Insights from Roots to Stems: Comparative Wood Anatomy and Dendroclimatic Investigation of Two Salix Species in Iceland
Abstract
:1. Introduction
2. Materials and Methods
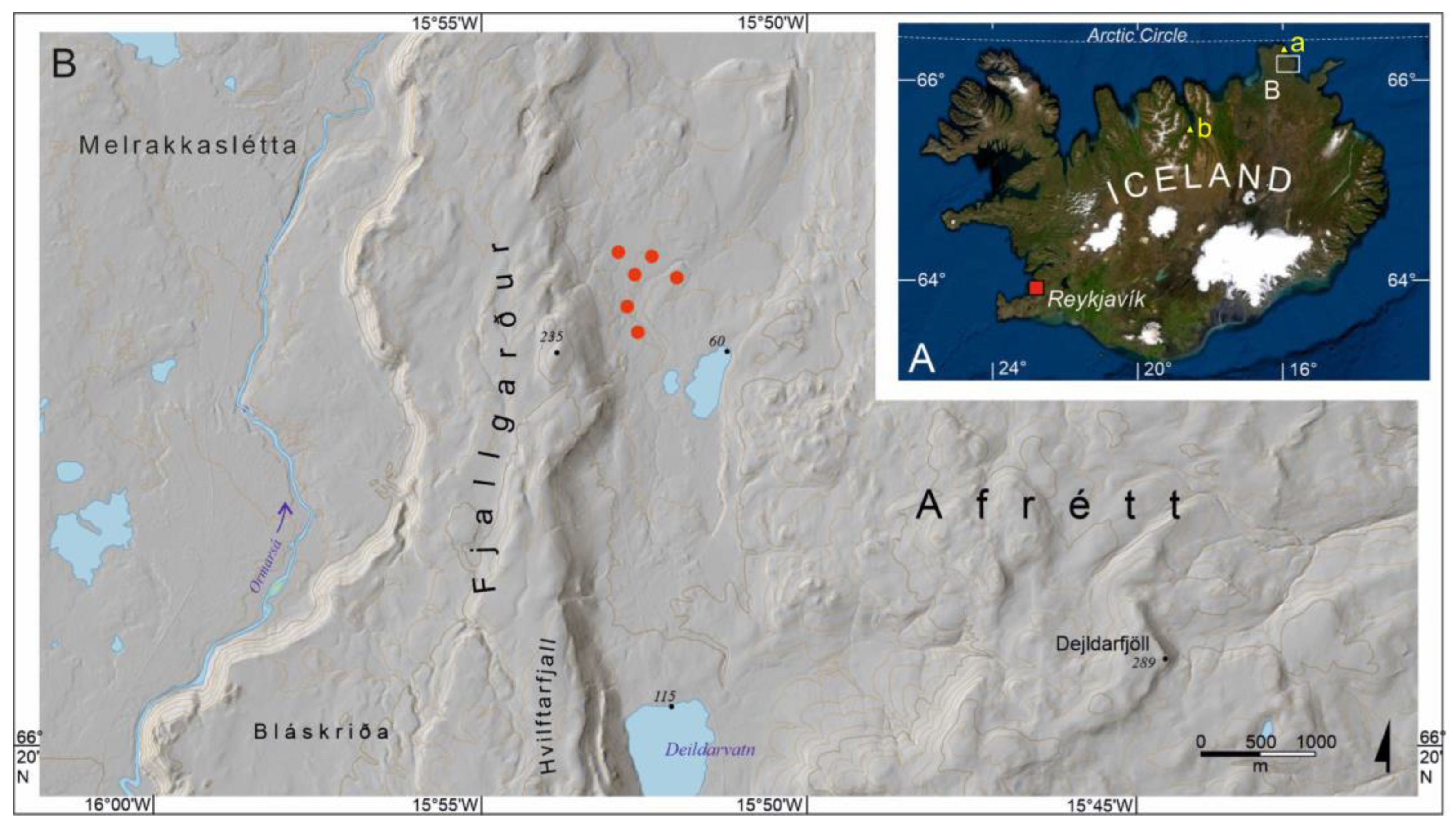
2.1. Study Area
2.2. Study Species
2.3. Sampling Method
2.4. Laboratory Method/Sample Preparation
2.5. Microscopic Analysis
2.6. Quantitative Wood Anatomy
2.7. Dendrochronological and Dendroclimatological Analyses
3. Results
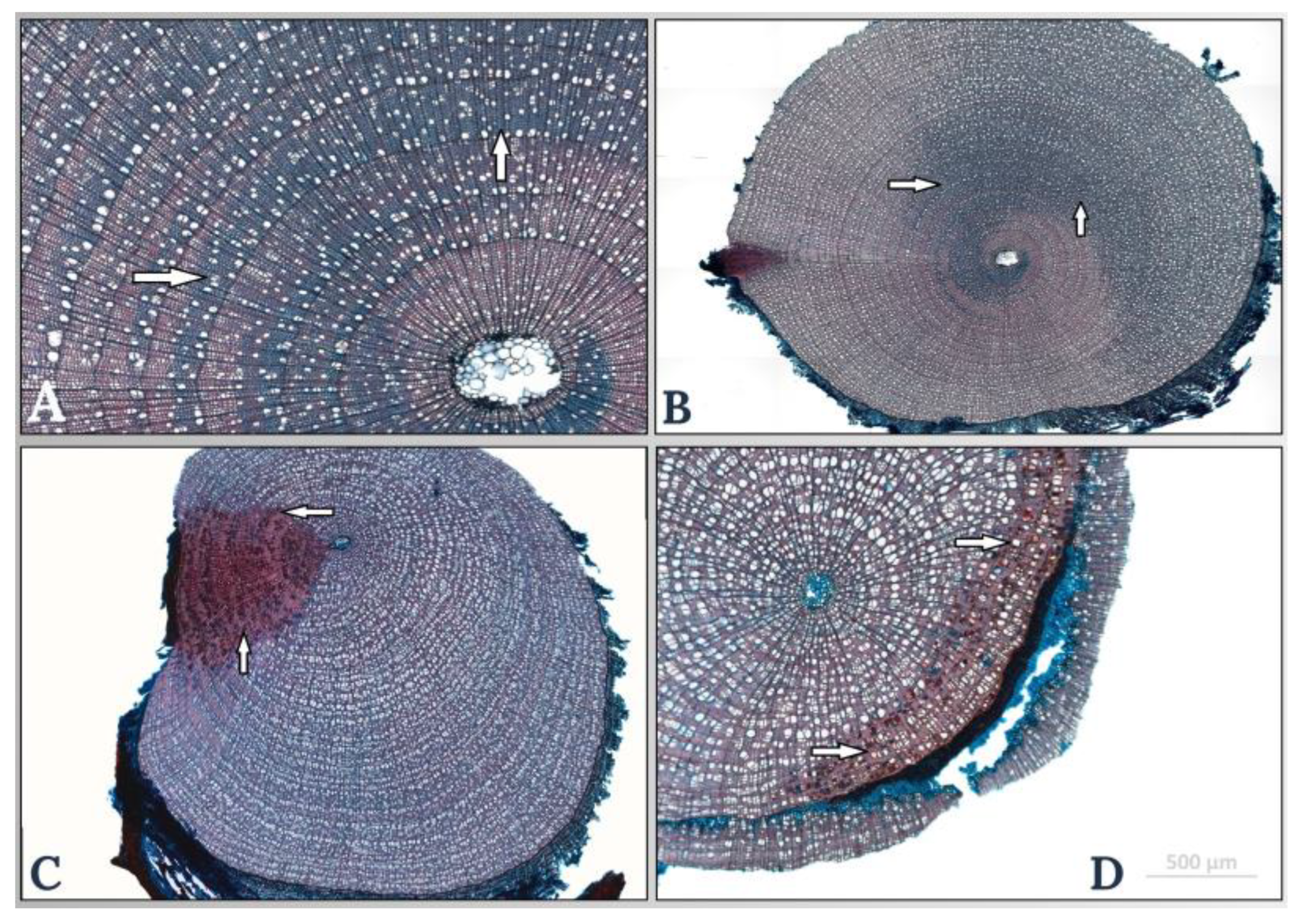
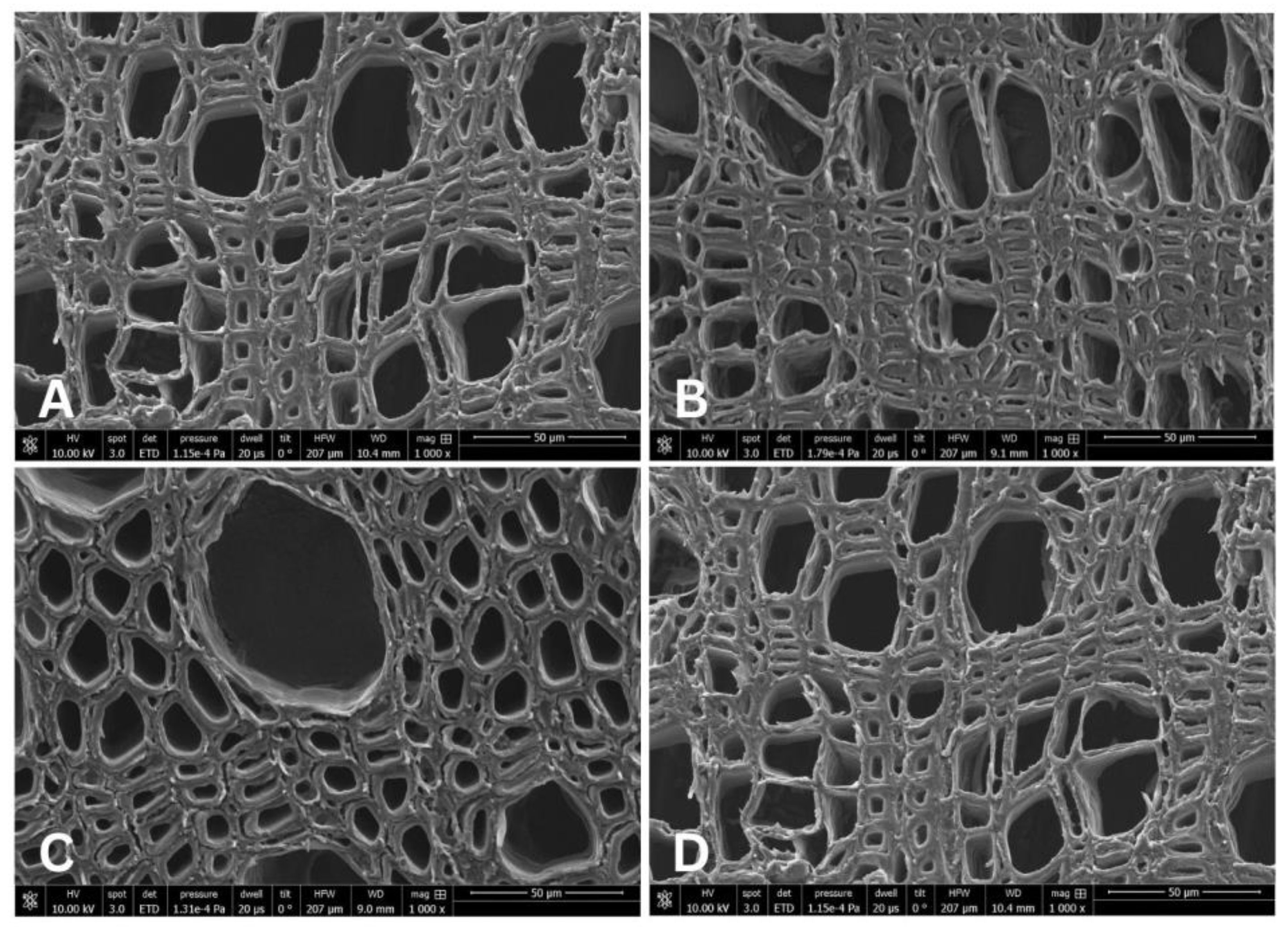
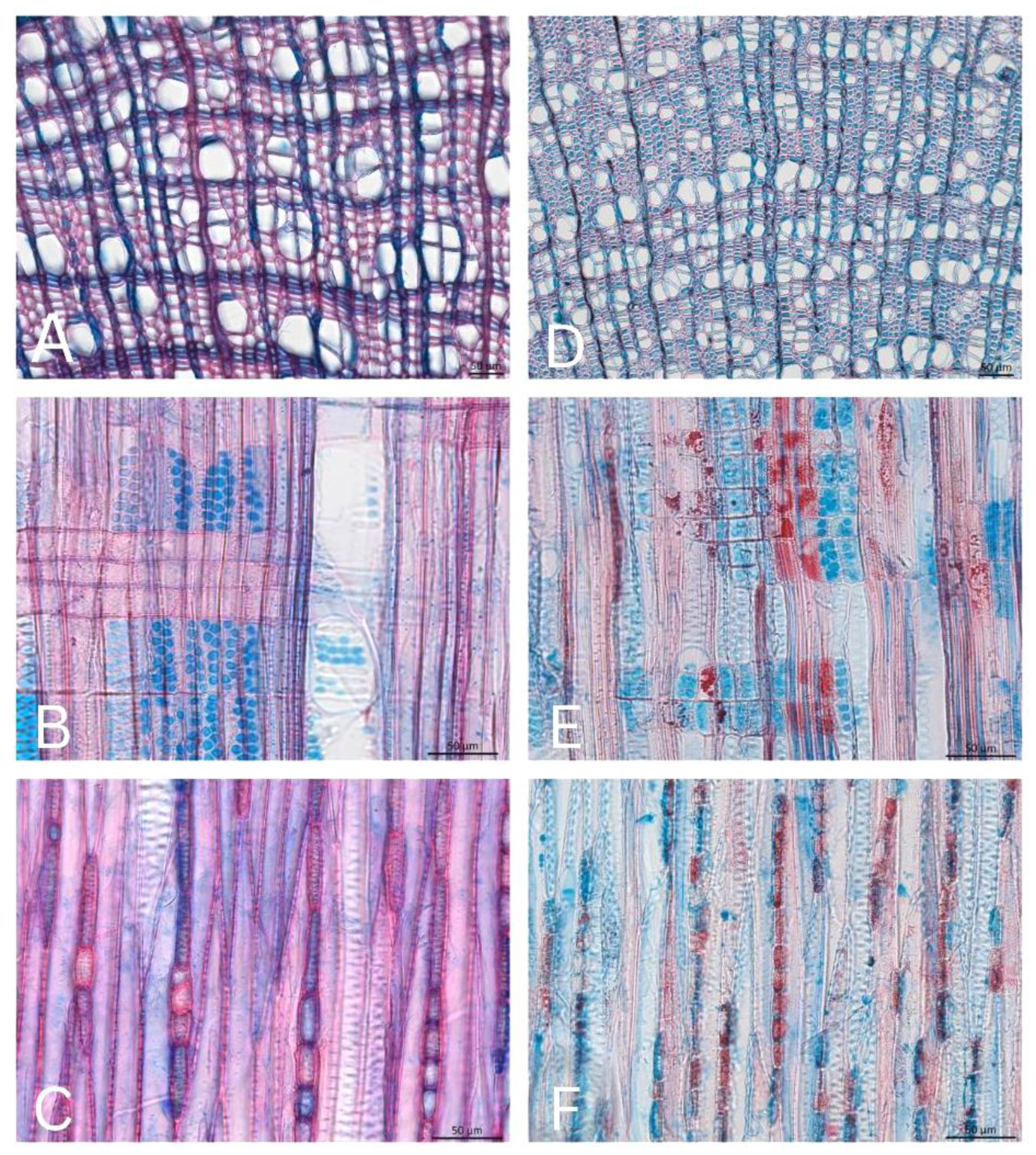
3.1. Anatomical Characteristics in the Stem and Root of Salix herbacea
3.2. Anatomical Characteristics of Salix arctica
3.3. Statistical Variabilities in the Anatomical Parameters
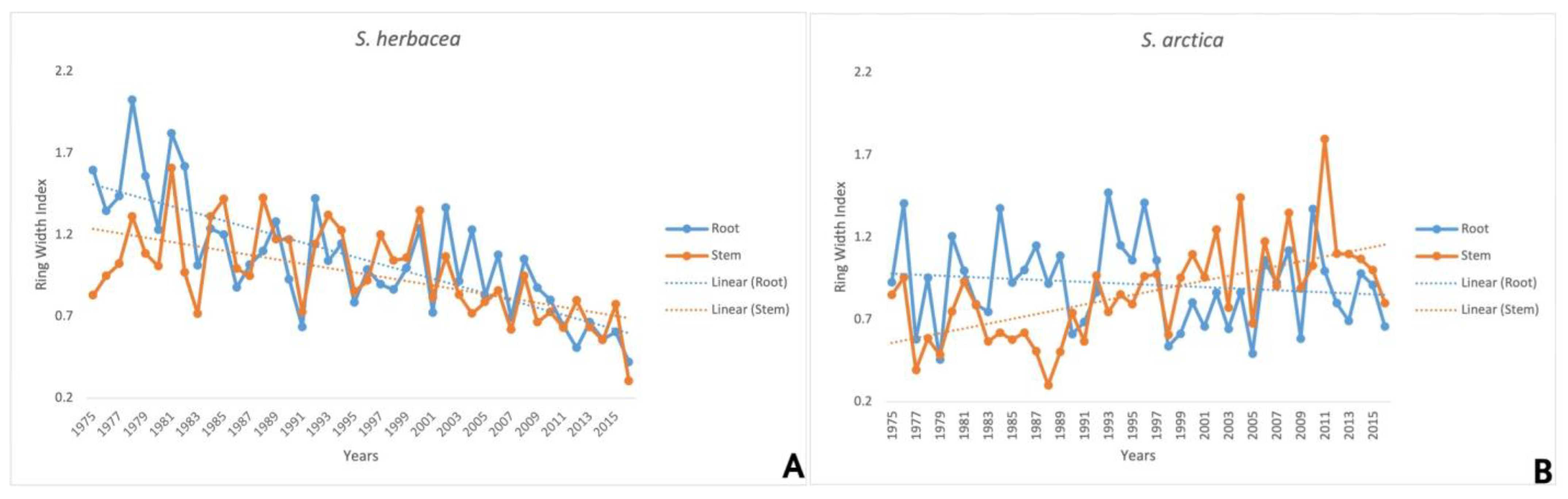
3.4. Stem and Root Growth Ring Chronologies
3.5. Climate Sensitivity of Root and Stem Parts
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elmendorf, S.C.; Henry, G.H.R.; Hollister, R.D.; Bjork, R.G.; Bjorkman, A.D.; Callaghan, T.V.; Collier, L.S.; Cooper, E.J.; Cornelissen, J.H.C.; Day, T.A.; et al. Global assessment of experimental climate warming on tundra vegetation: Heterogeneity over space and time. Ecol. Lett. 2012, 15, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Sturm, M.; Racine, C.; Tape, K. Climate change—Increasing shrub abundance in the Arctic. Nature 2001, 411, 546–547. [Google Scholar] [CrossRef] [PubMed]
- Buchwal, A.; Sullivan, P.F.; Macias-Fauria, M.; Post, E.; Myers-Smith, I.H.; Stroeve, J.C.; Blok, D.; Tape, K.D.; Forbes, B.C.; Ropars, P.; et al. Divergence of Arctic shrub growth associated with sea ice decline. Proc. Natl. Acad. Sci. USA 2020, 117, 33334–33344. [Google Scholar] [CrossRef]
- Myers-Smith, I.H.; Elmendorf, S.C.; Beck, P.S.A.; Wilmking, M.; Hallinger, M.; Blok, D.; Tape, K.D.; Rayback, S.A.; Macias-Fauria, M.; Forbes, B.C.; et al. Climate sensitivity of shrub growth across the tundra biome. Nat. Clim. Chang. 2015, 5, 887–891. [Google Scholar] [CrossRef]
- Opala-Owczarek, M.; Owczarek, P.; Lupikasza, E.; Boudreau, S.; Migala, K. Influence of climatic conditions on growth rings ofSalix uva-ursiPursh from the southeastern shore of Hudson Bay, Subarctic Canada. Arct. Antarct. Alp. Res. 2020, 52, 87–102. [Google Scholar] [CrossRef]
- Owczarek, P.; Latocha, A.; Wistuba, M.; Malik, I. Reconstruction of modern debris flow activity in the arctic environment with the use of dwarf shrubs (south-western Spitsbergen)—A new dendrochronological approach. Z. Geomorphol. 2013, 57, 75–95. [Google Scholar] [CrossRef]
- Elmendorf, S.C.; Henry, G.H.R.; Hollister, R.D.; Bjork, R.G.; Boulanger-Lapointe, N.; Cooper, E.J.; Cornelissen, J.H.C.; Day, T.A.; Dorrepaal, E.; Elumeeva, T.G.; et al. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat. Clim. Chang. 2012, 2, 453–457. [Google Scholar] [CrossRef]
