Molecular Cloning of QwMYB108 Gene and Its Response to Drought Stress in Quercus wutaishanica Mayr
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Main Reagents
2.3. RNA Extraction and cDNA Synthesis
2.4. Cloning and Sequencing of QwMYB108
2.5. Bioinformatics Analysis of QwMYB108
2.6. The Expression Profile of QwMYB108 of Different Tissues and the Leaves with Different Levels of Drought Stress in Q. wutaishanica
2.7. Subcellular Localization
3. Results
3.1. Gene Cloning of QwMYB108
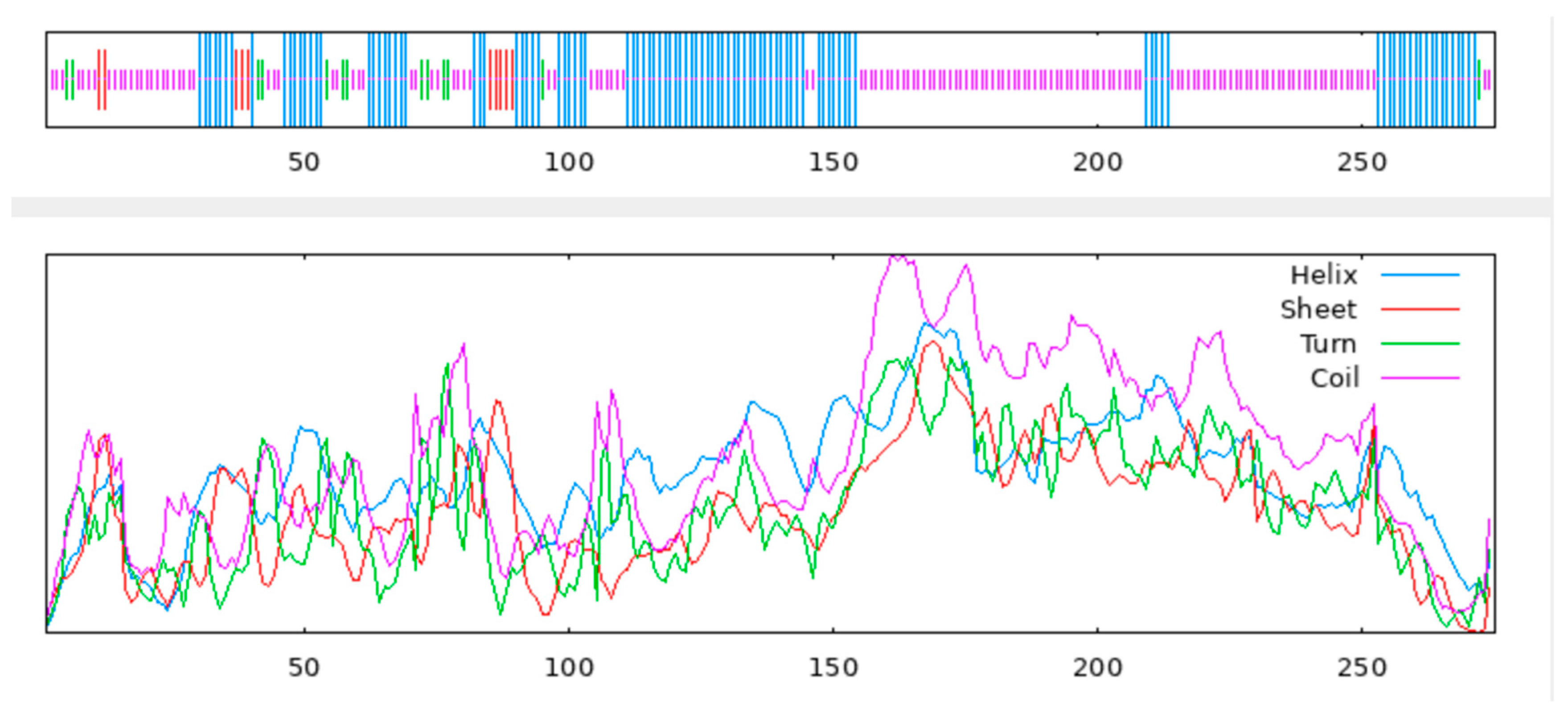
3.2. QwMYB108 Protein Sequence Analysis
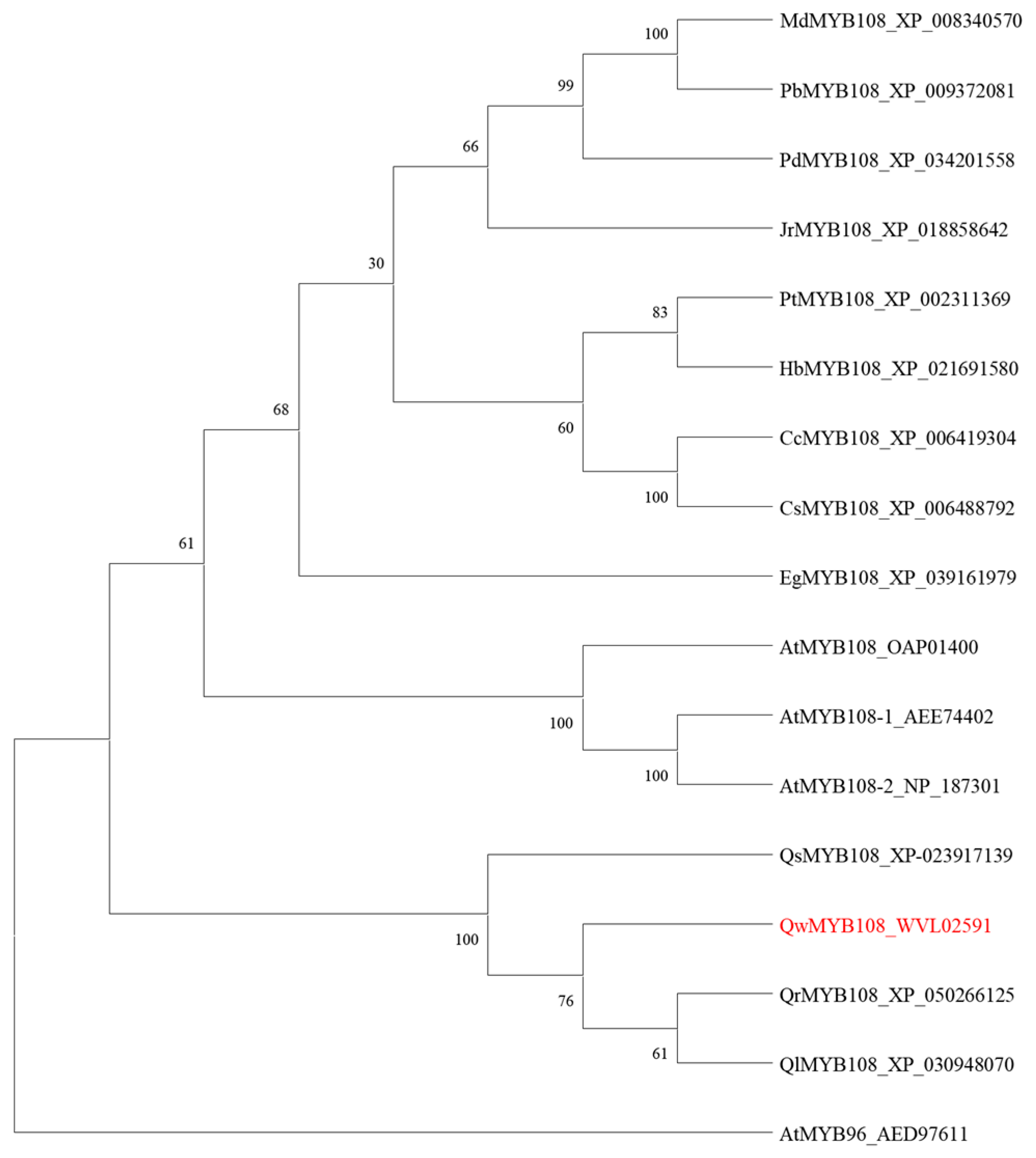
3.3. Phylogenetic Analysis of QwMYB108
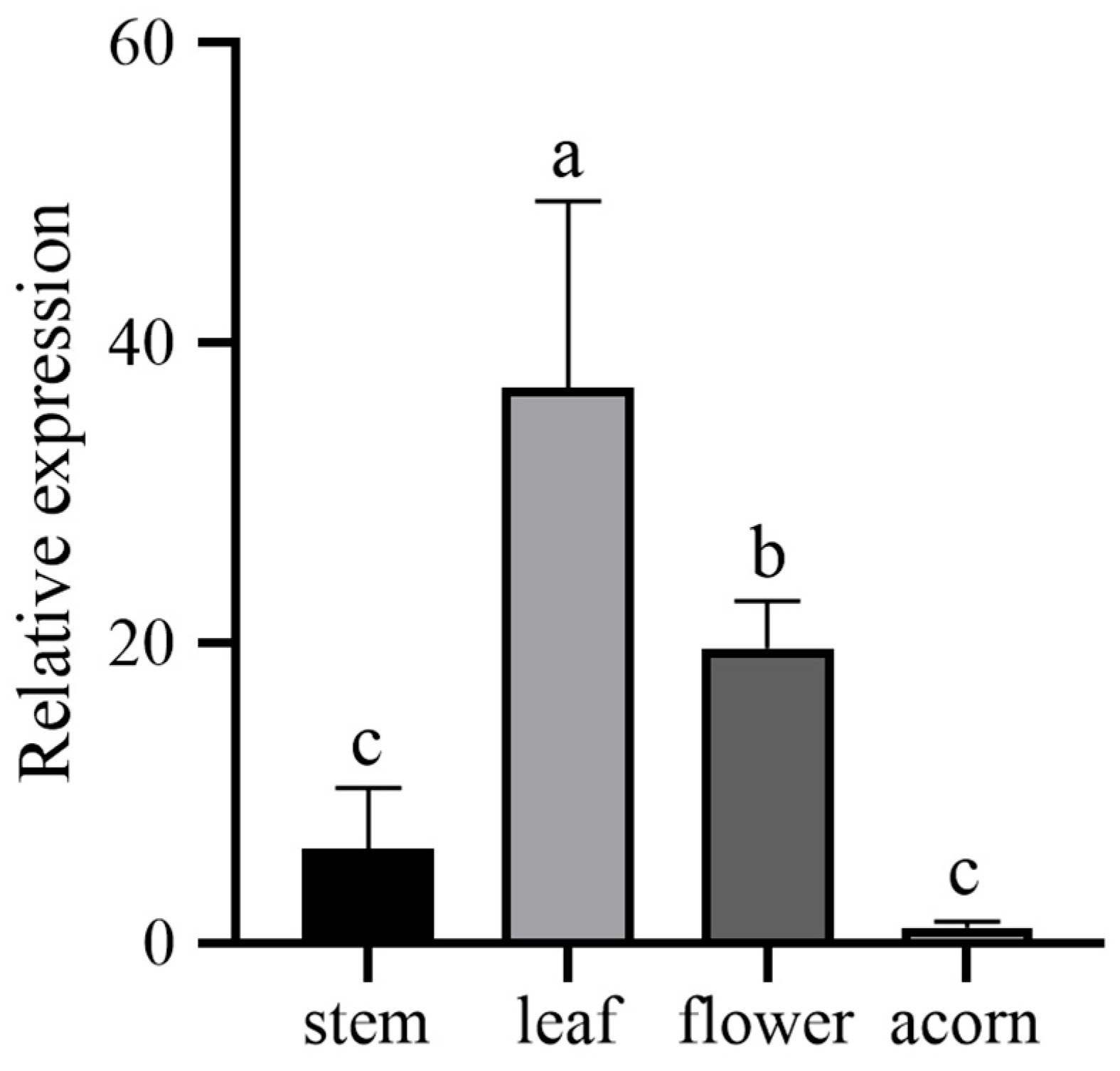
3.4. The Tissue Expression Profile of QwMYB108
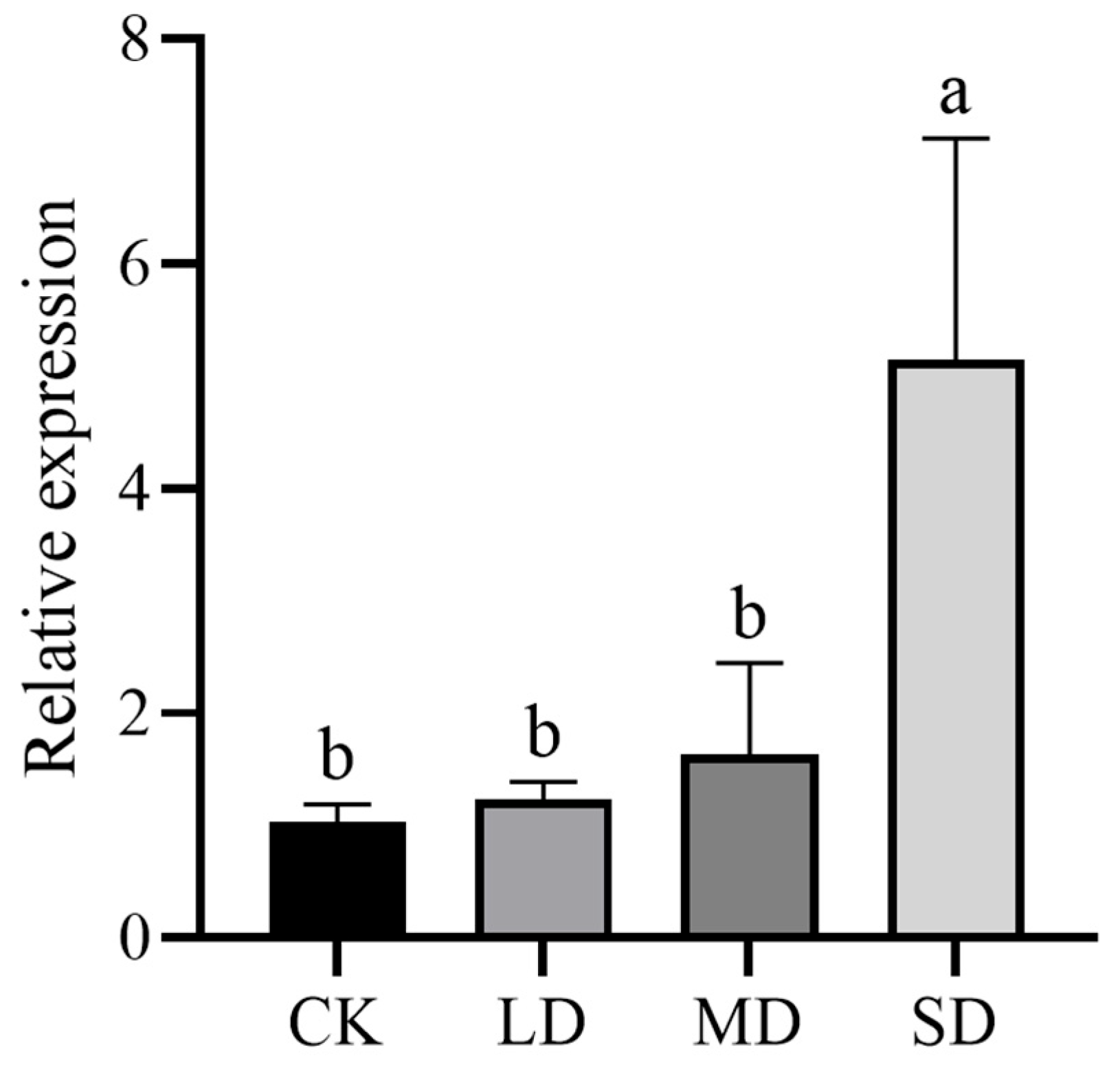
3.5. QwMYB108 Expression under Drought Stress
3.6. Subcellular Localization of QwMYB108
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jump, A.S.; Ruiz-Benito, P.; Greenwood, S.; Allen, C.D.; Kitzberger, T.; Fensham, R.; Martínez-Vilalta, J.; Lloret, F. Structural overshoot of tree growth with climate variability and the global spectrum of drought-induced forest dieback. Glob. Chang. Biol. 2017, 23, 3742–3757. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Serrano, S.M.; Peña-Angulo, D.; Beguería, S.; Domínguez-Castro, F.; Tomás-Burguera, M.; Noguera, I.; Gimeno-Sotelo, L.; El Kenawy, A. Global drought trends and future projections. Philos. Trans. A Math. Phys. Eng. Sci. 2022, 380, 20210285. [Google Scholar] [CrossRef]
- Baldoni, E.; Genga, A.; Cominelli, E. Plant MYB Transcription Factors: Their Role in Drought Response Mechanisms. Int. J. Mol. Sci. 2015, 16, 15811–15851. [Google Scholar] [CrossRef] [PubMed]
