Influence of Slope Aspect and Position on Xylem Formation Dynamics in Subtropical Chinese Fir Plantations
Abstract
:1. Introduction
2. Methods
2.1. Study Area
2.2. Samples Collecting and Lab Preparation
2.3. Data Analysis
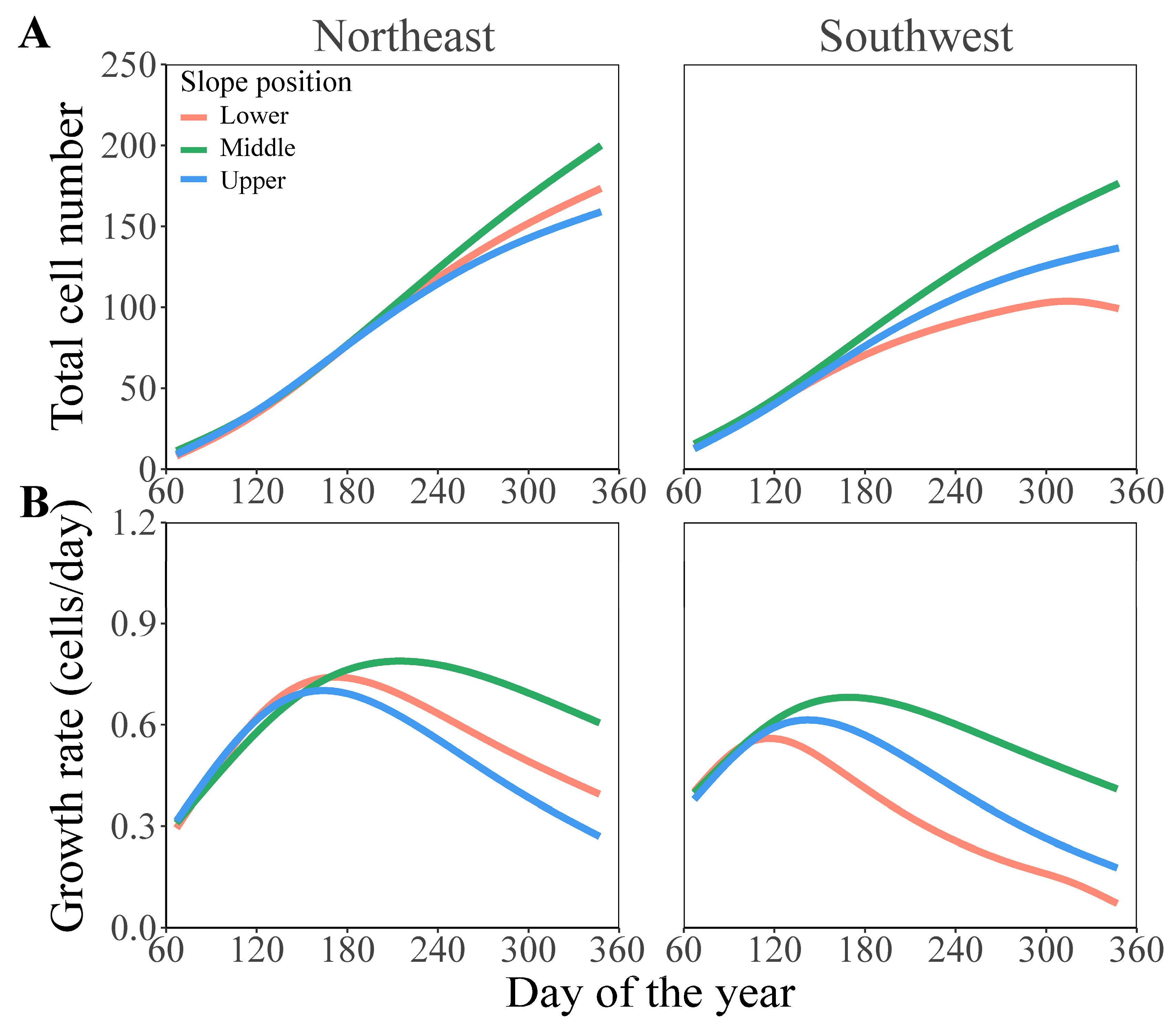
3. Results
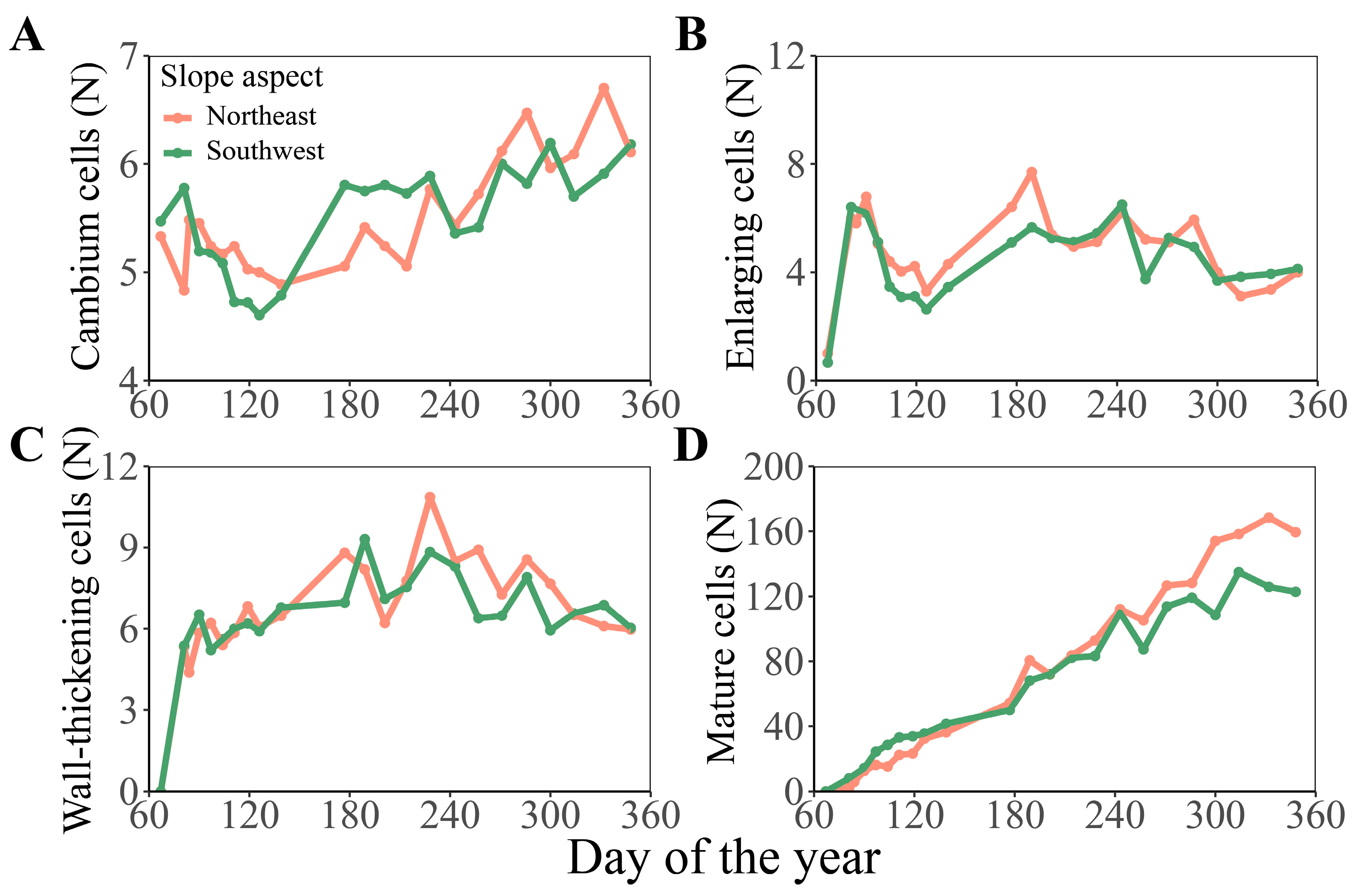
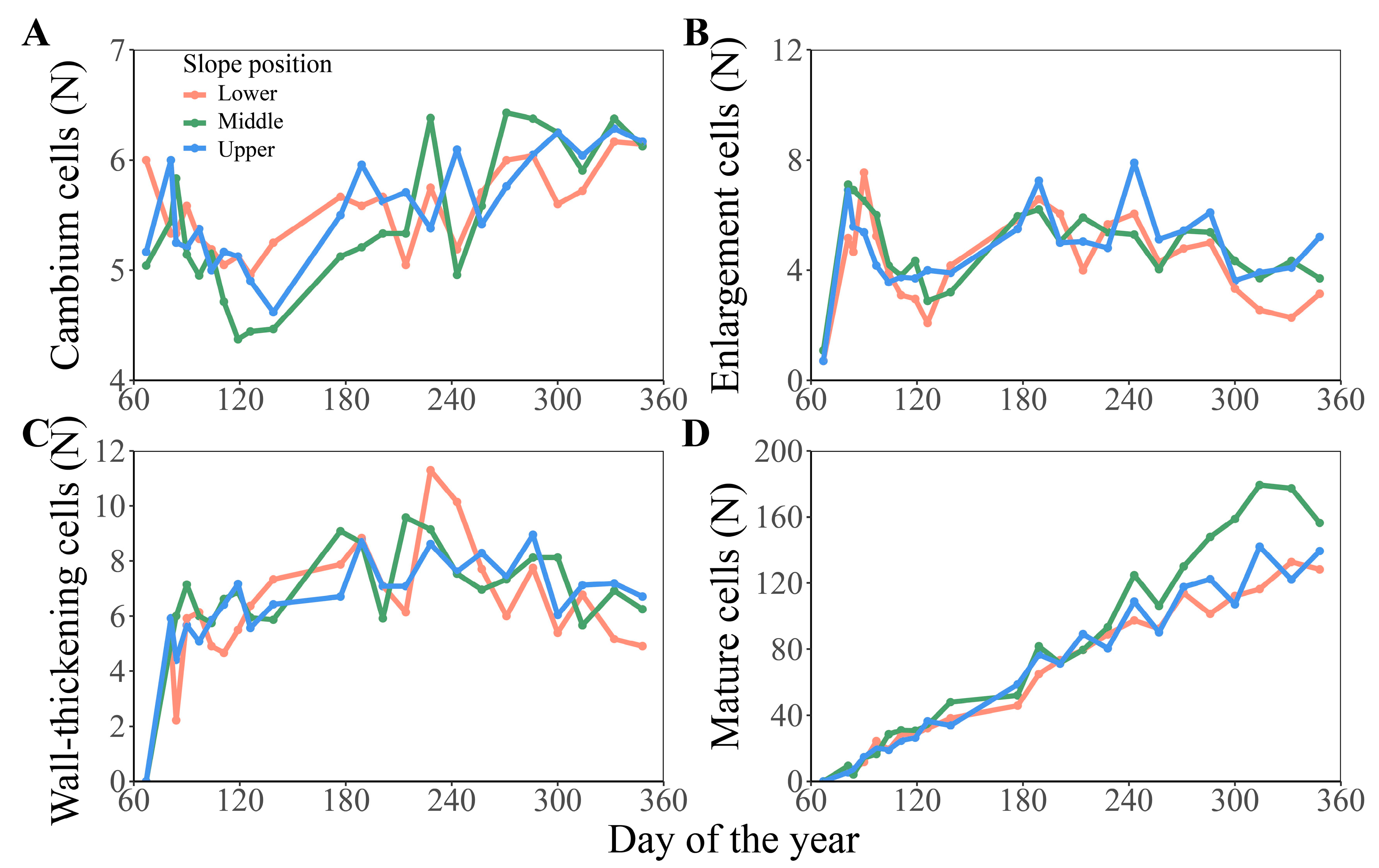
3.1. Comparison of Cambium Activity and Xylem Formation Dynamic of Chinese Fir in Different Slope Aspects and Slope Positions
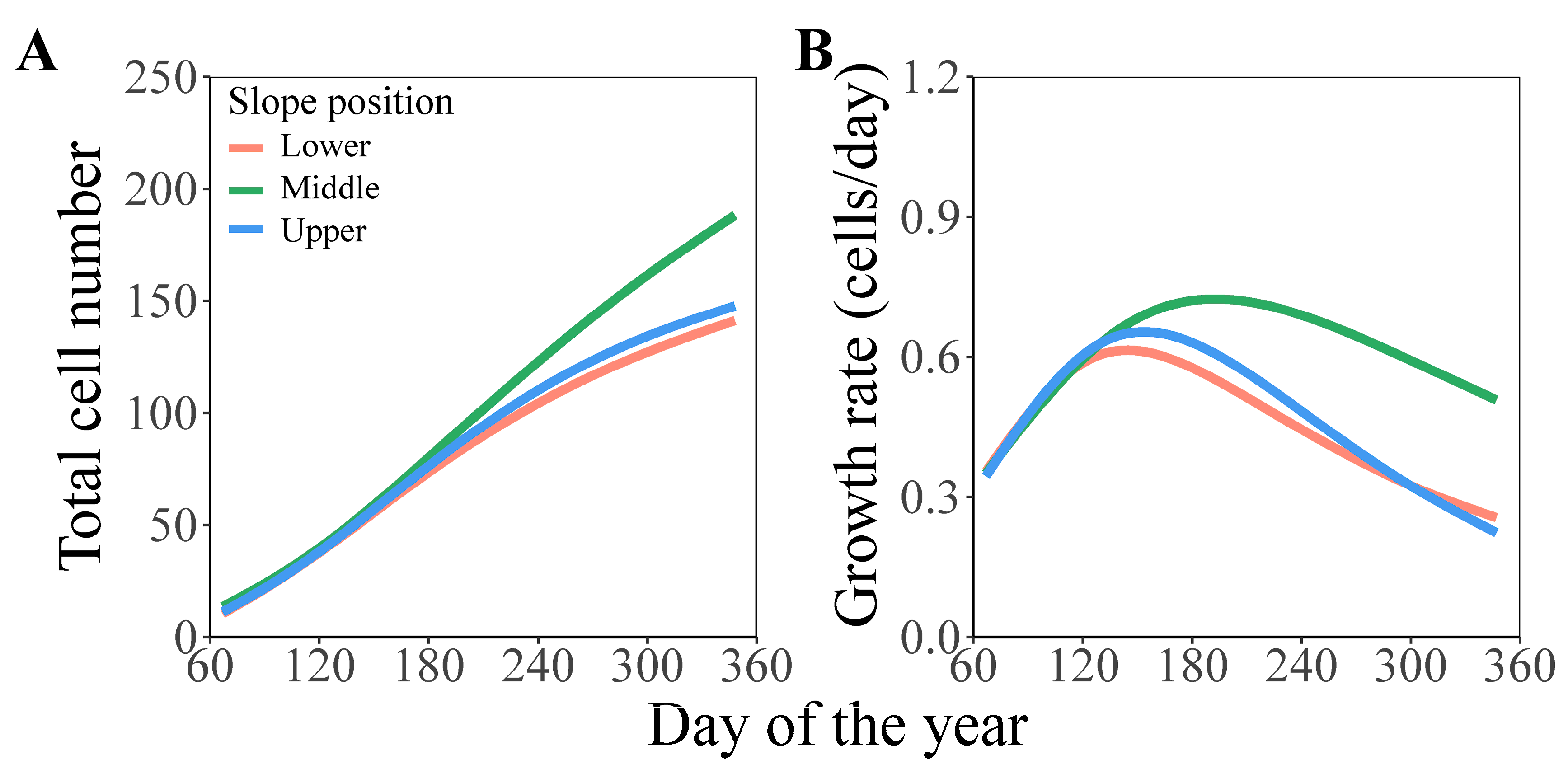
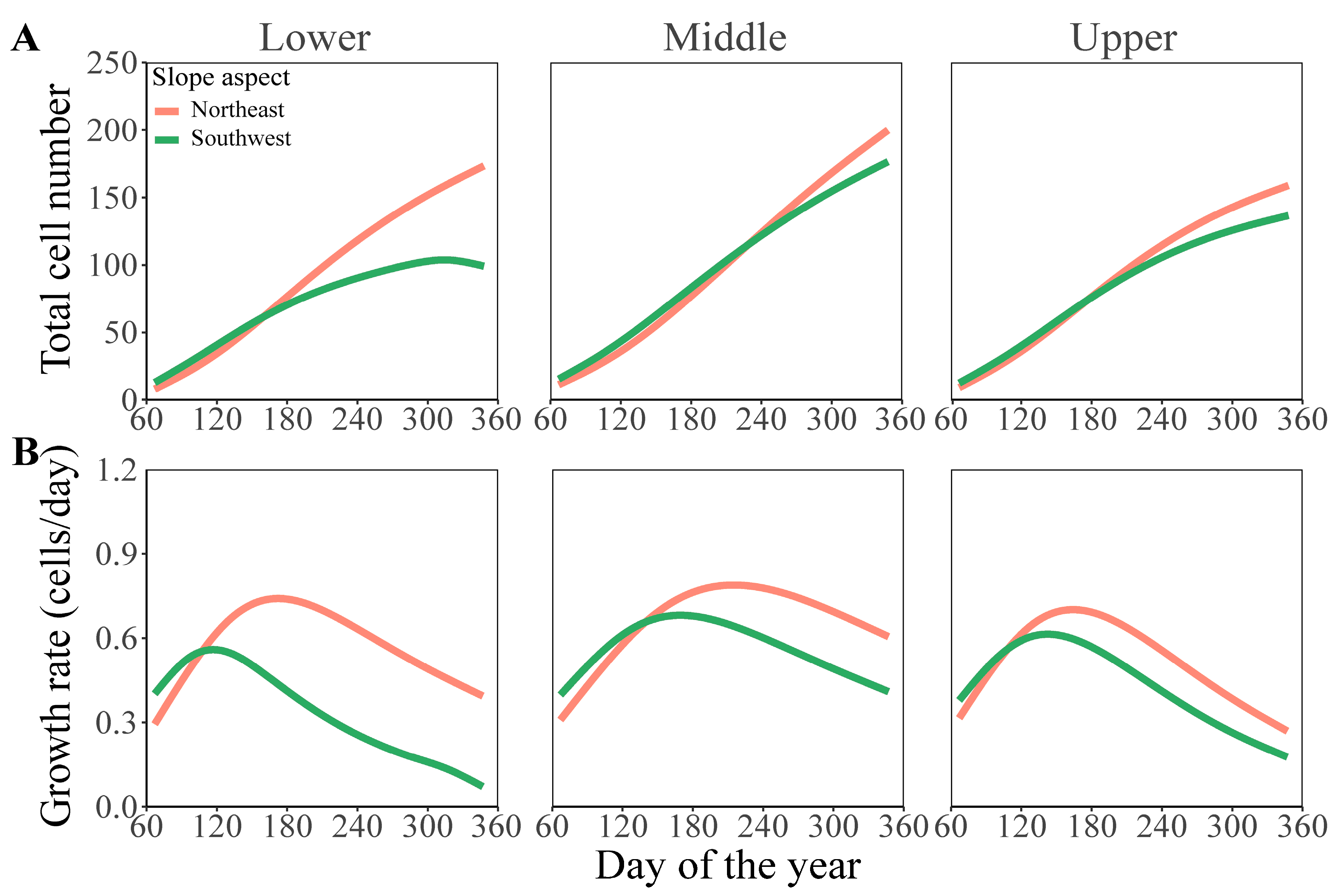
3.2. Comparison of Formation Dynamics and Development Patterns of Chinese Fir Xylem in Different Slope Aspects and Slope Positions
3.3. Comparison of Simulation Parameters of Chinese Fir Xylem Formation in Different Slope Aspects and Slope Positions
4. Discussion
4.1. Effect of Slope Aspect on Xylem Growth of Chinese Fir
4.2. Effect of Slope Position on Xylem Growth of Chinese Fir
4.3. The Application for Forest Management
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arias, P.; Bellouin, N.; Coppola, E.; Jones, R.; Krinner, G.; Marotzke, J.; Naik, V.; Palmer, M.; Plattner, G.-K.; Rogelj, J. Climate Change 2021: The Physical Science Basis. Contribution of Working Group14 I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Technical Summary; IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- Liu, H.; Williams, A.P.; Allen, C.D.; Guo, D.; Wu, X.; Anenkhonov, O.A.; Liang, E.; Sandanov, D.V.; Yin, Y.; Qi, Z.; et al. Rapid warming accelerates tree growth decline in semi-arid forests of Inner Asia. Glob. Chang. Biol. 2013, 19, 2500–2510. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Deslauriers, A.; Fonti, P.; Rossi, S.; Rathgeber, C.B.; Gričar, J. Ecophysiology and plasticity of wood and phloem formation. In Dendroecology; Springer: Cham, Switzerland, 2017; pp. 13–33. [Google Scholar]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- McDowell, N.G.; Michaletz, S.T.; Bennett, K.E.; Solander, K.C.; Xu, C.; Maxwell, R.M.; Middleton, R.S. Predicting Chronic Climate-Driven Disturbances and Their Mitigation. Trends Ecol. Evol. 2018, 33, 15–27. [Google Scholar] [CrossRef]
- Dinneny, J.R.; Yanofsky, M.F. Vascular Patterning: Xylem or Phloem? Curr. Biol. 2004, 14, R112–R114. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.S.; Hacke, U.G.; Oren, R.; Comstock, J.P. Water deficits and hydraulic limits to leaf water supply. Plant Cell Environ. 2002, 25, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Rathgeber, C.B.K.; Cuny, H.E.; Fonti, P. Biological basis of tree-ring formation: A crash course. Front. Plant Sci. 2016, 7, 734. [Google Scholar] [CrossRef]
