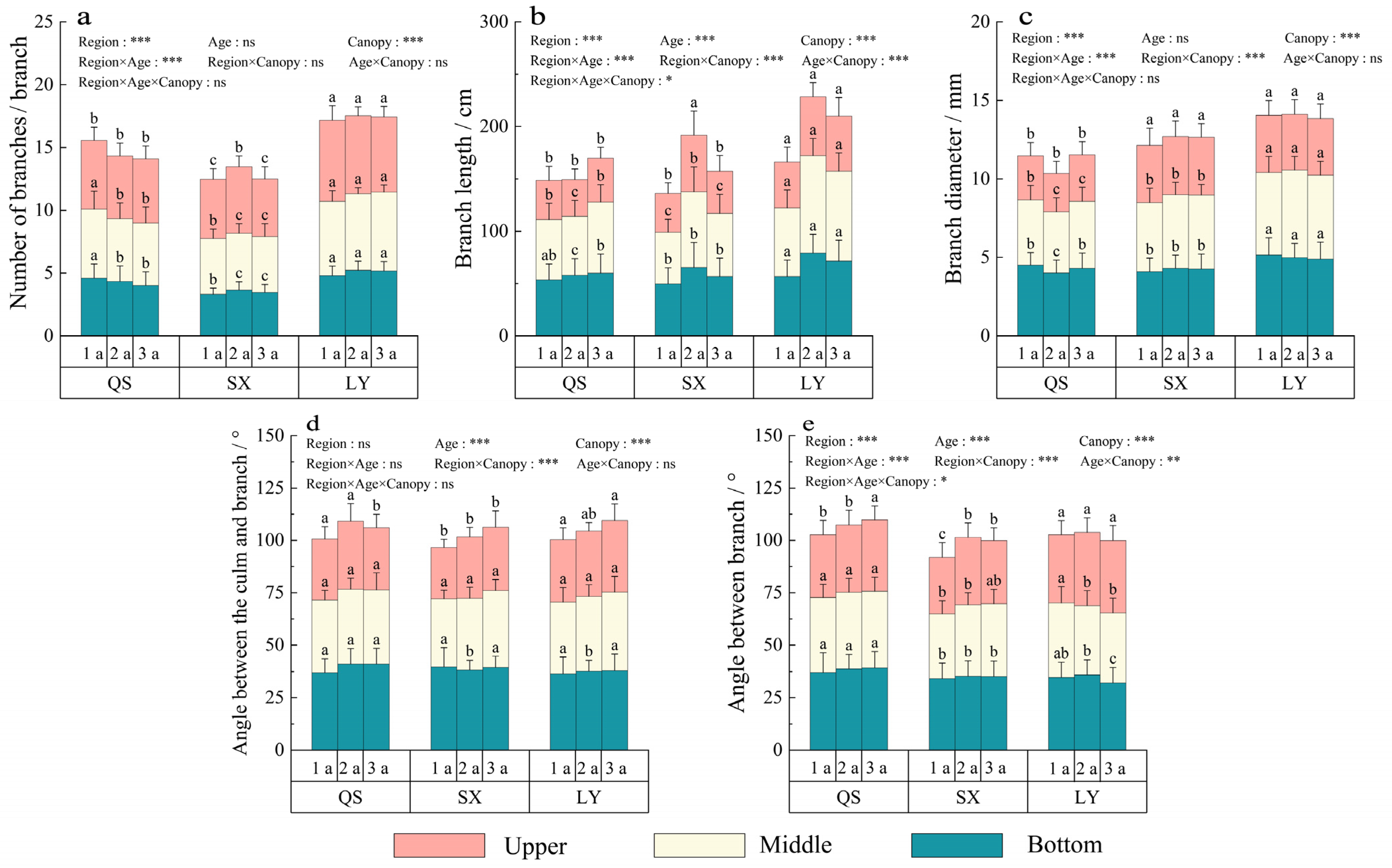
3.3. Branch Morphology within the Three Regions
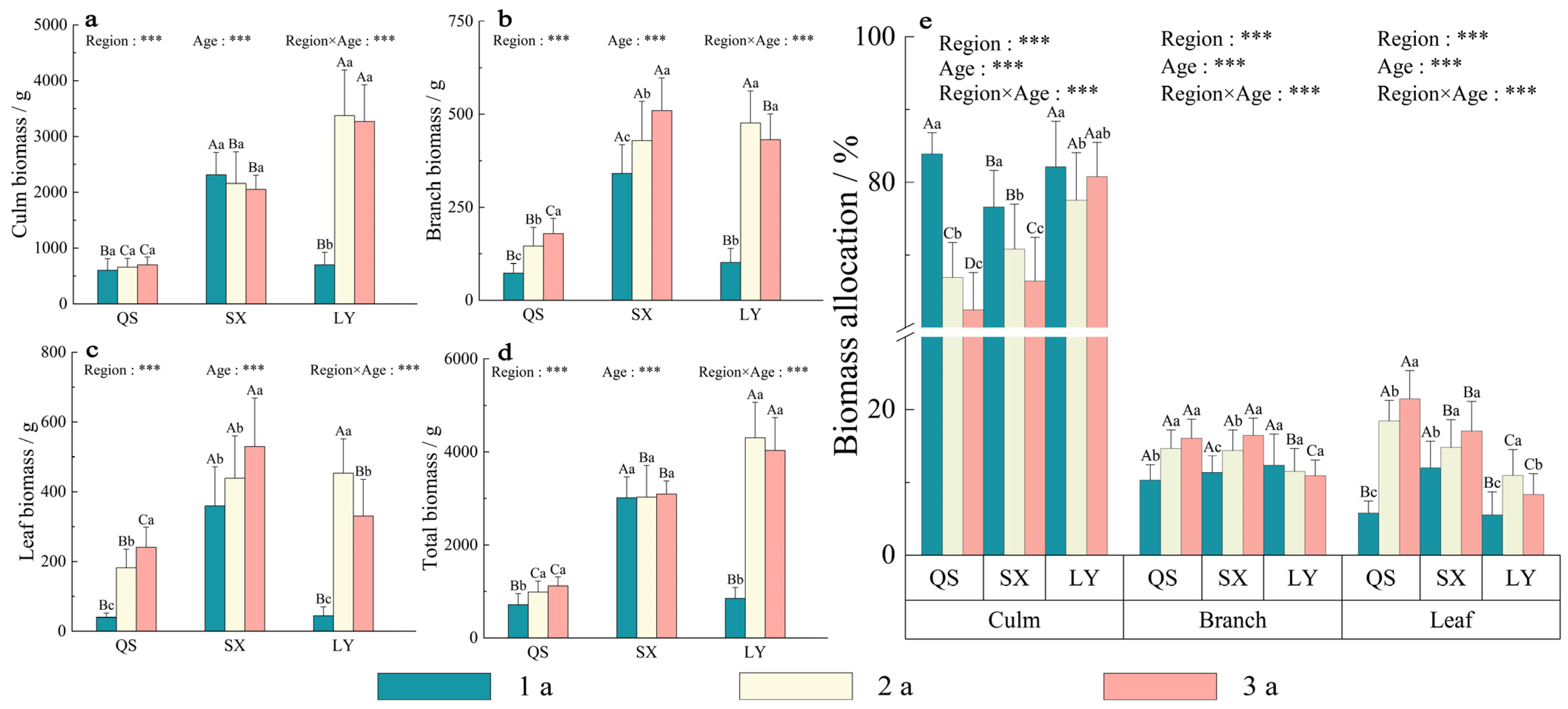
3.4. Biomass Accumulation and Allocation within the Three Regions
3.5. Explanatory Variable Contributions to Phenotypic Plasticity, Biomass Accumulation, and Allocation Using PCA
3.6. Environmental Factor Effects on Phenotypic Plasticity and Biomass Accumulation and Allocation
4. Discussion
4.1. Relationship between Aboveground Components and Environmental Factors
4.2. Relationship between Biomass Accumulation and Allocation and Environmental Factors
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, M.; Ding, G.; Gao, G.; Liu, Z.; Wang, C. Double effects of age and environment on resource allocation trade-offs of Salix psammophila in different microtopographic habitats of a sand dune. J. Plant Growth Regul. 2019, 39, 544–552. [Google Scholar] [CrossRef]
- Michaletz, S.T.; Cheng, D.; Kerkhoff, A.J.; Enquist, B.J. Convergence of terrestrial plant production across global climate gradients. Nature 2014, 512, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Raich, J.W.; Russell, A.E.; Kitayama, K.; Parton, W.J.; Vitousek, P.M. Temperature influences carbon accumulation in moist tropical forests. Ecology 2006, 87, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Lie, G.; Xue, L. Biomass allocation patterns in forests growing different climatic zones of China. Trees 2015, 30, 639–646. [Google Scholar] [CrossRef]
- Olivera Viciedo, D.; de Mello Prado, R.; Martinez, C.A.; Habermann, E.; de Cassia Piccolo, M.; Calero Hurtado, A.; Barreto, R.F.; Pena Calzada, K. Changes in soil water availability and air-temperature impact biomass allocation and C:N:P stoichiometry in different organs of Stylosanthes capitata Vogel. J. Environ. Manag. 2021, 278, 111540. [Google Scholar] [CrossRef] [PubMed]
- Olivera Viciedo, D.; de Mello Prado, R.; Martinez, C.A.; Habermann, E.; de Cassia Piccolo, M. Short-term warming and water stress affect Panicum maximum Jacq. stoichiometric homeostasis and biomass production. Sci. Total Environ. 2019, 681, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, Z.; Zhang, M.; Huang, P.; Dai, L.; Ma, Z.; Liu, J. Climate vs. nutrient control: A global analysis of driving environmental factors of wetland plant biomass allocation strategy. J. Clean. Prod. 2023, 406, 136983. [Google Scholar] [CrossRef]
- Wang, P.; Heijmans, M.M.P.D.; Mommer, L.; van Ruijven, J.; Maximov, T.C.; Berendse, F. Belowground plant biomass allocation in tundra ecosystems and its relationship with temperature. Environ. Res. Lett. 2016, 11, 055003. [Google Scholar] [CrossRef]
- Mao, W.; Allington, G.; Li, Y.L.; Zhang, T.H.; Wang, S.K. Life history strategy influences biomass allocation in response to limiting nutrients and water in an arid system. Pol. J. Ecol. 2012, 60, 545–557. [Google Scholar]
- Villar, R.; Veneklaas, E.J.; Jordano, P.; Lambers, H. Relative growth rate and biomass allocation in 20 Aegilops (Poaceae) species. New Phytol. 1998, 140, 425–437. [Google Scholar] [CrossRef]