- Abdullah-Zawawi, M.R.; Ahmad-Nizammuddin, N.F.; Govender, N.; Harun, S.; Mohd-Assaad, N.; Mohamed-Hussein, Z.A. Comparative genome-wide analysis of WRKY, MADS-box and MYB transcription factor families in Arabidopsis and rice. Sci. Rep. 2021, 11, 19678. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhang, L.; Liu, L.; Tang, X.F.; Yang, W.J.; Wu, Y.M.; Huang, Y.B.; Tang, Y.X. Biochemical and molecular characterization of plant MYB transcription factor family. Biochemistry 2009, 74, 1–11. [Google Scholar] [CrossRef]
- Bemer, M.; van Dijk, A.D.J.; Immink, R.G.H.; Angenent, G.C. Cross-Family Transcription Factor Interactions: An Additional Layer of Gene Regulation. Trends Plant Sci. 2017, 22, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Dong, Q.; Li, C.; Liu, C.; Ma, F. Genome Wide Identification and Characterization of Apple bHLH Transcription Factors and Expression Analysis in Response to Drought and Salt Stress. Front. Plant Sci. 2017, 8, 480. [Google Scholar] [CrossRef] [PubMed]
- Jolma, A.; Yin, Y.; Nitta, K.R.; Dave, K.; Popov, A.; Taipale, M.; Enge, M.; Kivioja, T.; Morgunova, E.; Taipale, J. DNA-dependent formation of transcription factor pairs alters their binding specificity. Nature 2015, 527, 384–388. [Google Scholar] [CrossRef]
- Uimari, A.; Strommer, J. Myb26: A MYB-like protein of pea flowers with affinity for promoters of phenylpropanoid genes. Plant J. 1997, 12, 1273–1284. [Google Scholar] [CrossRef]
- Li, C.; Ng, C.; Fan, L.M. MYB transcription factors, active players in abiotic stress signaling. Environ. Exp. Bot. 2015, 114, 80–91. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Niu, Y.; Zheng, Y. Multiple Functions of MYB Transcription Factors in Abiotic Stress Responses. Int. J. Mol. Sci. 2021, 22, 6125. [Google Scholar] [CrossRef]
- Paz-Ares, J.; Ghosal, D.; Wienand, U.; Peterson, P.A.; Saedler, H. The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J. 1987, 6, 3553–3558. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J. Myb transcription factors: Their role in growth, differentiation and disease. J. Proteins Cell Regul. 2004, 2, 1–2. [Google Scholar]
