Impact of Climate Change on the Potential Geographical Distribution Patterns of Luculia pinceana Hook. f. since the Last Glacial Maximum
Abstract
:1. Introduction
2. Materials and Methods
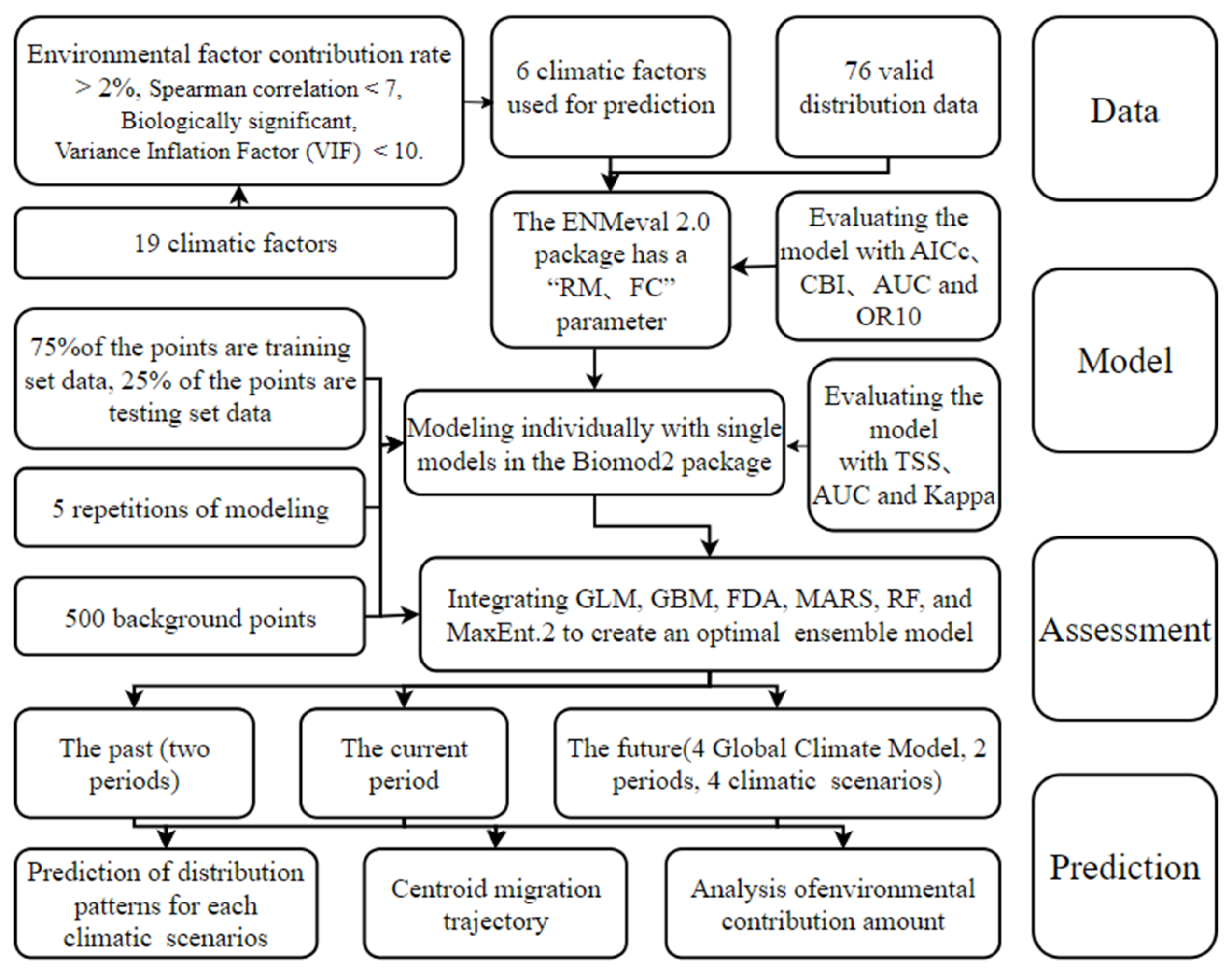
2.1. Workflow
2.2. Collection of Distribution Points
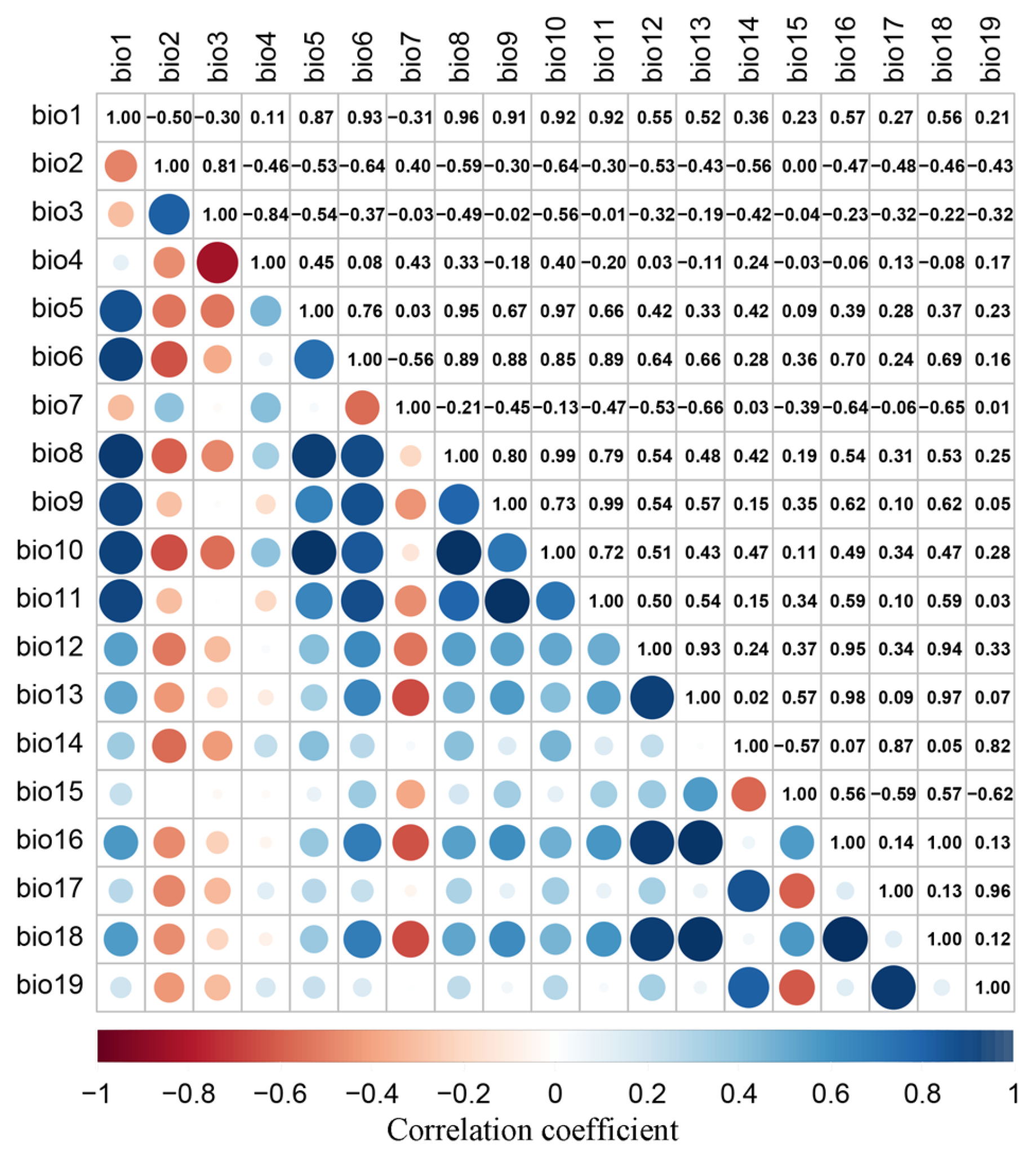
2.3. Acquisition of Environmental Variables
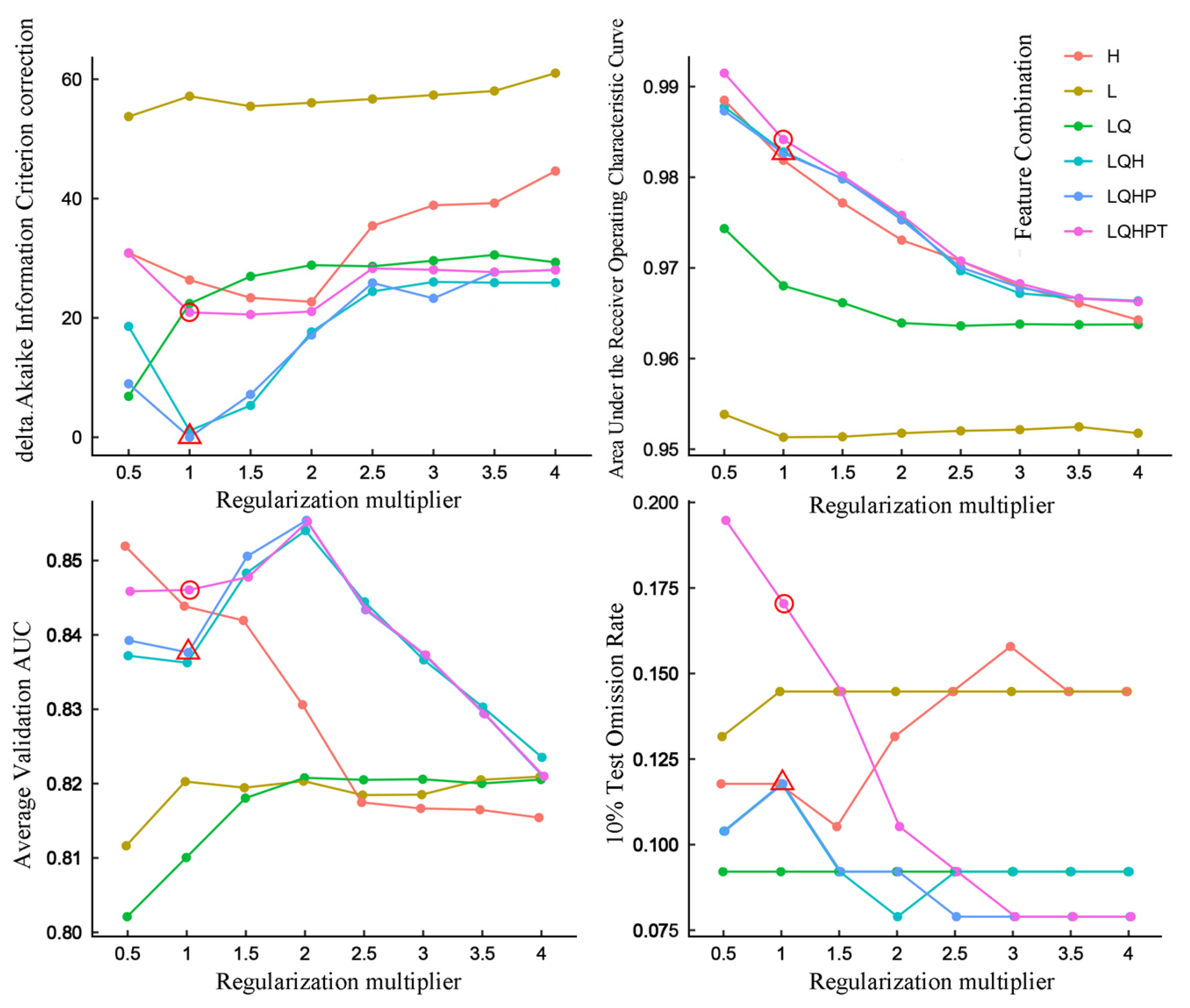
2.4. Model Evaluation and Construction
2.5. Analysis of Important Environmental Factors and Suitable Habitat Patterns
3. Results
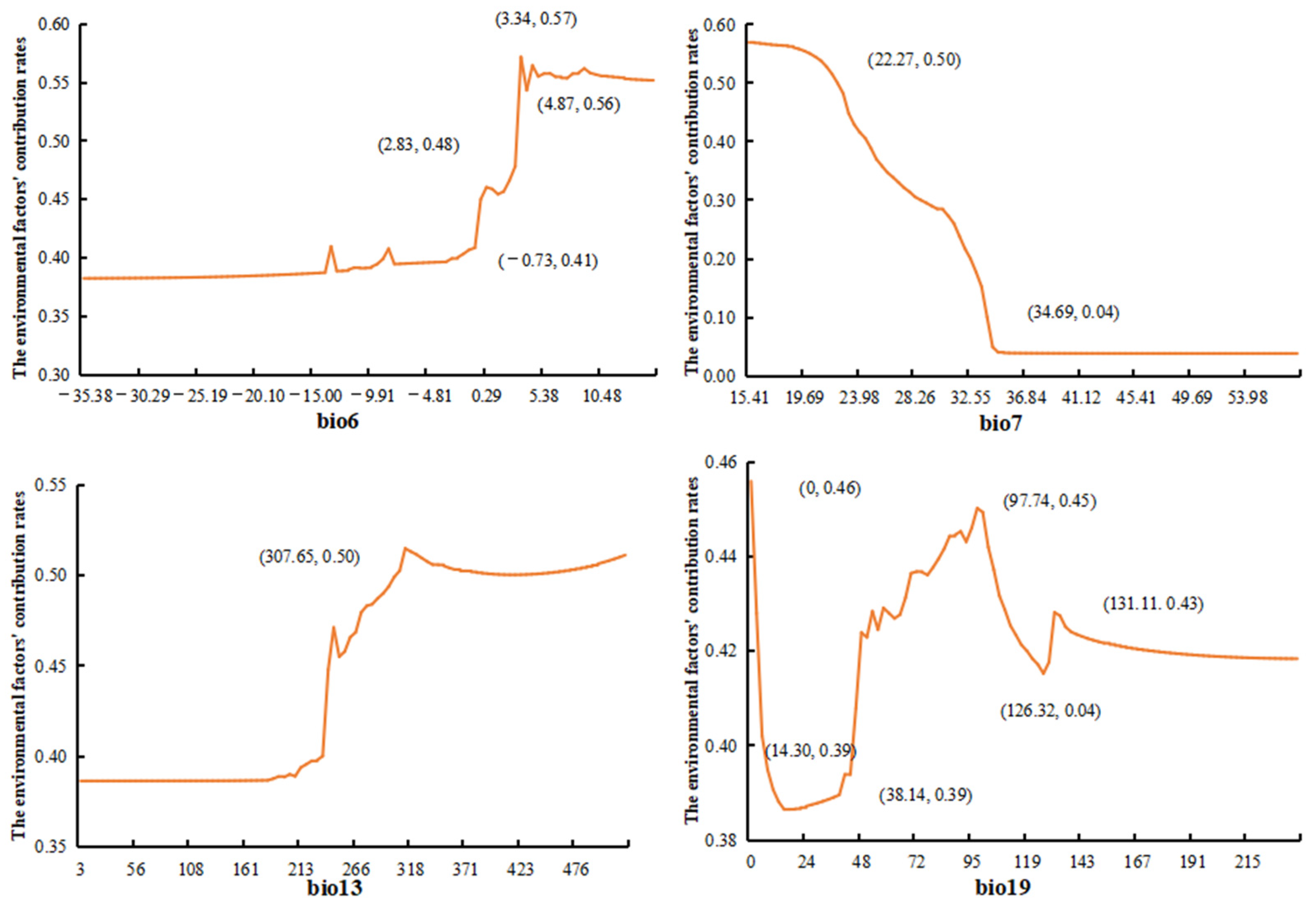
3.1. Model Construction and Important Environmental Factors
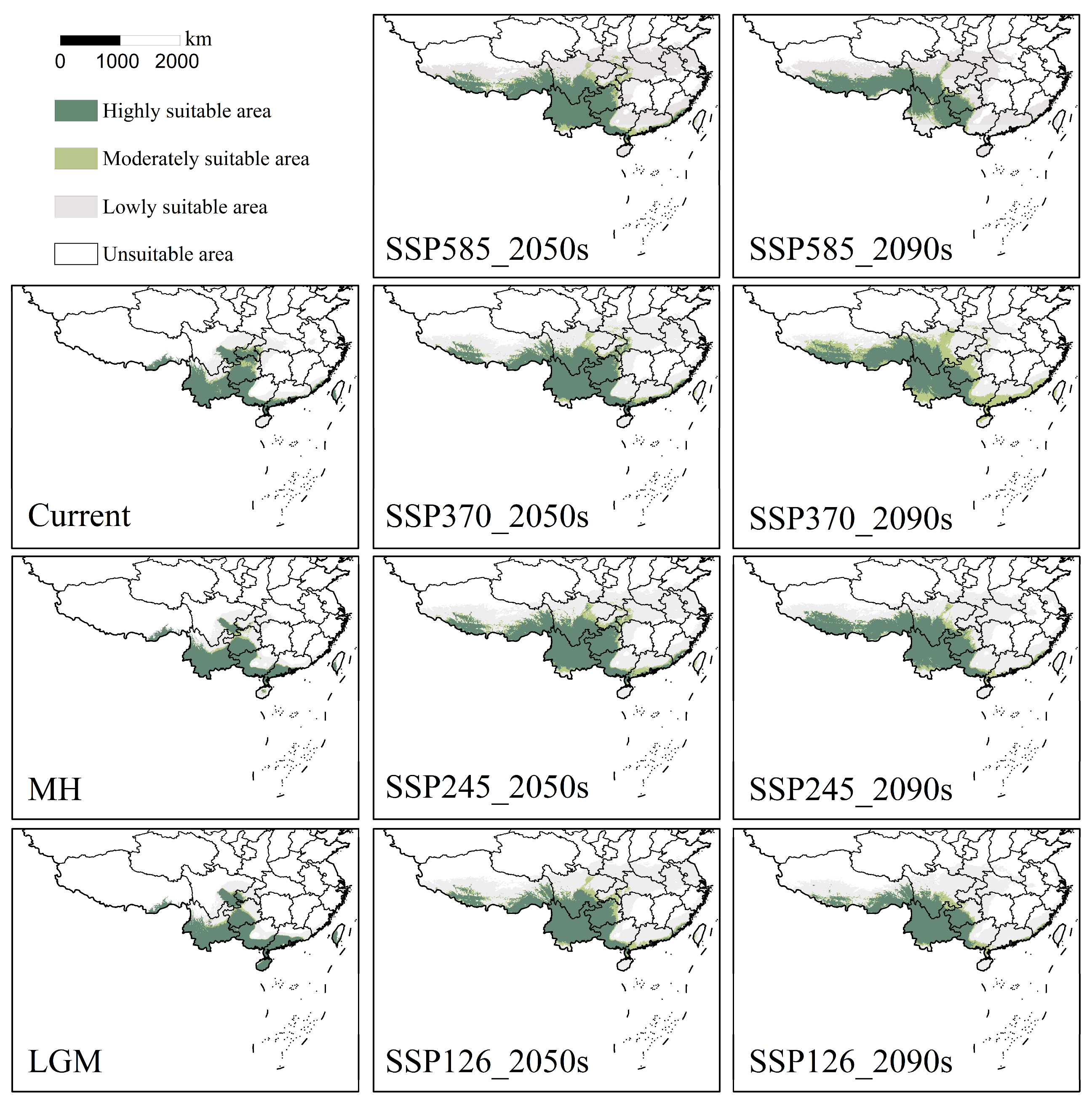
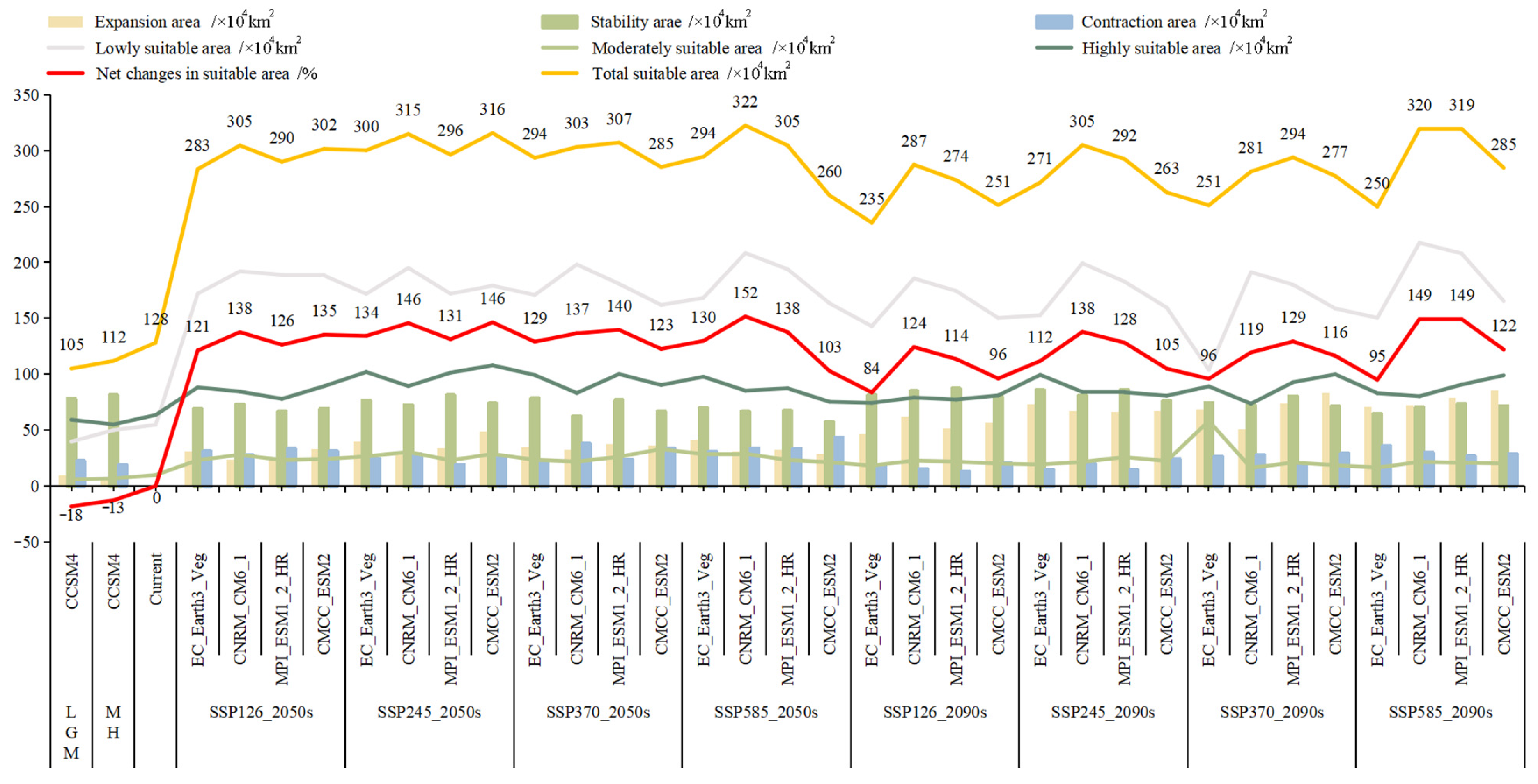
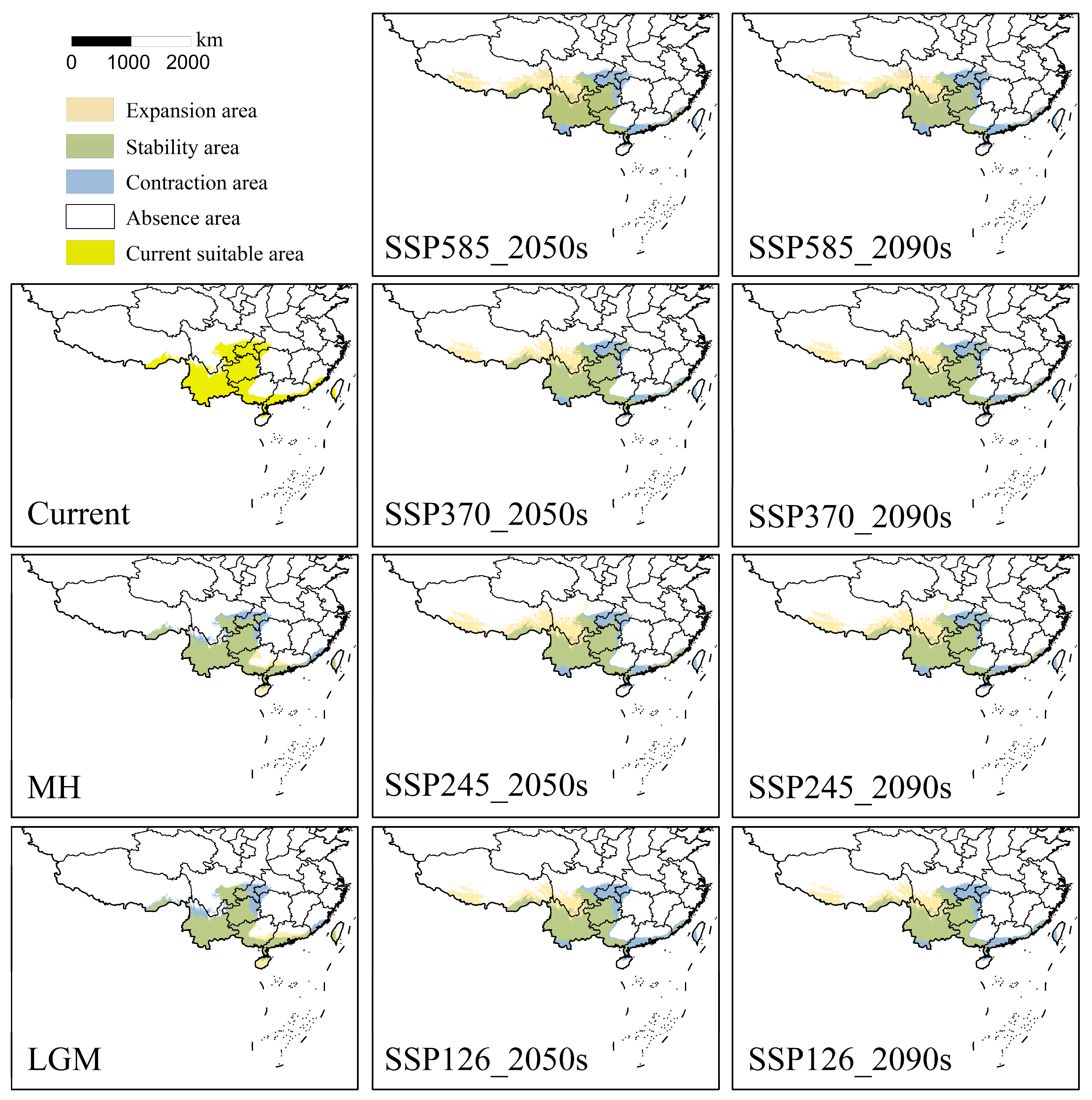
3.2. Analysis of Suitable Area Patterns
3.3. Analysis of Centroids Migration
4. Discussion
4.1. Application and Limitations of SDMs
4.2. Geographical Distribution Changes of L. pinceana
4.3. Analysis of Key Climatic Factors for L. pinceana
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Almeida, A.M.; Ribeiro, M.M.; Ferreira, M.R.; Roque, N.; Quintela-Sabarís, C.; Fernandez, P. Big data help to define climate change challenges for the typical Mediterranean species Cistus ladanifer L. Front. Ecol. Evol. 2023, 11, 1136224. [Google Scholar] [CrossRef]
- Wang, X.F.; Duan, Y.X.; Jin, L.L.; Wang, C.Y.; Peng, M.C.; Li, Y.; Wang, X.H.; Ma, Y.F. Prediction of historical, present and future distribution of Quercus sect. Heterobalanus based on the optimized MaxEnt model in China. Acta Ecol. Sin. 2023, 43, 6590–6604. [Google Scholar] [CrossRef]
- Zhang, A.P.; Wang, Y.; Xiong, Q.L.; Wu, X.G.; Sun, X.M.; Huang, Y.M.; Zhang, L.; Pan, K.W. Distribution changes and refugia of three spruce taxa since the last interglacial. Chin. J. Appl. Ecol. 2018, 29, 2411–2421. [Google Scholar] [CrossRef]
- Liu, D.T.; Chen, J.Y.; Sun, W.B. Distributional responses to climate change of two maple species in southern China. Ecol. Evol. 2023, 13, e10490. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, Z.Y.; Chen, L.D.; Peng, Y.S.; Wang, X. Changes in potential geographical distribution of Tsoongiodendron odorum since the Last Glacial Maximum. Chin. J. Plant Ecol. 2020, 44, 44–55. [Google Scholar] [CrossRef]
