Antifungal and Plant-Growth Promotion Effects of Bacillus velezensis When Applied to Coastal to Pine (Pinus thunbergii Parl.) Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biocontrol Fusariym Oxysporum and Alternaria Alternata
2.1.1. Preparation of Bacterial Strain and Cell Growth Pattern
2.1.2. Quantitative Analysis of Lytic Enzyme Production from the Bacterial Strain
2.1.3. Preparation of Crude Enzyme Fraction from Bacterial Strain
2.1.4. Preparation of Phytopathogenic Fungi from Coastal Pine Seedlings
2.1.5. The Effect of the Bacterial Strain on the Inhibition Spore Germination and Mycelium Growth
2.2. Growth Improvement in Coastal Pine Seedlings under Different Treatments
2.2.1. Analysis of Indole-Acetic Acid (IAA) Produced by the B. velezensis CE 100
2.2.2. Greenhouse Experiment Conditions
2.2.3. Nutrient Content in Coastal Pine Seedlings
2.2.4. Antifungal and Seedling Growth Promotion Effect of the Bacterial Strain
2.3. Statistical Analysis
3. Results
3.1. Antagonistic Activity of B. velezensis CE 100
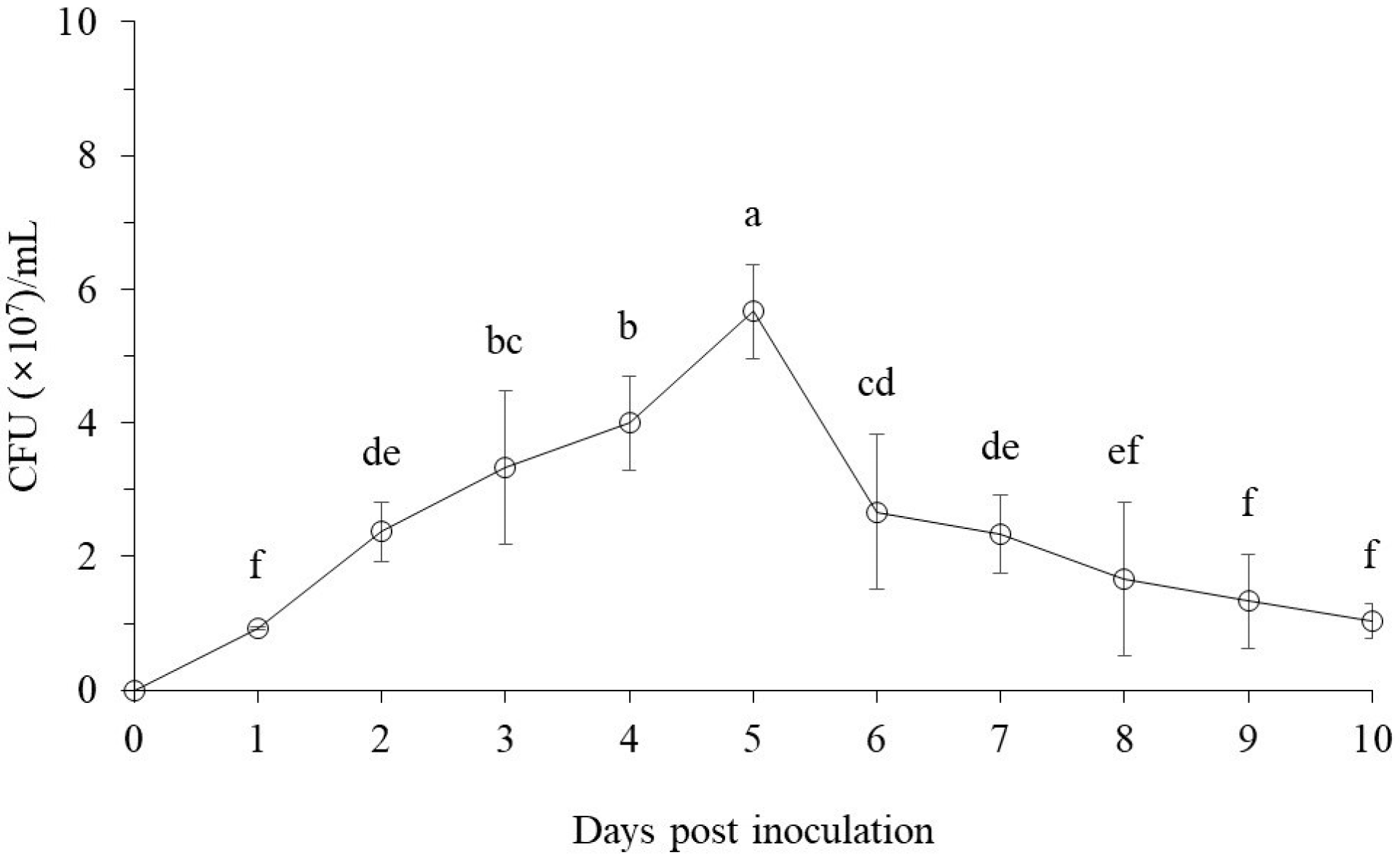
3.1.1. The Bacterial Cell Growth Pattern
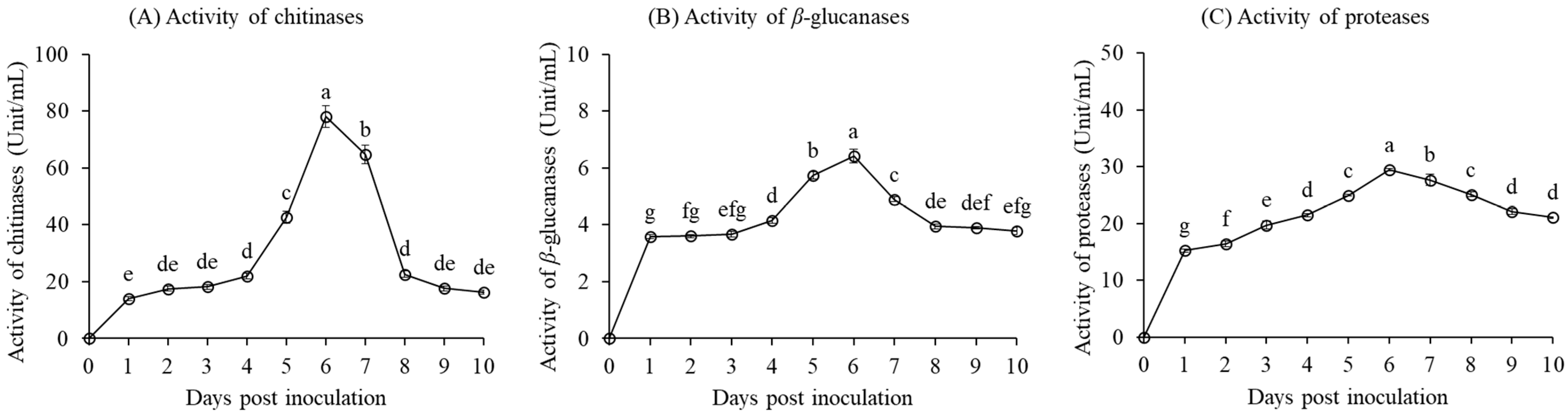
3.1.2. Lytic Enzyme Activity of the Bacterial Strain
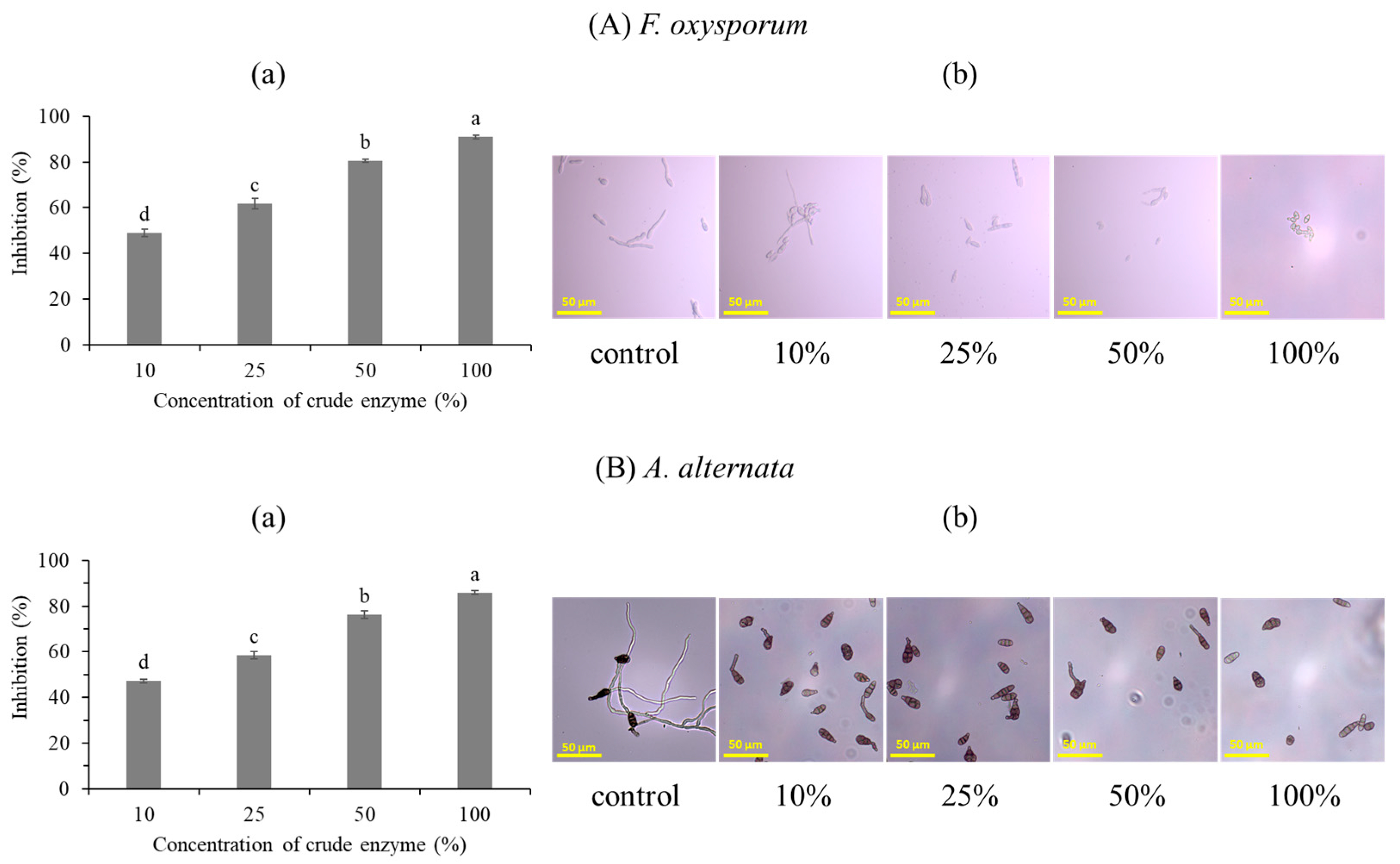
3.1.3. Antifungal Activity of the Bacterial Strain against F. oxysporum and A. alternata
3.1.4. The Survival Rate of Coastal Pine Seedling in Greenhouse
3.2. Effect of Bacterial Treatment on Coastal Pine Seedling Growth
3.2.1. The Production of Indole-3-Acetic Acid
3.2.2. Effect of Treatment on the Nutrient Content in Coastal Pine Seedlings
3.2.3. Effect Treatments on Coastal Pine Seedling Growth and Biomass Production
4. Discussion
4.1. Antifungal Effect of the Bacterial Strain against Phytopathogenic Fungi
4.2. Seedling Growth Promoting Effect of the Bacterial Strain
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lopes, A.I. Fighting drift sands with pine trees: Reforestation of coastal areas of NW Portugal at the end of eighteenth century. J. Coast. Conserv. 2023, 27, 42. [Google Scholar] [CrossRef]
- Zhu, J.; Gonda, Y.; Yu, L.; Li, F.; Yan, Q.; Sun, Y. Regeneration of a coastal pine (Pinus thunbergii Parl.) forest 11 years after thinning, Niigata, Japan. PLoS ONE 2012, 7, e47593. [Google Scholar] [CrossRef]