- Thakur, S.; Vasudev, P.G. MYB transcription factors and their role in Medicinal plants. Mol. Biol. Rep. 2022, 49, 10995–11008. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Cao, A.; Wen, Y.; Bao, W.; She, F.; Wu, W.; Zheng, S.; Yang, N. Characteristics and Functions of MYB (v-Myb avivan myoblastsis virus oncogene homolog)-Related Genes in Arabidopsis thaliana. Genes 2023, 14, 2026. [Google Scholar] [CrossRef]
- Cui, F.; Brosché, M.; Sipari, N.; Tang, S.; Overmyer, K. Regulation of ABA dependent wound induced spreading cell death by MYB108. New Phytol. 2013, 200, 634–640. [Google Scholar] [CrossRef]
- Cui, F.; Li, X.; Wu, W.; Luo, W.; Wu, Y.; Brosché, M.; Overmyer, K. Ectopic expression of BOTRYTIS SUSCEPTIBLE1 reveals its function as a positive regulator of wound-induced cell death and plant susceptibility to Botrytis. Plant Cell 2022, 34, 4105–4116. [Google Scholar] [CrossRef]
- Cheng, H.Q.; Han, L.B.; Yang, C.L.; Wu, X.M.; Zhong, N.Q.; Wu, J.H.; Wang, F.X.; Wang, H.Y.; Xia, G.X. The cotton MYB108 forms a positive feedback regulation loop with CML11 and participates in the defense response against Verticillium dahliae infection. J. Exp. Bot. 2016, 67, 1935–1950. [Google Scholar] [CrossRef]
- Chou, M.L.; Liao, W.Y.; Wei, W.C.; Li, A.Y.; Chu, C.Y.; Wu, C.L.; Liu, C.L.; Fu, T.H.; Lin, L.F. The Direct Involvement of Dark-Induced Tic55 Protein in Chlorophyll Catabolism and Its Indirect Role in the MYB108-NAC Signaling Pathway during Leaf Senescence in Arabidopsis thaliana. Int. J. Mol. Sci. 2018, 19, 1854. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Kan, B.; He, H.; Li, T.; Ding, Y.; Du, P.; Lai, W.; Hu, H.; Huang, J. Transcriptome analysis reveals MYB and WRKY transcription factors involved in banana (Musa paradisiaca AA) magnesium deficiency. Planta 2021, 254, 115. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, S.; Liu, H.; Hu, Q.; Liu, X.; Wang, J.; Wang, J.; Xin, W.; Chen, Q. Physiological and transcriptomic analysis of the mangrove species Kandelia obovata in response to flooding stress. Mar. Pollut. Bull. 2023, 196, 115598. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Cao, L.; Zhang, X.; Zhang, W.; Yang, T.; Zhang, J.; Che, D. An R2R3-MYB Transcription Factor RmMYB108 Responds to Chilling Stress of Rosa multiflora and Conferred Cold Tolerance of Arabidopsis. Front. Plant Sci. 2021, 12, 696919. [Google Scholar] [CrossRef]
- Ahmad, S.; Lu, C.; Wu, J.; Wei, Y.; Gao, J.; Jin, J.; Zheng, C.; Zhu, G.; Yang, F. Transcriptional Cascade in the Regulation of Flowering in the Bamboo Orchid Arundina graminifolia. Biomolecules 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liao, Z.; Zhang, B.; Yue, J.; Wang, Z.; Jie, X.; Liu, J. Transcriptome sequencing and screening of genes related to sex determination of Trichosanthes kirilowii Maxim. PLoS ONE 2020, 15, e0239230. [Google Scholar] [CrossRef]
- Ni, J.; Shah, F.A.; Liu, W.; Wang, Q.; Wang, D.; Zhao, W.; Lu, W.; Huang, S.; Fu, S.; Wu, L. Comparative transcriptome analysis reveals the regulatory networks of cytokinin in promoting the floral feminization in the oil plant Sapium sebiferum. BMC Plant Biol. 2018, 18, 96. [Google Scholar] [CrossRef]
- Xu, X.F.; Wang, B.; Feng, Y.F.; Xue, J.S.; Qian, X.X.; Liu, S.Q.; Zhou, J.; Yu, Y.H.; Yang, N.Y.; Xu, P.; et al. AUXIN RESPONSE FACTOR17 Directly Regulates MYB108 for Anther Dehiscence. Plant Physiol. 2019, 181, 645–655. [Google Scholar] [CrossRef]
- Mandaokar, A.; Browse, J. MYB108 acts together with MYB24 to regulate jasmonate-mediated stamen maturation in Arabidopsis. Plant Physiol. 2009, 149, 851–862. [Google Scholar] [CrossRef]
