Leaf Area Estimation of Yellow Oleander Thevetia peruviana (Pers.) K. Schum Using a Non-Destructive Allometric Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Allometry of Leaf Area
2.1.1. Model Construction
2.1.2. Test for Model Identity
2.1.3. Model Validation
2.1.4. Equation Generation
2.1.5. Root Mean Square Error (RMSE) and Akaike (AIC) Criteria
2.2. Statistical Data Analysis
3. Results
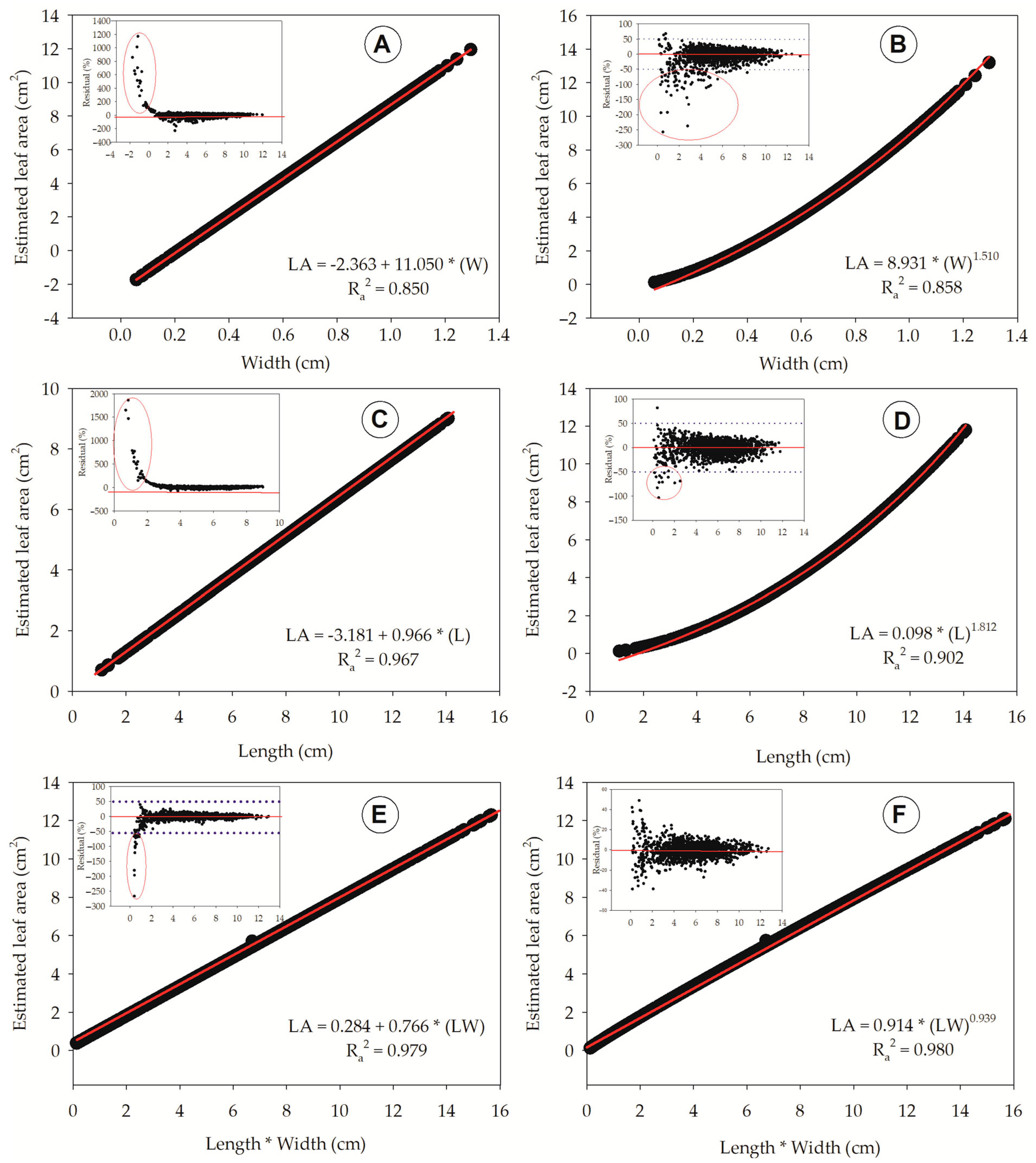
3.1. Equation Generation
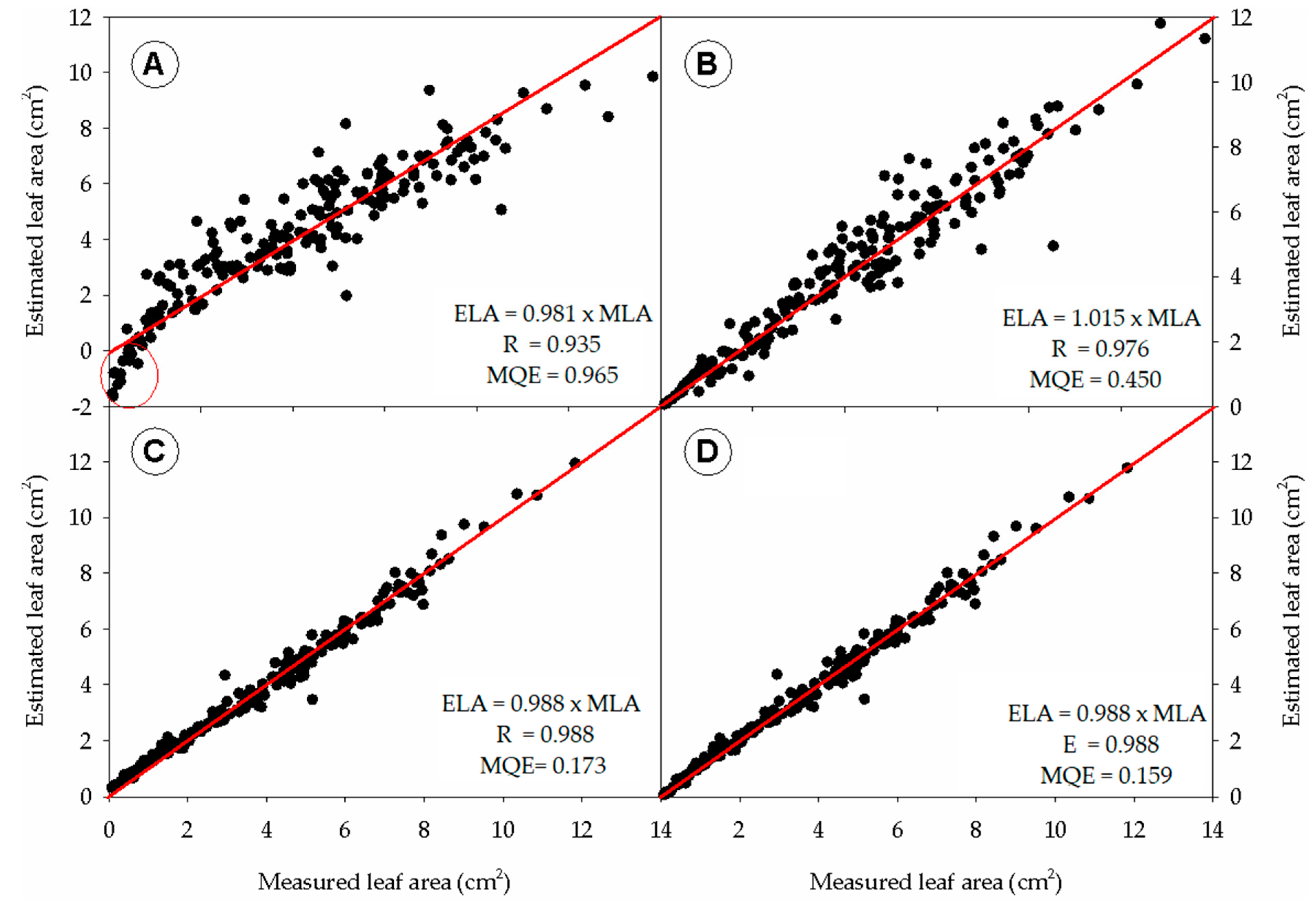
3.2. Analysis of Deviation from the Estimated to Observed Leaf Area
3.3. Analyzis of the Deviation from the Estimated to Observed Leaf Area
3.4. Confirming the Alometry Equation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adamu, F.A.; Jahun, B.G.; Daniel, C. Assessment and optimization of energy use of yellow oleander (Thevetia peruviana) for biodiesel blends in Nigeria. Int. J. Sci. Res. 2015, 4, 1277–1280. [Google Scholar]
- El Gizawy, H.A.; El Zanaty, S.A.; El Ghaly, E.M.; Seif-Eldein, N.A. Thevetia peruviana leaves, HPLC profile, isolation, and in vitro cytotoxicity. RSC Adv. 2023, 13, 12072. [Google Scholar] [CrossRef] [PubMed]
- Villa, F.A.; Hormaza, A.; Arias, Z.M. Thevetin B: Cardiotonic glycoside predominant in Thevetia peruviana. Sci. Tech. 2011, 9, 298–303. [Google Scholar]
- Zibbu, G.; Batra, A. Thevetia peruviana (Pers.) Schum: A plant with enormous therapeutic potential. J. Pharm. Res. 2011, 4, 4461–4464. [Google Scholar]
- Oluwaniyi, O.O.; Ibiyemi, S.A. Extractability of Thevetia peruviana glycosides with alcohol mixture. Afr. J. Biotechnol. 2007, 6, 2166–2170. [Google Scholar]
- Sharma, T.; Kaur, J.; Singh, G. Phytochemistry and pharmacological activities of Thevetia peruviana: A review. Int. J. Pharm. Sci. Res. 2022, 13, 2274–2282. [Google Scholar] [CrossRef]
