Early Dynamics of Carbon Accumulation as Influenced by Spacing of a Populus deltoides Planting
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site, Plant Material, and Establishment
2.2. Experimental Design and Measurements
2.3. Data Analysis and Modeling
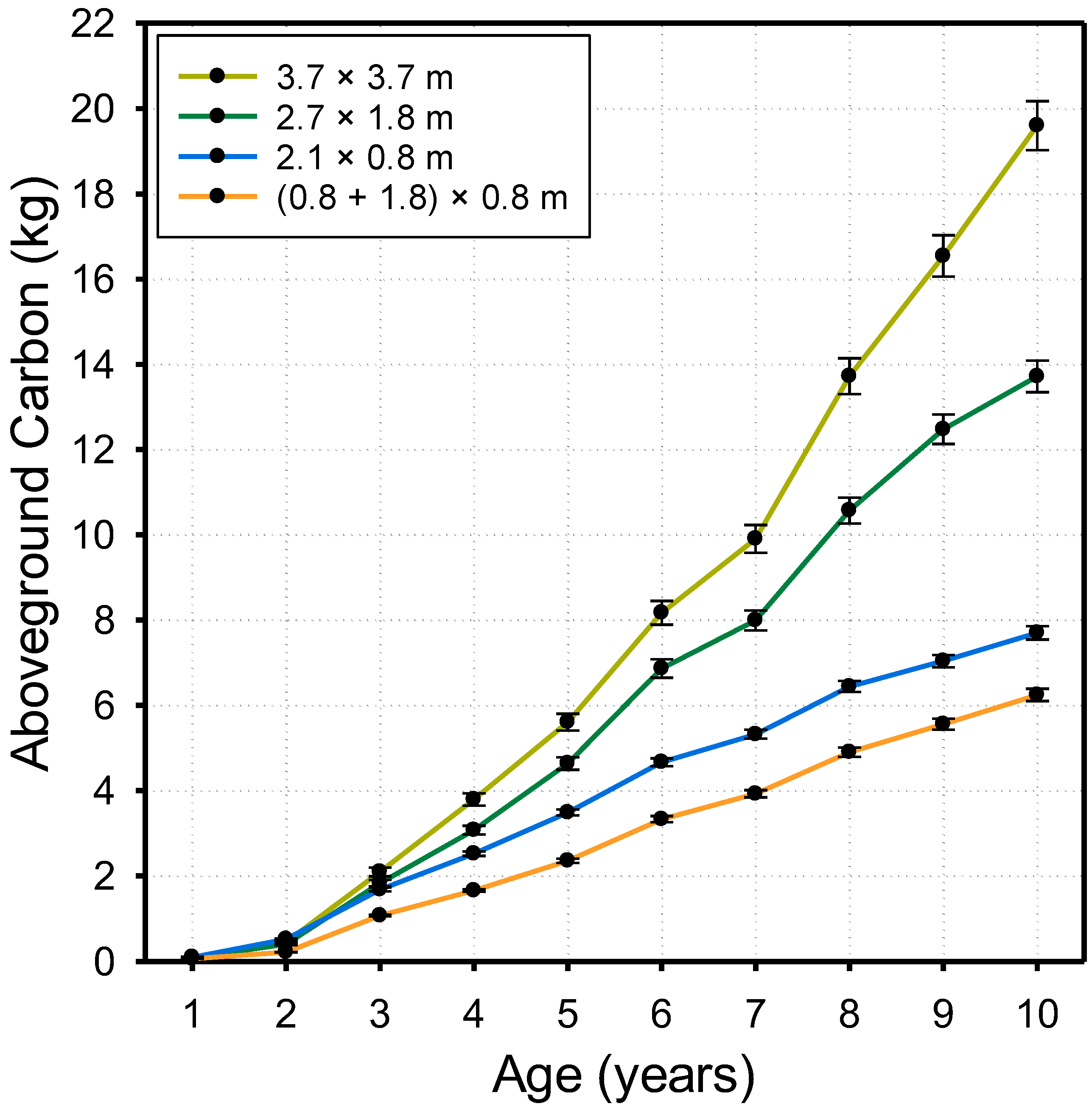
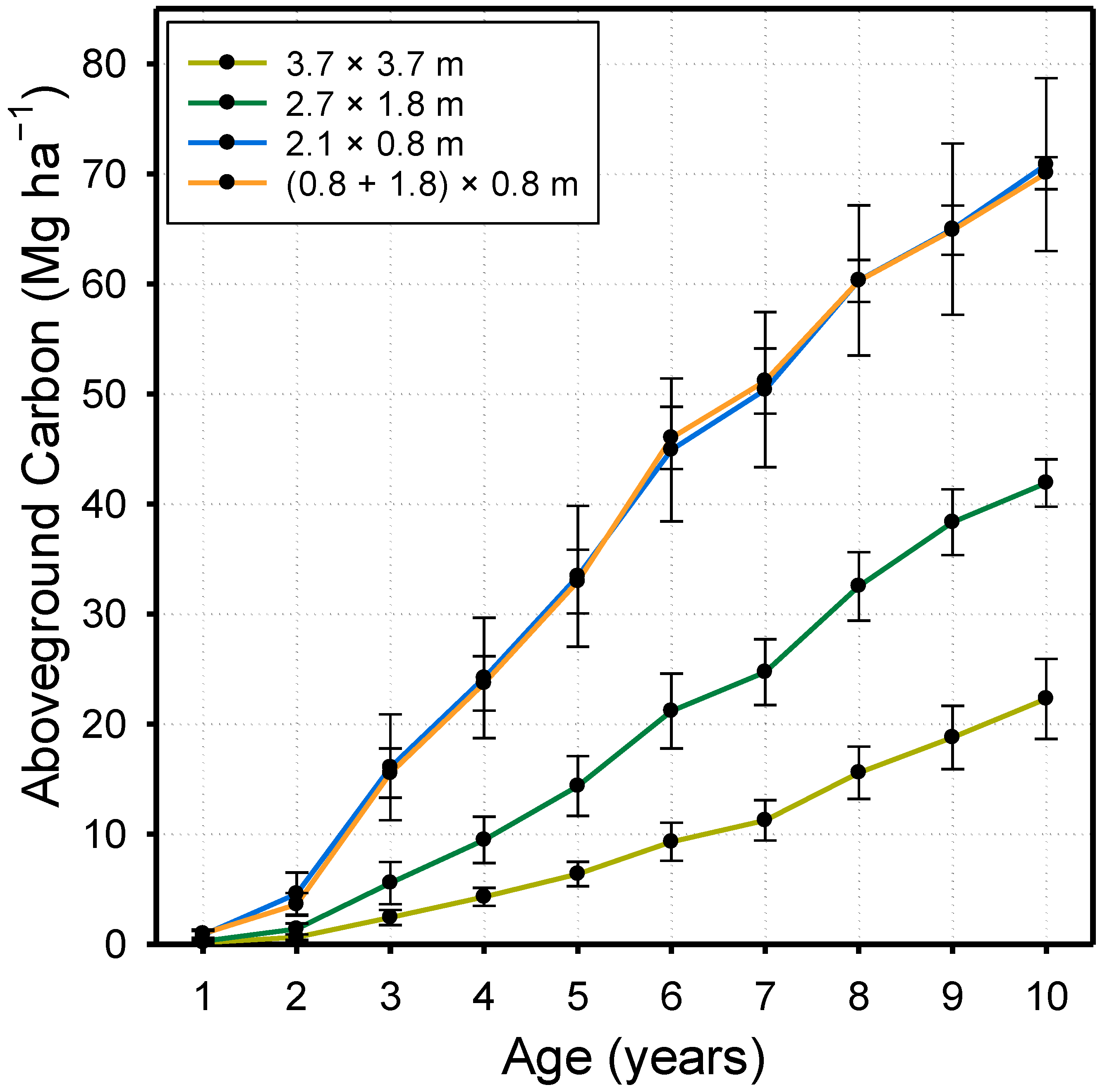
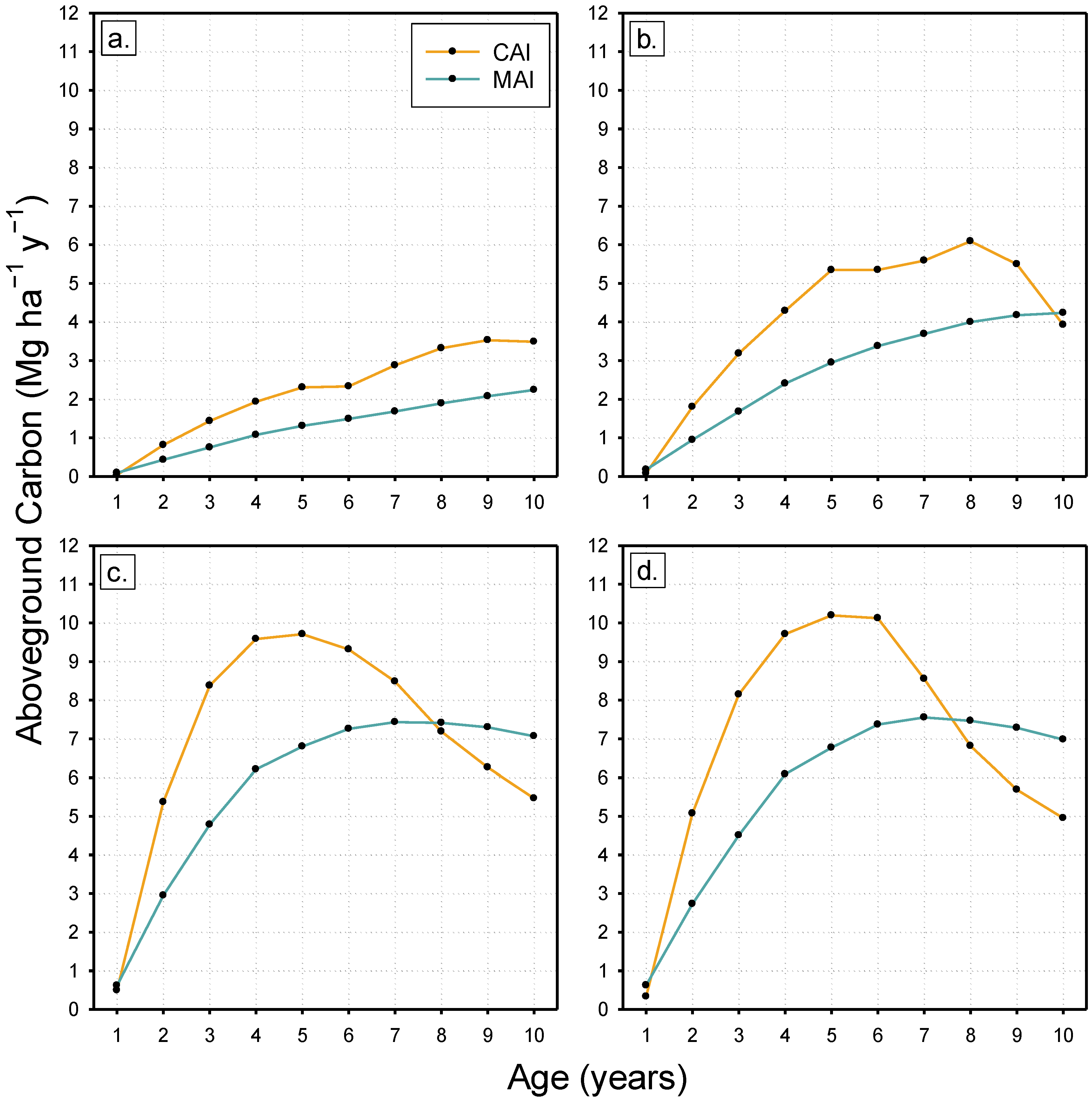
3. Results
4. Discussion
4.1. Cottonwood Survival and Growth Relative to Spacing on a Degraded, Low Productivity Site
4.2. Stand Development and Dynamics of Carbon Accumulation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, W.S.; Gorelik, S.R.; Cook-Patton, S.C.; Baccini, A.; Farina, M.K.; Solvik, K.K.; Ellis, P.W.; Sanderman, J.; Houghton, R.A.; Leavitt, S.M.; et al. The global potential for increased storage of carbon on land. Proc. Natl. Acad. Sci. USA 2022, 119, e2111312119. [Google Scholar] [CrossRef]
- Waring, B.; Neumann, M.; Prentice, I.C.; Adams, M.; Smith, P.; Siegert, M. Forests and decarbonization—Roles of natural and planted forests. Front. For. Glob. Chang. 2020, 3, 58. [Google Scholar] [CrossRef]
- Bastin, J.-F.; Finegold, Y.; Garcia, C.; Mollicone, D.; Rezende, M.; Routh, D.; Zohner, C.M.; Crowther, T.W. The global tree restoration potential. Science 2019, 365, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Nayak, N.; Mehrotra, R.; Mehrotra, S. Carbon biosequestration strategies: A review. Carbon. Capture Sci. Technol. 2022, 4, 100065. [Google Scholar] [CrossRef]