- Khan, I.A.; Cao, K.; Guo, J.; Li, Y.; Wang, Q.; Yang, X.; Wu, J.; Fang, W.; Wang, L. Identification of key gene networks controlling anthocyanin biosynthesis in peach flower. Plant Sci. 2022, 316, 111151. [Google Scholar] [CrossRef]
- Ye, L.; Bai, F.; Zhang, L.; Luo, M.; Gao, L.; Wang, Z.; Peng, J.; Chen, Q.; Luo, X. Transcriptome and metabolome analyses of anthocyanin biosynthesis in post-harvest fruits of a full red-type kiwifruit (Actinidia arguta) ‘Jinhongguan’. Front. Plant Sci. 2023, 14, 1280970. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Wang, Z.; Feng, A.; Li, H.; Qaseem, M.F.; Liu, L.; Deng, X.; Wu, A.M. Integrative analysis of the metabolome and transcriptome reveals the molecular regulatory mechanism of isoflavonoid biosynthesis in Ormosia henryi Prain. Int. J. Biol. Macromol. 2023, 246, 125601. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, H.; Sun, L.; Fan, G.; Ye, M.; Jiang, L.; Liu, X.; Ma, K.; Shi, C.; Bao, F.; et al. The genetic architecture of floral traits in the woody plant Prunus mume. Nat. Commun. 2018, 9, 1702. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Lin, Z.; Shen, J.; Luo, L.; Xu, Q.; Li, L.; Gui, J. OsTCP19 coordinates inhibition of lignin biosynthesis and promotion of cellulose biosynthesis to modify lodging resistance in rice. J. Exp. Bot. 2024, 75, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Torre, S.; Tattini, M.; Brunetti, C.; Fineschi, S.; Fini, A.; Ferrini, F.; Sebastiani, F. RNA-seq analysis of Quercus pubescens Leaves: De novo transcriptome assembly, annotation and functional markers development. PLoS ONE 2014, 9, e112487. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Wang, Q.; Jiang, Y.; Qin, L. Germplasm Resources of Oaks (Quercus L.) in China: Utilization and Prospects. Biology 2022, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.B.; Wang, Y.; Qin, L. Transcriptome sequencing and gene function annotation of Quercus mongolica leaves. Sci. Seric. 2020, 46, 560–565. [Google Scholar]