- García Criado, M.; Myers-Smith, I.H.; Bjorkman, A.D.; Lehmann, C.E.; Stevens, N. Woody plant encroachment intensifies under climate change across tundra and savanna biomes. Glob. Ecol. Biogeogr. 2020, 29, 925–943. [Google Scholar] [CrossRef]
- Myers-Smith, I.H.; Forbes, B.C.; Wilmking, M.; Hallinger, M.; Lantz, T.; Blok, D.; Tape, K.D.; Macias-Fauria, M.; Sass-Klaassen, U.; Lévesque, E. Shrub expansion in tundra ecosystems: Dynamics, impacts and research priorities. Environ. Res. Lett. 2011, 6, 045509. [Google Scholar] [CrossRef]
- Myers-Smith, I.H.; Grabowski, M.M.; Thomas, H.J.D.; Angers-Blondin, S.; Daskalova, G.N.; Bjorkman, A.D.; Cunliffe, A.M.; Assmann, J.J.; Boyle, J.S.; McLeod, E.; et al. Eighteen years of ecological monitoring reveals multiple lines of evidence for tundra vegetation change. Ecol. Monogr. 2019, 89, e01351. [Google Scholar] [CrossRef]
- Tape, K.; Sturm, M.; Racine, C. The evidence for shrub expansion in Northern Alaska and the Pan-Arctic. Glob. Chang. Biol. 2006, 12, 686–702. [Google Scholar] [CrossRef]
- Myers-Smith, I.H.; Kerby, J.T.; Phoenix, G.K.; Bjerke, J.W.; Epstein, H.E.; Assmann, J.J.; John, C.; Andreu-Hayles, L.; Angers-Blondin, S.; Beck, P.S.A.; et al. Complexity revealed in the greening of the Arctic. Nat. Clim. Chang. 2020, 10, 106–117. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Epstein, H.E.; Box, E.O.; Euskirchen, E.S.; Goswami, S.; Iversen, C.M.; Kattge, J.; Norby, R.J.; van Bodegom, P.M.; Xu, X. Plant functional types in Earth system models: Past experiences and future directions for application of dynamic vegetation models in high-latitude ecosystems. Ann. Bot. 2014, 114, 1–16. [Google Scholar] [CrossRef]
- Holbrook, N.M.; Zwieniecki, M.A. Embolism repair. Comp. Biochem. Phys. A 2005, 141, S302. [Google Scholar]
- Kim, H.K.; Park, J.; Hwang, I. Investigating water transport through the xylem network in vascular plants. J. Exp. Bot. 2014, 65, 1895–1904. [Google Scholar] [CrossRef]
- Ropars, P.; Angers-Blondin, S.; Gagnon, M.; Myers-Smith, I.H.; Levesque, E.; Boudreau, S. Different parts, different stories: Climate sensitivity of growth is stronger in root collars vs. stems in tundra shrubs. Glob. Chang. Biol. 2017, 23, 3281–3291. [Google Scholar] [CrossRef]
- Iversen, C.M.; Sloan, V.L.; Sullivan, P.F.; Euskirchen, E.S.; McGuire, A.D.; Norby, R.J.; Walker, A.P.; Warren, J.M.; Wullschleger, S.D. The unseen iceberg: Plant roots in arctic tundra. New Phytol. 2015, 205, 34–58. [Google Scholar] [CrossRef]
- Pajunen, A.M. Environmental and Biotic Determinants of Growth and Height of Arctic Willow Shrubs along a Latitudinal Gradient. Arct. Antarct. Alp. Res. 2009, 41, 478–485. [Google Scholar] [CrossRef]
- Beeckman, H. Wood Anatomy and Trait-Based Ecology. IAWA J. 2016, 37, 127–151. [Google Scholar] [CrossRef]