- Doelman, J.C.; Stehfest, E.; van Vuuren, D.P.; Tabeau, A.; Hof, A.F.; Braakhekke, M.C.; Gernaat, D.E.H.J.; van den Berg, M.; van Zeist, W.-J.; Daioglou, V.; et al. Afforestation for climate change mitigation: Potentials, risks and trade-offs. Glob. Chang. Biol. 2020, 26, 1576–1591. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Yin, G.; Piao, S.; Dybzinski, R.; Cong, N.; Li, X.; Wang, K.; Peñuelas, J.; Zeng, H.; Chen, A. Divergent responses of soil organic carbon to afforestation. Nat. Sustain. 2020, 3, 694–700. [Google Scholar] [CrossRef]
- Forster, E.J.; Healey, J.R.; Dymond, C.; Styles, D. Commercial afforestation can deliver effective climate change mitigation under multiple decarbonisation pathways. Nat. Commun. 2021, 12, 3831. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Abdalla, M.; Espenberg, M.; Hastings, A.; Hallett, P.; Smith, P. A systematic analysis and review of the impacts of afforestation on soil quality indicators as modified by climate zone, forest type and age. Sci. Total Environ. 2021, 757, 143824. [Google Scholar] [CrossRef]
- Zalesny, R.S., Jr.; Stanturf, J.A.; Gardiner, E.S.; Perdue, J.H.; Young, T.M.; Coyle, D.R.; Headlee, W.L.; Bañuelos, G.S.; Hass, A. Ecosystem services of woody crop production systems. BioEnergy Res. 2016, 9, 465–491. [Google Scholar] [CrossRef]