- Cuny, H.E.; Rathgeber, C.B.K.; Frank, D.; Fonti, P.; Mäkinen, H.; Prislan, P.; Rossi, S.; del Castillo, E.M.; Campelo, F.; Vavrčík, H.; et al. Woody biomass production lags stem-girth increase by over one month in coniferous forests. Nat. Plants 2015, 1, 15160. [Google Scholar] [CrossRef] [PubMed]
- Rigling, A.; Bräker, O.; Schneiter, G.; Schweingruber, F. Intra-annual tree-ring parameters indicating differences in drought stress of Pinus sylvestris forests within the Erico-Pinion in the Valais (Switzerland). Plant Ecol. 2002, 163, 105–121. [Google Scholar] [CrossRef]
- Balzano, A.; Battipaglia, G.; Cherubini, P.; De Micco, V. Xylem Plasticity in Pinus pinaster and Quercus ilex Growing at Sites with Different Water Availability in the Mediterranean Region: Relations between Intra-Annual Density Fluctuations and Environmental Conditions. Forests 2020, 11, 379. [Google Scholar] [CrossRef]
- Battipaglia, G.; Kabala, J.P.; Pacheco-Solana, A.; Niccoli, F.; Bräuning, A.; Campelo, F.; Cufar, K.; de Luis, M.; De Micco, V.; Klisz, M.; et al. Intra-annual density fluctuations in tree rings are proxies of air temperature across Europe. Sci. Rep. 2023, 13, 12294. [Google Scholar] [CrossRef] [PubMed]
- Campelo, F.; Vieira, J.; Nabais, C. Tree-ring growth and intra-annual density fluctuations of Pinus pinaster responses to climate: Does size matter? Trees 2013, 27, 763–772. [Google Scholar] [CrossRef]
- Rozas, V.; García-González, I.; Zas, R. Climatic control of intra-annual wood density fluctuations of Pinus pinaster in NW Spain. Trees 2011, 25, 443–453. [Google Scholar] [CrossRef]
- Yan, J.; Liu, X.; Tang, X.; Yu, G.; Zhang, L.; Chen, Q.; Li, K. Substantial amounts of carbon are sequestered during dry periods in an old-growth subtropical forest in South China. J. For. Res. 2013, 18, 21–30. [Google Scholar] [CrossRef]
- Deslauriers, A.; Morin, H.; Begin, Y. Cellular phenology of annual ring formation of Abies balsamea in the Quebec boreal forest (Canada). Can. J. For. Res. 2003, 33, 190–200. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Anfodillo, T. Assessment of cambial activity and xylogenesis by microsampling tree species: An example at the Alpine timberline. IAWA J. 2006, 27, 383–394. [Google Scholar] [CrossRef]
- Huang, J.; Bergeron, Y.; Zhai, L.; Denneler, B. Variation in intra-annual radial growth (xylem formation) of Picea mariana (Pinaceae) along a latitudinal gradient in western Quebec, Canada. Am. J. Bot. 2011, 98, 792–800. [Google Scholar] [CrossRef]