- Opała-Owczarek, M.; Owczarek, P.; Phulara, M.; Bielec-Bąkowska, Z.; Wawrzyniak, Z. Dendrochronology and extreme climate signals recorded in seven Icelandic shrubs: A multi-species approach in the sub-Arctic. Dendrochronologia 2024, 85, 126207. [Google Scholar] [CrossRef]
- Owczarek, P.; Dagsson-Waldhauserova, P.; Opala-Owczarek, M.; Migala, K.; Arnalds, O.; Schaetzl, R.J. Anatomical changes in dwarf shrub roots provide insight into aeolian erosion rates in northeastern Iceland. Geoderma 2022, 428, 116173. [Google Scholar] [CrossRef]
- Phulara, M.; Opala-Owczarek, M.; Owczarek, P. Climatic Signals on Growth Ring Variation in Salix herbacea: Comparing Two Contrasting Sites in Iceland. Atmosphere 2022, 13, 718. [Google Scholar] [CrossRef]
- Arnalds, O. Soils of Iceland; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–183. [Google Scholar] [CrossRef]
- Chen, W.; Tape, K.D.; Euskirchen, E.S.; Liang, S.; Matos, A.; Greenberg, J.; Fraterrigo, J.M. Impacts of Arctic Shrubs on Root Traits and Belowground Nutrient Cycles across a Northern Alaskan Climate Gradient. Front. Plant Sci. 2020, 11, 588098. [Google Scholar] [CrossRef] [PubMed]
- Savile, D.B.O. Ring Counts in Salix-Arctica from Northern Ellesmere Island. Can. Field Nat. 1979, 93, 81–82. [Google Scholar] [CrossRef]
- Wilson, J.W. Annual growth of Salix arctica in the High-Arctic. Ann. Bot. 1964, 28, 71–76. [Google Scholar] [CrossRef]
- Woodcock, H.; Bradley, R.S. Salix arctica (Pall.): Its potential for dendroclimatological studies in the High Arctic. Dendrochronologia 1994, 12, 11–22. [Google Scholar]
- Schmidt, N.M.; Baittinger, C.; Forchhammer, M.C. Reconstructing century-long snow regimes using estimates of high arctic Salix arctica radial growth. Arct. Antarct. Alp. Res. 2006, 38, 257–262. [Google Scholar] [CrossRef]
- Schmidt, N.M.; Baittinger, C.; Kollmann, J.; Forchhammer, M.C. Consistent dendrochronological response of the dioecious Salix arctica to variation in local snow precipitation across gender and vegetation types. Arct. Antarct. Alp. Res. 2010, 42, 471–475. [Google Scholar] [CrossRef]
- Wilson, J.W. Vegetation patterns associated with soil movement on Jan Mayen Island. J. Ecol. 1952, 249–264. [Google Scholar] [CrossRef]
- Bjorkman, A.D.; Myers-Smith, I.H.; Elmendorf, S.C.; Normand, S.; Ruger, N.; Beck, P.S.A.; Blach-Overgaard, A.; Blok, D.; Cornelissen, J.H.C.; Forbes, B.C.; et al. Plant functional trait change across a warming tundra biome. Nature 2018, 562, 57–62. [Google Scholar] [CrossRef]
- Arnalds, O. The Icelandic ‘rofabard’soil erosion features. Earth Surf. Process. Landf. J. Br. Geomorphol. Res. Group. 2000, 25, 17–28. [Google Scholar] [CrossRef]
- Arnalds, O.; Thorarinsdottir, E.F.; Metusalemsson, S.; Jonsson, A.; Arnason, A. Soil Erosion in Iceland; Agricultural Research Institute, Soil Conservation Service: Washington, DC, USA, 2001. [Google Scholar]