- Poorter, H.; Sack, L. Pitfalls and possibilities in the analysis of biomass allocation patterns in plants. Front. Plant Sci. 2012, 3, 259. [Google Scholar] [CrossRef] [PubMed]
- Padilla, F.M.; Miranda, J.D.; Jorquera, M.J.; Pugnaire, F.I. Variability in amount and frequency of water supply affects roots but not growth of arid shrubs. Plant Ecolog. 2009, 204, 261–270. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, M.; Li, G.; Wang, M.; Li, Z.; De Boeck, H.J. Changes of aboveground and belowground biomass allocation in four dominant grassland species across a precipitation gradient. Front. Plant Sci. 2021, 12, 650802. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.S.; Goldstein, G.; Meinzer, F.C.; Fisher, J.B.; Machado, K.; Woodruff, D.; Jones, T. Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. Oecologia 2004, 140, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.-M.; Wang, G.-X.; Morris, E.C.; Wei, X.-P.; Li, D.-X.; Chen, B.-M.; Zhao, C.-M.; Liu, J.; Wang, Y.U.N. Plant mass-density relationship along a moisture gradient in north-west China. J. Ecol. 2006, 94, 953–958. [Google Scholar] [CrossRef]
- Wang, J.; Gao, J.; Wu, Y.; Xu, B.; Shi, F.; Zhou, H.; Bisht, N.; Wu, N. Effects of heterogeneous environment after deforestation on plant phenotypic plasticity of three shrubs based on leaf traits and biomass allocation. Front. Ecol. Evol. 2021, 9, 608663. [Google Scholar] [CrossRef]
- Schellenberger Costa, D.; Classen, A.; Ferger, S.; Helbig-Bonitz, M.; Peters, M.; Bohning-Gaese, K.; Steffan-Dewenter, I.; Kleyer, M. Relationships between abiotic environment, plant functional traits, and animal body size at Mount Kilimanjaro, Tanzania. PLoS ONE 2017, 12, e0174157. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Chen, J.; Feng, L.; Li, F.; Yu, L. Study on the relationship between functional characteristics and environmental factors in karst plant communities. Ecol. Evol. 2022, 12, e9335. [Google Scholar] [CrossRef]
- Mizutani, M.; Kanaoka, M.M. Environmental sensing and morphological plasticity in plants. Semin. Cell Dev. Biol. 2018, 83, 69–77. [Google Scholar] [CrossRef]
- Pigliucci, M. Evolution of phenotypic plasticity: Where are we going now? Trends Ecol. Evol. 2005, 20, 481–486. [Google Scholar] [CrossRef]
- Gratani, L. Plant phenotypic plasticity in response to environmental factors. Adv. Bot. 2014, 2014, 1–17. [Google Scholar] [CrossRef]
- Aribal, L.G.; Parlucha, J.A.; Gelaga, J.N.; Aguinsatan, R.G. Influence of abiotic factors on growth and development of giant Bamboo (Dendrocalamus Asper) in bukidnon, philippines. J. Trop. For. Sci. 2022, 34, 63–73. [Google Scholar] [CrossRef]
- Lihavainen, J.; Ahonen, V.; Keski-Saari, S.; Kontunen-Soppela, S.; Oksanen, E.; Keinanen, M. Low vapour pressure deficit affects nitrogen nutrition and foliar metabolites in silver birch. J. Exp. Bot. 2016, 67, 4353–4365. [Google Scholar] [CrossRef] [PubMed]