- Kohls, S.; Scholz-Böttcher, B.M.; Teske, J.; Zark, P.; Rullkötter, J. Cardiac glycosides from yellow oleander (Thevetia peruviana) seeds sarah kohls. Phytochemistry 2012, 75, 114–127. [Google Scholar] [CrossRef]
- Said, I.M.; Rahman, Z.A. The cardenolides of Thevetia peruviana grown in Malasya. Asean J. Sci. Technol. Dev. 1986, 3, 83–86. [Google Scholar] [CrossRef]
- Bandara, V.; Weinstein, S.A.; White, J.; Eddleston, M. A review of the natural history, toxinology, diagnosis and clinical management of Nerium oleander (common oleander) and Thevetia peruviana (yellow oleander) poisoning. Toxicon 2010, 56, 273–281. [Google Scholar] [CrossRef]
- Terra, S.G.; Washam, J.B.; Dunham, G.D.; Gattis, W.A. Therapeutic range of digoxin’s efficacy in heart failure: What is the evidence? Pharmacotherapy 1999, 19, 1123–1126. [Google Scholar] [CrossRef]
- Prabhasankar, P.; Raguputhi, G.; Sundaravadivel, B.; Annapoorani, K.S.; Damodaran, C. Enzyme-linked immunosorbent assay for the phytotoxin thevetin. J. Immunoass. 1993, 14, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M.; Ariaratnam, C.A.; Meyer, W.P.; Perera, G.; Kularatne, A.M.; Attapattu, S.; Sheriff, M.H.; Warrell, D.A. Epidemic of selfpoisoning with seeds of the yellow oleander tree (Thevetia peruviana) in northern Sri Lanka. Trop. Med. Int. Health 1999, 4, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-de-Gyves, E.; Cristofori, V.; Fallovo, C.; Rouphael, Y.; Bignami, C. Accurate and rapid technique for leaf area measurement in medlar (Mespilus germanica L.). Adv. Hortic. Sci. 2008, 22, 223–226. [Google Scholar]