- Zachar, D. Soil Erosion; Elsevier: New York, NY, USA, 1982. [Google Scholar]
- Arnalds, O.; Dagsson-Waldhauserova, P.; Olafsson, H. The Icelandic volcanic aeolian environment: Processes and impacts—A review. Aeolian Res. 2016, 20, 176–195. [Google Scholar] [CrossRef]
- Arnalds, O.; Olafsson, H.; Dagsson-Waldhauserova, P. Quantification of iron-rich volcanogenic dust emissions and deposition over the ocean from Icelandic dust sources. Biogeosciences 2014, 11, 6623–6632. [Google Scholar] [CrossRef]
- Bullard, J.E.; Austin, M.J. Dust generation on a proglacial floodplain, West Greenland. Aeolian Res. 2011, 3, 43–54. [Google Scholar] [CrossRef]
- Butwin, M.K.; von Löwis, S.; Pfeffer, M.A.; Thorsteinsson, T. The effects of volcanic eruptions on the frequency of particulate matter suspension events in Iceland. J. Aerosol Sci. 2019, 128, 99–113. [Google Scholar] [CrossRef]
- Dagsson-Waldhauserova, P.; Arnalds, O.; Olafsson, H. Long-term frequency and characteristics of dust storm events in Northeast Iceland (1949–2011). Atmos. Environ. 2013, 77, 117–127. [Google Scholar] [CrossRef]
- Dagsson-Waldhauserova, P.; Arnalds, O.; Olafsson, H. Long-term variability of dust events in Iceland (1949–2011). Atmos. Chem. Phys. 2014, 14, 13411–13422. [Google Scholar] [CrossRef]
- Dagsson-Waldhauserova, P.; Renard, J.B.; Olafsson, H.; Vignelles, D.; Berthet, G.; Verdier, N.; Duverger, V. Vertical distribution of aerosols in dust storms during the Arctic winter. Sci. Rep. 2019, 9, 16122. [Google Scholar] [CrossRef]
- Gärtner, H.; Schweingruber, F.H. Microscopic Preparation Techniques for Plant Stem Analysis; Kessel: Lenting, Germany, 2013. [Google Scholar]
- Schweingruber, F.H.; Poschlod, P.; la Neige et le Paysage (Birmensdorf) Institut Fédéral de Recherches sur la Forêt. Growth Rings in Herbs and Shrubs: Life Span, Age Determination and Stem Anatomy; Swiss Federal Research Institute WSL: Birmensdorf, Switzerland, 2005; Volume 79. [Google Scholar]
- Myers-Smith, I.H.; Hallinger, M.; Blok, D.; Sass-Klaassen, U.; Rayback, S.A.; Weijers, S.; Trant, A.J.; Tape, K.D.; Naito, A.T.; Wipf, S.; et al. Methods for measuring arctic and alpine shrub growth: A review. Earth-Sci. Rev. 2015, 140, 1–13. [Google Scholar] [CrossRef]
- Bär, A.; Bräuning, A.; Löffler, J. Dendroecology of dwarf shrubs in the high mountains of Norway—A methodological approach. Dendrochronologia 2006, 24, 17–27. [Google Scholar] [CrossRef]
- Balzano, A.; Merela, M.; Cufar, K. Scanning Electron Microscopy Protocol for Studying Anatomy of Highly Degraded Waterlogged Archaeological Wood. Forests 2022, 13, 161. [Google Scholar] [CrossRef]
- Ioraș, F.; Gurău, L.; Timar, M.C.; Porojan, M. Image Processing Method as a Supporting Tool for Wood Species Identification. Wood Fiber Sci. 2013, 45, 303–313. [Google Scholar]