- Lu, H.D.; Xue, J.Q.; Guo, D.W. Efficacy of planting date adjustment as a cultivation strategy to cope with drought stress and increase rainfed maize yield and water-use efficiency. J. Agric. Water Manag. 2016, 179, 227–235. [Google Scholar] [CrossRef]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef]
- Krizek, B.A.; Meyerowitz, E.M. The Arabidopsis homeotic genes APETALA3 and PISTILLATA are sufficient to provide the B class organ identity function. Development 1996, 122, 11–22. [Google Scholar] [CrossRef]
- Sun, P.; Zhu, X.; Huang, X.; Liu, J.H. Overexpression of a stress-responsive MYB transcription factor of Poncirus trifoliata confers enhanced dehydration tolerance and increases polyamine biosynthesis. Plant Physiol. Biochem. 2014, 78, 71–79. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Xu, L.; Wang, J.; Qi, H.; Guo, J.; Zhang, L.; Shen, J.; Wang, H.; Zhang, F.; et al. The OsFTIP6-OsHB22-OsMYBR57 module regulates drought response in rice. Mol. Plant 2022, 15, 1227–1242. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Indoliya, Y.; Chauhan, A.S.; Pande, V.; Chakrabarty, D. Overexpression of rice R1-type MYB transcription factor confers different abiotic stress tolerance in transgenic Arabidopsis. Ecotoxicol. Environ. Saf. 2020, 206, 111361. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.K.; Cao, Z.H.; Hao, Y.J. Overexpression of a R2R3 MYB gene MdSIMYB1 increases tolerance to multiple stresses in transgenic tobacco and apples. Physiol. Plant. 2014, 150, 76–87. [Google Scholar] [CrossRef]
- Yu, Y.T.; Wu, Z.; Lu, K.; Bi, C.; Liang, S.; Wang, X.F.; Zhang, D.P. Overexpression of the MYB37 transcription factor enhances abscisic acid sensitivity, and improves both drought tolerance and seed productivity in Arabidopsis thaliana. Plant Mol. Biol. 2016, 90, 267–279. [Google Scholar] [CrossRef]
