Stress Responses to Bark Beetle Infestations among Pine (Pinus sylvestris), Fir (Abies alba), and Beech (Fagus sylvatica) Trees
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Plant Material Sampling
2.3. Enzyme Extraction
2.4. Antioxidative Enzyme Activity Assays
2.5. Chlorophyll and Carotenoids Measurement
2.6. Preparation of Sample Extract for Detection of Antioxidative Compounds
2.7. Total Phenol Content
2.8. Total Flavonoid Content
2.9. Evaluation of Antioxidant Capacity
2.10. Phytohormone Analysis
2.11. Statistical Evaluation
3. Results
3.1. Photosynthetic Pigments
3.2. Antioxidative Parameters
3.3. Antioxidant Enzymes
3.4. Phytohormones
4. Discussion
4.1. Photosynthetic Pigments
4.2. Antioxidative Parameters
4.3. Antioxidant Enzymes
4.4. Phytohormones
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Huang, J.; Kautz, M.; Trowbridge, A.M.; Hammerbacher, A.; Raffa, K.F.; Adams, H.D.; Goodsman, D.W.; Xu, C.; Meddens, A.J.H.; Kandasamy, D.; et al. Tree defence and bark beetles in a drying world: Carbon partitioning, functioning and modelling. New Phytol. 2020, 225, 26–36. [Google Scholar] [CrossRef]
- Jactel, H.; Petit, J.; Desprez-Loustau, M.-L.; Delzon, S.; Piou, D.; Battisti, A.; Koricheva, J. Drought effects on damage by forest insects and pathogens: A meta-analysis. Glob. Change Biol. 2012, 18, 267–276. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.W.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C.; et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef]
- Sommerfeld, A.; Rammer, W.; Heurich, M.; Hilmers, T.; Müller, J.; Seidl, R. Do bark beetle outbreaks amplify or dampen future bark beetle disturbances in Central Europe? J. Ecol. 2021, 109, 737–749. [Google Scholar] [CrossRef]
- Netherer, S.; Panassiti, B.; Pennerstorfer, J.; Matthews, B. Acute Drought Is an Important Driver of Bark Beetle Infestation in Austrian Norway Spruce Stands. Front. For. Glob. Change 2019, 2, 39. [Google Scholar] [CrossRef]
- Lubojacký, J.; Lorenc, F.; Liška, J.; Knížek, M. Hlavní problémy v ochraně lesa v Česku v roce 2018 a prognóza na rok 2019. In Zpravodaj Ochrany Lesa Knížek M (Hrsg.); Forest Protection Service and Forestry and Game Management Research Institute: Prague, Czech Republic, 2019; pp. 14–19. Available online: www.vulhm.cz/files/uploads/2019/06/ZOL_22_2019.pdf (accessed on 7 October 2024).
- Hlásny, T.; König, L.; Krokene, P.; Lindner, M.; Montagné-Huck, C.; Müller, J.; Qin, H.; Raffa, K.F.; Schelhaas, M.-J.; Svoboda, M.; et al. Bark Beetle Outbreaks in Europe: State of Knowledge and Ways Forward for Management. Curr. Forestry Rep. 2021, 7, 138–165. [Google Scholar] [CrossRef]
- Dobor, L.; Hlásny, T.; Rammer, W.; Zimová, S.; Barka, I.; Seidl, R. Is salvage logging effectively dampening bark beetle outbreaks and preserving forest carbon stocks? J. Appl. Ecol. 2020, 57, 67–76. [Google Scholar] [CrossRef]
- Messier, C.; Bauhus, J.; Sousa-Silva, R.; Auge, H.; Baeten, L.; Barsoum, N.; Bruelheide, H.; Caldwell, B.; Cavender-Bares, J.; Dhiedt, E.; et al. For the sake of resilience and multifunctionality, let’s diversify planted forests! Conserv. Lett. 2022, 15, e12829. [Google Scholar] [CrossRef]
- Bose, A.K.; Gessler, A.; Büntgen, U.; Rigling, A. Tamm review: Drought-induced Scots pine mortality—Trends, contributing factors, and mechanisms. For. Ecol. Manag. 2024, 561, 121873. [Google Scholar] [CrossRef]