- Wheeler, E.A. Vessels per square millimetre or vessel groups per square millimetre? IAWA J. 1986, 7, 73–74. [Google Scholar] [CrossRef]
- Kolishchuk, V. Dendroclimatological study of prostrate woody plants. Methods Dendrochronol. 1990, 353, 51–55. [Google Scholar]
- Holmes, R. Dendrochronology Program Library User’s Manual; Laboratory of Tree-Ring, Research University of Arizona: Tucson, AZ, USA, 1994. [Google Scholar]
- Cook, E.R.; Peters, K. The Smoothing Spline: A New Approach to Standardizing Forest Interior Tree-Ring Width Series for Dendroclimatic Studies. Tree-Ring Bull. 1981, 41, 45–53. [Google Scholar]
- Schweingruber, F.H. Dendrochronology—An extremely exact measuring tool for the study of environmental and human history. Naturwissenschaften 1996, 83, 370–377. [Google Scholar] [CrossRef]
- Boulanger-Lapointe, N.; Levesque, E.; Baittinger, C.; Schmidt, N.M. Local variability in growth and reproduction of Salix arctica in the High Arctic. Polar Res. 2016, 35, 24126. [Google Scholar] [CrossRef]
- Buchwal, A.; Rachlewicz, G.; Fonti, P.; Cherubini, P.; Gartner, H. Temperature modulates intra-plant growth of Salix polaris from a high Arctic site (Svalbard). Polar Biol. 2013, 36, 1305–1318. [Google Scholar] [CrossRef]
- Buchwal, A.; Weijers, S.; Blok, D.; Elberling, B. Temperature sensitivity of willow dwarf shrub growth from two distinct High Arctic sites. Int. J. Biometeorol. 2019, 63, 167–181. [Google Scholar] [CrossRef]
- Opala-Owczarek, M.; Piroznikow, E.; Owczarek, P.; Szymanski, W.; Luks, B.; Kepski, D.; Szymanowski, M.; Wojtun, B.; Migala, K. The influence of abiotic factors on the growth of two vascular plant species (Saxifraga oppositifolia and Salix polaris) in the High Arctic. Catena 2018, 163, 219–232. [Google Scholar] [CrossRef]
- Owczarek, P.; Opala-Owczarek, M.; Migala, K. Post-1980s shift in the sensitivity of tundra vegetation to climate revealed by the first dendrochronological record from Bear Island (Bjornoya), western Barents Sea. Environ. Res. Lett. 2021, 16, 014031. [Google Scholar] [CrossRef]
- Prevéy, J.; Vellend, M.; Rüger, N.; Hollister, R.D.; Bjorkman, A.D.; Myers-Smith, I.H.; Elmendorf, S.C.; Clark, K.; Cooper, E.J.; Elberling, B. Greater temperature sensitivity of plant phenology at colder sites: Implications for convergence across northern latitudes. Glob. Chang. Biol. 2017, 23, 2660–2671. [Google Scholar] [CrossRef] [PubMed]
- Abeli, T.; Vamosi, J.C.; Orsenigo, S. The importance of marginal population hotspots of cold-adapted species for research on climate change and conservation. J. Biogeogr. 2018, 45, 977–985. [Google Scholar] [CrossRef]
- Alsos, I.G.; Alm, T.; Normand, S.; Brochmann, C. Past and future range shifts and loss of diversity in dwarf willow (Salix herbacea L.) inferred from genetics, fossils and modelling. Glob. Ecol. Biogeogr. 2009, 18, 223–239. [Google Scholar] [CrossRef]