- Ni, J.; Yu, G.; Harrison, S.P.; Prentice, I.C. Palaeovegetation in China during the late Quaternary: Biome reconstructions based on a global scheme of plant functional types. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 289, 44–61. [Google Scholar] [CrossRef]
- Tian, Z.P.; Jiang, D.B. Mid-Holocene and last glacial maximum changes in monsoon area and precipitation over China. Chin. Sci. Bull. 2015, 60, 400–410. [Google Scholar] [CrossRef]
- Zou, X.; Peng, Y.; Wang, L.; Li, Y.; Zhang, W.X.; Liu, X. Impact of climate change on the distribution pattern of Malus baccata (L.) Borkh. in China since the Last Glacial Maximum. Plant Sci. J. 2018, 36, 676–686. [Google Scholar] [CrossRef]
- Tang, Z.H.; Liu, X.A.; Peng, P.H.; Li, X.Y. Prediction of potential geographical distribution patterns of Emmenopterys henryi since the Last Glacial Maximum. Acta Ecol. Sin. 2023, 43, 3339–3347. [Google Scholar] [CrossRef]
- Shi, X.W.; Zhang, L.; Zhang, J.J.; Ouyang, Z.Y.; Xiao, Y. Priority area of biodiversity conservation in Southwest China. Chin. J. Ecol. 2018, 37, 3721–3728. [Google Scholar] [CrossRef]
- Fraga, H.; Molitor, D.; Leolini, L.; Santos, J.A. What is the impact of heatwaves on European viticulture? A modelling assessment. Appl. Sci. 2020, 10, 3030. [Google Scholar] [CrossRef]
- Dagtekin, D.; Şahan, E.A.; Denk, T.; Köse, N.; Dalfes, H.N. Past, present and future distributions of Oriental beech (Fagus orientalis) under climate change projections. PLoS ONE 2020, 15, e0242280. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Fan, Z.F.; Zhou, B.J.; Ma, C.L.; Gao, C.; Han, D.N.; Chai, Y. Impacts of climate change on species distribution patterns of Polyspora sweet in China. Ecol. Evol. 2022, 12, e9516. [Google Scholar] [CrossRef]
- Boisvenue, C.; Running, S.W. Impacts of climate change on natural forest productivity—Evidence since the middle of the 20th century. Glob. Chang. Biol. 2006, 12, 862–882. [Google Scholar] [CrossRef]
- Wang, X.Y.; Chen, Y.; Yi, Y. Features of floral odor and nectar in the distylous Luculia pinceana (Rubiaceae) promote compatible pollination by hawkmoths. Ecol. Evol. 2023, 13, e9920. [Google Scholar] [CrossRef]