- Cristofori, V.; Rouphael, Y.; Mendoza-de Gyves, E.; Bignami, C. A simple model for estimating leaf area of hazelnut from linear measurements. Sci. Hortic. 2007, 113, 221–225. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Antunes, W.C.; Ferreira, D.T.R.G.; Cavalcante, P.P.G.S.; Wanderley-Filho, H.C.L.; Endres, L. Allometric models for non-destructive leaf area estimation of the Jatropha curcas. Biomass Bioenerg. 2012, 36, 77–85. [Google Scholar] [CrossRef]
- Antunes, W.C.; Pompelli, M.F.; Carretero, D.M.; DaMatta, F.M. Allometric models for non-destructive leaf area estimation in coffee (Coffea arabica and Coffea canephora). Ann. Appl. Biol. 2008, 153, 33–40. [Google Scholar] [CrossRef]
- Schrader, J.; Pillar, G.; Kreft, H. Leaf-IT: An Android application for measuring leaf area. Ecol. Evol. 2017, 7, 9731–9738. [Google Scholar] [CrossRef]
- Yang, J.; Alley, M. A mechanistic model for describing corn plant leaf area distribution. Agron. J. 2005, 97, 41–48. [Google Scholar] [CrossRef]
- Toebe, M.; de Melo, P.J.; de Souza, R.R.; Mello, A.C.; Tartaglia, F.L. Leaf area estimation in triticale by leaf dimensions. Rev. Bras. Cienc. Agrar. 2019, 14, e5656. [Google Scholar] [CrossRef]
- Souza, M.C.; Amaral, C.L. Non-destructive linear model for leaf area estimation in Vernonia ferruginea Less. Braz. J. Biol. 2015, 75, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.; Kliewer, W.M. Estimation of Thompson seedless grapevine leaf area. Am. J. Enol. Vitic. 1984, 35, 16–22. [Google Scholar] [CrossRef]
- Pandey, S.K. A simple, cost-effective method for leaf area estimation. J. Bot. 2011, 2011, 658240. [Google Scholar] [CrossRef]
- Adhikari, R.; Li, C.; Kalbaugh, K.; Nemali, K. A low-cost smartphone controlled sensor based on image analysis for estimating whole-plant tissue nitrogen (N) content in floriculture crops. Comput. Electron. Agric. 2020, 169, 205173. [Google Scholar] [CrossRef]