- Prislan, P.; Čufar, K.; Koch, G.; Schmitt, U.; Gričar, J. Review of cellular and subcellular changes in the cambium. IAWA J. 2013, 34, 391–407. [Google Scholar] [CrossRef]
- Rossi, S.; DesLauriers, A.; Griçar, J.; Seo, J.-W.; Rathgeber, C.B.K.; Anfodillo, T.; Morin, H.; Levanic, T.; Oven, P.; Jalkanen, R. Critical temperatures for xylogenesis in conifers of cold climates. Glob. Ecol. Biogeogr. 2008, 17, 696–707. [Google Scholar] [CrossRef]
- Huang, J.; Deslauriers, A.; Rossi, S. Xylem formation can be modeled statistically as a function of primary growth and cambium activity. New Phytol. 2014, 203, 831–841. [Google Scholar] [CrossRef]
- Crowther, T.W.; Glick, H.B.; Covey, K.R.; Bettigole, C.; Maynard, D.S.; Thomas, S.M.; Smith, J.R.; Hintler, G.; Duguid, M.C.; Amatulli, G.; et al. Mapping tree density at a global scale. Nature 2015, 525, 201–205. [Google Scholar] [CrossRef]
- Rossi, S.; Anfodillo, T.; Čufar, K.; Cuny, H.E.; Deslauriers, A.; Fonti, P.; Frank, D.; Gričar, J.; Gruber, A.; Huang, J.; et al. Pattern of xylem phenology in conifers of cold ecosystems at the Northern Hemisphere. Glob. Chang. Biol. 2016, 22, 3804–3813. [Google Scholar] [CrossRef] [PubMed]
- Amthor, J.S. Efficiency of Lignin Biosynthesis: A Quantitative Analysis. Ann. Bot. 2003, 91, 673–695. [Google Scholar] [CrossRef] [PubMed]
- Aloni, R. Ecophysiological implications of vascular differentiation and plant evolution. Trees 2015, 29, 1–16. [Google Scholar] [CrossRef]
- Zhang, S.; Rossi, S.; Huang, J.-G.; Jiang, S.; Yu, B.; Zhang, W.; Ye, Q. Intra-annual Dynamics of Xylem Formation in Liquidambar formosana Subjected to Canopy and Understory N Addition. Front. Plant Sci. 2018, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, R.; Zimmermann, L.; Zeugin, F.; Newbery, D.M. Intra-annual radial growth and water relations of trees: Implications towards a growth mechanism. J. Exp. Bot. 2006, 57, 1445–1459. [Google Scholar] [CrossRef]
- Jackson, S.D. Plant responses to photoperiod. New Phytol. 2009, 181, 517–531. [Google Scholar] [CrossRef]
- Bosio, F.; Rossi, S.; Marcati, C.R. Periodicity and environmental drivers of apical and lateral growth in a Cerrado woody species. Trees 2016, 30, 1495–1505. [Google Scholar] [CrossRef]
- Takahashi, K.; Hirai, T. Seasonal change in xylem growth of Pinus densiflora in central Japan. Landsc. Ecol. Eng. 2016, 12, 231–237. [Google Scholar] [CrossRef]