- Bjorkman, A.D.; Myers-Smith, I.H.; Elmendorf, S.C.; Normand, S.; Ruger, N.; Beck, P.S.A.; Blach-Overgaard, A.; Blok, D.; Cornelissen, J.H.C.; Forbes, B.C.; et al. Plant functional trait change across a warming tundra biome. Nature 2018, 562, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Zhang, Y.; Wang, R.; Guo, Z.; Xie, Z. Impacts of drought stress on the morphology, physiology, and sugar content of Lanzhou lily (Lilium davidii var. unicolor). Acta Physiol. Plant. 2020, 42, 127. [Google Scholar] [CrossRef]
- Kessler, M.; Siorak, Y.; Wunderlich, M.; Wegner, C. Patterns of morphological leaf traits among pteridophytes along humidity and temperature gradients in the Bolivian Andes. Funct. Plant Biol. 2007, 34, 963–971. [Google Scholar] [CrossRef]
- Sugiyama, S. Geographical distribution and phenotypic differentiation in populations of Dactylis glomerata L. in Japan. Plant Ecolog. 2002, 169, 295–305. [Google Scholar] [CrossRef]
- Ellison, A.M.; Buckley, H.L.; Miller, T.E.; Gotelli, N.J. Morphological variation in Sarracenia purpurea (Sarraceniaceae): Geographic, environmental, and taxonomic correlates. Am. J. Bot. 2004, 91, 1930–1935. [Google Scholar] [CrossRef]
- Ruedas, M.; Valverde, T.; Zavala-Hurtado, J.A. Analysis of the factors that affect the distribution and abundance of three Neobuxbaumia species (Cactaceae) that differ in their degree of rarity. Acta Oecologica 2006, 29, 155–164. [Google Scholar] [CrossRef]
- Uribe-Salas, D.; Sáenz-Romero, C.; González-Rodríguez, A.; Téllez-Valdéz, O.; Oyama, K. Foliar morphological variation in the white oak Quercus rugosa Née (Fagaceae) along a latitudinal gradient in Mexico: Potential implications for management and conservation. For. Ecol. Manage 2008, 256, 2121–2126. [Google Scholar] [CrossRef]
- Pyakurel, A.; Wang, J.R. Leaf morphological variation among paper birch (Betula papyrifera Marsh.) genotypes across Canada. Open J. Ecol. 2013, 3, 284–295. [Google Scholar] [CrossRef]
- Arroyo-Cosultchi, G.; Arias, S.; López-Mata, L.; Terrazas, T. Morphological plasticity of an endemic widespread columnar cactus and its congener. Braz. J. Bot. 2017, 40, 1029–1040. [Google Scholar] [CrossRef]
- Dorken, M.E.; Barrett, S.C. Phenotypic plasticity of vegetative and reproductive traits in monoecious and dioecious populations of Sagittaria latifolia (Alismataceae): A clonal aquatic plant. J. Ecol. 2004, 92, 32–44. [Google Scholar] [CrossRef]
- Milla, R.; Reich, P.B. Multi-trait interactions, not phylogeny, fine-tune leaf size reduction with increasing altitude. Ann. Bot. 2011, 107, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Mou, P.; Jones, R.H.; Tan, Z.; Bao, Z.; Chen, H. Morphological and physiological plasticity of plant roots when nutrients are both spatially and temporally heterogeneous. Plant Soil 2013, 364, 373–384. [Google Scholar] [CrossRef]
- Wang, R.; Yu, G.; He, N.; Wang, Q.; Zhao, N.; Xu, Z. Latitudinal variation of leaf morphological traits from species to communities along a forest transect in eastern China. J. Geogr. Sci 2016, 26, 15–26. [Google Scholar] [CrossRef]
