Exploring Functional Gene XsPDAT1’s Involvement in Xanthoceras sorbifolium Oil Synthesis and Its Acclimation to Cold Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions, and Low-Temperature Treatments
2.2. Gene Cloning and qRT-PCR
2.3. Plant Transformation
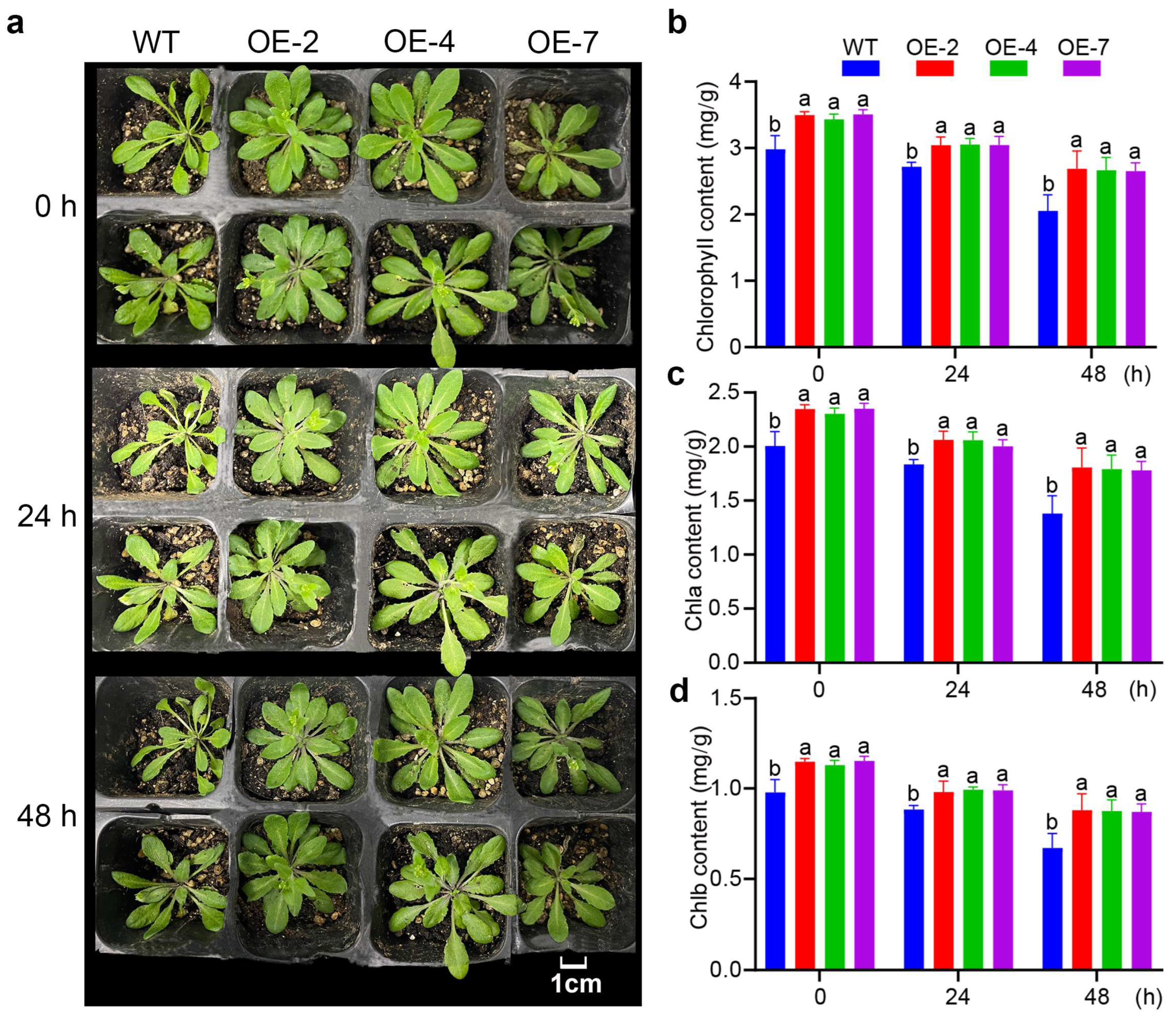
2.4. Chlorophyll Content Measurements
2.5. Cell Membrane Permeability Assays
2.6. Plant Peroxidase Activity Analysis
2.7. Free Proline Content Determination
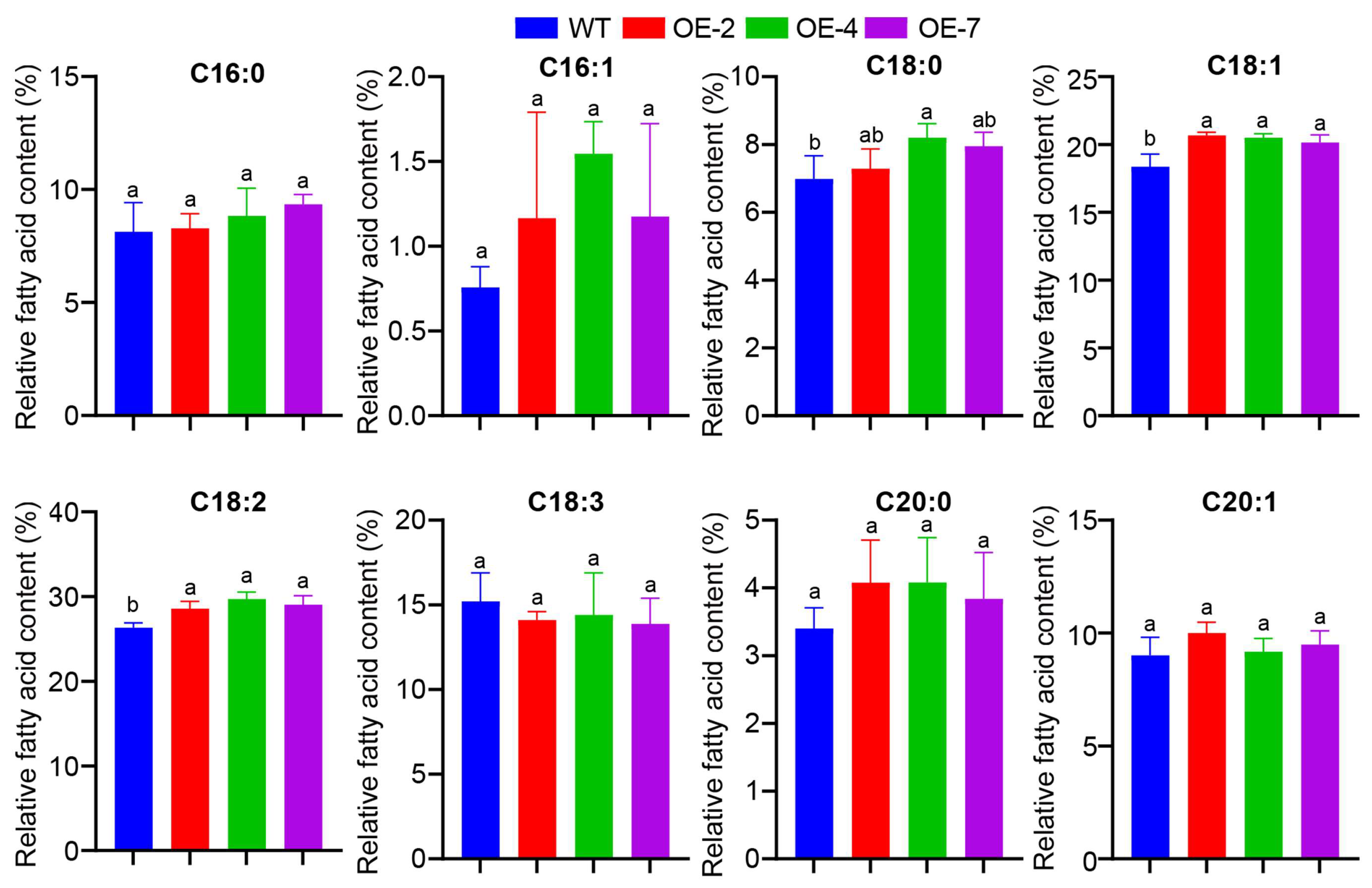
2.8. Oil Content and Fatty Acid Determination
2.9. Statistical Analyses
3. Results
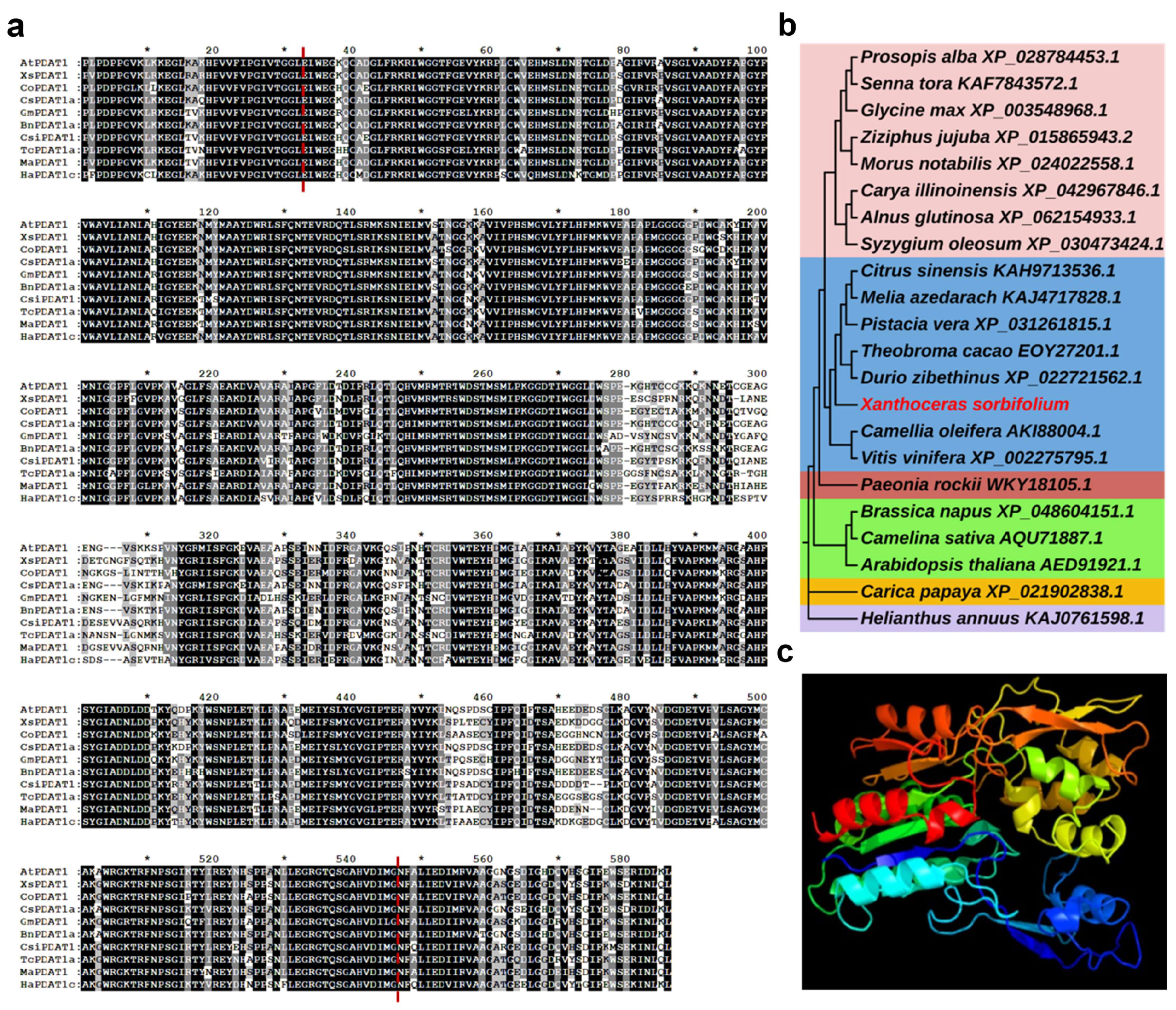
3.1. Gene Cloning and Sequence Analysis of XsPDAT1
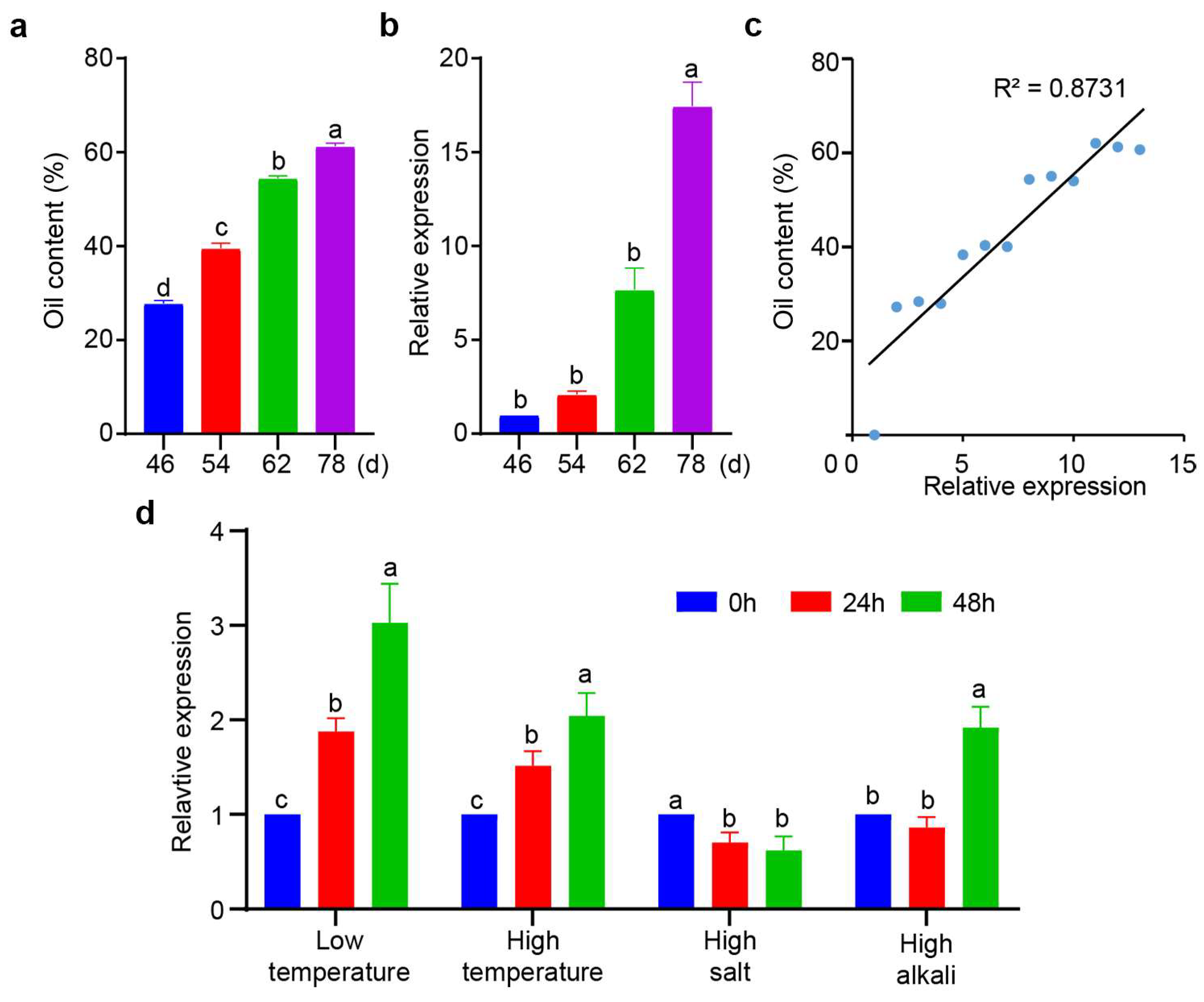
3.2. Expression Pattern of XsPDAT1
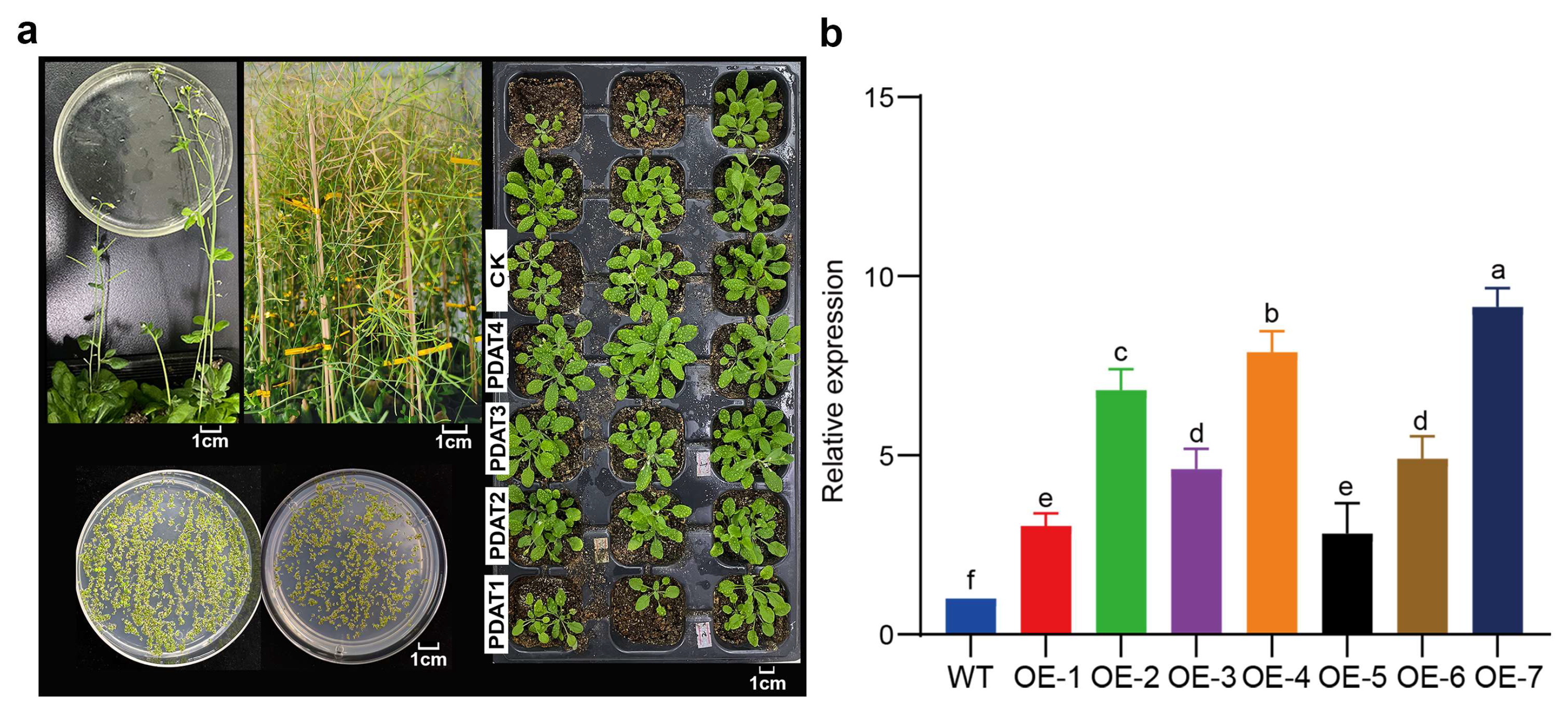
3.3. Genetic Transformation and Screening
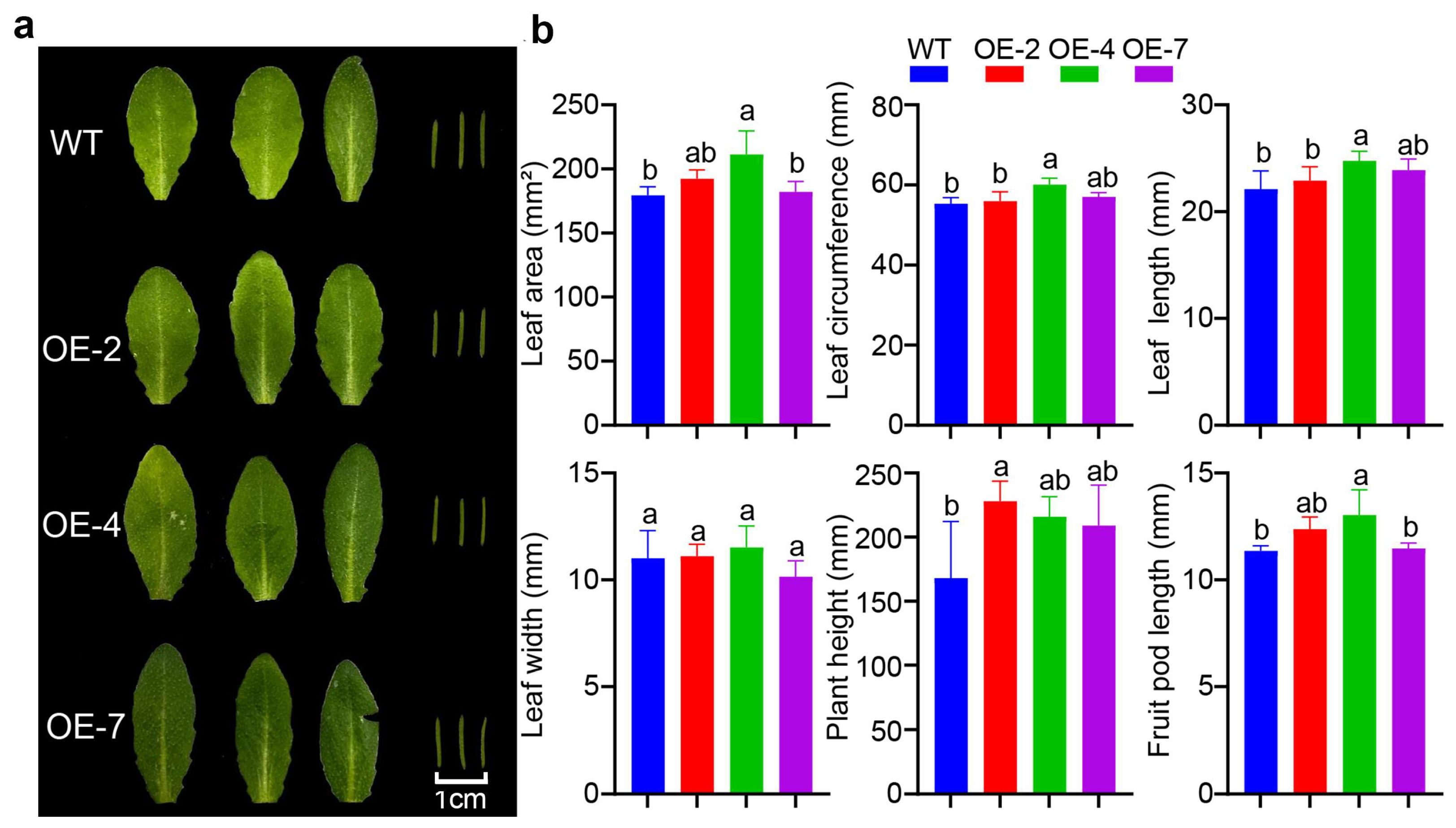
3.4. Changes in Morphology of Transgenic A. thaliana Lines
3.5. XsPDAT1 Confers Low-Temperature Resistance in Transgenic A. thaliana
3.6. XsPDAT1 Enhances Cellular Homeostasis of A. thaliana in Response to Low-Temperature Stress
3.7. Analysis of Fatty Acid Composition of Transgenic A. thaliana Seeds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, C.Y.; Ha, W.; Lin, Y.; Jiang, K.; Yang, J.L.; Shi, Y.P. Polyphenols isolated from Xanthoceras sorbifolia husks and their anti-tumor and radical-scavenging activities. Molecules 2016, 21, 1694. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lei, Z.; Cao, J.; Zhang, W.; Wu, R.; Cao, F.; Guo, Q.; Wang, J. Traditional uses, phytochemistry, pharmacology and current uses of underutilized Xanthoceras sorbifolium bunge: A review. J. Ethnopharmacol. 