- Schweingruber, F.H.; Hellmann, L.; Tegel, W.; Braun, S.; Nievergelt, D.; Büntgen, U. Evaluating the wood anatomical and dendroecological potential of arctic dwarf shrub communities. IAWA J. 2013, 34, 485–497. [Google Scholar] [CrossRef]
- Friedman, J.M.; Vincent, K.R.; Shafroth, P.B. Dating floodplain sediments using tree-ring response to burial. Earth Surf. Proc. Land. 2005, 30, 1077–1091. [Google Scholar] [CrossRef]
- Marin, P.; Filion, L. Recent Dynamics of Sub-Arctic Dunes as Determined by Tree-Ring Analysis of White Spruce, Hudson-Bay, Quebec. Quat. Res. 1992, 38, 316–330. [Google Scholar] [CrossRef]
- Matisons, R.; Brūmelis, G. Effect of burial by sand on Scots pine (Pinus sylvestris L.) radial growth on seacoast wooded dunes at Cape Kolka, Latvia. Acta Univ. Latv. 2008, 745, 131–144. [Google Scholar]
- Sigafoos, R.S. Botanical Evidence of Floods and Flood-Plain Deposition; US Government Printing Office: Washington, DC, USA, 1964; Volume 485. [Google Scholar]
- Den Ouden, J.; Sass-Klaassen, U.; Copini, P. Dendrogeomorphology—A new tool to study drift-sand dynamics. Neth. J. Geosci. 2007, 86, 355. [Google Scholar] [CrossRef]
- Knowlson, H. A Long-Term Experiment on the Radial Growth of the Oak; CABI Digital Library: Wallingford, UK, 1939. [Google Scholar]
- Cournoyer, L.; Bégin, Y. Effet de l’érosion riveraine sur les structures anatomiques de Fraxinus pennsylvanica Marsh. dans le haut estuaire du Saint-Laurent, Québec, Canada. Dendrochronologia 1992, 10, 107–119. [Google Scholar]
- Fayle, D.C.F. Radial Growth in Tree Roots; University of Toronto. Faculty of Forestry: Toronto, ON, Canada, 1968. [Google Scholar]
- Hitz, O.M.; Gärtner, H.; Heinrich, I.; Monbaron, M. Wood anatomical changes in roots of European ash (Fraxinus excelsior L.) after exposure. Dendrochronologia 2008, 25, 145–152. [Google Scholar] [CrossRef]
- Gärtner, H.; Schweingruber, F.H.; Dikau, R. Determination of Erosion Rates by Analyzing Structural Changes in the Growth Pattern of Exposed Roots. Dendrochronologia 2001, 19, 81–91. [Google Scholar]
- Nakashima, M.; Dagsson-Waldhauserová, P. A 60 year examination of dust day activity and its contributing factors from ten Icelandic weather stations from 1950 to 2009. Front. Earth Sci. 2019, 6, 245. [Google Scholar] [CrossRef]
- Beryl Beakbane, A. Anatomical studies of stems and roots of hardy fruit trees iii. The anatomical structure of some clonal and seedling apple rootstocks stem-and root-grafted with a scion variety. J. Pomol. Hortic. Sci. 1941, 18, 344–367. [Google Scholar] [CrossRef]
- Campioli, M.; Leblans, N.; Michelsen, A. Stem secondary growth of tundra shrubs: Impact of environmental factors and relationships with apical growth. Arct. Antarct. Alp. Res. 2012, 44, 16–25. [Google Scholar] [CrossRef]