- Liu, G.; Freschet, G.T.; Pan, X.; Cornelissen, J.H.; Li, Y.; Dong, M. Coordinated variation in leaf and root traits across multiple spatial scales in Chinese semi-arid and arid ecosystems. New Phytol. 2010, 188, 543–553. [Google Scholar] [CrossRef]
- Moller, A.P.; Merilä, J. Analysis and interpretation of long-term studies investigating responses to climate change. In Birds and Climate Change; Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 2004; Volume 35, pp. 111–130. [Google Scholar]
- Du, H.; Mao, F.; Li, X.; Zhou, G.; Xu, X.; Han, N.; Sun, S.; Gao, G.; Cui, L.; Li, Y.; et al. Mapping global bamboo forest distribution using multisource remote sensing data. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2018, 11, 1458–1471. [Google Scholar] [CrossRef]
- Li, W.; Shi, C.; Li, K.; Zhang, Q.J.; Tong, Y.; Zhang, Y.; Wang, J.; Clark, L.; Gao, L.Z. Draft genome of the herbaceous bamboo Raddia distichophylla. G3 Genes Genome Genetics 2021, 11, jkaa049. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Singhwane, A.; Dhangar, M.; Mili, M.; Gorhae, N.; Naik, A.; Prashant, N.; Srivastava, A.K.; Verma, S. Bamboo for producing charcoal and biochar for versatile applications. Biomass Convers. Biorefin. 2023, 1–27. [Google Scholar] [CrossRef]
- Takano, K.T.; Hibino, K.; Numata, A.; Oguro, M.; Aiba, M.; Shiogama, H.; Takayabu, I.; Nakashizuka, T. Detecting latitudinal and altitudinal expansion of invasive bamboo Phyllostachys edulis and Phyllostachys bambusoides (Poaceae) in Japan to project potential habitats under 1.5 °C -4.0 °C global warming. Ecol. Evol. 2017, 7, 9848–9859. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Preisler, H.K.; Quinn, B.K.; Zhao, J.; Huang, W.; Röll, A.; Cheng, X.; Li, H.; Hölscher, D. Precipitation is the most crucial factor determining the distribution of moso bamboo in Mainland China. Global Ecol. Conserv. 2020, 22, e00924. [Google Scholar] [CrossRef]
- Guo, W.; Cherubini, P.; Zhang, J.; Hu, X.; Li, M.-H.; Qi, L. Soil physicochemical properties determine leaf traits but not size traits of moso bamboo (Phyllostachys edulis). Environ. Res. Lett. 2022, 17, 114061. [Google Scholar] [CrossRef]
- Guo, Z.; Lin, H.; Chen, S.; Yang, Q. Altitudinal patterns of leaf traits and leaf allometry in bamboo Pleioblastus amarus. Front. Plant Sci. 2018, 9, 1110. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Yan, J.; Liu, S.; Chen, S.; Zou, L.; Yang, Y.; He, L.; Li, S.; Liu, A.; Zhao, K. Extraction, isolation and identification of four phenolic compounds from Pleioblastus amarus shoots and their antioxidant and anti-inflammatory properties in vitro. Food Chem. 2022, 374, 131743. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-T.; Tu, L.-H.; Peng, Y.; Hu, H.-L.; Hu, T.-X.; Xu, Z.-F.; Liu, L.; Tang, Y. Effect of nitrogen additions on root morphology and chemistry in a subtropical bamboo forest. Plant Soil 2016, 412, 441–451. [Google Scholar] [CrossRef]
- Shi, J.; Chen, S.; Guo, Z.; Zhang, C. Influence of altitude on culm form, crown, phylliform of Phyllostachys prominens. J. Northeast. For. Univ. 2018, 46, 13–17. [Google Scholar] [CrossRef]