2022, 283, 114747. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gao, P.; Wang, S.; Ruan, Y.; Zhong, W.; Hu, C.; He, D. Comparison of different extraction processes on the physicochemical properties, nutritional components and antioxidant ability of Xanthoceras sorbifolia Bunge kernel oil. Molecules 2022, 27, 4185. [Google Scholar] [CrossRef]
- Ruan, C.J.; Yan, R.; Wang, B.X.; Mopper, S.; Guan, W.K.; Zhang, J. The importance of yellow horn (Xanthoceras sorbifolia) for restoration of arid habitats and production of bioactive seed oils. Ecol. Eng. 2017, 99, 504–512. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, J.N.; Fang, H.; Dong, Y.; Wang, C.; Bao, Y.; Hou, W.; Zhou, R.; Ma, X.; Gai, S.; et al. Genomic and transcriptomic analyses provide insights into valuable fatty acid biosynthesis and environmental adaptation of yellowhorn. Front. Plant Sci. 2022, 13, 991197. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Li, X.; Zhang, H.; Zhou, D.; Wang, W.; Li, W.; Zhang, X.; Li, X.; Hou, Y.; et al. Bioactive phenols as potential neuroinflammation inhibitors from the leaves of Xanthoceras sorbifolia Bunge. Bioorg. Med. Chem. Lett. 2016, 26, 5018–5023. [Google Scholar] [CrossRef]
- Dahlqvist, A.; Stahl, U.; Lenman, M.; Banas, A.; Lee, M.; Sandager, L.; Ronne, H.; Stymne, S. Phospholipid: Diacylglycerol acyltransferase: An enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc. Natl. Acad. Sci. USA 2000, 97, 6487–6492. [Google Scholar] [CrossRef]