- Graybill, F.A. Theory and Application of the Linear Model; Duxbury Press: Pacific Grove, CA, USA, 2000. [Google Scholar]
- Zuur, A.F.; Elena, N.I.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2009, 1, 3–14. [Google Scholar] [CrossRef]
- Williams, L., III; Martinson, T.E. Nondestructive leaf area estimation of ‘Niagara’ and ‘DeChaunac’ grapevines. Sci. Hortic. 2003, 98, 493–498. [Google Scholar] [CrossRef]
- Whitworth, J.L.; Mauromoustakos, A.; Smith, M.W. A nondestructive method for estimation of leaf area in pecan. HortScience 1992, 27, 851. [Google Scholar] [CrossRef]
- Tsialtas, J.T.; Maslaris, N. Leaf aea estimation in a sugar beet cultivar by linear models. Photosynthetica 2005, 43, 477–479. [Google Scholar] [CrossRef]
- Torri, S.I.; Descalzi, C.; Frusso, E. Estimation of leaf area in pecan cultivars (Carya illinoinensis). Cienc. Investig. Agrar. 2009, 36, 53–58. [Google Scholar] [CrossRef]
- Shabani, A.; Sepaskhah, A.R. Leaf area estimation by a simple and non-destructive method. Iran. Agric. Res. 2017, 36, 101–105. [Google Scholar]
- Salazar, J.C.S.; Melgarejo, L.M.; Bautista, E.H.D.; Rienzo, J.A.; Casanoves, F. Non-destructive estimation of the leaf weight and leaf area in cacao (Theobroma cacao L.). Sci. Hortic. 2018, 229, 19–24. [Google Scholar] [CrossRef]
- Santos, J.N.B.; Jarma-Orozco, A.; Antunes, W.C.; Mendes, K.R.; Figueiroa, J.M.; Pessoa, L.M.; Pompelli, M.F. New approaches to predict leaf area in woody tree species from the Atlantic Rainforest, Brazil. Austral Ecol. 2021, 46, 613–626. [Google Scholar] [CrossRef]