- Liu, X.X.; Ma, H.; Wan, Y.M.; Zhao, Z.G.; Li, Z.H. A new Luculia pinceana cultivar ‘Jinyudian’. Acta Hortic. Sin. 2019, 46, 199–200. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.J.; Wan, Y.M.; Liu, X.X.; Li, Z.H. Breeding of a New Luculia gratissima Cultivar ‘Xiangbo’. N. Hortic. 2021, 45, 179–180. [Google Scholar] [CrossRef]
- Ma, H.; Wan, Y.M.; Liu, X.F.; Liu, X.X.; Li, Z.H. ‘Xianghun’, a New Luculia Hybrid Cultivar. Acta Hortic. Sin. 2020, 47, 3097–3098. [Google Scholar] [CrossRef]
- Li, Y.Y.; Xu, H.Y.; Ma, H.; Li, Z.H. Temporal variation of floral scent emission of a hybrid variety of Luculia. J. Shanxi Agric. Univ. 2020, 40, 8. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wan, Y.M.; Sun, Z.H.; Li, T.Q.; Liu, X.F.; Ma, H.; Liu, X.X.; He, R.; Ma, Y.; Li, Z.H. Floral scent chemistry of Luculia yunnanensis (Rubiaceae), a species endemic to China with sweetly fragrant flowers. Molecules 2017, 22, 879. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.Y.; Zhang, L.; Song, Y.L. α-Glucosidase inhibitors from Luculia pinciana. China J. Chin. Mater. Medica 2009, 34, 406–409. [Google Scholar]
- Li, H.Y.; Li, S.F.; Li, Y.F.; Kong, L.F. Study of Preservative Technology of Fresh Cut Flowers of Luculia pinceana. J. Dali Univ. 2023, 8, 96–100. [Google Scholar] [CrossRef]
- Sharma, M.K.; Ram, B.; Chawla, A. Ensemble modelling under multiple climate change scenarios predicts reduction in highly suitable range of habitats of Dactylorhiza hatagirea (D.Don) Soo in Himachal Pradesh, western Himalaya. S. Afr. J. Bot. 2023, 154, 203–218. [Google Scholar] [CrossRef]
- Bi, B.; Chen, Q.; Chang, E.F.; Li, P.R.; Yin, A.P. Phenological observation and value of ornamentation and utilization of Luculia intermedia. Guangxi For. Sci. 2005, 34, 135–136. [Google Scholar] [CrossRef]
- Kong, L.F.; Yang, Z.Y.; Li, S.F. Effect of low temperature stress on physiological effect and resistance induction on Luculia pinceana. Mol. Plant Breed. 2022, 20, 3069–3075. [Google Scholar] [CrossRef]
- Ma, H.; Wang, Y.; Li, Z.H.; Wan, Y.M.; Liu, X.X.; Liang, N. A study on the breeding system of Luculia pinciana. For. Res. 2009, 22, 373–378. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.Y.; You, X.S.; Hu, D.M.; Yao, R.X.; Tang, X.X.; Wang, X.Y. A study of pollination accuracy in distylous Rubiaceae species (Luculia pinceana). Acta Ecol. Sin. 2021, 41, 6654–6664. [Google Scholar] [CrossRef]