- Liu, X.Y.; Ouyang, L.L.; Zhou, Z.G. Phospholipid: Diacylglycerol acyltransferase contributes to the conversion of membrane lipids into triacylglycerol in Myrmecia incisa during the nitrogen starvation stress. Sci. Rep. 2016, 6, 26610. [Google Scholar] [CrossRef]
- Mueller, S.P.; Unger, M.; Guender, L.; Fekete, Á.; Mueller, M.J. Phospholipid: Diacylglycerol acyltransferase-mediated triacylglyerol synthesis augments basal thermotolerance. Plant Physiol. 2017, 175, 486–497. [Google Scholar] [CrossRef]
- Niazian, M.; Sadat-Noori, S.A.; Tohidfar, M.; Mortazavian, S.M.M.; Sabbatini, P. Betaine aldehyde dehydrogenase (BADH) vs. Flavodoxin (Fld): Two important genes for enhancing plants stress tolerance and productivity. Front. Plant Sci. 2021, 12, 650215. [Google Scholar] [CrossRef]
- Yoon, K.; Han, D.; Li, Y.; Sommerfeld, M.; Hu, Q. Phospholipid: Diacylglycerol acyltransferase is a multifunctional enzyme involved in membrane lipid turnover and degradation while synthesizing triacylglycerol in the unicellular green microalga Chlamydomonas reinhardtii. Plant Cell 2012, 24, 3708–3724. [Google Scholar] [CrossRef] [PubMed]
- Klińska-Bachor, S.; Kdzierska, S.; Demski, K.; Banaś, A. Phospholipid: Diacylglycerol acyltransferase1-overexpression stimulates lipid turnover, oil production and fitness in cold-grown plants. BMC Plant Biol. 2023, 23, 370. [Google Scholar] [CrossRef] [PubMed]
- Stahl, U.; Carlsson, A.S.; Lenman, M.; Dahlqvist, A.; Huang, B.; Banaś, W.; Banaś, A.; Stymne, S. Cloning and functional characterization of a phospholipid: Diacylglycerol acyltransferase from Arabidopsis. Plant Physiol. 2004, 135, 1324–1335. [Google Scholar] [CrossRef]