- Fujihara, M.; Ohnishi, M.; Miura, H.; Sawada, Y. Conservation and Management of the Coastal Pine Forest as a Cultural Landscape. In Landscape Ecology in Asian Cultures. Ecological Research Monographs; Hong, S., Kim, J.E., Wu, J., Nakagoshi, N., Eds.; Springer: Tokyo, Japan, 2011; pp. 235–248. [Google Scholar] [CrossRef]
- Zhu, J.; Matsuzaki, T.; Gonda, Y. Wind profiles in a coastal forest of Japanese black pine (Pinus thunbergii Parl.) with different thinning intensities. J. For. Res. 2001, 6, 287–296. [Google Scholar] [CrossRef]
- Sarmati, S.; Bonari, G.; Angiolini, C. Conservation status of Mediterranean coastal dune habitats: Anthropogenic disturbance may hamper habitat assignment. Rend. Lincei Sci. Fis. 2019, 30, 623–636. [Google Scholar] [CrossRef]
- Drius, M.; Carranza, M.L.; Stanisci, A.; Jones, L. The role of Italian coastal dunes as carbon sinks and diversity sources. A multi-service perspective. Appl. Geogr. 2016, 75, 127–136. [Google Scholar] [CrossRef]
- Rodrigues-Honda, K.C.D.S.; Junkes, C.F.D.O.; Lima, J.C.D.; Waldow, V.D.A.; Rocha, F.S.; Sausen, T.L.; Bayer, C.; Talamini, E.; Fett-Neto, A.G. Carbon sequestration in resin-tapped slash pine (Pinus elliottii Engelm.) subtropical plantations. Biology 2023, 12, 324. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Kim, T.; Ham, S.; Choi, S.; Park, C.R. Importance of urban green at reduction of particulate matters in Sihwa Industrial Complex, Korea. Sustainability 2020, 12, 7647. [Google Scholar] [CrossRef]
- Shin, H.S.; Lee, S.C.; Choi, S.H.; Kang, H.M. Ecological characteristic and vegetation structure of Pinus thunbergii community in coastal forest of Busan metropolitan city, Korea. Korean J. Environ. Ecol. 2019, 33, 539–551. [Google Scholar] [CrossRef]
- Hocking, D.; Setliff, E.; Jaffer, A. Potential pathogenicities of fungi associated with damped-off pine seedlings in East African pine nurseries. Trans. Br. Mycol. Soc. 1968, 51, 227–232. [Google Scholar] [CrossRef]
- Zhang, M.J.; Zheng, X.R.; Li, H.; Chen, F.M. Alternaria alternata, the causal agent of a new needle blight disease on Pinus bungeana. J. Fungi 2023, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yu, C. First report of damping-off disease caused by Fusarium oxysporum in Pinus massoniana in China. J. Plant Dis Prot. 2020, 127, 401–409. [Google Scholar] [CrossRef]