- Taylor, G.; Donnison, I.S.; Murphy-Bokern, D.; Morgante, M.; Bogeat-Triboulot, M.-B.; Bhalerao, R.; Hertzberg, M.; Polle, A.; Harfouche, A.; Alasia, F.; et al. Sustainable bioenergy for climate mitigation: Developing drought-tolerant trees and grasses. Ann. Bot. 2019, 124, 513–520. [Google Scholar] [CrossRef]
- Englund, O.; Dimitriou, I.; Dale, V.H.; Kline, K.L.; Mola-Yudego, B.; Murphy, F.; English, B.; McGrath, J.; Busch, G.; Negri, M.C.; et al. Multifunctional perennial production systems for bioenergy: Performance and progress. WIREs Energy Environ. 2020, 9, e375. [Google Scholar] [CrossRef]
- Volk, T.A.; Heavy, J.P.; Eisenbies, M.H. Advances in shrub-willow crops for bioenergy, renewable products, and environmental benefits. Food Energy Secur. 2016, 5, 97–106. [Google Scholar] [CrossRef]
- Mola-Yudego, B.; Pelkonen, P. Pulling effects of district heating plants on the adoption and spread of willow plantations for biomass: The power plant in Enköping (Sweden). Biomass Bioenergy 2011, 35, 2986–2992. [Google Scholar] [CrossRef]
- Sabatti, M.; Fabbrini, F.; Harfouche, A.; Beritognolo, I.; Mareschi, L.; Carlini, M.; Paris, P.; Scarascia-Mugnozza, G. Evaluation of biomass production potential and heating value of hybrid poplar genotypes in a short-rotation culture in Italy. Ind. Crops Prod. 2014, 61, 62–73. [Google Scholar] [CrossRef]