- Li, Y.Y.; Ma, H.; Wan, Y.M.; Li, T.Q.; Liu, X.X.; Sun, Z.H.; Li, Z.H. Volatile organic compounds emissions from Luculia pinceana flower and its changes at different stages of flower development. Molecules 2016, 21, 531. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Q.; Xu, J. Application of integrated species distribution models in parasitic diseases prevention and control: A review. Chin. J. Schistosomiasis Control 2022, 34, 659–664. [Google Scholar] [CrossRef]
- Hutchinson, G.E. Concluding remarks. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Araújo, M.B.; Guisan, A. Five (or so) challenges for species distribution modelling. J. Biogeogr. 2006, 33, 1677–1688. [Google Scholar] [CrossRef]
- Chen, S.S.; Liu, X.; Tong, X.Y.; Guan, B.C. Prediction of Camellia edithae species distribution based on multi-model combination. Ecol. Sci. 2020, 39, 58–66. [Google Scholar] [CrossRef]
- Guo, Y.L.; Zhao, Z.F.; Qiao, H.J.; Wang, R.; Wei, H.Y.; Wang, L.K.; Gu, W.; Li, X. Challenges and development trend of species distribution model. Adv. Earth Sci. 2020, 35, 1292–1305. [Google Scholar] [CrossRef]
- Bai, J.J.; Hou, P.; Zhao, Y.H.; Xu, H.T.; Zhang, B. Research progress of species habitat suitability models and their verification. Chin. J. Ecol. 2022, 41, 1423–1432. [Google Scholar] [CrossRef]
- Zhu, G.P.; Liu, G.Q.; Bu, W.J.; Gao, Y.B. Ecological niche modeling and its applications in biodiversity conservation. Biodivers. Sci. 2013, 21, 90–98. [Google Scholar] [CrossRef]
- Han, J.L.; Williams, G.M.; Zou, Q.X.; Dong, B.N. The range and habitat suitability of François’ langur (Trachypithecus francoisi) in Mayanghe Nature Reserve, China. Environ. Sci. Pollut. Res. 2023, 30, 40952–40960. [Google Scholar] [CrossRef] [PubMed]
- Adão, F.; Campos, J.C.; Santos, J.A.; Malheiro, A.C.; Fraga, H. Relocation of bioclimatic suitability of Portuguese grapevine varieties under climate change scenarios. Front. Plant Sci. 2023, 14, 974020. [Google Scholar] [CrossRef]