- Dar, G.H.; Beig, M.A.; Ahanger, F.A.; Ganai, N.A.; Ahangar, M.A. Management of root rot caused by Rhizoctonia solani and Fusarium oxysporum in blue pine (Pinus wallichiana) through use of fungal antagonists. Asian J. Plant Pathol. 2011, 5, 62–67. [Google Scholar] [CrossRef]
- Stewart, J.E.; Kim, M.S.; James, R.L.; Dumroese, R.K.; Klopfenstein, N.B. Molecular characterization of Fusarium oxysporum and Fusarium commune isolates from a conifer nursery. Phytopathology 2006, 96, 1124–1133. [Google Scholar] [CrossRef]
- Hrunyk, N.; Gout, R.; Kovaleva, V. Regulation of gene expression for defensins and lipid transfer protein in Scots pine seedlings by necrotrophic pathogen Alternaria alternata (Fr.). Folia For. Pol. A. 2017, 59, 152–158. [Google Scholar] [CrossRef]
- Yanfei, H.; Hancheng, W.; Qingyuan, C.; Jin, W.; Changqing, Z.; Hongxue, L. Inhibitory activities of six fungicides against mycelial growth and conidial germination of Alternaria alternata. Chin. J. Pestic. Sci. 2016, 18, 263–267. [Google Scholar] [CrossRef]
- Avenot, H.; Morgan, D.; Michailides, T. Resistance to pyraclostrobin, boscalid and multiple resistance to Pristine® (pyraclostrobin+boscalid) fungicide in Alternaria alternata causing Alternaria late blight of pistachios in California. Plant Pathol. 2008, 57, 135–140. [Google Scholar] [CrossRef]
- He, M.H.; Wang, Y.P.; Wu, E.J.; Shen, L.L.; Yang, L.N.; Wang, T.; Shang, L.P.; Zhu, W.; Zhan, J. Constraining evolution of Alternaria alternata resistance to a demethylation inhibitor (DMI) fungicide difenoconazole. Front. Microbiol. 2019, 10, 1609. [Google Scholar] [CrossRef]
- Rajput, A.Q.; Arain, M.H.; Pathan, M.A.; Jiskani, M.M.; Lodhi, A.M. Efficacy of different fungicides against Fusarium wilt of cotton caused by Fusarium oxysporum f. sp. vasinfectum. Pak. J. Bot. 2006, 38, 875. [Google Scholar]
- Bozdogan, A.M. Assessment of total risk on non-target organisms in fungicide application for agricultural sustainability. Sustainability 2014, 6, 1046–1058. [Google Scholar] [CrossRef]
- Pirozzi, A.V.A.; Stellavato, A.; La Gatta, A.; Lamberti, M.; Schiraldi, C. Mancozeb, a fungicide routinely used in agriculture, worsens nonalcoholic fatty liver disease in the human HepG2 cell model. Toxicol. Lett. 2016, 249, 1–4. [Google Scholar] [CrossRef]
- Sharma, M.; Maheshwari, N.; Khan, F.H.; Mahmood, R. Carbendazim toxicity in different cell lines and mammalian tissues. J. Biochem. Mol. Toxicol. 2022, 36, e23194. [Google Scholar] [CrossRef]