- Malik, R.; Rossi, S.; Sukumar, R. Variations in the timing of different phenological stages of cambial activity in Abies pindrow (Royle) along an elevation gradient in the north-western Himalaya. Dendrochronologia 2020, 59, 125660. [Google Scholar] [CrossRef]
- Park, T.; Chen, C.; Macias-Fauria, M.; Tømmervik, H.; Choi, S.; Winkler, A.; Bhatt, U.S.; Walker, D.A.; Piao, S.; Brovkin, V.; et al. Changes in timing of seasonal peak photosynthetic activity in northern ecosystems. Glob. Chang. Biol. 2019, 25, 2382–2395. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Yang, Z.; Yu, B.; Ma, Q.; Jiang, S.; Shishov, V.V.; Zhou, P.; Huang, J.-G.; Ding, X. An earlier start of growing season can affect tree radial growth through regulating cumulative growth rate. Agric. For. Meteorol. 2023, 342, 109738. [Google Scholar] [CrossRef]
- Adams, H.D.; Germino, M.J.; Breshears, D.D.; Barron-Gafford, G.A.; Guardiola-Claramonte, M.; Zou, C.B.; Huxman, T.E. Nonstructural leaf carbohydrate dynamics of Pinus edulis during drought-induced tree mortality reveal role for carbon metabolism in mortality mechanism. New Phytol. 2013, 197, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Ziaco, E.; Rossi, S.; Biondi, F.; Prislan, P.; Liang, E. Growth rate rather than growing season length determines wood biomass in dry environments. Agric. For. Meteorol. 2019, 271, 46–53. [Google Scholar] [CrossRef]
- Coonen, E.J.; Sillett, S.C. Separating effects of crown structure and competition for light on trunk growth of Sequoia sempervirens. For. Ecol. Manag. 2015, 358, 26–40. [Google Scholar] [CrossRef]
- de Lara, N.O.T.; Da Silva, M.R.; Nogueira, A.; Marcati, C.R. Duration of cambial activity is determined by water availability while cambial stimulus is day-length dependent in a Neotropical evergreen species. Environ. Exp. Bot. 2017, 141, 50–59. [Google Scholar] [CrossRef]
- Häusser, M.; Aryal, S.; Barth, J.A.C.; Bendix, J.; Garel, E.; van Geldern, R.; Huneau, F.; Juhlke, T.R.; Knerr, I.; Santoni, S.; et al. Xylem formation patterns from Mediterranean to subalpine climate conditions reveal high growth plasticity of pine species on Corsica. Trees 2023, 37, 1027–1039. [Google Scholar] [CrossRef]
- Kraus, C.; Zang, C.; Menzel, A. Elevational response in leaf and xylem phenology reveals different prolongation of growing period of common beech and Norway spruce under warming conditions in the Bavarian Alps. Eur. J. For. Res. 2016, 135, 1011–1023. [Google Scholar] [CrossRef]