- Durand-Gillmann, M.; Cailleret, M.; Boivin, T.; Nageleisen, L.-M.; Davi, H. Individual vulnerability factors of Silver fir (Abies alba Mill.) to parasitism by two contrasting biotic agents: Mistletoe (Viscum album L. ssp. abietis) and bark beetles (Coleoptera: Curculionidae: Scolytinae) during a decline process. Ann. For. Sci. 2014, 71, 659–673. [Google Scholar] [CrossRef]
- Foit, J.; Čermák, V. Colonization of disturbed Scots pine trees by bark- and wood-boring beetles. Agr. Forest Entomol. 2014, 16, 184–195. [Google Scholar] [CrossRef]
- Hlávková, D.; Doležal, P. Cambioxylophagous Pests of Scots Pine: Ecological Physiology of European Populations—A Review. Front. For. Glob. Change 2022, 5, 864651. [Google Scholar] [CrossRef]
- Háva, J.; Holuša, J. First record of the siricid Urocerus albicornis, an invasive alien pest, in the Czech Republic. J. Appl. Entomol. 2019, 143, 487–491. [Google Scholar] [CrossRef]
- Rouault, G.; Candau, J.-N.; Lieutier, F.; Nageleisen, L.-M.; Martin, J.C.; Warzée, N. Effects of drought and heat on forest insect populations in relation to the 2003 drought in Western Europe. Ann. For. Sci. 2006, 63, 613–624. [Google Scholar] [CrossRef]
- Stauder, C.M.; Utano, N.M.; Kasson, M.T. Resolving host and species boundaries for perithecia-producing nectriaceous fungi across the central Appalachian Mountains. Fungal Ecol. 2020, 47, 100980. [Google Scholar] [CrossRef]
- Perlík, M.; Kraus, D.; Bußler, H.; Neudam, L.; Pietsch, S.; Mergner, U.; Seidel, D.; Sebek, P.; Thorn, S. Canopy openness as the main driver of aculeate Hymenoptera and saproxylic beetle diversity following natural disturbances and salvage logging. For. Ecol. Manag. 2023, 540, 121033. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New phytol. 2005, 167, 353–376. [Google Scholar] [CrossRef] [PubMed]
- Gaylord, M.L.; Kolb, T.E.; Pockman, W.T.; Plaut, J.A.; Yepez, E.A.; Macalady, A.K.; Pangle, R.E.; McDowell, N.G. Drought predisposes piñon–juniper woodlands to insect attacks and mortality. New Phytol. 2013, 198, 567–578. [Google Scholar] [CrossRef]
- Arango-Velez, A.; El Kayal, W.; Copeland, C.C.J.; Zaharia, L.I.; Lusebrink, I.; Cooke, J.E.K. Differences in defence responses of Pinus contorta and Pinus banksiana to the mountain pine beetle fungal associate Grosmannia clavigera are affected by water deficit. Plant Cell Environ. 2016, 39, 726–744. [Google Scholar] [CrossRef]
- Netherer, S.; Kandasamy, D.; Jirosová, A.; Kalinová, B.; Schebeck, M.; Schlyter, F. Interactions among Norway spruce, the bark beetle Ips typographus and its fungal symbionts in times of drought. J. Pest. Sci. 2021, 94, 591–614. [Google Scholar] [CrossRef] [PubMed]
- Erbilgin, N.; Galvez, D.A.; Zhang, B.; Najar, A. Resource availability and repeated defoliation mediate compensatory growth in trembling aspen (Populus tremuloides) seedlings. PeerJ 2014, 2, e491. [Google Scholar] [CrossRef] [PubMed]
- Kolb, T.; Keefover-Ring, K.; Burr, S.J.; Hofstetter, R.; Gaylord, M.; Raffa, K.F. Drought-Mediated Changes in Tree Physiological Processes Weaken Tree Defenses to Bark Beetle Attack. J. Chem. Ecol. 2019, 45, 888–900. [Google Scholar] [CrossRef]
- Sallé, A.; Monclus, R.; Yart, A.; Lieutier, F. Effect of phenolic compounds on the in vitro growth of two fungi associated with Ips typographus. Forest Pathol. 2005, 35, 298–304. [Google Scholar] [CrossRef]
- Klutsch, J.; Shamoun, S.; Erbilgin, N. Drought stress leads to systemic induced susceptibility to a necrotrophic fungus associated with mountain pine beetle in Pinus banksiana seedlings. PLoS ONE 2017, 12, e0189203. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.E.; Koricheva, J. The Ontogeny of Plant Defense and Herbivory: Characterizing General Patterns Using Meta-Analysis. Am. Nat. 2010, 175, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Forkner, R.E.; Marquis, R.J.; Lill, J.T. Feeny revisited: Condensed tannins as anti-herbivore defences in leaf-chewing herbivore communities of Quercus. Ecol. Entomol. 2004, 29, 174–187. [Google Scholar] [CrossRef]