- Van Erp, H.; Bates, P.D.; Burgal, J.; Shockey, J.; Browse, J. Castor phospholipid: Diacylglycerol acyltransferase facilitates efficient metabolism of hydroxy fatty acids in transgenic Arabidopsis. Plant Physiol. 2011, 15, 683–693. [Google Scholar] [CrossRef]
- Pan, X.; Siloto, R.M.P.; Wickramarathna, A.D.; Mietkiewska, E.; Weselake, R.J. Identification of a pair of phospholipid: Diacylglycerol acyltransferases from developing flax (Linum usitatissimum L.) seed catalyzing the selective production of trilinolenin. J. Biol. Chem. 2013, 288, 24173–24188. [Google Scholar] [CrossRef]
- Yuan, L.; Mao, X.; Zhao, K.; Ji, X.; Ji, C.; Xue, J.; Li, R. Characterisation of phospholipid: Diacylglycerol acyltransferases (PDATs) from Camelina sativa and their roles in stress responses. Biol. Open 2017, 6, 1024–1034. [Google Scholar]
- Zang, X.; Geng, X.; Ma, L.; Wang, N.; Pei, W.; Wu, M.; Zhang, J.; Yu, J. A genome-wide analysis of the phospholipid: Diacylglycerol acyltransferase gene family in Gossypium. BMC Genom. 2019, 20, 402. [Google Scholar] [CrossRef]
- Zhou, B.; Fei, W.; Yang, S.; Yang, F.; Qu, G.; Tang, W.; Ou, J.; Peng, D. Alteration of the fatty acid composition of Brassica napus L. via overexpression of phospholipid: Diacylglycerol acyltransferase 1 from Sapium sebiferum (L.) Roxb. Plant Sci. 2020, 298, 110562. [Google Scholar] [CrossRef]
- Voelker, T. Secrets of palm oil biosynthesis revealed. Proc. Natl. Acad. Sci. USA 2011, 108, 12193–12194. [Google Scholar] [CrossRef]