- Lan, M.; Xue, C.; Yang, J.; Wang, N.; Sun, C.; Wu, G.; Chen, H.; Su, Z. Changes in plant diversity and soil factors under different rocky desertification degrees in Northern Guangdong, China. Forests 2023, 14, 694. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Zhang, H.; Liber, K. A comprehensive support vector machine-based classification model for soil quality assessment. Soil Tillage Res. 2016, 155, 19–26. [Google Scholar] [CrossRef]
- Huang, D.; Luo, T.; Li, Y.; Qiu, X. Tebeigai soil conditioner can increase eggplant yield, reduce heavy metal content and improve soil acidification. J. Phys. Conf. Ser. 2020, 1622, 012077. [Google Scholar] [CrossRef]
- Neyeloff, J.L.; Fuchs, S.C.; Moreira, L.B. Meta-analyses and forest plots using a microsoft excel spreadsheet: Step-by-step guide focusing on descriptive data analysis. BMC Res. Notes 2012, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, Z.; Sharifi, G. Comparing the effect of resistance, aerobic, and concurrent exercise program on the level of resistin and high reactive protein C of overweight and obese women. Int. Arch. Health Sci. 2017, 4, 1–6. [Google Scholar] [CrossRef]
- Lee, B.-H.; De Jesús Pérez, J.J.; Moiseenkova-Bell, V.; Rohacs, T. Structural basis of the activation of TRPV5 channels by long-chain acyl-Coenzyme-A. Nat. Commun. 2023, 14, 5883. [Google Scholar] [CrossRef] [PubMed]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Extract and Visualize the Results of Multivariate Data Analyses [R Package Factoextra Version 1.0.7]. 2020. Available online: https://scirp.org/reference/referencespapers?referenceid=3067217 (accessed on 14 June 2023).
- Wong, K.K.-K. Partial least squares structural equation modeling (PLS-SEM) techniques using SmartPLS. Mark. Bull. 2013, 24, 1–32. [Google Scholar]
- Hu, B.; Ni, H.; Xie, M.; Li, H.; Wen, Y.; Chen, S.; Zhou, Y.; Teng, H.; Bourennane, H.; Shi, Z. Mapping soil organic matter and identifying potential controls in the farmland of Southern China: Integration of multi-source data, machine learning and geostatistics. Land Degrad. Dev. 2023, 34, 5468–5485. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Lu, X.; Wang, Q.; Han, H.; Zhang, X.; Ma, Y.; Gan, X. Leaf phenotypic variation of endangered plant Tetracentron sinense Oliv. and influence of geographical and climatic factors. J. For. Res. 2021, 32, 623–636. [Google Scholar] [CrossRef]
- Henn, J.J.; Buzzard, V.; Enquist, B.J.; Halbritter, A.H.; Klanderud, K.; Maitner, B.S.; Michaletz, S.T.; Potsch, C.; Seltzer, L.; Telford, R.J.; et al. Intraspecific trait variation and phenotypic plasticity mediate alpine plant species response to climate change. Front. Plant Sci. 2018, 9, 1548. [Google Scholar] [CrossRef]
- Mulatu, Y. Growth, Morphology and Biomass of Arundinaria alpina (Highland Bamboo) (Poaceae) as Affected by Landrace, Environment and Silvicultural Management in the Choke Mountain, Northwestern Ethiopia. 2012. Available online: https://www.efd.gov.et/wp-content/uploads/2016/01/PhD-Thesis-on-bamboo-management.-Yirgadu-Mulatu.pdf (accessed on 14 June 2023).