- Chavan, S.B.; Dhillon, R.S.; Sirohi, C.; Uthappa, A.R.; Jinger, D.; Jatav, H.S.; Chichaghare, A.R.; Kakade, V.; Paramesh, V.; Kumari, S.; et al. Carbon sequestration potential of commercial agroforestry systems in Indo-Gangetic Plains of India: Poplar and eucalyptus-based agroforestry systems. Forests 2023, 14, 559. [Google Scholar] [CrossRef]
- McGrath, J.F.; Goss, K.F.; Brown, M.W.; Bartle, J.R.; Abadi, A. Aviation biofuel from integrated woody biomass in southern Australia. WIREs Energy Environ. 2016, 6, e221. [Google Scholar] [CrossRef]
- Langholtz, M.H.; Stokes, B.J.; Eaton, L.M.; Brandt, C.C.; Davis, M.R.; Theiss, T.J.; Turhollow, A.F., Jr.; Webb, E.; Coleman, A.; Wigmosta, M.; et al. 2016 Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy, Volume 1: Economic Availability of Feedstocks; Oak Ridge National Lab (ORNL): Oak Ridge, TN, USA, 2016. [CrossRef]
- Shukla, P.R.; Skea, J.; Slade, R.; Al Khourdajie, A.; van Diemen, R.; McCollum, D.; Pathak, M.; Some, S.; Vyas, P.; Fradera, R.; et al. Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- Gul, T.; Cozzi, L.; Havlik, P. What Does Net-Zero Emissions by 2050 Mean for Bioenergy and Land Use? International Energy Agency. Available online: https://www.iea.org/articles/what-does-net-zero-emissions-by-2050-mean-for-bioenergy-and-land-use (accessed on 25 August 2023).