- Yang, L.N.; He, M.H.; Ouyang, H.B.; Zhu, W.; Pan, Z.C.; Sui, Q.J.; Shang, L.P.; Zhan, J. Cross-resistance of the pathogenic fungus Alternaria alternata to fungicides with different modes of action. BMC Microbiol. 2019, 19, 205. [Google Scholar] [CrossRef]
- Chung, W.H.; Chung, W.C.; Ting, P.F.; Ru, C.C.; Huang, H.C.; Huang, J.W. Nature of resistance to methyl benzimidazole carbamate fungicides in Fusarium oxysporum f. sp. lilii and F. oxysporum f. sp. gladioli in Taiwan. J. Phytopathol. 2009, 157, 742–747. [Google Scholar] [CrossRef]
- González-Oviedo, N.A.; Iglesias-Andreu, L.G.; Flores-de la Rosa, F.R.; Rivera-Fernández, A.; Luna-Rodríguez, M. Genetic analysis of the fungicide resistance in Fusarium oxysporum associated to Vanilla planifolia. Rev. Mex. fitopatol. 2022, 40, 330–348. [Google Scholar] [CrossRef]
- Choub, V.; Ajuna, H.B.; Won, S.J.; Moon, J.H.; Choi, S.I.; Maung, C.E.H.; Kim, C.W.; Ahn, Y.S. Antifungal activity of Bacillus velezensis CE 100 against anthracnose disease (Colletotrichum gloeosporioides) and growth promotion of walnut (Juglans regia L.) trees. Int. J. Mol. Sci. 2021, 22, 10438. [Google Scholar] [CrossRef]
- Hong, S.; Kim, T.Y.; Won, S.-J.; Moon, J.-H.; Ajuna, H.B.; Kim, K.Y.; Ahn, Y.S. Control of fungal diseases and fruit yield improvement of strawberry using Bacillus velezensis CE 100. Microorganisms 2022, 10, 365. [Google Scholar] [CrossRef]
- Won, S.-J.; Moon, J.-H.; Ajuna, H.B.; Choi, S.-I.; Maung, C.E.H.; Lee, S.; Ahn, Y.S. Biological control of leaf blight disease caused by Pestalotiopsis maculans and growth promotion of Quercus acutissima Carruth container seedlings using Bacillus velezensis CE 100. Int. J. Mol. Sci. 2021, 22, 11296. [Google Scholar] [CrossRef]
- Ahmed, M.; El-Fiki, I. Effect of biological control of root rot diseases of strawberry using Trichoderma spp. Middle East J. Appl. Sci. 2017, 7, 482–492. [Google Scholar]
- Jochum, C.; Osborne, L.; Yuen, G. Fusarium head blight biological control with Lysobacter enzymogenes strain C3. Biol. Control 2006, 39, 336–344. [Google Scholar] [CrossRef]
- Yu, C.; Luo, X. Trichoderma koningiopsis controls Fusarium oxysporum causing damping-off in Pinus massoniana seedlings by regulating active oxygen metabolism, osmotic potential, and the rhizosphere microbiome. Biol. Control 2020, 150, 104352. [Google Scholar] [CrossRef]