- Boyle, J.S.; Angers-Blondin, S.; Assmann, J.J.; Myers-Smith, I.H. Summer temperature—but not growing season length—influences radial growth of Salix arctica in coastal Arctic tundra. Polar Biol. 2022, 45, 1257–1270. [Google Scholar] [CrossRef]
- Kraus, G. Einige bemerkungen über alter und wachstum verhältnisse ostgrönländischer holzgewächse. In Die Zweite Deutsche Nordpolarfahrt in den Jahren 1869 und 1870 unter Führung des Kapitän Karl Koldewey; Zweiter Band. Wissenschaftliche Ergebnisse. f.a. Brockhaus; F.A. Brockhaus: Leipzig, Germany, 1874; pp. 133–137. [Google Scholar]

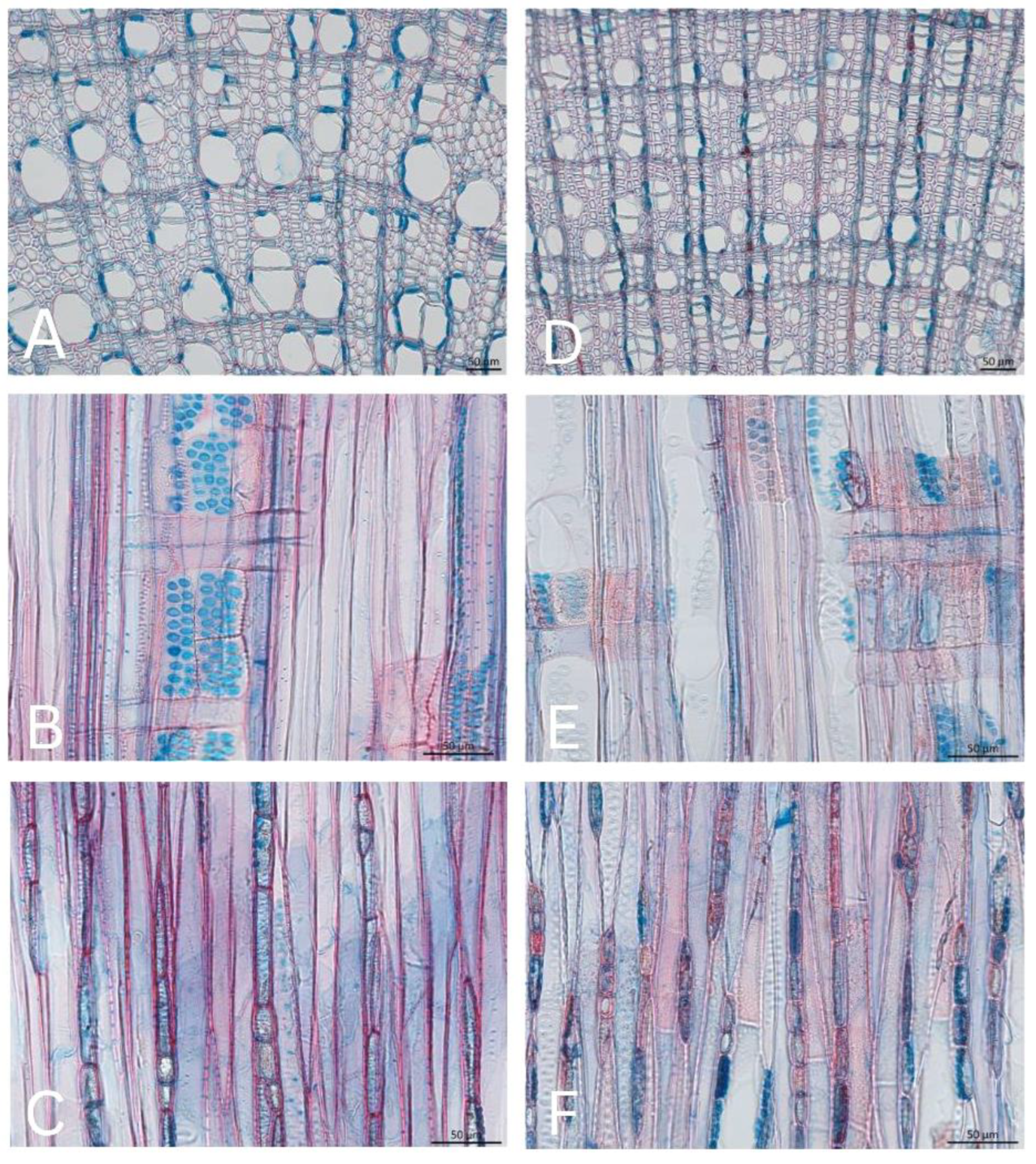
| Pant Species | No. of Plants | Stem | Root | Slides/Sections |
|---|---|---|---|---|
| S. herbacea | 3 | 05 | 10 | 15 |
| S. arctica | 3 | 19 | 06 | 25 |
| Anatomical Characters | Salix herbacea (SAHE) | Salix arctica (SAAR) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stems | Roots | Stems | Roots | |||||||||||
| No. | Features | No. | Features | No. | Features | No. | Features | |||||||
| Growth rings | 1–2 | Less distinct | 1 | Distinct | ||||||||||
| Porosity | 4 | Semi ring porous | ||||||||||||
| Vessel Arrangement | 6 | Vessels in tangential bands | ||||||||||||
| Vessel Grouping | 0 | Radial multiples of 2 to 4 with a variable proportion of solitary vessels | 10 | Radial multiples of 4 or more | ||||||||||
| Solitary vessel outline | 12 | Solitary vessel elements outline Angular | ||||||||||||
| Perforation plates | 13 | Simple perforation plates | ||||||||||||
| Inter-vessel pits: arrangement and size | 22, 23 | Inter-vessels pits alternate; Shapes of alternate pits polygonal | 23,29 | Shapes of alternate pits polygonal; Vestured inter-vessel pits | ||||||||||
| 26, 27 | Medium to large (7–10 um; >10 um) | 27,29 | Vestured pits | |||||||||||
| Vessel ray pitting | 31 | Vessel ray pits with much-reduced borders to apparently simple: pits rounded or angular | ||||||||||||
| Tangential dia. of vessel l. | 40 | 25.20 µm | 28.24 µm | 40 | 26.24 µm | 46.97 µm | ||||||||
| Vessels per square mm | 50 | 471/mm2 | 359/mm2 | 50 | 232/mm2 | 130/mm2 | ||||||||
| Tyloses and deposits | 58 | Gums and other deposits in heartwood vessels are common | 0 | No tyloses | ||||||||||
| Tracheids and fibers | 60 | Vascular tracheids | ||||||||||||
| Ground tissue fibers | 61 | Fibers with simple to minutely bordered pits | ||||||||||||
| Fiber wall thickness | 70 | Fibers very thick-walled | 69 | thin- to thick-walled | 69 | thin- to thick-walled | 68 | Fibers very thin-walled | ||||||
| Axial parenchyma absent or extremely rare | 75–76 | Axial parenchyma absent or extremely rare Axial parenchyma diffuse | 76, 78 | Axial parenchyma scanty diffuse and scanty paratracheal | ||||||||||
| Banded parenchyma | 89 | Axial parenchyma in marginal or in seemingly marginal bands | ||||||||||||