- Stanturf, J.A.; Young, T.M.; Perdue, J.H.; Doughetry, D.; Pigott, M.; Guo, Z.; Huang, X. Potential profitability zones for Populus spp. biomass plantings in the eastern United States. For. Sci. 2017, 63, 586–595. [Google Scholar] [CrossRef]
- Chudy, R.P.; Busby, G.M.; Binkley, C.S.; Stanton, B.J. The economics of dedicated hybrid poplar biomass plantations in the western U.S. Biomass Bioenergy 2019, 124, 114–124. [Google Scholar] [CrossRef]
- Ghezehei, S.B.; Nichols, E.G.; Hazel, D.W. Productivity and cost-effectiveness of short-rotation hardwoods on various land types in the southeastern USA. Int. J. Phytoremediat. 2019, 22, 98–110. [Google Scholar] [CrossRef]
- Bressler, A.; Vidon, P.; Hirsch, P.; Volk, T. Valuation of ecosystem services of commercial shrub willow (Salix spp.) woody biomass crops. Environ. Monit. Assess. 2017, 189, 137. [Google Scholar] [CrossRef]
- Ile, O.J.; McCormick, H.; Skrabacz, S.; Bhattacharya, S.; Aguilos, M.; Carvalho, H.D.R.; Idassi, J.; Baker, J.; Heitman, J.L.; King, J.S. Integrating short rotation woody crops into conventional agricultural practices in the southeastern United States: A review. Land 2022, 12, 10. [Google Scholar] [CrossRef]
- Mei, B. Carbon offset as another driver of timberland investment returns in the United States. J. For. Bus. Res. 2023, 2, 1–19. [Google Scholar]
- Adhikari, R.K.; Grala, R.K.; Grado, S.C.; Grebner, D.L.; Petrolia, D.R. Landowner satisfaction with conservation programs in the southern United States. Sustainability 2022, 14, 5513. [Google Scholar] [CrossRef]
- Hellerstein, D.M. The US Conservation Reserve Program: The evolution of an enrollment mechanism. Land. Use Policy 2017, 63, 601–610. [Google Scholar] [CrossRef]
- Van Voorhis, C. The rise of natural capitalism and the new frontier of the restoration economy. For Landowner, November/December 2012; pp. 18–25. [Google Scholar]
- Fahrenkrog, A.M.; Neves, L.G.; Resende, M.F.R., Jr.; Vazquez, A.I.; de los Campos, G.; Dervinis, C.; Sykes, R.; Davis, M.; Davenport, R.; Barbazuk, W.B.; et al. Genome-wide association study reveals putative regulators of bioenergy traits in Populus deltoides. New Phytol. 2017, 213, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, M.; Tuskan, G.A.; Muchero, W.; Chen, J.-G. Recent advances in the transcriptional regulation of secondary cell wall biosynthesis of the woody plants. Front. Plant Sci. 2018, 9, 1535. [Google Scholar] [CrossRef] [PubMed]
- Balmant, K.M.; Noble, J.D.; Alves, F.C.; Dervinis, C.; Conde, D.; Schmidt, H.W.; Vazquez, A.I.; Barbazuk, W.B.; de los Campos, G.; Resende, M.F.R., Jr.; et al. Xylem systems genetics analysis reveals a key regulator of lignin biosynthesis in Populus deltoides. Genome Res. 2020, 30, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Zalesny, R.S., Jr.; Stanturf, J.A.; Gardiner, E.S.; Bañuelos, G.S.; Hallett, R.A.; Hass, A.; Stange, C.M.; Perdue, J.H.; Young, T.M.; Coyle, D.R.; et al. Environmental technologies of woody crop production systems. BioEnergy Res. 2016, 9, 492–506. [Google Scholar] [CrossRef]
- DeBell, D.S.; Harrington, C.A. Productivity of Populus in monoclonal and polyclonal blocks at three spacings. Can. J. For. Res. 1997, 27, 978–985. [Google Scholar] [CrossRef]
- Gabira, M.M.; Girona, M.M.; DesRochers, A.; Kratz, D.; da Silva, R.B.G.; Duarte, M.M.; deAguiar, N.S.; Wendling, I. The impact of planting density on forest monospecific plantations: An overview. For. Ecol. Manag. 2023, 534, 120882. [Google Scholar] [CrossRef]