- Balderas-Ruíz, K.A.; Bustos, P.; Santamaria, R.I.; González, V.; Cristiano-Fajardo, S.A.; Barrera-Ortíz, S.; Mezo-Villalobos, M.; Aranda-Ocampo, S.; Guevara-García, Á.A.; Galindo, E.; et al. Bacillus velezensis 83 a bacterial strain from mango phyllosphere, useful for biological control and plant growth promotion. AMB Express 2020, 10, 163. [Google Scholar] [CrossRef]
- Bouchard-Rochette, M.; Machrafi, Y.; Cossus, L.; Nguyen, T.T.A.; Antoun, H.; Droit, A.; Tweddell, R.J. Bacillus pumilus PTB180 and Bacillus subtilis PTB185: Production of lipopeptides, antifungal activity, and biocontrol ability against Botrytis cinerea. Biol. Control 2022, 170, 104925. [Google Scholar] [CrossRef]
- Choi, S.H.; Ahn, J.B.; Kozukue, N.; Levin, C.E.; Friedman, M. Distribution of free amino acids, flavonoids, total phenolics, and antioxidative activities of jujube (Ziziphus jujuba) fruits and seeds harvested from plants grown in Korea. J. Agric. Food Chem. 2011, 59, 6594–6604. [Google Scholar] [CrossRef]
- Plaza, V.; Silva-Moreno, E.; Castillo, L. Breakpoint: Cell wall and glycoproteins and their crucial role in the phytopathogenic fungi infection. Curr. Protein Pept. Sci. 2020, 21, 227–244. [Google Scholar] [CrossRef]
- Oliveira-Garcia, E.; Deising, H.B. Infection structure–specific expression of β-1, 3-glucan synthase is essential for pathogenicity of Colletotrichum graminicola and evasion of β-glucan–triggered immunity in maize. Plant Cell 2013, 25, 2356–2378. [Google Scholar] [CrossRef]
- Budi, S.W.; Van Tuinen, D.; Arnould, C.; Dumas-Gaudot, E.; Gianinazzi-Pearson, V.; Gianinazzi, S. Hydrolytic enzyme activity of Paenibacillus sp. strain B2 and effects of the antagonistic bacterium on cell integrity of two soil-borne pathogenic fungi. Appl. Soil Ecol. 2000, 15, 191–199. [Google Scholar] [CrossRef]
- Enders, T.A.; Strader, L.C. Auxin activity: Past, present, and future. Am. J. Bot. 2015, 102, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Majda, M.; Robert, S. The role of auxin in cell wall expansion. Int. J. Mol. Sci. 2018, 19, 951. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-H.; Won, S.-J.; Maung, C.E.H.; Choi, J.-H.; Choi, S.-I.; Ajuna, H.B.; Ahn, Y.S. Bacillus velezensis CE 100 Inhibits root rot diseases (Phytophthora spp.) and promotes growth of Japanese cypress (Chamaecyparis obtusa Endlicher) seedlings. Microorganisms 2021, 9, 821. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liu, X.; Zhu, T.H.; Liu, G.H.; Mao, C. Isolation and characterization of phosphate-solubilizing bacteria from walnut and their effect on growth and phosphorus mobilization. Biol. Fertil. Soils 2011, 47, 437–446. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Wang, J.; Wen, X.; Zhang, X.; Li, S.; Zhang, D.Y. Co-regulation of photosynthetic capacity by nitrogen, phosphorus and magnesium in a subtropical Karst forest in China. Sci. Rep. 2018, 8, 7406. [Google Scholar] [CrossRef]
- Park, H.G.; Lee, Y.S.; Kim, K.Y.; Park, Y.S.; Park, K.H.; Han, H.O.; Park, C.M.; Ahn, Y.S. Inoculation with Bacillus licheniformis MH48 promotes nutrient uptake in seedlings of the ornamental plant Camellia japonica grown in Korean reclaimed coastal lands. Hortic. Sci. Technol. 2017, 35, 11–20. [Google Scholar] [CrossRef]
- Kuan, K.B.; Othman, R.; Abdul Rahim, K.; Shamsuddin, Z.H. Plant growth-promoting rhizobacteria inoculation to enhance vegetative growth, nitrogen fixation and nitrogen remobilisation of maize under greenhouse conditions. PLoS ONE 2016, 11, e0152478. [Google Scholar] [CrossRef]
- Davis, A.S.; Jacobs, D.F. Quantifying root system quality of nursery seedlings and relationship to outplanting performance. New For. 2005, 30, 295–311. [Google Scholar] [CrossRef]
- Choi, T.G.; Maung, C.E.H.; Lee, D.R.; Henry, A.B.; Lee, Y.S.; Kim, K.Y. Role of bacterial antagonists of fungal pathogens, Bacillus thuringiensis KYC and Bacillus velezensis CE 100 in control of root-knot nematode, Meloidogyne incognita and subsequent growth promotion of tomato. Biocontrol. Sci. Technol. 2020, 30, 685–700. [Google Scholar] [CrossRef]
- Ajuna, H.B.; Kim, I.; Han, Y.S.; Maung, C.E.H.; Kim, K.Y. Aphicidal activity of Bacillus thuringiensis strain AH-2 against cotton aphid (Aphis gossypii). Entomol. Res. 2021, 51, 151–160. [Google Scholar] [CrossRef]
- Lingappa, Y. Chitin media for selective isolation and culture of actinomycetes. Phytopathology 1962, 52, 317–323. [Google Scholar]
- Liang, Z.C.; Hseu, R.S.; Wang, H.H. Partial purification and characterization of a 1, 3-β-D-glucanase from Ganoderma tsugae. J. Ind. Microbiol. Biotechnol. 1995, 14, 5–9. [Google Scholar] [CrossRef]