- Guo, H.; Li, Q.; Wang, T.; Hu, Q.; Deng, W.; Xia, X.; Gao, H. XsFAD2 gene encodes the enzyme responsible for the high linoleic acid content in oil accumulated in Xanthoceras sorbifolia seeds. J. Sci. Food Agric. 2014, 94, 482–488. [Google Scholar] [CrossRef]
- Guo, H.H.; Wang, T.T.; Li, Q.Q.; Zhao, N.; Zhang, Y.; Liu, D.; Hu, Q.; Li, F.L. Two novel diacylglycerol acyltransferase genes from Xanthoceras sorbifolia are responsible for its seed oil content. Gene 2013, 527, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Han, S.; Yang, Q.; Wang, J.; Guo, H. Cloning and expression analysis of LEC1 gene from Xanthoceras sorbifolia embryos. J. Beijing For. Univ. 2018, 40, 8–16. [Google Scholar]
- Wang, J.; Zhang, Y.; Yan, X.; Guo, J. Physiological and transcriptomic analyses of yellow horn (Xanthoceras sorbifolia) provide important insights into salt and saline-alkali stress tolerance. PLoS ONE 2020, 15, e0244365. [Google Scholar] [CrossRef]
- Wang, J.; Guo, J.; Zhang, Y.; Yan, X. Integrated transcriptomic and metabolomic analyses of yellow horn (Xanthoceras sorbifolia) in response to cold stress. PLoS ONE 2020, 15, e0236588. [Google Scholar] [CrossRef]
- Wang, J.; Hu, H.F.; Liang, X.Z.; Qamar, M.T.; Zhang, Y.X.; Zhao, J.G.; Ren, H.Q.; Yan, Y.R.; Ding, B.P.; Guo, J.P. High-quality genome assembly and comparative genomic profiling of yellowhorn (Xanthoceras sorbifolia) revealed environmental adaptation footprints and seed oil contents variations. Front. Plant Sci. 2023, 14, 1147946. [Google Scholar] [CrossRef]