- Armstrong, A.; Johns, C.; Tubby, I. Effects of spacing and cutting cycle on the yield of poplar grown as an energy crop. Biomass Bioenergy 1999, 17, 305–314. [Google Scholar] [CrossRef]
- Hall, R.B.; Hart, E.R.; Peszlen, I. Modifying Populus environmental responses: Impacts on wood quantity and quality. In Molecular Breeding of Woody Plants, 1st ed.; Morohoshi, N., Komamine, A., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2001; pp. 229–238. [Google Scholar]
- Sang, Y.; Gao, P.; Kang, X.; Zhang, P. Effect of initial planting density on growth traits and wood properties of triploid Chinese white poplar (Populus tomentosa) plantation. Forests 2021, 12, 1676. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Y.; Ding, S.; Lv, Y.; Samjhana, W.; Fang, S. Growth, carbon storage, and optimal rotation in poplar plantations: A case study on clone and planting spacing effects. Forests 2020, 11, 842. [Google Scholar] [CrossRef]
- Fang, S.; Xue, J.; Tang, L. Biomass production and carbon sequestration potential in poplar plantations with different management patterns. J. Environ. Manag. 2007, 85, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Truax, B.; Fortier, J.; Gagnon, D.; Lambert, F. Planting density and site effects on stem dimensions, stand productivity, biomass partitioning, carbon stocks and soil nutrient supply in hybrid poplar plantations. Forests 2018, 9, 293. [Google Scholar] [CrossRef]
- Qian, Z.; Ge, X.; Bai, Y.; Tian, Y.; Zhuang, S.; Fang, S.; Tang, L. Effects of different planting configurations and clones on biomass and carbon storage of a 12-year-old poplar ecosystem in southern China. Can. J. For. Res. 2022, 52, 70–78. [Google Scholar] [CrossRef]
- Dahal, B.; Poudel, K.P.; Renninger, H.J.; Granger, J.J.; Leininger, T.D.; Gardiner, E.S.; Souter, R.A.; Rousseau, R.J. Aboveground biomass equations for black willow (Salix nigra Marsh.) and eastern cottonwood (Populus deltoides Bartr. ex Marsh.). Trees For. People 2022, 7, 100195. [Google Scholar] [CrossRef]
- USDA Natural Resources Conservation Service, Web Soil Survey. Available online: https://websoilsurvey.sc.egov.usda.gov (accessed on 15 August 2023).
- USDOC National Oceanic and Atmospheric Administration, National Centers for Environmental Information, 2006–2020 Normals. Available online: http://www.ncdc.noaa.gov/cdo-web/datatools/normals (accessed on 15 August 2023).
- Broadfoot, W.M. Hardwood Suitability for and Properties of Important Midsouth Soils; Southern Forest Experiment Station: New Orleans, LA, USA, 1976; USDA Forest Service Research Paper SO-127; 84p. [Google Scholar]
- Robison, T.L.; Rousseau, R.R. Cottonwood leaf beetle control with imidacloprid soaked cuttings. In Proceedings, 15th Central Hardwood Forest Conference; Buckley, D.S., Clatterbuck, W.K., Eds.; USDA Forest Service, Southern Research Station, e-GTR-SRS-101; USDA: Asheville, NC, USA, 2007; pp. 198–205. [Google Scholar]
- Lynch, T.B.; Hamlin, D.; Ducey, M.J.; Borders, B.E. Design-unbiased estimation and alternatives for sampling trees on plantation rows. Forests 2018, 9, 362. [Google Scholar] [CrossRef]
- SAS. Version 9.4 for Windows; SAS Institute, Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Vera, I.; Hoefnagels, R.; Junginger, M.; van der Hilst, F. Supply potential of lignocellulosic energy crops grown on marginal land and greenhouse gas footprint of advanced biofuels—A spatially explicit assessment under the sustainability criteria of the Renewable Energy Directive Recast. GCB-Bioenergy 2021, 13, 1425–1447. [Google Scholar] [CrossRef]