- Bussotti, F.; Gravano, E.; Grossoni, P.; Tani, C. Occurrence of tannins in leaves of beech trees (Fagus sylvatica) along an ecological gradient, detected by histochemical and ultrastructural analyses. New Phytol. 1998, 138, 469–479. [Google Scholar] [CrossRef]
- Dubeler, A.; Voltmer, G.; Gora, V.; Lunderstadt, J.; Zeeck, A. Phenols from Fagus sylvatica and their role in defence against Cryptococcus fagisuga. Phytochem. 1997, 45, 51–57. [Google Scholar] [CrossRef]
- Mämmelä, P. Phenolics in selected European hardwood species by liquid chromatography-electrospray ionisation mass spectrometry. Analyst 2001, 126, 1535–1538. [Google Scholar] [CrossRef]
- Feeny, P. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 1970, 51, 565–581. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Walker, A.C.; Uddin, F. Antioxidants in the midgut fluids of a tannin-tolerant and a tannin-sensitive caterpillar: Effects of seasonal changes in tree leaves. J. Chem. Ecol. 2003, 29, 1099–1116. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Stuchlikova, L.R.; Skalova, L.; Szotakova, B.; Syslova, E.; Vokral, I.; Vanek, T.; Podlipna, R. Biotransformation of flubendazole and fenbendazole and their effects in the ribwort plantain (Plantago lanceolata). Ecotoxicol. Environ. Saf. 2018, 147, 681–687. [Google Scholar] [CrossRef]
- El-Shabrawi, H.; Kumar, B.; Kaul, T.; Reddy, M.K.; Singla-Pareek, S.L.; Sopory, S.K. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 2010, 245, 85–96. [Google Scholar] [CrossRef]
- Drotar, A.; Phelps, P.; Fall, R. Evidence for glutathione-peroxidase activities in cultured plant-cells. Plant Sci. 1985, 42, 35–40. [Google Scholar] [CrossRef]
- Verma, S.; Dubey, R.S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Vanacker, H.; Carver, T.L.W.; Foyer, C.H. Pathogen-induced changes in the antioxidant status of the apoplast in barley leaves. Plant Physiol. 1998, 117, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione-S-Transferases—First enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Matić, P.; Sabljić, M.; Jakobek, L. Validation of Spectrophotometric Methods for the Determination of Total Polyphenol and Total Flavonoid Content. J. AOAC Int. 2017, 100, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Langhansova, L.; Pumprova, K.; Haisel, D.; Ekrt, L.; Pavicic, A.; Zajíčková, M.; Vanek, T.; Dvorakova, M. European ferns as rich sources of antioxidants in the human diet. Food Chem. 2021, 356, 129637. [Google Scholar] [CrossRef] [PubMed]
- Prerostova, S.; Dobrev, P.I.; Kramna, B.; Gaudinova, A.; Knirsch, V.; Spichal, L.; Zatloukal, M.; Vankova, R. Heat acclimation and inhibition of cytokinin degradation positively affect heat stress tolerance of Arabidopsis. Front. Plant Sci. 2020, 11, 87. [Google Scholar] [CrossRef]
- Baldy, R.W.; DeBenedictis, J.A.; Johnson, L.; Weber, E.; Baldy, M.; Osborn, B.; Burleigh, J. Leaf colour and vine size are related to yield in a phylloxera-infested vineyard. Vitis 1996, 35, 201–205. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Wang, J.; Chen, X.; Yang, Z.; Liang, J. Photosynthetic traits and antioxidative defense responses of Pinus yunnanensis after joint attack by bark beetles Tomicus yunnanensis and T. minor. J. For. Res. 2019, 30, 2031–2038. [Google Scholar] [CrossRef]