- Méndez-Toribio, M.; Meave, J.A.; Zermeño-Hernández, I.; Ibarra-Manríquez, G. Effects of slope aspect and topographic position on environmental variables, disturbance regime and tree community attributes in a seasonal tropical dry forest. J. Veg. Sci. 2016, 27, 1094–1103. [Google Scholar] [CrossRef]
- Méndez-Toribio, M.; Ibarra-Manríquez, G.; Navarrete-Segueda, A.; Paz, H. Topographic position, but not slope aspect, drives the dominance of functional strategies of tropical dry forest trees. Environ. Res. Lett. 2017, 12, 085002. [Google Scholar] [CrossRef]
- Li, X.; Song, X.; Zhao, J.; Lu, H.; Qian, C.; Zhao, X. Shifts and plasticity of plant leaf mass per area and leaf size among slope aspects in a subalpine meadow. Ecol. Evol. 2021, 11, 14042–14055. [Google Scholar] [CrossRef] [PubMed]
- Måren, I.E.; Karki, S.; Prajapati, C.; Yadav, R.K.; Shrestha, B.B. Facing north or south: Does slope aspect impact forest stand characteristics and soil properties in a semiarid trans-Himalayan valley? J. Arid. Environ. 2015, 121, 112–123. [Google Scholar] [CrossRef]
- Dutcă, I.; Cernat, A.; Stăncioiu, P.T.; Ioraș, F.; Niță, M.D. Does Slope Aspect Affect the Aboveground Tree Shape and Volume Allometry of European Beech (Fagus sylvatica L.) Trees? Forests 2022, 13, 1071. [Google Scholar] [CrossRef]
- Cullen, L.E.; Palmer, J.G.; Duncan, R.P.; Stewart, G.H. Climate change and tree-ring relationships of Nothofagus menziesii tree-line forests. Can. J. For. Res. 2001, 31, 1981–1991. [Google Scholar] [CrossRef]
- Wang, L.; Wei, S.; Horton, R.; Shao, M. Effects of vegetation and slope aspect on water budget in the hill and gully region of the Loess Plateau of China. CATENA 2011, 87, 90–100. [Google Scholar] [CrossRef]
- Sternberg, M.; Shoshany, M. Influence of slope aspect on Mediterranean woody formations: Comparison of a semiarid and an arid site in Israel. Ecol. Res. 2001, 16, 335–345. [Google Scholar] [CrossRef]
- Galicia, L.; López-Blanco, J.; Zarco-Arista, A.; Filips, V.; García-Oliva, F. The relationship between solar radiation interception and soil water content in a tropical deciduous forest in Mexico. CATENA 1999, 36, 153–164. [Google Scholar] [CrossRef]
- Ebel, B.A. Simulated unsaturated flow processes after wildfire and interactions with slope aspect. Water Resour. Res. 2013, 49, 8090–8107. [Google Scholar] [CrossRef]
- Holland, P.G.; Steyn, D.G. Vegetational responses to latitudinal variations in slope angle and aspect. J. Biogeogr. 1975, 2, 179–183. [Google Scholar] [CrossRef]
- Daws, M.I.; Mullins, C.E.; Burslem, D.F.; Paton, S.R.; Dalling, J.W. Topographic position affects the water regime in a semideciduous tropical forest in Panamá. Plant Soil 2002, 238, 79–89. [Google Scholar] [CrossRef]