- Zhang, B.; Lan, K.; Harris, T.B.; Ashton, M.S.; Yao, Y. Climate-smart forestry through innovative wood products and commercial afforestation and reforestation on marginal land. Proc. Natl. Acad. Sci. USA 2023, 120, e2221840120. [Google Scholar] [CrossRef]
- Čomić, D.R. Regression models of carbon and CO2 sequestration of hybrid poplar plantations in northern Serbia. Can. J. For. Res. 2021, 51, 1859–1874. [Google Scholar] [CrossRef]
- Bazrgar, A.B.; Sidders, D.; Thevathasan, N. Predicting aboveground biomass carbon sequestration potential in hybrid poplar clones under afforestation plantation management in southern Ontario, Canada. For. Chron. 2022, 98, 89–102. [Google Scholar] [CrossRef]
- Stanturf, J.A.; Gardiner, E.S.; Hamel, P.B.; Devall, M.S.; Leininger, T.D.; Warren, M.E., Jr. Restoring bottomland hardwood ecosystems in the Lower Mississippi Alluvial Valley. J. For. 2000, 98, 10–16. [Google Scholar] [CrossRef]
- Krinard, R.M.; Kennedy, H.E., Jr. Five-year-old cottonwood plantation on a clay site: Growth, yield, and soil properties. South. J. Appl. For. 1980, 4, 80–83. [Google Scholar] [CrossRef]
- Krinard, R.M.; Johnson, R.L. Ten-Year Results in a Cottonwood Plantation Spacing Study; USDA Forest Service Research Paper SO-106; Southern Forest Experiment Station: New Orleans, LA, USA, 1975; 10p.
- Krinard, R.M. Cottonwood Development through 19 Years in a Nelder’s Design; USDA Forest Service Research Note SO-322; Southern Forest Experiment Station: New Orleans, LA, USA, 1985; 4p.
- Cao, Q.V.; Durand, K.M. Site index curves for eastern cottonwood plantations in the Lower Mississippi Delta. South. J. Appl. For. 1991, 15, 28–30. [Google Scholar] [CrossRef]
- DeBell, D.S.; Clendenen, G.W.; Harrington, C.A.; Zasada, J.C. Tree growth and stand development in short-rotation Populus plantings: 7-year results for two clones at three spacings. Biomass Bioenergy 1996, 11, 253–269. [Google Scholar] [CrossRef]
- Kaczmarek, D.J.; Coyle, D.R.; Coleman, M.D. Survival and growth of a range of Populus clones in central South Carolina USA through age ten: Do early assessments reflect longer-term survival and growth trends? Biomass Bioenergy 2013, 49, 260–272. [Google Scholar] [CrossRef]
- DeBell, D.S.; Harrington, C.A.; Clendenen, G.W.; Zasada, J.C. Tree growth and stand development of four Populus clones in large monoclonal plots. New For. 1997, 14, 1–18. [Google Scholar] [CrossRef]
- Larocque, G.R. Performance and morphological response of the hybrid poplar DN-74 (Populus deltoides × nigra) under different spacings on a 4-year rotation. Ann. For. Sci. 1999, 56, 275–287. [Google Scholar] [CrossRef]
- Bo, H.; Zhu, J.; Zhang, R.; Nie, L.; Song, L. Effects of planting density and clone type on growth and wood volume of Populus tomentosa stands. J. For. Res. 2020, 25, 444–449. [Google Scholar] [CrossRef]
- Fang, S.; Liu, Y.; Yue, J.; Tian, Y.; Xu, X. Assessments of growth performance, crown structure, stem form and wood property of introduced poplar clones: Results from a long-term field experiment at a lowland site. For. Ecol. Manag. 2021, 479, 118586. [Google Scholar] [CrossRef]
- Benomar, L.; DesRochers, A.; Larocque, G.R. The effects of spacing on growth, morphology and biomass production and allocation in two hybrid poplar clones growing in the boreal region of Canada. Trees 2012, 26, 939–949. [Google Scholar] [CrossRef]