- Banik, R.L. Morphology and growth. In Bamboo: The Plant and Its Uses; Springer: Berlin/Heidelberg, Germany, 2015; pp. 43–89. [Google Scholar]
- Dai, E.; Wu, Z.; Ge, Q.; Xi, W.; Wang, X. Predicting the responses of forest distribution and aboveground biomass to climate change under RCP scenarios in southern China. Global Change Biol. 2016, 22, 3642–3661. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, H.; Dong, Z.; Duan, K.; Wang, H.; Han, Y. East Asian summer monsoon and topography co-determine the Holocene migration of forest-steppe ecotone in northern China. Global Planet. Change 2020, 187, 103135. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Y.; Liu, T.; Yan, F. Impact of recent vegetation greening on temperature and precipitation over China. Agric. For. Meteorol. 2020, 295, 108197. [Google Scholar] [CrossRef]
- Tao, G.-Y.; Ramakrishnan, M.; Vinod, K.K.; Yrjälä, K.; Satheesh, V.; Cho, J.; Fu, Y.; Zhou, M. Multi-omics analysis of cellular pathways involved in different rapid growth stages of moso bamboo. Tree Physiol. 2020, 40, 1487–1508. [Google Scholar] [CrossRef] [PubMed]
- Nurmadina; Nugroho, N.; Bahtiar, E.T. Structural grading of Gigantochloa apus bamboo based on its flexural properties. Constr. Build. Mater. 2017, 157, 1173–1189. [Google Scholar] [CrossRef]
- Zhang, Z.; Rao, F.; Wang, Y. Morphological, Chemical, and Physical–mechanical properties of a clumping bamboo (Thyrsostachys oliveri) for construction applications. Polymers 2022, 14, 3681. [Google Scholar] [CrossRef] [PubMed]
- Hunter, I.R. Bamboo resources, uses and trade: The future? J. Bamboo Ratt. 2003, 2, 319–326. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, Y.; Xu, K.; Cao, M.; Hu, S.; Yang, Q.; Liu, X.; Ma, Q.; Hu, T.; Kelly, M.; et al. Canopy structure: An intermediate factor regulating grassland diversity-function relationships under human disturbances. Fundam. Res. 2023, 3, 179–187. [Google Scholar] [CrossRef]
- Holder, C.D.; Gibbes, C. Influence of leaf and canopy characteristics on rainfall interception and urban hydrology. Hydrol. Sci. J. 2016, 62, 182–190. [Google Scholar] [CrossRef]
- Zhang, J.; Gou, Z.; Zhang, F.; Shutter, L. A study of tree crown characteristics and their cooling effects in a subtropical city of Australia. Ecol. Eng. 2020, 158, 106027. [Google Scholar] [CrossRef]
- Ferrante, A.; Mariani, L. Agronomic management for enhancing plant tolerance to abiotic stresses: High and low values of temperature, light intensity, and relative humidity. Horticulturae 2018, 4, 21. [Google Scholar] [CrossRef]
- Su, Y.; Hu, T.; Wang, Y.; Li, Y.; Dai, J.; Liu, H.; Jin, S.; Ma, Q.; Wu, J.; Liu, L.; et al. Large-scale geographical variations and climatic controls on crown architecture traits. J. Geophys. Res. Biogeosci. 2020, 125, e2019JG005306. [Google Scholar] [CrossRef]