- Fetene, E.; Teka, G.; Dejene, H.; Mandefro, D.; Teshome, T.; Temesgen, D.; Negussie, H.; Mulatu, T.; Jaleta, M.B.; Leta, S. Modeling the spatial distribution of Culicoides species (Diptera: Ceratopogonidae) as vectors of animal diseases in Ethiopia. Sci. Rep. 2022, 12, 12904. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.C.; Kang, B.; Fan, W.; Xu, L.L.; Zhang, S.M.; Cui, X.S.; Dai, Y. Spatio-temporal variations in the potential habitat distribution of Pacific sardine (Sardinops sagax) in the northwest Pacific Ocean. Fishes 2023, 8, 86. [Google Scholar] [CrossRef]
- Mallen-Cooper, M.; Rodríguez-Caballero, E.; Eldridge, D.J.; Weber, B.; Büdel, B.; Höhne, H.; Cornwell, W.K. Towards an understanding of future range shifts in lichens and mosses under climate change. J. Biogeogr. 2023, 50, 406–417. [Google Scholar] [CrossRef]
- Li, Y.P.; Gao, X.; An, Q.; Sun, Z.; Wang, H.B. Ecological niche modeling based on ensemble algorithms to predicting current and future potential distribution of African swine fever virus in China. Sci. Rep. 2022, 12, 15614. [Google Scholar] [CrossRef]
- Pecchi, M.; Marchi, M.; Burton, V.; Giannetti, F.; Moriondo, M.; Bernetti, I.; Bindi, M.; Chirici, G. Species distribution modelling to support forest management. A literature review. Ecol. Model. 2019, 411, 108817. [Google Scholar] [CrossRef]
- Brown, J.L.; Yoder, A.D. Shifting ranges and conservation challenges for lemurs in the face of climate change. Ecol. Evol. 2015, 5, 1131–1142. [Google Scholar] [CrossRef]
- Lv, Y.Y.; Sun, Y.; Yi, S.H.; Meng, B.P. Human activities dominant the distribution of Kobresia pygmaea community in alpine meadow grassland in the east source region of Yellow River, China. Front. Ecol. Evol. 2023, 11, 1127973. [Google Scholar] [CrossRef]
- Qiao, H.J.; Hu, J.H.; Huang, J.H. Theoretical basis, future directions, and challenges for ecological niche models. Sci. Sin. 2013, 43, 915–927. [Google Scholar] [CrossRef]
- Rana, S.K.; Rana, H.K.; Luo, D.; Sun, H. Estimating climate-induced ‘Nowhere to go’ range shifts of the Himalayan Incarvillea Juss. using multi-model median ensemble species distribution models. Ecol. Indic. 2020, 121, 107127. [Google Scholar] [CrossRef]
- Zurell, D.; Franklin, J.; König, C.; Bouchet, P.J.; Dormann, C.F.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A.; et al. A standard protocol for reporting species distribution models. Ecography 2020, 43, 1261–1277. [Google Scholar] [CrossRef]