- Ghorbel-Frikha, B.; Sellami-Kamoun, A.; Fakhfakh, N.; Haddar, A.; Manni, L.; Nasri, M. Production and purification of a calcium-dependent protease from Bacillus cereus BG1. J. Ind. Microbiol. Biotechnol. 2005, 32, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-H.; Won, S.-J.; Maung, C.E.H.; Choi, J.-H.; Choi, S.-I.; Ajuna, H.B.; Ahn, Y.S.; Jo, Y.H. The Role of Lysobacter antibioticus HS124 on the control of fall webworm (Hyphantria cunea Drury) and growth promotion of Canadian poplar (Populus canadensis Moench) at Saemangeum reclaimed land in Korea. Microorganisms 2021, 9, 1580. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Sitepu, I.R.; Tang, S.Y.; Hashidoko, Y. Salkowski’s reagent test as a primary screening index for functionalities of rhizobacteria isolated from wild dipterocarp saplings growing naturally on medium-strongly acidic tropical peat soil. Biosci. Biotechnol. Biochem. 2010, 74, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Widyastuti, S.M.; Tasik, S.; Harjono, H. Infection process of Fusarium oxysporum fungus: A cause of damping-off on Acacia mangium seedlings. Agrivita, J. Agric. Sci. 2013, 35, 110–118. [Google Scholar] [CrossRef]
- Hong, C.; Moorman, G. Plant pathogens in irrigation water: Challenges and opportunities. CRC Crit. Rev. Plant. Sci. 2005, 24, 189–208. [Google Scholar] [CrossRef]
- Bashan, Y.; Levanony, H.; Or, R. Wind dispersal of Alternaria alternata, a cause of leaf blight of cotton. J. Phytopathol. 1991, 133, 225–238. [Google Scholar] [CrossRef]
- Rice, K.C.; Bayles, K.W. Molecular control of bacterial death and lysis. Microbiol. Mol. Biol. 2008, 72, 85–109. [Google Scholar] [CrossRef]
- Gow, N.A.; Latge, J.P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Beauvais, A.; Latgé, J.-P. Fungal cell wall. J. Fungi 2018, 4, 91. [Google Scholar] [CrossRef]
- Geoghegan, I.; Steinberg, G.; Gurr, S. The role of the fungal cell wall in the infection of plants. Trends Microbiol. 2017, 25, 957–967. [Google Scholar] [CrossRef]
- Latgé, J.P. The cell wall: A carbohydrate armour for the fungal cell. Mol. Microbiol. 2007, 66, 279–290. [Google Scholar] [CrossRef]
- Paramanandham, P.; Rajkumari, J.; Pattnaik, S.; Busi, S. Biocontrol potential against Fusarium oxysporum f. sp. lycopersici and Alternaria solani and tomato plant growth due to plant growth–promoting rhizobacteria. Int. J. Veg. Sci. 2017, 23, 294–303. [Google Scholar] [CrossRef]
- Haruta, M.; Gray, W.M.; Sussman, M.R. Regulation of the plasma membrane proton pump (H+-ATPase) by phosphorylation. Curr. Opin. Plant Biol. 2015, 28, 68–75. [Google Scholar] [CrossRef]