- Craine, J.M.; Dybzinski, R.; Robinson, D. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 2013, 27, 833–840. [Google Scholar] [CrossRef]
- Iqbal, N.; Fatma, M.; Gautam, H.; Umar, S.; Sofo, A.; D’Ippolito, I.; Khan, N.A. The crosstalk of melatonin and hydrogen sulfide determines photosynthetic performance by regulation of carbohydrate metabolism in wheat under heat stress. Plants 2021, 10, 1778. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Jiang, Y.; Lü, X.; Wang, X.; Li, M.-H.; Bai, E.; Han, X.; Xu, Z. Patterns of plant biomass allocation in temperate grasslands across a 2500-km transect in northern China. PLoS ONE 2013, 8, e71749. [Google Scholar] [CrossRef] [PubMed]
- Pichler, P.; Oberhuber, W. Radial growth response of coniferous forest trees in an inner Alpine environment to heat-wave in 2003. For. Ecol. Manag. 2007, 242, 688–699. [Google Scholar] [CrossRef]
- Blessing, C.H.; Werner, R.A.; Siegwolf, R.; Buchmann, N. Allocation dynamics of recently fixed carbon in beech saplings in response to increased temperatures and drought. Tree Physiol. 2015, 35, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; He, W.; Huang, K.; Otieno, D.; Zhou, C.; He, C.; Huang, Y. Transpiration of Moso bamboo in southern China is influenced by ramet age, phenology, and drought. For. Ecol. Manag. 2019, 450, 117526. [Google Scholar] [CrossRef]
- Zaeem, M.; Nadeem, M.; Pham, T.H.; Ashiq, W.; Ali, W.; Gilani, S.S.M.; Elavarthi, S.; Kavanagh, V.; Cheema, M.; Galagedara, L.; et al. The potential of corn-soybean intercropping to improve the soil health status and biomass production in cool climate boreal ecosystems. Sci. Rep. 2019, 9, 13148. [Google Scholar] [CrossRef]
- Shen, X.; Jiang, M.; Lu, X.; Liu, X.; Liu, B.; Zhang, J.; Wang, X.; Tong, S.; Lei, G.; Wang, S.; et al. Aboveground biomass and its spatial distribution pattern of herbaceous marsh vegetation in China. Sci. China Earth Sci. 2021, 64, 1115–1125. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, T.; Chen, X.; Xu, W.; Dong, T.; Liu, Q.; Xu, X. Twigs of dove tree in high-latitude region tend to increase biomass accumulation in vegetative organs but decrease it in reproductive organs. Front. Plant Sci. 2022, 13, 1088955. [Google Scholar] [CrossRef]
- Zheng, Y.; Feng, Y.; Fan, S.; Zhang, M.; Zhang, X.; Zhou, X.; Guan, F. Effects of different harvesting methods on aboveground nutrient pools of Moso Bamboo (Phyllostachys edulis) forest in China. Forests 2022, 13, 2138. [Google Scholar] [CrossRef]
- Qing, L.; Yunxiang, L.; Zhangcheng, Z. Effects of moisture availability on clonal growth in bamboo Pleioblastus maculata. Plant Ecolog. 2004, 173, 107–113. [Google Scholar] [CrossRef]