- Xu, Y.D.; Huang, Y.; Zhao, H.R.; Yang, M.L.; Zhuang, Y.Q.; Ye, X.P. Modelling the effects of climate change on the distribution of endangered Cypripedium japonicum in China. Forests 2021, 12, 429. [Google Scholar] [CrossRef]
- Yang, X.L.; Zhou, B.T.; Xu, Y.; Han, Z.Y. CMIP6 evaluation and projection of temperature and precipitation over China. Adv. Atmos. Sci. 2021, 38, 817–830. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, Z.; Li, L. Projection of climate extremes in China, an incremental exercise from CMIP5 to CMIP6. Sci. Bull. 2021, 66, 2528–2537. [Google Scholar] [CrossRef] [PubMed]
- Döscher, R.; Acosta, M.; Alessandri, A.; Anthoni, P.; Arsouze, T.; Bergman, T.; Bernardello, R.; Boussetta, S.; Caron, L.P.; Carver, G.; et al. The EC-Earth3 Earth system model for the Coupled Model Intercomparison Project 6. Geosci. Model Dev. 2022, 15, 2973–3020. [Google Scholar] [CrossRef]
- Salinas-Ramos, V.B.; Ancillotto, L.; Cistrone, L.; Nastasi, C.; Bosso, L.; Smeraldo, S.; Sánchez Cordero, V.; Russo, D. Artificial illumination influences niche segregation in bats. Environ. Pollut. 2021, 284, 117187. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Jiang, C.; Liu, S.R.; Long, W.X.; Sun, J.X. Spatial distribution patterns in potential species richness of foraging plants for Hainan gibbons. Chin. J. Plant Ecol. 2023, 47, 491–505. [Google Scholar] [CrossRef]
- Resquin, F.; Duque-Lazo, J.; Acosta-Muñoz, C.; Rachid-Casnati, C.; Carrasco-Letelier, L.; Navarro-Cerrillo, R.M. Modelling current and future potential habitats for plantations of Eucalyptus grandis Hill ex Maiden and E. dunnii Maiden in Uruguay. Forests 2020, 11, 948. [Google Scholar] [CrossRef]
- Zhao, G.H.; Cui, X.Y.; Sun, J.J.; Li, T.T.; Wang, Q.; Ye, X.Z.; Fan, B.G. Analysis of the distribution pattern of Chinese Ziziphus jujuba under climate change based on optimized Biomod2 and MaxEnt models. Ecol. Indic. 2021, 132, 108256. [Google Scholar] [CrossRef]
- Chakraborty, D.; Móricz, N.; Rasztovits, E.; Dobor, L.; Schueler, S. Provisioning forest and conservation science with high-resolution maps of potential distribution of major European tree species under climate change. Ann. For. Sci. 2021, 78, 26. [Google Scholar] [CrossRef]