- Steel, M.; Penny, D. Parsimony, likelihood, and the role of models in molecular phylogenetics. Mol. Biol. Evol. 2000, 17, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Cumming, G.; Fidler, F.; Vaux, D.L. Error bars in experimental biology. J. Cell Biol. 2007, 177, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A new look at the statistical model identification. Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Lozano-Isla, F.; Alfaro, O.B.; Pompelli, M.F. GerminaR: An R package for germination analysis with the interactive web application ‘GerminaQuant for R’. Ecol. Res. 2019, 34, 339–346. [Google Scholar] [CrossRef]
- Lima Filho, O.P.; Malavolta, E. Estimativa de Área Foliar em Estevia (Stevia rebaudiana (Bert.) Bertoni). Available online: http://www.lni.unipi.it/stevia/stevia/estimativadearea.htm (accessed on 17 May 2020).
- Ramesh, K.; Ramawat, N.; Singh, V. Leaf area distribution pattern and non-destructive estimation methods of leaf area for Stevia rebaudiana (Bert.) Bertoni. Asian J. Plant Sci. 2007, 6, 1037–1043. [Google Scholar] [CrossRef]
- Achten, W.M.J.; Maes, W.H.; Reubens, B.; Mathijs, E.; Singh, V.P.; Verchot, L.; Muys, B. Biomass production and allocation in Jatropha curcas L. seedlings under different levels of drought stress. Biomass Bioenerg. 2010, 34, 667–676. [Google Scholar] [CrossRef]
- Blanco, F.F.; Folegatti, M.V. Estimation of leaf area for greenhouse cucumber by linear measurements under salinity and grafting. Sci. Agric. 2005, 62, 305–309. [Google Scholar] [CrossRef]
- Schmildt, E.R.; Amaral, J.T.; Santos, J.S.; Schmildt, O. Allometric model for estimating leaf area in clonal varieties of coffee (Coffea canephora). Rev. Cien Agronon. 2015, 46, 740–748. [Google Scholar] [CrossRef]