- Aldea, M.; Hamilton, J.G.; Resti, J.P.; Zangerl, A.R.; Berenbaum, M.R.; DeLucia, E.H. Indirect effects of insect herbivory on leaf gas exchange in soybean. Plant Cell Environ. 2005, 28, 402–411. [Google Scholar] [CrossRef]
- Tang, J.Y.; Zielinski, R.E.; Zangerl, A.R.; Crofts, A.R.; Berembaum, M.R.; DeLucia, E.H. The differential effects of herbivory by first and fourth instars of Trichoplusia ni (Lepidoptera: Noctuidae) on photosynthesis in Arabidopsis thaliana. J. Exp. Bot. 2006, 57, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Arpiwi, N.L.; Muksin, I.K.; Nio, S.A. Drought Stress Decreases Morphophysiological Characteristics of Pongamia pinnata (L.) Pierre a Biodiesel Tree. Pak. J. Biol. Sci. 2023, 26, 463–471. [Google Scholar] [CrossRef]
- Bown, A.W.; Hall, D.E.; MacGregor, K.B. Insect footsteps leaves stimulate the accumulation of 4-aminobutyrate and can be visualized through increased chlorophyll fluorescence and superoxide production. Plant Physiol. 2002, 129, 1430–1434. [Google Scholar] [CrossRef]
- Cárdenas, A.; Gallardo, P. Relationship between insect damage and chlorophyll content in mediterranean oak species. Appl. Ecol. Environ. Res. 2016, 14, 477–491. [Google Scholar] [CrossRef]
- Abdullah, H.; Darvishzadeh, R.; Skidmore, A.K.; Groen, T.A.; Heurich, M. European spruce bark beetle (Ips typographus L.) green attack affects foliar reflectance and biochemical properties. Int. J. Appl. Earth Obs. Geoinf. 2018, 64, 199–209. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Pastierovič, F.; Kalyniukova, A.; Hradecký, J.; Dvořák, O.; Vítámvás, J.; Mogilicherla, K.; Tomášková, I. Biochemical Responses in Populus tremula: Defending against Sucking and Leaf-Chewing Insect Herbivores. Plants 2024, 13, 1243. [Google Scholar] [CrossRef] [PubMed]
- Golan, K.; Rubinowska, K.; Kmieć, K.; Kot, I.; Górska-Drabik, E.; Łagowska, B.; Michałek, W. Impact of scale insect infestation on the content of photosynthetic pigments and chlorophyll fluorescence in two host plant species. Arthropod-Plant Inte. 2015, 9, 55–65. [Google Scholar] [CrossRef]
- Othmen, S.B.; Boussaa, F.; Hajji-Hedfi, L. Effects of nymphal density (Bactericera trigonica) and feeding on photosynthetic pigments, proline content and phenolic compounds in car-rot plants. Eur. J. Plant. Pathol. 2022, 163, 51–59. [Google Scholar] [CrossRef]
- Krajnc, A.U. A temporal analysis of antioxidative defense responses in the phloem of Picea abies after attack by Ips typographus. Tree Physiol. 2009, 29, 1059–1068. [Google Scholar] [CrossRef]
- Krajnc, A.U.; Kristl, J.; Ivancic, A. Application of salicylic acid induces antioxidant defense responses in the phloem of Picea abies and inhibits colonization by Ips typographus. For. Ecol. Manag. 2011, 261, 416–426. [Google Scholar] [CrossRef]
- Wainhouse, D.; Rose, D.R.; Peace, A.J. The influence of preformed defences on the dynamic wound response in Spruce bark. Funct. Ecol. 1997, 11, 564–572. [Google Scholar] [CrossRef]
- Korolyova, N.; Buechling, A.; Lieutier, F.; Yart, A.; Cudlín, P.; Turčáni, M.; Jakuš, R. Primary and secondary host selection by Ips typographus depends on Norway spruce crown characteristics and phenolic-based defenses. Plant Sci. 2022, 321, 111319. [Google Scholar] [CrossRef]
- Nagel, R.; Hammerbacher, A.; Kunert, G.; Phillips, M.A.; Gershenzon, J.; Schmidt, A. Bark Beetle Attack History Does Not Influence the Induction of Terpene and Phenolic Defenses in Mature Norway Spruce (Picea abies) Trees by the Bark Beetle-Associated Fungus Endoconidiophora polonica. Front. Plant Sci. 2022, 13, 892907. [Google Scholar] [CrossRef]