- Qiu, Y.; Fu, B.; Wang, J.; Chen, L. Soil moisture variation in relation to topography and land use in a hillslope catchment of the Loess Plateau, China. J. Hydrol. 2001, 240, 243–263. [Google Scholar] [CrossRef]
- Watts, S.H.; Jump, A.S. The benefits of mountain woodland restoration. Restor. Ecol. 2022, 30, e13701. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W.; Zhou, M.; Zhuo, Z.; Zhang, Y.; Jiang, F.; Huang, Y.; Lin, J. Runoff and sediment characteristics of a typical watershed after continuous soil erosion control in the red soil region of Southern China. CATENA 2023, 233, 107484. [Google Scholar] [CrossRef]
- Bao, W.; Qu, Y.; Shan, X.; Wan, Y. Screening and Validation of Housekeeping Genes of the Root and Cotyledon of Cunninghamia lanceolata under Abiotic Stresses by Using Quantitative Real-Time PCR. Int. J. Mol. Sci. 2016, 17, 1198. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Seely, B.; Wang, G.; Innes, J.; Zheng, D.; Chen, P.; Wang, T.; Li, Q. Evaluating management tradeoffs between economic fiber production and other ecosystem services in a Chinese-fir dominated forest plantation in Fujian Province. Sci. Total. Environ. 2016, 557–558, 80–90. [Google Scholar] [CrossRef]
- Wang, H.; Sun, J.; Duan, A.; Zhu, A.; Wu, H.; Zhang, J. Dendroclimatological Analysis of Chinese Fir Using a Long-Term Provenance Trial in Southern China. Forests 2022, 13, 1348. [Google Scholar] [CrossRef]
- Guan, Y.; Huang, Y.; Qin, H.; Zhang, X. Prediction technology of Chinese fir forest ecological value based on remote sensing inversion. Appl. Nanosci. 2023, 13, 2093–2101. [Google Scholar] [CrossRef]
- Rossi, S.; Anfodillo, T.; Menardi, R. Trephor: A new tool for sampling microcores from tree stems. IAWA J. 2006, 27, 89–97. [Google Scholar] [CrossRef]
- Camarero, J.J.; Guerrero-Campo, J.; Gutierrez, E. Tree-ring growth and structure of Pinus uncinata and Pinus sylvestris in the Central Spanish Pyrenees. Arct. Alp. Res. 1998, 30, 1–10. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Morin, H. Application of the Gompertz equation for the study of xylem cell development. Dendrochronologia 2003, 21, 33–39. [Google Scholar] [CrossRef]
- Oberhuber, W.; Gruber, A.; Kofler, W.; Swidrak, I. Radial stem growth in response to microclimate and soil moisture in a drought-prone mixed coniferous forest at an inner Alpine site. Eur. J. For. Res. 2014, 133, 467–479. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 20 December 2022).