- Al-Barzinji, I.M.; Amin, B.M. Non-destructive method of leaf area estimation for oleander (Nerium oleander L.) cultivated in the Iraqi Kurdistan region. Sci. J. Koya Univ. 2016, IV, 22–26. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hadi, A.S. Regression Analysis by Example; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Kandiannan, K.; Kailassam, C.; Chandaragiri, K.K.; Sankaran, N. Allometric model for leaf area estimation in black pepper (Piper nigrum L.). J. Agron. Crop Sci. 2002, 188, 138–140. [Google Scholar] [CrossRef]
- Kandiannan, K.; Parthasarathy, U.; Krishnamurthy, K.S.; Thankamani, C.K.; Srinivasan, V. Modeling individual leaf area of ginger (Zingiber officinale Roscoe) using leaf length and width. Sci. Hortic. 2009, 120, 532–537. [Google Scholar] [CrossRef]
- Sousa, E.F.; Araújo, M.C.; Posse, R.P.; Detmann, E.; Bernardo, S.; Berbert, P.A.; Santos, P.A. Estimating the total leaf area of the green dwarf coconut tree (Cocos nucifera L.). Sci. Agric. 2005, 62, 597–600. [Google Scholar] [CrossRef]
- Toebe, M.; Souza, R.R.; Mello, A.C.; Melo, P.J.; Segatto, A.; Castanha, A.C. Leaf area estimation of squash ‘Brasileirinha’ by leaf dimensions. Ciência Rural 2019, 49, e20180932. [Google Scholar] [CrossRef]
- Peksen, E. Non-destructive leaf area estimation model for faba bean (Vicia faba L.). Sci. Hortic. 2007, 113, 322–328. [Google Scholar] [CrossRef]
- Keramatlou, I.; Sharifani, M.; Sabouri, H.; Alizadeh, M.; Kamkar, B. A simple linear model for leaf area estimation in Persian walnut (Juglans regia L.). Sci. Hortic. 2015, 184, 36–39. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.; Li, F.; Jin, G. Non-destructively predicting leaf area, leaf mass and specific leaf area based on a linear mixed-effect model for broadleaf species. Ecol. Indic. 2017, 78, 340–350. [Google Scholar] [CrossRef]