- Manzoor, M.; Yang, L.; Wu, S.; El-Shafie, H.; Haider, M.S.; Ahmad, J.N. Feeding preference of Rhynchophorus ferrugineus (Oliver) (Coleoptera: Curculionidae) on different date palm cultivars and host biochemical responses to its infestation. Bull. Entom. Res., Lond. 2022, 112, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Sau, A.K.; Dhillon, M.K.; Trivedi, N. Activation of antioxidant defense in maize in response to attack by Sesamia inferens (Walker). Phytoparasitica 2022, 50, 1043–1058. [Google Scholar] [CrossRef]
- Khan, A.L.; Asaf, S.; Khan, A.; Khan, A.; Imran, M.; Al-Harrasi, A.; Lee, I.-J.; Al-Rawahi, A. Transcriptomic analysis of Dubas bug (Ommatissus lybicus Bergevin) infestation to Date Palm. Sci. Rep. 2020, 10, 11505. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, S.; Xu, X.; Korpelainen, H.; Li, C. Changes in antioxidant enzyme activities and isozyme profiles in leaves of male and female Populus cathayana infected with Melampsora larici-populina. Tree Physiol. 2010, 30, 116–128. [Google Scholar] [CrossRef]
- Li, L.M.; Men, X.Y.; Ye, B.H.; Yu, Y.; Zhang, A.S.; Li, L.L.; Zhou, X.H.; Zhuang, Q.Y. Defense enzyme activity of Winter Jujube at different stages induced by the damage of Apolygus lucorum. Sci. Agric. Sin. 2014, 47, 191–198. [Google Scholar] [CrossRef]
- Kumari, A.; Goyal, M.; Mittal, A.; Kumar, R. Defensive capabilities of contrasting sorghum genotypes against Atherigona soccata (Rondani) infestation. Protoplasma 2022, 259, 809–822. [Google Scholar] [CrossRef]
- Nikolic, N.; Pilipovic, A.; Drekic, M.; Kojic, D.; Poljakovic-Pajnik, L.; Orlovic, S.; Arsenov, D. Physiological responses of pedunculate oak (Quercus robur L.) to Corythucha arcuata (Say, 1832) attack. Arch. Biol. Sci. 2019, 71, 167–176. [Google Scholar] [CrossRef]
- Kunkel, B.N.; Brooks, D.M. Cross talk between signaling pathways in pathogen defense. Curr. Opin. Plant Biol. 2002, 5, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Eldhuset, T.D.; Nagy, N.E.; Volařík, D.; Børja, I.; Gebauer, R.; Yakovlev, I.A.; Krokene, P. Drought affects tracheid structure, dehydrin expression, and above- and belowground growth in 5-year-old Norway spruce. Plant Soil. 2013, 366, 305–320. [Google Scholar] [CrossRef]
- Jackson, G.E.; Irvine, J.; Grace, J.; Khalil, A.A.M. Abscisic acid concentrations and fluxes in droughted conifer saplings. Plant Cell Environ. 1995, 18, 13–22. [Google Scholar] [CrossRef]
- Tan, W.; Blake, T.J. Drought tolerance, abscisic acid and electrolyte leakage in fast-and slow-growing black spruce (Picea mariana) progenies. Physiol. Plant. 1993, 89, 817–823. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, G.; Sun, T.; Wang, L.; Xu, Q.; Liu, J.; Gao, B. Metabolites and Plant Hormones Related to the Resistance Response to Feeding Stimulation and Leaf Clipping Control in Chinese Pine (Pinus tabuliformis Carr.). Curr. Issues Mol. Biol. 2023, 45, 1086–1099. [Google Scholar] [CrossRef] [PubMed]
- Hudgins, J.W.; Franceschi, V.R. Methyl jasmonate-induced ethylene production is responsible for conifer phloem defense responses and reprogramming of stem cambial zone for traumatic resin duct formation. Plant Physiol. 2004, 135, 2134–2149. [Google Scholar] [CrossRef] [PubMed]
- Zlobin, I.E.; Vankova, R.; Pashkovskiy, P.P.; Dobrev, P.; Kartashov, A.V.; Ivanov, Y.V.; Kuznetsov, V.V. Profiles of endogenous phytohormones and expression of some hormone-related genes in Scots pine and Norway spruce seedlings under water deficit. Plant Physiol. Biochem. 2020, 151, 457–468. [Google Scholar] [CrossRef]