- Ding, B.; Li, X.; Lin, Y.; Hu, C.; Yang, R.; Wei, B.; Cao, Y.; Bai, Y.; Yan, R.; Li, L. Study on cloning and bioinformatics of auxin nicotinamide synthase gene GH3 in ‘Yuluxiangli’. J. Shanxi Agric. Univ. (Nat. Sci.) 2024, 44, 14–23. [Google Scholar]
- Ding, B.; Hu, H.; Cao, Y.; Xu, R.; Lin, Y.; Muhammad, T.U.Q.; Song, Y.; He, G.; Han, Y.; Guo, H.; et al. Pear genomes display significant genetic diversity and provide novel insights into the fruit quality traits differentiation. Hortic. Plant J. 2024, 10, 1274–1290. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Jiang, L.; Li, X.X.; Lv, K.; Wang, H.; Li, Z.Y.; Qi, W.; Zhang, L.; Cao, Y.P. Rosaceae phylogenomic studies provide insights into the evolution of new genes. Horti. Plant J. 2024, in press. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, S.; Du, W.; Yue, A.; Li, G.; Ding, Q. The relationship between variation and quality and the accumulation of protein and fat with different-maturing soybean cultivars. Acta Agric. Boreali-Sin. 2004, 19, 33–35. [Google Scholar]
- Zhao, G.; Song, Z.B.; Liu, M.L.; Long, H.X.; Li, Z.; Zhang, L. Cloning and expression analysis of a phospholipid: Diacylglycerol acyltransferase (PDAT) gene in Camellia oleifera. Plant Physiol. 2017, 53, 1619–1628. [Google Scholar]
- Kim, H.U.; Lee, K.R.; Go, Y.S.; Jung, J.H.; Suh, M.C.; Kim, J.B. Endoplasmic reticulum-located PDAT1-2 from Castor bean enhances hydroxy fatty acid accumulation in transgenic plants. Plant Cell Physiol. 