- López-Tirado, J.; Moreno-García, M.; Romera-Romera, D.; Zarco, V.; Hidalgo, P.J. Forecasting the circum-Mediterranean firs (Abies spp., Pinaceae) distribution, an assessment of a threatened conifers’ group facing climate change in the twenty-first century. New For. 2024, 55, 143–156. [Google Scholar] [CrossRef]
- Zhao, Z.F.; Wei, H.Y.; Guo, Y.L.; Luan, W.F.; Zhao, Z.B. Impact of climate change on the suitable habitat distribution of Gymnocarpos przewalskii, a relict plant. J. Desert Res. 2020, 40, 125–133. [Google Scholar] [CrossRef]
- Igawa, T.K.; Toledo, P.M.; Anjos, L.J.S. Climate change could reduce and spatially reconfigure cocoa cultivation in the Brazilian Amazon by 2050. PLoS ONE 2022, 17, e0262729. [Google Scholar] [CrossRef]
- Rana, S.K.; Rana, H.K.; Stöcklin, J.; Ranjitkar, S.; Sun, H.; Song, B. Global warming pushes the distribution range of the two alpine ‘glasshouse’ Rheum species north- and upwards in the Eastern Himalayas and the Hengduan Mountains. Front. Plant Sci. 2022, 13, 925296. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Xu, X.T. Effects of climate change on the distribution of wild population of Metasequoia glyptostroboides, an endangered and endemic species in China. Chin. J. Ecol. 2019, 38, 1629–1636. [Google Scholar] [CrossRef]
- Oduor, A.M.O.; Yang, B.F.; Li, J.M. Alien ornamental plant species cultivated in Taizhou, southeastern China, may experience greater range expansions than native species under future climates. Glob. Ecol. Conserv. 2023, 41, e02371. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, J.E.; Jiang, Y.P.; Wang, R.L.; Wu, R.S. Predicting the potential distribution of Pseudomonas syringae pv. actinidiae in China using ensemble models. Plant Pathol. 2020, 69, 120–131. [Google Scholar] [CrossRef]
- Wang, Y.G.; Zhang, B.R.; Zhao, R. Influence of species interaction on species distribution simulation and modeling methods. Chin. J. Appl. Ecol. 2022, 33, 837–843. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Guo, K.Q.; Jiang, X.L.; Xu, G.B. Potential suitable distribution area of Quercus lamellosa and the influence of climate change. Chin. J. Ecol. 2021, 40, 2563–2574. [Google Scholar] [CrossRef]