| Tree | Locality | Coordinates | Area (ha) | Annual Temperature * (°C) | Annual Precipitation ** (mm) |
|---|---|---|---|---|---|
| Beech (Fagus sylvatica) | Buková hora | N 50.67662 E 14.21815 | 5.54 | 9.6 8.6 1.0 | 524 640 82 |
| Vrabinec | N 50.71306 E 14.2183 | 7.53 | |||
| Pine (Pinus sylvestris) | Stará Boleslav | N 50.21140 E 14.70668 | 1.66 | 10.0 9.0 1.0 | 618 583 106 |
| Podbrahy | N 50.22586 E 14.74161 | 0.64 | |||
| Fir (Abies alba) | Rychnov nad Kněžnou | N 50.15704 E 16.33344 | 1.32 | 9.0 8.2 0.8 | 679 732 93 |
| Nové Město nad Metují | N 50.34794 E 16.16489 | 1.49 |
| Pine | Beech | Fir | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Infested | Uninfested | p | Infested | Uninfested | p | Infested | Uninfested | p | ||
| Parameter | Unit | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| neoxanthin | μg/g DW | 42.0 (20.8) | 67.0 (34.9) | 28.7 (5.75) | 35.5 (7.18) | 21.0 (3.31) | 26.9 (3.40) | |||
| violaxanthin | μg/g DW | 4.36 (1.26) | 6.60 (2.10) | 23.4 (6.16) | 31.7 (5.66) | 9.57 (1.32) | 9.46 (1.01) | |||
| antheraxanthin | μg/g DW | 0.705 (0.212) | 0.941 (0.197) | 4.76 (1.22) | 4.33 (0.826) | 1.20 (0.303) | 0.751 (0.143) | |||
| zeaxanthin | μg/g DW | 0.953 (0.443) | 0.853 (0.180) | 8.39 (1.96) | 6.27 (1.03) | 2.51 (0.670) | 2.30 (0.526) | |||
| b-carotene | μg/g DW | 75.6 (21.5) | 110 (49.0) | 12.1 (2.60) | 14.6 (2.19) | 3.72 (0.685) | 4.01 (1.04) | |||
| chl a/b ratio | 2.08 (0.246) | 2.19 (0.373) | 2.37 (0.143) | 2.38 (0.161) | 2.49 (0.112) | 2.74 (0.143) | ||||
| de-epoxidation state | 0.225 (0.087) | 0.170 (0.057) | 0.300 (0.050) | 0.205 (0.030) | 0.233 (0.034) | 0.213 (0.032) | ||||
| xanthophyll cycle pigments | μg/g DW | 6.02 (1.26) | 8.39 (2.04) | 36.5 (7.45) | 42.5 (6.19) | 13.3 (1.98) | 12.5 (1.36) | |||
| lutein | μg/g DW | 80.3 (20.9) | 97.3 (17.9) | 138 (16.4) | 156 (18.3) | 70.5 (8.16) | 85.3 (7.69) | |||
| chlorophyll a | μg/g DW | 101 (17.9) | 123 (20.6) | 199 (22.2) | 231 (33.1) | 51.3 (6.27) | 60.0 (5.66) | |||
| chlorophyll b | μg/g DW | 208 (39.9) | 269 (60.9) | 469 (45.1) | 547 (82.0) | 128 (15.6) | 164 (14.2) | |||
| total chlorophyll | μg/g DW | 310 (55.4) | 392 (76.7) | 667 (65.2) | 777 (113) | 179 (21.6) | 224 (19.2) | |||
| total carotenoids | μg/g DW | 204 (58.3) | 283 (93.6) | 216 (20.1) | 249 (23.4) | 108 (12.6) | 130 (12.2) | |||
| total phenol | µg GAE/g DW | 19.1 (4.39) | 19.5 (3.65) | 12.6 (3.91) | 12.2 (4.25) | 13.5 (4.07) | 11.7 (2.89) | |||
| DPPH radical scavenging capacity | µM TE/g DW | 90.4 (18.5) | 86.3 (22.5) | 75.9 (16.9) | 78.8 (11.5) | 81.6 (11.8) | 81.7 (8.43) | |||
| flavonoids (alkalic matrix) | µg R/g DW | 24.9 (8.70) | 25.1 (6.29) | 51.7 (21.7) | 44.4 (18.6) | 28.3 (10.2) | 30.1 (7.93) | |||
| flavonoids (acidic matrix) | µg R/g DW | 11.8 (3.06) | 10.1 (3.28) | 12.8 (3.21) | 11.8 (2.02) | 11.8 (1.73) | 12.5 (1.33) | |||
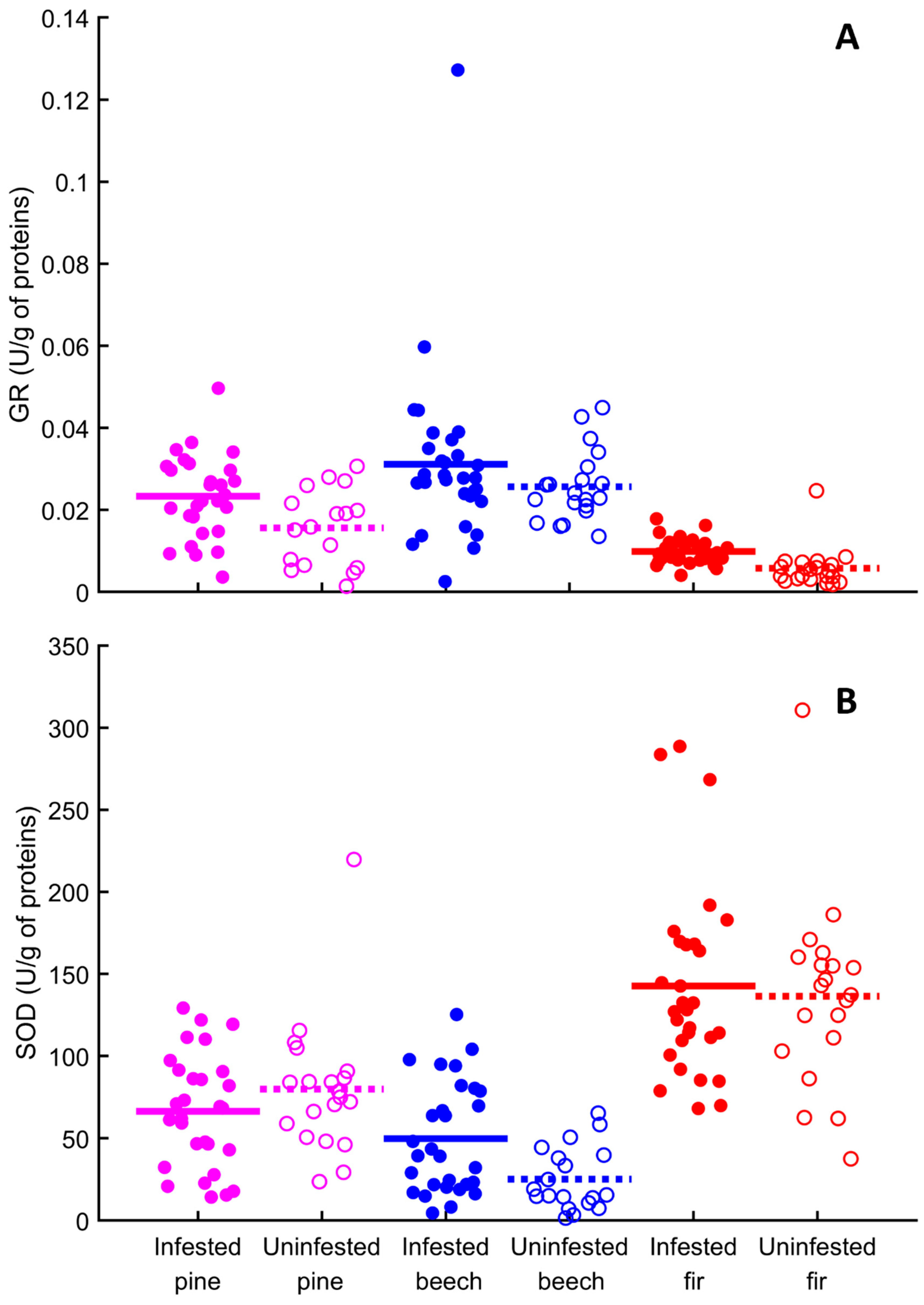
| GST | U/mg proteins | 0.021 (0.010) | 0.022 (0.010) | 0.033 (0.019) | 0.026 (0.014) | 0.016 (0.005) | 0.015 (0.004) | |||
| GR | U/mg proteins | 0.023 (0.010) | 0.016 (0.009) | 0.031 (0.021) | 0.026 (0.009) | 0.010 (0.003) | 0.006 (0.005) | |||
| GPX (POX) | U/mg proteins | 0.002 (0.003) | 0.003 (0.002) | 0.007 (0.003) | 0.006 (0.005) | 0.004 (0.003) | 0.003 (0.001) | |||
| APX | U/mg proteins | 0.065 (0.044) | 0.068 (0.058) | 0.093 (0.043) | 0.098 (0.042) | 0.176 (0.058) | 0.162 (0.054) | |||
| CAT | U/mg proteins | 8.59 (5.13) | 9.11 (6.78) | 34.6 (22.8) | 31.8 (8.94) | 5.62 (1.63) | 4.68 (2.13) | |||