2011, 52, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.L.; Moretti, S.; Sicardo, M.D.; García, Ú.; Pérez, A.; Sebastiani, L.; Martínez-Rivas, J.M. Distinct physiological roles of three phospholipid: Diacylglycerol acyltransferase genes in olive fruit with respect to oil accumulation and the response to abiotic stress. Front. Plant Sci. 2021, 12, 751959. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Guo, D.; Xing, G.; Yang, C.; Zhang, Y.; Yang, J.; Niu, L.; Zhong, X.; Zhao, Q.; Cui, Y.; et al. Complete chloroplast genome sequence and comparative and phylogenetic analyses of the cultivated Cyperus esculentus. Diversity 2021, 13, 405. [Google Scholar] [CrossRef]
- Bana’s, W.; Carlsson, A.S.; Bana´s, A. Effect of overexpression of PDAT gene on Arabidopsis growth rate and seed oil content. J. Agric. Sci. 2014, 6, 65–79. [Google Scholar] [CrossRef]
- Demski, K.; Łosiewska, A.; Jasieniecka-Gazarkiewicz, K.; Klińska, S.; Banaś, A. Phospholipid: Diacylglycerol acyltransferase1 overexpression delays senescence and enhances post-heat and cold exposure fitness. Front. Plant Sci. 2020, 11, 611897. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Zang, D.; Cui, H.; Li, R. Identification and expression analysis of phospholipid-diacylglycerol acyltransferase gene (HpPDAT) from Haematococcus pluvialis. J. Shanxi Agric. Sci. 2022, 50, 319–326. [Google Scholar]
- Liao, P.; Lechon, T.; Romsdahl, T.; Woodfield, H.; Fenyk, S.; Fawcett, T.; Wallington, E.; Bates, R.E.; Chye, M.L.; Chapman, K.D.; et al. Transgenic manipulation of triacylglycerol biosynthetic enzymes in B. napus alters lipid-associated gene expression and lipid metabolism. Sci. Rep. 2022, 12, 3352. [Google Scholar] [CrossRef]