- Saderi, S.; Rathgeber, C.B.K.; Rozenberg, P.; Fournier, M. Phenology of wood formation in larch (Larix decidua Mill.) trees growing along a 1000-m elevation gradient in the French Southern Alps. Ann. For. Sci. 2019, 76, 89. [Google Scholar] [CrossRef]
- Miller, T.W.; Stangler, D.F.; Larysch, E.; Seifert, T.; Spiecker, H.; Kahle, H.-P. Plasticity of seasonal xylem and phloem production of Norway spruce along an elevational gradient. Trees 2020, 34, 1281–1297. [Google Scholar] [CrossRef]
- Wheeler, E.A.; Baas, P.; Gasson, P.E. IAWA List of Microscopic Features for Hardwood Identification: With an Appendix on Non-Anatomical Information; IAWA: Leiden, The Netherlands, 1989. [Google Scholar]
- Guo, B.D.; Zhang, Y.D.; Wang, X.C. Response of Picea purpurea and Abies faxoniana tree rings at different slope aspects to rapid warming in western Sichuan, China. Chin. J. Appl. Ecol. 2016, 27, 354–364. [Google Scholar]
- Iszkuło, G.; Jasin, A.K.; Romo, À.; Tomaszewski, D.; Szmyt, J. The Greater Growth Rate of Male over Female of the Dioecious Tree Juniperus thurifera Only in Worse Habitat Conditions; Polish Academy of Sciences: Warsaw, Poland, 2011. [Google Scholar]
- Cantlon, J.E. Vegetation and Microclimates on North and South Slopes of Cushetunk Mountain, New Jersey. Ecol. Monogr. 1953, 23, 241–270. [Google Scholar] [CrossRef]
- Bennie, J.; Huntley, B.; Wiltshire, A.; Hill, M.O.; Baxter, R. Slope, aspect and climate: Spatially explicit and implicit models of topographic microclimate in chalk grassland. Ecol. Model. 2008, 216, 47–59. [Google Scholar] [CrossRef]
- Burnett, B.; Meyer, G.; McFadden, L. Aspect-related microclimatic influences on slope forms and processes, northeastern Arizona. J. Geophys. Res. 2008, 113, F03002. [Google Scholar] [CrossRef]
- Hernández-Hernández, V.; Benítez, M.; Boudaoud, A. Interplay between turgor pressure and plasmodesmata during plant development. J. Exp. Bot. 2019, 71, 768–777. [Google Scholar] [CrossRef]
- Woodruff, D.R.; Meinzer, F.C. Water stress, shoot growth and storage of non-structural carbohydrates along a tree height gradient in a tall conifer. Plant Cell Environ. 2011, 34, 1920–1930. [Google Scholar] [CrossRef]
- Deslauriers, A.; Huang, J.-G.; Balducci, L.; Beaulieu, M.; Rossi, S. The Contribution of Carbon and Water in Modulating Wood Formation in Black Spruce Saplings. Plant Physiol. 2016, 170, 2072–2084. [Google Scholar] [CrossRef]
- Kaewmano, A.; Fu, P.-L.; Fan, Z.-X.; Pumijumnong, N.; Zuidema, P.A.; Bräuning, A. Climatic influences on intra-annual stem radial variations and xylem formation of Toona ciliata at two Asian tropical forest sites with contrasting soil water availability. Agric. For. Meteorol. 2022, 318, 108906. [Google Scholar] [CrossRef]
- Deslauriers, A.; Morin, H. Intra-annual tracheid production in balsam fir stems and the effect of meteorological variables. Trees 2005, 19, 402–408. [Google Scholar] [CrossRef]
- Cuny, H.E.; Rathgeber, C.B.K.; LeBourgeois, F.; Fortin, M.; Fournier, M. Life strategies in intra-annual dynamics of wood formation: Example of three conifer species in a temperate forest in north-east France. Tree Physiol. 2012, 32, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Rossi, S.; Gricar, J.; Liang, E.; Cufar, K. Is precipitation a trigger for the onset of xylogenesis in Juniperus przewalskii on the north-eastern Tibetan Plateau? Ann. Bot. 2015, 115, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Brueck, H.; Giese, K.; Zhang, L.; Sattelmacher, B.; Lin, S. Slope aspect has effects on productivity and species composition of hilly grassland in the Xilin River Basin, Inner Mongolia, China. J. Arid. Environ. 2008, 72, 483–493. [Google Scholar] [CrossRef]
- Yimer, F.; Ledin, S.; Abdelkadir, A. Soil organic carbon and total nitrogen stocks as affected by topographic aspect and vegetation in the Bale Mountains, Ethiopia. Geoderma 2006, 135, 335–344. [Google Scholar] [CrossRef]