| SOD | U/mg proteins | 66.4 (34.4) | 79.9 (41.0) | 49.8 (33.7) | 25.1 (19.2) | 143 (58.5) | 136 (57.0) | |||
| ABA | nmol/g FW | 5.83 (4.32) | 10.77 (4.56) | 0.451 (0.281) | 0.357 (0.284) | 2.54 (2.12) | 1.81 (1.16) | |||
| ABA-Me | pmol/g FW | 12.6 (8.29) | 7.43 (6.26) | 1.02 (1.55) | 0.854 (0.807) | 1.13 (2.48) | 0.317 (0.341) | |||
| ABA-GE | nmol/g FW | 49.08 (25.83) | 65.41 (28.13) | 2.49 (1.15) | 2.32 (1.46) | 27.77 (21.66) | 19.66 (10.13) | |||
| PA | pmol/g FW | 88.4 (54.6) | 90.8 (82.1) | 31.1 (49.3) | 26.0 (31.3) | 1536 (1414) | 1105 (634) | |||
| DPA | nmol/g FW | 0.040 (0.040) | 0.039 (0.037) | 0.125 (0.136) | 0.207 (0.227) | 11.20 (13.40) | 17.18 (42.59) | |||
| 7OH-ABA | pmol/g FW | 116 (68.9) | 166 (112) | 28.6 (43.6) | 27.7 (37.3) | 1139 (927) | 809 (401) | |||
| 9OH-ABA | pmol/g FW | 61.8 (37.8) | 65.9 (40.5) | 15.6 (16.5) | 13.6 (14.2) | 1417 (1305) | 1020 (585) | |||
| JA | pmol/g FW | 106 (111) | 219 (180) | 1431 (1292) | 970 (911) | 79.4 (95.0) | 32.0 (42.4) | |||
| JA-Ile | pmol/g FW | 88.7 (87.9) | 162 (129) | 337 (341) | 460 (514) | 31.8 (35.5) | 10.9 (22.6) | |||
| JA-Me | pmol/g FW | 74.1 (47.9) | 106 (58.7) | 95.2 (66.0) | 68.3 (53.6) | 32.9 (29.1) | 23.3 (18.7) | |||
| DiH-JA | pmol/g FW | 21.9 (18.4) | 28.1 (20.3) | 36.8 (29.1) | 46.0 (61.3) | 17.7 (17.4) | 15.8 (18.0) | |||
| tZ | pmol/g FW | 0.643 (0.540) | 0.534 (0.525) | 0.494 (0.382) | 0.370 (0.173) | 0.647 (0.528) | 0.545 (0.184) | |||
| iP | pmol/g FW | 2.05 (1.39) | 2.26 (1.49) | 3.31 (1.48) | 3.30 (1.46) | 1.11 (0.693) | 0.819 (0.618) | |||
| DZ | pmol/g FW | 2.01 (1.57) | 1.83 (1.97) | 0.718 (0.675) | 0.966 (1.03) | 1.61 (1.33) | 1.34 (1.14) | |||
| cZ | pmol/g FW | 1.31 (0.809) | 0.913 (0.695) | 0.471 (0.814) | 0.283 (0.185) | 0.446 (0.238) | 0.443 (0.440) | |||
| tZR | pmol/g FW | 5.27 (2.09) | 4.35 (2.15) | 1.21 (0.691) | 1.25 (0.681) | 7.89 (2.29) | 6.77 (2.40) | |||
| iPR | pmol/g FW | 6.36 (3.31) | 6.21 (4.25) | 4.66 (1.75) | 5.83 (1.58) | 1.98 (1.57) | 1.28 (0.963) | |||
| DZR | pmol/g FW | 5.98 (2.82) | 6.51 (3.35) | 0.913 (0.942) | 0.648 (0.374) | 6.27 (2.47) | 7.01 (3.49) | |||
| cZR | pmol/g FW | 2.34 (1.39) | 2.38 (0.918) | 0.694 (0.375) | 0.748 (0.387) | 3.03 (1.39) | 2.28 (0.915) | |||
| tZOG | pmol/g FW | 2.23 (1.59) | 0.770 (0.648) | 1.26 (1.02) | 1.36 (1.18) | 1.08 (0.685) | 0.934 (0.510) | |||
| tZROG | pmol/g FW | 26.9 (15.6) | 16.1 (16.8) | 0.821 (0.790) | 1.12 (1.29) | 10.4 (4.37) | 9.97 (4.44) | |||
| DZOG | pmol/g FW | 11.6 (6.74) | 3.68 (3.06) | 0.844 (0.598) | 0.822 (0.720) | 2.07 (1.06) | 2.09 (1.25) | |||
| DZROG | pmol/g FW | 5.71 (2.44) | 4.32 (2.37) | 2.52 (2.17) | 2.88 (3.10) | 5.66 (3.54) | 6.31 (4.42) | |||
| cZOG | pmol/g FW | 1.17 (0.720) | 1.18 (0.727) | 0.488 (0.415) | 0.547 (0.577) | 0.993 (1.17) | 0.834 (1.60) | |||
| cZROG | pmol/g FW | 31.8 (13.2) | 28.8 (14.9) | 0.360 (0.261) | 0.472 (0.306) | 21.5 (10.6) | 20.8 (8.48) | |||
| DZ7G | pmol/g FW | 0.749 (1.02) | 0.323 (0.533) | 11.5 (10.4) | 8.68 (8.94) | 4.89 (8.43) | 3.06 (2.77) | |||
| tZRMP | pmol/g FW | 1.71 (1.83) | 0.905 (1.19) | 21.7 (12.5) | 20.0 (12.3) | 4.20 (2.34) | 4.55 (2.97) | |||
| iPRMP | pmol/g FW | 4.30 (2.41) | 2.33 (1.19) | 3.11 (3.18) | 5.09 (4.62) | 9.09 (3.17) | 10.1 (5.30) | |||
| DZRMP | pmol/g FW | 5.87 (4.52) | 4.46 (3.92) | 2.39 (2.60) | 4.00 (4.05) | 4.90 (3.00) | 4.97 (3.41) | |||
| cZRMP | pmol/g FW | 7.53 (4.58) | 6.96 (3.59) | 5.01 (3.81) | 6.72 (5.51) | 16.1 (7.01) | 15.4 (5.01) | |||