- Mir, A.R.; Siddiqui, H.; Alam, P.; Hayat, S. Foliar spray of Auxin/IAA modulates photosynthesis, elemental composition, ROS localization and antioxidant machinery to promote growth of Brassica juncea. Physiol. Mol. Biol. Plants. 2020, 26, 2503–2520. [Google Scholar] [CrossRef]



| Treatment | Survival Rate (%) | ||||||
|---|---|---|---|---|---|---|---|
| March | April | May | June | July | August | September | |
| Control | 100.0 ± 0.0 | 85.0 ± 5.6 * | 78.3 ± 5.8 * | 66.7 ± 7.6 * | 61.7 ± 7.6 * | 56.7 ± 3.5 * | 52.8 ± 1.8 * |
| Bacterial inoculation | 100.0 ± 0.0 | 100.0 ± 0.0 * | 100.0 ± 0.0 * | 100.0 ± 0.0 * | 100.0 ± 0.0 * | 100.0 ± 0.0 * | 100.0 ± 0.0 * |
| Treatment | Concentration (%) | Content (mg) | ||
|---|---|---|---|---|
| Total N | Total P | Total N | Total P | |
| Control | 0.54 ± 0.01 * | 0.13 ± 0.01 * | 0.65 ± 0.01 * | 0.15 ± 0.01 * |
| Bacterial inoculation | 1.43 ± 0.04 * | 0.15 ± 0.01 * | 43.55 ± 1.11 * | 4.28 ± 0.07 * |
| Treatment | Stem Girth (mm) | Length (cm) | Dry Weight (cm) | ||
|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | ||
| Control | 1.35 ± 0.49 * | 8.81 ± 2.54 * | 13.28 ± 7.56 * | 0.26 ± 0.21 * | 0.20 ± 0.15 * |
| Bacterial inoculation | 5.99 ± 1.06 * | 27.71 ± 5.23 * | 18.07 ± 4.55 * | 8.21 ± 2.91 * | 2.21 ± 0.60 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, J.-Y.; Kim, H.-S.; Moon, J.-H.; Won, S.-J.; Choub, V.; Choi, S.-I.; Ajuna, H.B.; Lee, P.S.-H.; Ahn, Y.S. Antifungal and Plant-Growth Promotion Effects of Bacillus velezensis When Applied to Coastal to Pine (Pinus thunbergii Parl.) Seedlings. Forests 2024, 15, 62. https://doi.org/10.3390/f15010062
Yun J-Y, Kim H-S, Moon J-H, Won S-J, Choub V, Choi S-I, Ajuna HB, Lee PS-H, Ahn YS. Antifungal and Plant-Growth Promotion Effects of Bacillus velezensis When Applied to Coastal to Pine (Pinus thunbergii Parl.) Seedlings. Forests. 2024; 15(1):62. https://doi.org/10.3390/f15010062
Chicago/Turabian StyleYun, Ju-Yeol, Hyun-Seop Kim, Jae-Hyun Moon, Sang-Jae Won, Vantha Choub, Su-In Choi, Henry B. Ajuna, Peter Sang-Hoon Lee, and Young Sang Ahn. 2024. "Antifungal and Plant-Growth Promotion Effects of Bacillus velezensis When Applied to Coastal to Pine (Pinus thunbergii Parl.) Seedlings" Forests 15, no. 1: 62. https://doi.org/10.3390/f15010062