| Ray width | 96 | Rays exclusively uniseriate | ||||||||||||
| Rays: cellular compos’n | 104 | All ray cells procumbent | ||||||||||||
| Mineral Inclusions: Prismatic crystals | 136 | More frequent | Present | |||||||||||
| Druses | 144 | More frequent | Present | |||||||||||
| Geo. distribution | 164, 165, 182 | |||||||||||||
| Habit | 190 | Shrubs (Arctic) | ||||||||||||
| Family, genus, species, authority | Family-Salicaceae Genus-Salix Species-herbacea | Family-Salicaceae Genus-Salix Species-arctica | ||||||||||||
| Statistical Parameter | Tan. Diameter (SAAR Stems) | Tan. Diameter (SAHE-Stems) | Tan. Diameter (SAAR-Roots) | Tan. Diameter (SAHE-Roots) | Vessel Frequency (SAAR-Stems) | Vessel Frequency (SAHE-Stems) | Vessel Frequency (SAAR-Roots) | Vessel Frequency (SAHE-Roots) |
|---|---|---|---|---|---|---|---|---|
| Mean | 26.24 mm | 25.203 mm | 46.969 mm | 28.243 mm | 232.667 | 471.667 | 130.333 | 359.375 |
| Std. dev. | 6.729 | 1.988 | 1.926 | 5.244 | 66.521 | 111.044 | 19.242 | 63.817 |
| Std. error | 2.747 | 0.811 | 0.786 | 1.854 | 27.157 | 45.333 | 7.856 | 22.563 |
| t-statistic | 0.362 | 9.299 | −4.523 | −9.587 | ||||
| p-value | 0.730 | 0.0000051 | 0.001837 | 0.0000069 | ||||
| Species | Chronology Length (Years) | Number of Samples Collected/Included in Chronology | Mean Correlation between Samples | Mean Ring Width | Standard Deviation | Mean Sensitivity |
|---|---|---|---|---|---|---|
| SAAR stem | 1939–2016 (77) | 10/8 | 0.468 | 72.3 | 45.7 | 0.454 |
| SAAR root | 1940–2016 (76) | 12/7 | 0.552 | 98.5 | 54.0 | 0.532 |
| SAHE stem | 1969–2016 (47) | 10/9 | 0.437 | 95.3 | 11.7 | 0.335 |
| SAHE root | 1975–2016 (41) | 10/7 | 0.429 | 81.4 | 19.5 | 0.382 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phulara, M.; Balzano, A.; Opała-Owczarek, M.; Owczarek, P.; Merela, M. Insights from Roots to Stems: Comparative Wood Anatomy and Dendroclimatic Investigation of Two Salix Species in Iceland. Forests 2024, 15, 1707. https://doi.org/10.3390/f15101707
Phulara M, Balzano A, Opała-Owczarek M, Owczarek P, Merela M. Insights from Roots to Stems: Comparative Wood Anatomy and Dendroclimatic Investigation of Two Salix Species in Iceland. Forests. 2024; 15(10):1707. https://doi.org/10.3390/f15101707
Chicago/Turabian StylePhulara, Mohit, Angela Balzano, Magdalena Opała-Owczarek, Piotr Owczarek, and Maks Merela. 2024. "Insights from Roots to Stems: Comparative Wood Anatomy and Dendroclimatic Investigation of Two Salix Species in Iceland" Forests 15, no. 10: 1707. https://doi.org/10.3390/f15101707
APA StylePhulara, M., Balzano, A., Opała-Owczarek, M., Owczarek, P., & Merela, M. (2024). Insights from Roots to Stems: Comparative Wood Anatomy and Dendroclimatic Investigation of Two Salix Species in Iceland. Forests, 15(10), 1707. https://doi.org/10.3390/f15101707