| MeS-Z | pmol/g FW | 0.492 (0.847) | 1.16 (2.25) | 0.957 (1.34) | 0.748 (1.13) | 0.479 (0.940) | 0.409 (0.503) | |||
| MeS-ZR | pmol/g FW | 21.1 (7.60) | 21.6 (5.64) | 59.8 (18.3) | 49.3 (15.6) | 15.0 (6.31) | 13.6 (4.51) | |||
| MeS-iP | pmol/g FW | 0.297 (0.562) | 1.00 (2.17) | 0.849 (1.22) | 0.599 (0.833) | 0.531 (1.35) | 0.315 (0.518) | |||
| SA | pmol/g FW | 229 (122) | 206 (78.3) | 763 (267) | 587 (271) | 341 (506) | 336 (393) | |||
| BzA | pmol/g FW | 344 (171) | 465 (215) | 1046 (933) | 722 (408) | 495 (711) | 395 (271) | |||
| PAA | pmol/g FW | 547 (213) | 468 (175) | 488 (222) | 410 (225) | 543 (381) | 650 (369) | |||
| PAAM | pmol/g FW | 2.97 (1.57) | 3.93 (2.00) | 4.67 (2.34) | 4.33 (1.44) | 8.21 (10.4) | 7.47 (8.62) | |||
| IAA | pmol/g FW | 140 (91.4) | 162 (90.8) | 87.1 (64.1) | 55.1 (55.5) | 20.8 (16.0) | 13.3 (6.91) | |||
| IAA-Me | pmol/g FW | 30.3 (30.5) | 118 (75.0) | 161 (168) | 86.8 (69.1) | 141 (155) | 489 (341) | |||
| IAA-Glu | pmol/g FW | 0.774 (2.12) | 1.48 (2.58) | 0.421 (0.636) | 0.550 (0.692) | 0.098 (0.101) | 0.053 (0.045) | |||
| IAA-Ala | pmol/g FW | 0.380 (0.545) | 0.209 (0.345) | 4.70 (5.54) | 5.82 (6.84) | 4.34 (2.98) | 4.05 (2.13) | |||
| IAA-GE | pmol/g FW | 11.6 (10.3) | 11.0 (9.46) | 5.58 (4.05) | 5.59 (3.49) | 15.1 (6.93) | 16.3 (6.34) | |||
| OxIAA | pmol/g FW | 51.5 (42.3) | 56.2 (31.8) | 18.8 (19.0) | 15.8 (17.6) | 1.04 (1.68) | 0.695 (1.11) | |||
| 5OH-IAA | pmol/g FW | 0.591 (0.639) | 0.771 (1.20) | 2.78 (1.61) | 3.98 (2.10) | 3.65 (5.32) | 1.31 (3.92) | |||
| OxIAA-GE | pmol/g FW | 108 (105) | 63.6 (57.6) | 78.4 (31.2) | 111 (52.2) | 13.6 (6.56) | 14.0 (3.12) | |||
| I3A | pmol/g FW | 15.5 (14.2) | 19.5 (9.25) | 22.7 (14.2) | 22.4 (10.5) | 2.63 (2.21) | 2.66 (2.80) | |||
| ILacA | pmol/g FW | 1.03 (1.50) | 1.35 (1.03) | 1.37 (1.38) | 1.77 (1.61) | 1.74 (1.30) | 1.73 (0.919) | |||
| OxIAA-Glu | pmol/g FW | 1.15 (1.80) | 0.830 (0.772) | 41.9 (28.2) | 45.9 (20.1) | 1.11 (1.18) | 1.05 (1.29) | |||
| OxIAA-Asp | pmol/g FW | 7.11 (7.12) | 5.35 (4.39) | 3808 (1487) | 5380 (2310) | 6.02 (3.79) | 5.97 (2.57) | |||
| extremely significant increase in infected plants | p ≤ 0.001 | significant decrease in infected plants | p ≤ 0.05 | ||
| very significant increase in infected plants | p ≤ 0.01 | very significant decrease in infected plants | p ≤ 0.01 | ||
| significant increase in infected plants | p ≤ 0.05 | extremely significant decrease in infected plants | p ≤ 0.001 | ||
| insignificant result | p > 0.05 | white |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soudek, P.; Podlipná, R.; Langhansová, L.; Moťková, K.; Dvořáková, M.; Petrová, Š.; Haisel, D.; Satarova, T.M.; Dobrev, P.I.; Gaudinová, A.; et al. Stress Responses to Bark Beetle Infestations among Pine (Pinus sylvestris), Fir (Abies alba), and Beech (Fagus sylvatica) Trees. Forests 2024, 15, 1761. https://doi.org/10.3390/f15101761
Soudek P, Podlipná R, Langhansová L, Moťková K, Dvořáková M, Petrová Š, Haisel D, Satarova TM, Dobrev PI, Gaudinová A, et al. Stress Responses to Bark Beetle Infestations among Pine (Pinus sylvestris), Fir (Abies alba), and Beech (Fagus sylvatica) Trees. Forests. 2024; 15(10):1761. https://doi.org/10.3390/f15101761
Chicago/Turabian StyleSoudek, Petr, Radka Podlipná, Lenka Langhansová, Kateřina Moťková, Marcela Dvořáková, Šárka Petrová, Daniel Haisel, Tetiana M. Satarova, Petre I. Dobrev, Alena Gaudinová, and et al. 2024. "Stress Responses to Bark Beetle Infestations among Pine (Pinus sylvestris), Fir (Abies alba), and Beech (Fagus sylvatica) Trees" Forests 15, no. 10: 1761. https://doi.org/10.3390/f15101761