- Arora, G.; Chaturvedi, S.; Kaushal, R.; Nain, A.; Tewari, S.; Alam, N.M.; Chaturvedi, O.P. Growth, biomass, carbon stocks, and sequestration in an age series of Populus deltoides plantations in Tarai region of central Himalaya. Turk. J. Agric. For. 2014, 38, 550–560. [Google Scholar] [CrossRef]
- Dănilă, I.-C.; Mititelu, C.; Palaghianu, C. Productivity of short-rotation poplar crops: A case study in the NE of Romania. Forests 2022, 13, 1089. [Google Scholar] [CrossRef]
- Ghezehei, S.B.; Wright, J.; Zalesny, R.S., Jr.; Nichols, E.G.; Hazel, D.W. Matching site-suitable poplars to rotation length for optimized productivity. For. Ecol. Manag. 2019, 457, 117670. [Google Scholar] [CrossRef]
- Verlinden, M.S.; Broeckx, L.S.; Van den Bulcke, J.; Van Acker, J.; Ceulemans, R. Comparative study of biomass determinants of 12 poplar (Populus) genotypes in a high-density short-rotation culture. For. Ecol. Manag. 2013, 307, 101–111. [Google Scholar] [CrossRef]
- Zenone, T.; Fischer, M.; Arriga, N.; Broeckx, L.S.; Verlinden, M.S.; Vanbeveren, S.; Zona, D.; Ceulemans, R. Biophysical drivers of the carbon dioxide, water vapor, and energy exchanges of a short-rotation poplar coppice. Agric. For. Meteorol. 2015, 209–210, 22–35. [Google Scholar] [CrossRef]
- Strong, T.; Hansen, E. Hybrid poplar spacing/productivity relations in short rotation intensive culture plantations. Biomass Bioenergy 1993, 4, 255–261. [Google Scholar] [CrossRef]
- Ghezehei, S.B.; Ewald, A.L.; Hazel, D.W.; Zalesny, R.S., Jr.; Nichols, E.G. Productivity and profitability of poplars on fertile and marginal sandy soils under different density and fertilization treatments. Forests 2021, 12, 869. [Google Scholar] [CrossRef]
- Fang, S.; Xu, X.; Lu, S.; Tang, L. Growth dynamics and biomass production in short-rotation poplar plantations: 6-year results for three clones at four spacings. Biomass Bioenergy 1999, 17, 415–425. [Google Scholar] [CrossRef]



| Spacing | ||||
|---|---|---|---|---|
| Variable | 3.7 × 3.7 m | 2.7 × 1.8 m | 2.1 × 0.8 m | (0.8 + 1.8) × 0.8 m |
| Survival (%) | 73 ± 4 a | 77 ± 5 a | 76 ± 5 a | 64 ± 3 a |
| Height (m) | 12.8 ± 0.7 a | 11.2 ± 0.7 ab | 9.6 ± 0.9 ab | 8.4 ± 0.2 b |
| DBH (cm) | 11.9 ± 0.6 a | 9.4 ± 0.7 b | 6.3 ± 0.5 c | 5.4 ± 0.2 c |
| Stand BA (sq m ha−1) | 14.8 ± 2.1 c | 24.2 ± 0.9 b | 33.4 ± 3.4 a | 31.9 ± 0.4 a |
| Tree AGB (kg) | 39.2 ± 1.1 a | 27.4 ± 0.7 b | 15.4 ± 0.3 c | 12.5 ± 0.3 c |
| Stand AGB (Mg ha−1) | 44.6 ± 7.2 c | 83.8 ± 4.3 b | 141.7 ± 15.7 a | 140.1 ± 2.9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gardiner, E.S.; Poudel, K.P.; Leininger, T.D.; Souter, R.A.; Rousseau, R.J.; Dahal, B. Early Dynamics of Carbon Accumulation as Influenced by Spacing of a Populus deltoides Planting. Forests 2024, 15, 226. https://doi.org/10.3390/f15020226
Gardiner ES, Poudel KP, Leininger TD, Souter RA, Rousseau RJ, Dahal B. Early Dynamics of Carbon Accumulation as Influenced by Spacing of a Populus deltoides Planting. Forests. 2024; 15(2):226. https://doi.org/10.3390/f15020226
Chicago/Turabian StyleGardiner, Emile S., Krishna P. Poudel, Theodor D. Leininger, Ray A. Souter, Randall J. Rousseau, and Bini Dahal. 2024. "Early Dynamics of Carbon Accumulation as Influenced by Spacing of a Populus deltoides Planting" Forests 15, no. 2: 226. https://doi.org/10.3390/f15020226
APA StyleGardiner, E. S., Poudel, K. P., Leininger, T. D., Souter, R. A., Rousseau, R. J., & Dahal, B. (2024). Early Dynamics of Carbon Accumulation as Influenced by Spacing of a Populus deltoides Planting. Forests, 15(2), 226. https://doi.org/10.3390/f15020226