- Dolph, G.E. The effect of different calculational techniques on the estimation of leaf area and the construction of leaf size distributions. Torrey Bot. Soc. 1977, 104, 264–269. [Google Scholar] [CrossRef]
- Villar, R.; Ruiz-Robleto, J.; Ubera, J.L.; Poorter, H. Exploring variation in leaf mass per area (LMA) from leaf to cell: An anatomical analysis of 26 woody species. Am. J. Bot. 2013, 100, 1969–1980. [Google Scholar] [CrossRef]
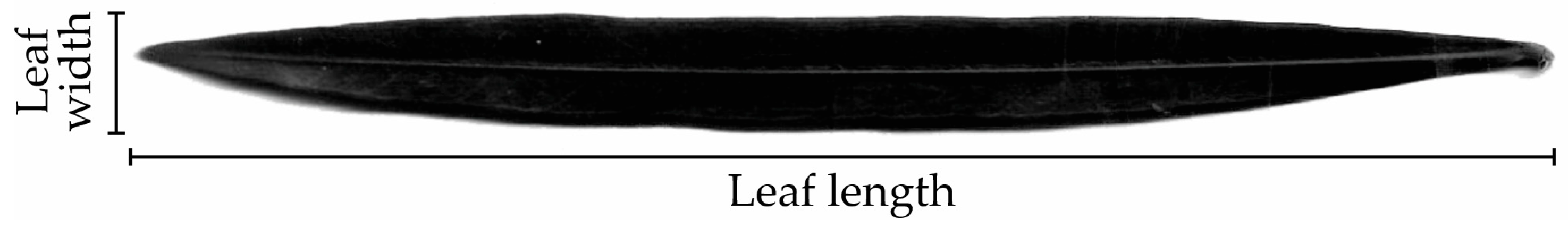
| Samples | Leaf Length (cm) | Leaf Width (cm) | Leaf Length × Width (cm2) | Leaf Length × Width Ratio | Leaf Area (cm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ẍ | Min | Max | ẍ | Min | Max | ẍ | Min | Max | ẍ | Min | Max | ẍ | Min | Max | |
| Rainy | 9.16 ± 2.25 | 1.10 | 13.77 | 0.74 ± 0.19 | 0.06 | 1.18 | 7.09 ± 2.97 | 0.13 | 15.67 | 12.65 ± 2.26 | 5.02 | 38.99 | 5.72 ± 2.31 | 0.14 | 12.36 |
| Dry | 9.24 ± 2.26 | 1.33 | 14.08 | 0.72 ± 0.20 | 0.08 | 1.29 | 7.05 ± 3.04 | 0.13 | 16.53 | 13.16 ± 2.70 | 7.13 | 33.87 | 5.68 ± 2.34 | 0.11 | 12.89 |
| Both | 9.20 ± 2.26 | 1.10 | 14.08 | 0.73 ± 0.19 | 0.06 | 1.29 | 7.07 ± 3.01 | 0.13 | 16.53 | 12.90 ± 2.50 | 5.02 | 38.99 | 5.70 ± 2.33 | 0.11 | 12.89 |
| SV | DF | SS | MQ | Fcalc |
|---|---|---|---|---|
| Parameters | 3.000 | 913.811 | - | |
| Reduction (βs) | 1.000 | 913.802 | - | |
| Reduction (H0) | 2.000 | 0.009 | 0.005 | 0.074 |
| Residual | 1797.000 | 115.569 | 0.064 | |
| Total | 1800.000 | 1029.380 |
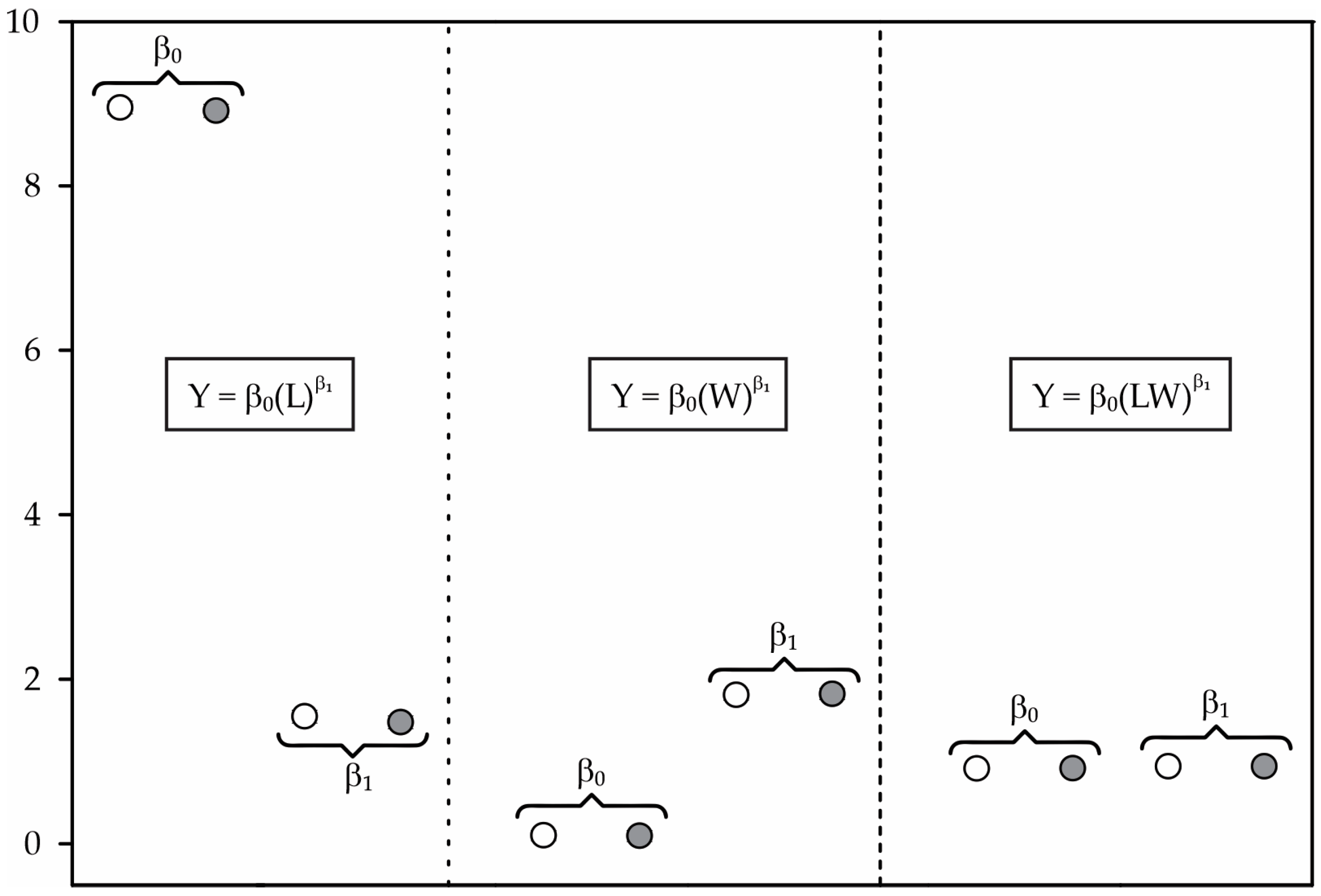
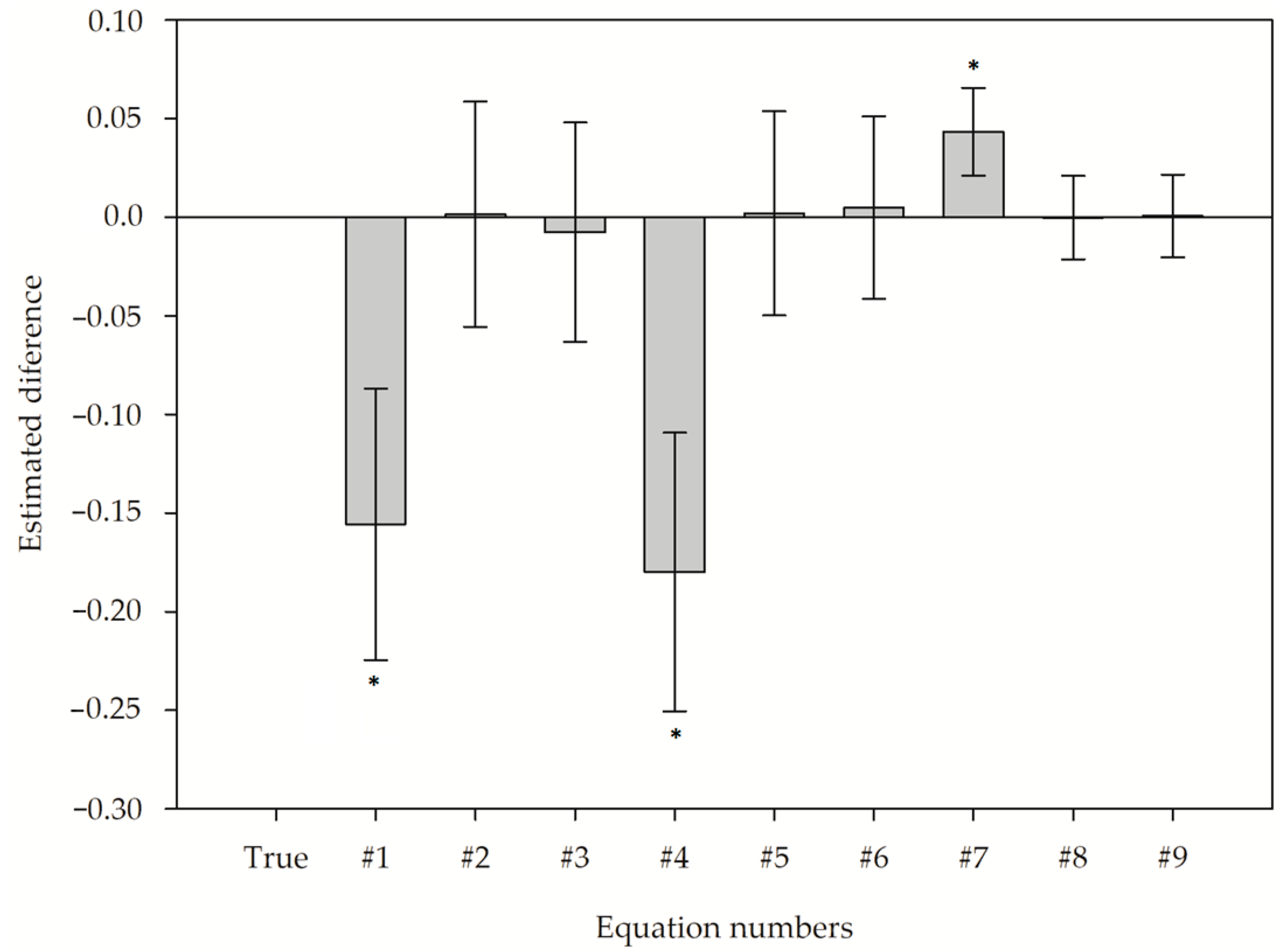
| Equation | Model | Coefficients | SE | MSres | Ra2 | Fcalc | Estimator | |
|---|---|---|---|---|---|---|---|---|
| Number | β0 | β1 | ||||||
| # 1 | --- | 8.025 | 1.086 | 1.179 | 0.968 | 51,513.588 | LA = 8.025 × (W) | |
| # 2 | 2.363 | 11.050 | 0.900 | 0.810 | 0.850 | 9396.222 | LA = −2.363 + 11.050 × (W) | |