- Gutiérrez-Jurado, H.A.; Vivoni, E.R.; Istanbulluoglu, E.; Bras, R.L. Ecohydrological response to a geomorphically significant flood event in a semiarid catchment with contrasting ecosystems. Geophys. Res. Lett. 2007, 34, L24S25. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Lin, S.; Li, Y.; Li, Y.; Chen, Q.; Wang, Q.; He, K. Influence of tree size, local forest structure, topography, and soil resource availability on plantation growth in Qinghai Province, China. Ecol. Indic. 2021, 120, 106957. [Google Scholar] [CrossRef]
- Rasmussen, L.V.; Jepsen, M.R. Monitoring systems to improve forest conditions. Curr. Opin. Environ. Sustain. 2018, 32, 29–37. [Google Scholar] [CrossRef]
- Yousefpour, R.; Jacobsen, J.B.; Thorsen, B.J.; Meilby, H.; Hanewinkel, M.; Oehler, K. A review of decision-making approaches to handle uncertainty and risk in adaptive forest management under climate change. Ann. For. Sci. 2012, 69, 1–15. [Google Scholar] [CrossRef]


| Tree Number | Slope Aspect | Slope Position | Plot Number | Elevation (m a.s.l) | Gradient (°) | DBHc (m) | Height (m) |
|---|---|---|---|---|---|---|---|
| 1 | NE | U | 1 | 257 | 23 | 11.2 | 8.8 |
| 2 | NE | U | 2 | 258 | 23 | 11.0 | 8.8 |
| 3 | NE | U | 3 | 258 | 24 | 10.3 | 8.1 |
| 4 | NE | U | 4 | 259 | 23 | 11.8 | 9.0 |
| 5 | NE | M | 5 | 242 | 24 | 11.3 | 8.3 |
| 6 | NE | M | 6 | 243 | 22 | 14.1 | 10.7 |
| 7 | NE | M | 7 | 241 | 25 | 10.8 | 7.2 |
| 8 | NE | M | 8 | 242 | 25 | 10.9 | 8.0 |
| 9 | NE | L | 9 | 226 | 24 | 13.0 | 9.8 |
| 10 | NE | L | 10 | 227 | 24 | 12.5 | 8.9 |
| 11 | NE | L | 11 | 226 | 25 | 13.6 | 9.3 |
| 12 | NE | L | 12 | 225 | 25 | 13.0 | 9.3 |
| 13 | SW | U | 13 | 257 | 25 | 10.7 | 7.6 |
| 14 | SW | U | 14 | 258 | 24 | 10.2 | 7.3 |
| 15 | SW | U | 15 | 257 | 24 | 10.2 | 7.5 |
| 16 | SW | U | 16 | 260 | 25 | 11.0 | 8.0 |
| 17 | SW | M | 17 | 243 | 24 | 10.0 | 7.1 |
| 18 | SW | M | 18 | 243 | 23 | 11.4 | 8.5 |
| 19 | SW | M | 19 | 240 | 23 | 11.7 | 8.4 |
| 20 | SW | M | 20 | 242 | 24 | 11.1 | 8.6 |
| 21 | SW | L | 21 | 225 | 25 | 12.1 | 8.5 |
| 22 | SW | L | 22 | 225 | 24 | 12.0 | 9 |
| 23 | SW | L | 23 | 227 | 25 | 13.5 | 9.6 |
| 24 | SW | L | 24 | 227 | 23 | 11.4 | 8.3 |
| Variables | F Value | ||||
|---|---|---|---|---|---|
| Slope Aspect | Slope Position | ||||
| NE | SW | U | M | L | |
| Onset (DOY) | 59 ± 10 A | 42 ± 13 B | 50 ± 15 a | 51 ± 9 a | 50 ± 20 a |
| End (DOY) | 322 ± 14 A | 304 ± 29 A | 313 ± 16 ab | 325 ± 11 a | 300 ± 34 b |
| Growing season length (days) | 262 ± 20 A | 262 ± 37 A | 262 ± 24 a | 274 ± 11 a | 250 ± 41 a |
| Average growth rate (cells/day) | 0.67 ± 0.11 A | 0.53 ± 0.10 B | 0.56 ± 0.11 b | 0.69 ± 0.07 a | 0.56 ± 0.15 b |
| Xylem cell production (N) | 176.98 ± 34.52 A | 140.19 ± 36.07 B | 147.35 ± 29.08 b | 187.89 ± 19.81 a | 140.51 ± 48.36 b |
| Variables | F Value | |||||
|---|---|---|---|---|---|---|
| NE | SW | |||||
| U | M | L | U | M | L | |
| Onset (DOY) | 57 ± 15 Aa | 59 ± 3 Aa | 62 ± 10 Aa | 44 ± 14 Aa | 43 ± 2 Ab | 38 ± 19 Aa |
| End (DOY) | 316 ± 16 Aa | 330 ± 8 Aa | 319 ± 15 Aa | 309 ± 17 Aa | 321 ± 14 Aa | 280 ± 37 Aa |
| Growing season length (days) | 260 ± 27 Aa | 271 ± 5 Aa | 257 ± 24 Aa | 265 ± 24 Aa | 278 ± 15 Aa | 243 ± 57 Aa |
| Average growth rate (cells/day) | 0.61 ± 0.12 Aa | 0.74 ± 0.03 Aa | 0.67 ± 0.14 Aa | 0.52 ± 0.09 Ba | 0.63 ± 0.05 Ab | 0.45 ± 0.04 Bb |
| Xylem cell production (N) | 158.41 ± 35.73 Aa | 199.50 ± 6.63 Aa | 173.03 ± 43.48 Aa | 136.29 ± 19.22 ABa | 176.29 ± 22.64 Aa | 108.00 ± 27.35 Bb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Li, Q.; Bai, C.; Zhao, W.; Rodríguez-Hernández, D.I.; Guo, X. Influence of Slope Aspect and Position on Xylem Formation Dynamics in Subtropical Chinese Fir Plantations. Forests 2024, 15, 1193. https://doi.org/10.3390/f15071193
Huang Y, Li Q, Bai C, Zhao W, Rodríguez-Hernández DI, Guo X. Influence of Slope Aspect and Position on Xylem Formation Dynamics in Subtropical Chinese Fir Plantations. Forests. 2024; 15(7):1193. https://doi.org/10.3390/f15071193
Chicago/Turabian StyleHuang, Yingni, Qianlin Li, Chunmei Bai, Wendi Zhao, Diego Ismael Rodríguez-Hernández, and Xiali Guo. 2024. "Influence of Slope Aspect and Position on Xylem Formation Dynamics in Subtropical Chinese Fir Plantations" Forests 15, no. 7: 1193. https://doi.org/10.3390/f15071193