- Yao, L.; Jiang, Y.; Lu, X.; Wang, B.; Zhou, P.; Wu, T. A R2R3-MYB transcription factor from Lablab purpureus induced by drought increases tolerance to abiotic stress in Arabidopsis. Mol. Biol. Rep. 2016, 43, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lei, J.; Jin, X.J.; Chai, S.S.; Jiao, C.H.; Yang, X.S.; Wang, L.J. A Sweet Potato MYB Transcription Factor IbMYB330 Enhances Tolerance to Drought and Salt Stress in Transgenic Tobacco. Genes 2024, 15, 693. [Google Scholar] [CrossRef]
- Zhu, Z.; Quan, R.; Chen, G.; Yu, G.; Li, X.; Han, Z.; Xu, W.; Li, G.; Shi, J.; Li, B. An R2R3-MYB transcription factor VyMYB24, isolated from wild grape Vitis yanshanesis J. X. Chen., regulates the plant development and confers the tolerance to drought. Front. Plant Sci. 2022, 13, 966641. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, Y.; Liang, Y.; Chen, L.; Chen, W.; Cheng, B. Expression of the maize MYB transcription factor ZmMYB3R enhances drought and salt stress tolerance in transgenic plants. Plant Physiol. Biochem. 2019, 137, 179–188. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Ma, R.; An, X.; Pan, F.; Zhang, S.; Fu, Y. Genome-wide identification and expression analysis of MYB gene family in Cajanus cajan and CcMYB107 improves plant drought tolerance. Physiol. Plant. 2023, 175, e13954. [Google Scholar] [CrossRef]







| Bioinformatics Analysis | Tools | Website |
|---|---|---|
| Open reading frame (ORF) and amino acid sequence | NCBI | https://www.ncbi.nlm.nih.gov/orffinder/ (accessed on 23 October 2023) |
| Genes encode the physicochemical properties of proteins | Expasy | https://web.expasy.org/protparam/ (accessed on 23 October 2023) |
| The hydrophobic and hydrophilic | ProtScale | https://web.expasy.org/protscale/ (accessed on 23 October 2023) |
| Domain | NCBI | https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi (accessed on 23 October 2023) |