- Umaña, M.N.; Arellano, G.; Swenson, N.G.; Zambrano, J. Tree seedling trait optimization and growth in response to local-scale soil and light variability. Ecology 2021, 102, e03252. [Google Scholar] [CrossRef] [PubMed]
- Enebe, M.C.; Babalola, O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: A survival strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Niu, W.; Cao, X.; Zhang, M.; Wang, J.; Zhang, Z. Growth response of greenhouse-produced muskmelon and tomato to sub-surface drip irrigation and soil aeration management factors. BMC Plant Biol. 2020, 20, 141. [Google Scholar] [CrossRef]
- Lin, C.; Hang, T.; Jiang, C.; Yang, P.; Zhou, M. Effects of different phosphorus levels on tiller bud development in hydroponic Phyllostachys edulis seedlings. Tree Physiol. 2023, 43, 1416–1431. [Google Scholar] [CrossRef]
- Tewari, R.K.; Yadav, N.; Gupta, R.; Kumar, P. Oxidative stress under macronutrient deficiency in plants. J. Soil Sci. Plant Nutr. 2021, 21, 832–859. [Google Scholar] [CrossRef]
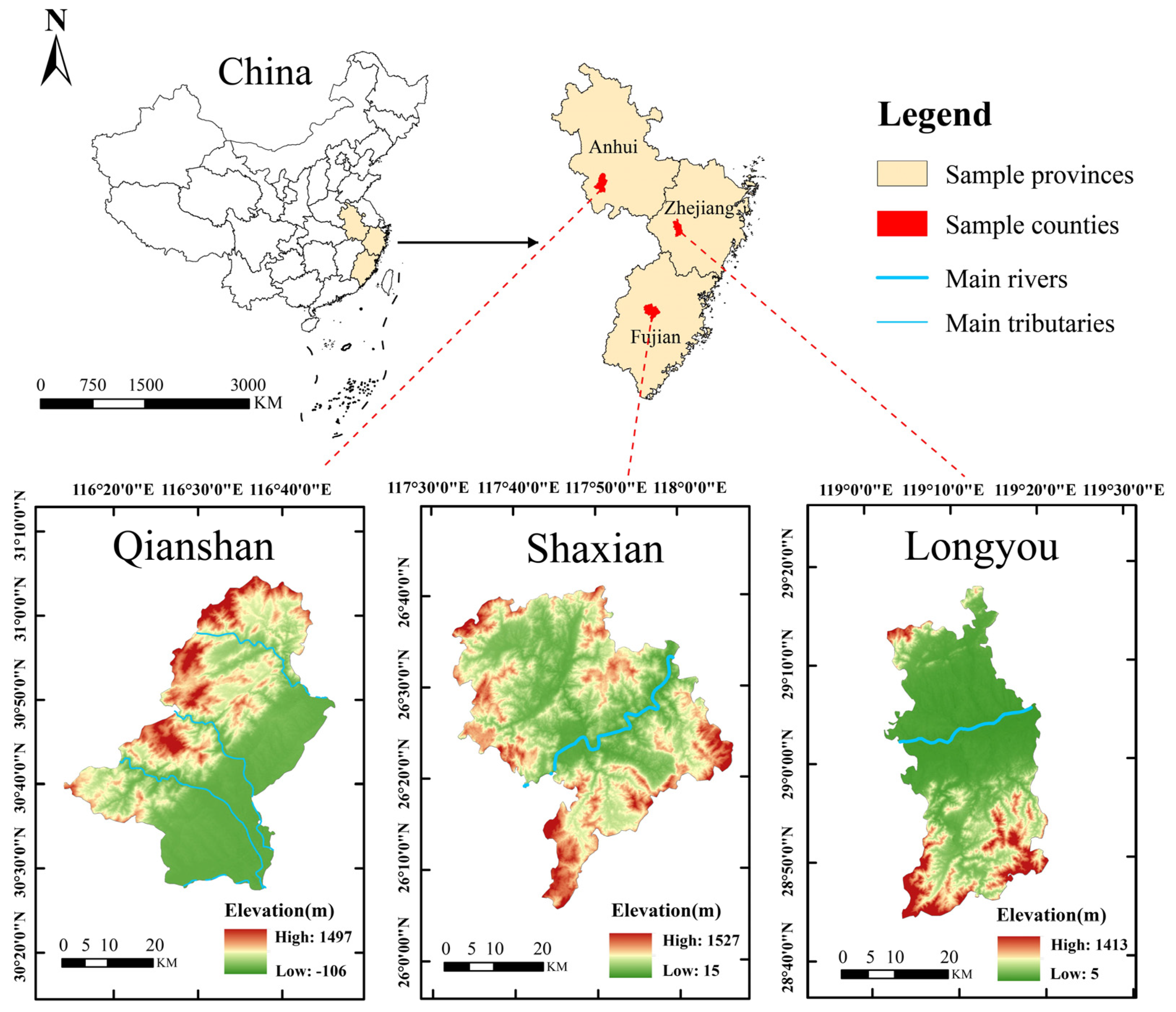




| Region | LNG | LAT | Elevation (m) | Slope (°) | Density (Plant · ha−1) | Age Ratio (1 a:2 a:3 a) | MAP (mm) | MAT (°C) | MAXT (°C) | MINT (°C) | ASH (h) | AFP (d) | MAH (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QS | 116°10′ E | 30°44′ N | 323.37 | 22 | 79,166 | 13:146:126 | 1596.06 | 17.13 | 37.45 | −5.78 | 1740.70 | 343.3 | 74.9 |
| LY | 119°11′ E | 28°51′ N | 215.53 | 35 | 29,125 | 18:206:309 | 1823.82 | 18.28 | 39.05 | −4.92 | 1719.00 | 261.5 | 77.6 |
| SX | 117°43′ E | 26°20′ N | 192.42 | 21 | 25,666 | 3:57:94 | 1778.65 | 20.59 | 39.19 | −2.01 | 1652.46 | 358.1 | 79.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, K.; Fan, L.; Guo, Z.; Zhang, J.; Duan, Y.; Zhang, L.; Chen, S.; Lin, H.; Hu, R. Aboveground Biomass Component Plasticity and Allocation Variations of Bamboo (Pleioblastus amarus) of Different Regions. Forests 2024, 15, 43. https://doi.org/10.3390/f15010043
Zuo K, Fan L, Guo Z, Zhang J, Duan Y, Zhang L, Chen S, Lin H, Hu R. Aboveground Biomass Component Plasticity and Allocation Variations of Bamboo (Pleioblastus amarus) of Different Regions. Forests. 2024; 15(1):43. https://doi.org/10.3390/f15010043
Chicago/Turabian StyleZuo, Keyi, Lili Fan, Ziwu Guo, Jingrun Zhang, Yiyang Duan, Le Zhang, Shuanglin Chen, Hua Lin, and Ruicai Hu. 2024. "Aboveground Biomass Component Plasticity and Allocation Variations of Bamboo (Pleioblastus amarus) of Different Regions" Forests 15, no. 1: 43. https://doi.org/10.3390/f15010043
APA StyleZuo, K., Fan, L., Guo, Z., Zhang, J., Duan, Y., Zhang, L., Chen, S., Lin, H., & Hu, R. (2024). Aboveground Biomass Component Plasticity and Allocation Variations of Bamboo (Pleioblastus amarus) of Different Regions. Forests, 15(1), 43. https://doi.org/10.3390/f15010043