| # 3 | 8.931 | 1.510 | 0.878 | 0.771 | 0.858 | 9960.266 | LA = 8.931 × (W)1.510 | |
| # 4 | --- | 0.639 | 1.114 | 1.241 | 0.967 | 48,870.145 | LA = 0.639 × (L) | |
| # 5 | −3.181 | 0.966 | 0.817 | 0.667 | 0.877 | 11,775.236 | LA = −3.181 + 0.966 × (L) | |
| # 6 | 0.098 | 1.812 | 0.730 | 0.533 | 0.902 | 15,145.687 | LA = 0.098 × (L)1.812 | |
| # 7 | --- | 0.800 | 0.352 | 0.124 | 0.996 | 505,248.150 | LA = 0.800 × (LW) | |
| # 8 | 0.284 | 0.766 | 0.334 | 0.111 | 0.997 | 78,688.275 | LA = 0.284 + 0.766 × (LW) | |
| # 9 | 0.914 | 0.939 | 0.331 | 0.109 | 0.998 | 80,236.492 | LA = 0.914 × (LW)0.939 | |
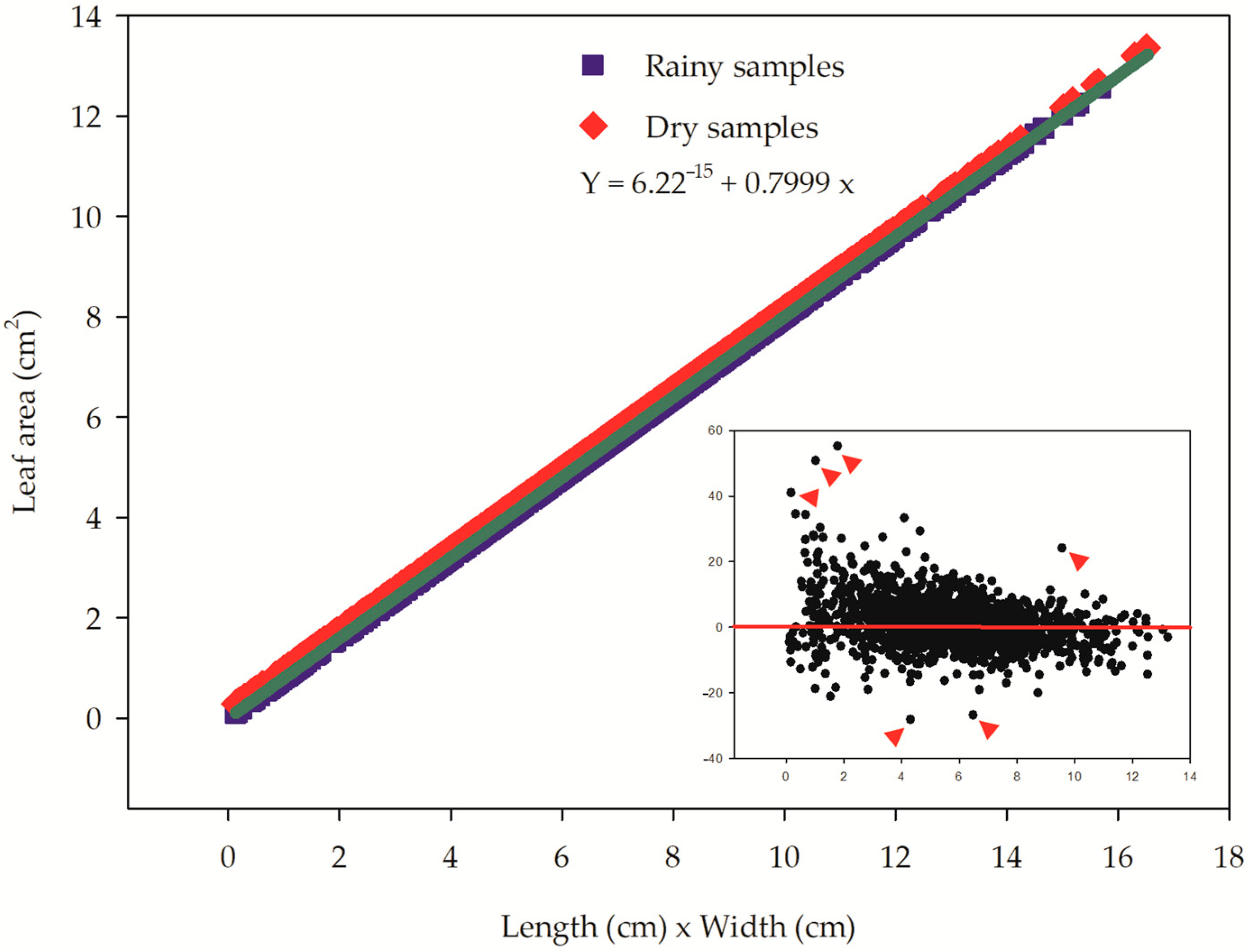
| Model | Equation | RMSE | AIC |
|---|---|---|---|
| Y = β0 + β1 × LW + εi | Ŷ = 0.284 + 0.766 × (LW) | 0.429 | 2764.085 |
| Y = β0 × LWβ1 + εi | Ŷ = 0.914 × (LW)0.939 | 0.425 | 2764.742 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Paez, L.A.; Jarma-Orozco, A.; Jaraba-Navas, J.d.D.; Pineda-Rodriguez, Y.Y.; Pompelli, M.F. Leaf Area Estimation of Yellow Oleander Thevetia peruviana (Pers.) K. Schum Using a Non-Destructive Allometric Model. Forests 2024, 15, 57. https://doi.org/10.3390/f15010057
Rodriguez-Paez LA, Jarma-Orozco A, Jaraba-Navas JdD, Pineda-Rodriguez YY, Pompelli MF. Leaf Area Estimation of Yellow Oleander Thevetia peruviana (Pers.) K. Schum Using a Non-Destructive Allometric Model. Forests. 2024; 15(1):57. https://doi.org/10.3390/f15010057
Chicago/Turabian StyleRodriguez-Paez, Luis Alfonso, Alfredo Jarma-Orozco, Juan de Dios Jaraba-Navas, Yirlis Yadeth Pineda-Rodriguez, and Marcelo F. Pompelli. 2024. "Leaf Area Estimation of Yellow Oleander Thevetia peruviana (Pers.) K. Schum Using a Non-Destructive Allometric Model" Forests 15, no. 1: 57. https://doi.org/10.3390/f15010057