| Transmembrane prediction | TMHMM | http://www.cbs.dtu.dk/services/TMHMM/ (accessed on 23 October 2023) |
| Subcellular location | ProtComp | http://www.softberry.com/berry.phtml?topic=index&group=programs&subgroup=proloc (accessed on 23 October 2023) |
| Protein structure | SOPMA | https://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_sopma.html (accessed on 23 October 2023) |
| 3D structure of protein | SWISS-MODEL | https://swissmodel.expasy.org/interactive (accessed on 23 October 2023) |
| Primers | Sequence (5′→3′) |
|---|---|
| EF1α -F | CAGGTTTGAAGCGAACTGGC |
| EF1α -R | ACCACCAAACCCATGCTCAA |
| RT-QwMYB108-F | CCGACCCAATTGACCCGTAT |
| RT-QwMYB108-R | ACCACCAAACCCATGCTCAA |
| Primers | Sequence (5′→3′) |
|---|---|
| M-QwMYB108-F | GGACTAGTATGGATTTTCACGTGAGAGC |
| M-QwMYB108-R | CGTTCGAATCAGATCTCATCGCAAAGCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Sun, Y.; Wang, Y.; Shao, D.; Chen, G.; Jiang, Y.; Qin, L. Molecular Cloning of QwMYB108 Gene and Its Response to Drought Stress in Quercus wutaishanica Mayr. Forests 2024, 15, 1557. https://doi.org/10.3390/f15091557
Zhao X, Sun Y, Wang Y, Shao D, Chen G, Jiang Y, Qin L. Molecular Cloning of QwMYB108 Gene and Its Response to Drought Stress in Quercus wutaishanica Mayr. Forests. 2024; 15(9):1557. https://doi.org/10.3390/f15091557
Chicago/Turabian StyleZhao, Xuefei, Ying Sun, Yong Wang, Di Shao, Gang Chen, Yiren Jiang, and Li Qin. 2024. "Molecular Cloning of QwMYB108 Gene and Its Response to Drought Stress in Quercus wutaishanica Mayr" Forests 15, no. 9: 1557. https://doi.org/10.3390/f15091557
APA StyleZhao, X., Sun, Y., Wang, Y., Shao, D., Chen, G., Jiang, Y., & Qin, L. (2024). Molecular Cloning of QwMYB108 Gene and Its Response to Drought Stress in Quercus wutaishanica Mayr. Forests, 15(9), 1557. https://doi.org/10.3390/f15091557