- Liu, F.; Yu, S.; Liu, G. Mechanism and research progress of plant lipid regulation under biotic and abiotic stresses. Chin. J. Oil Crop. Sci. 2023, 45, 1062–1072. [Google Scholar]
- Saini, R.; Kumar, S. Genome-wide identification characterization and insilico profiling of genes encoding FAD (fatty acid desaturase) proteins in chickpea (Cicer arietinum L.). Plant Gene 2019, 18, 100180. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Ren, H.; Shi, Z.; Phillip, F.O.; Liu, S.; Zhang, W.; Wang, X.; Bao, X.; Guo, J. Exploring Functional Gene XsPDAT1’s Involvement in Xanthoceras sorbifolium Oil Synthesis and Its Acclimation to Cold Stress. Forests 2024, 15, 1822. https://doi.org/10.3390/f15101822
Wang J, Ren H, Shi Z, Phillip FO, Liu S, Zhang W, Wang X, Bao X, Guo J. Exploring Functional Gene XsPDAT1’s Involvement in Xanthoceras sorbifolium Oil Synthesis and Its Acclimation to Cold Stress. Forests. 2024; 15(10):1822. https://doi.org/10.3390/f15101822
Chicago/Turabian StyleWang, Juan, Hongqian Ren, Zetao Shi, Fesobi Olumide Phillip, Sisi Liu, Weiyang Zhang, Xingqiang Wang, Xueping Bao, and Jinping Guo. 2024. "Exploring Functional Gene XsPDAT1’s Involvement in Xanthoceras sorbifolium Oil Synthesis and Its Acclimation to Cold Stress" Forests 15, no. 10: 1822. https://doi.org/10.3390/f15101822
APA StyleWang, J., Ren, H., Shi, Z., Phillip, F. O., Liu, S., Zhang, W., Wang, X., Bao, X., & Guo, J. (2024). Exploring Functional Gene XsPDAT1’s Involvement in Xanthoceras sorbifolium Oil Synthesis and Its Acclimation to Cold Stress. Forests, 15(10), 1822. https://doi.org/10.3390/f15101822