- Mainali, K.P.; Warren, D.L.; Dhileepan, K.; McConnachie, A.; Strathie, L.; Hassan, G.; Karki, D.; Shrestha, B.B.; Parmesan, C. Projecting future expansion of invasive species: Comparing and improving methodologies for species distribution modeling. Glob. Chang. Biol. 2015, 21, 4464–4480. [Google Scholar] [CrossRef] [PubMed]
- Landman, W. Climate change 2007: The physical science basis. S. Afr. Geogr. J. 2010, 92, 86–87. [Google Scholar] [CrossRef]
- Ye, J.W.; Zhang, Y.; Wang, X.J. Phylogeographic history of broad-leaved forest plants in subtropical China. Acta Ecol. Sin. 2017, 37, 5894–5904. [Google Scholar] [CrossRef]
- Zhou, W.; Barrett, S.C.H.; Wang, H.; Li, D.Z. Loss of floral polymorphism in heterostylous Luculia pinceana (Rubiaceae): A molecular phylogeographic perspective. Mol. Ecol. 2012, 21, 4631–4645. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.R.; Jouzel, J.; Raynaud, D.; Barkov, N.I.; Barnola, J.M.; Basile, I.; Bender, M.; Chappellaz, J.; Davis, M.; Delaygue, G.; et al. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature 1999, 399, 429–436. [Google Scholar] [CrossRef]
- Zhang, X.J.; Gao, X.M.; Ji, C.J.; Kang, M.Y.; Wang, R.Y.; Yue, M.; Zhang, F.; Tang, Z.Y. Response of abundance distribution of five species of Quercus to climate change in northern China. Chin. J. Plant Ecol. 2019, 43, 774–782. [Google Scholar] [CrossRef]
- Zhou, R.; Ci, X.Q.; Xiao, J.H.; Cao, G.L.; Li, J. Effects and conservation assessment of climate change on the dominant group—The genus Cinnamomum of subtropical evergreen broad-leaved forests. Biodivers. Sci. 2021, 29, 697–711. [Google Scholar] [CrossRef]
- Ma, D.X.; Yin, X.J.; Zhou, S.Y.; Lu, S.F.; Chen, Z. Pattern of main tree species richness in Southwest China based on Chinese vegetation map. J. Fujian Agric. For. Univ. 2022, 51, 249–257. [Google Scholar] [CrossRef]



| Variable | Description | Unit |
|---|---|---|
| Bio1 | Mean annual air temperature | °C |
| Bio2 | Mean diurnal range | °C |
| Bio3 | Isothermality (bio3 = (bio1/bio7) × 100) | - |
| Bio4 | Variation in temperature seasonlity | - |
| Bio5 | Maximum temperature of warmest month | °C |
| Bio6 | Minimum temperature of coldest month | °C |
| Bio7 | Temperature annual range | °C |
| Bio8 | Mean temperature of wettest quarter | °C |
| Bio9 | Mean temperature of driest quarter | °C |
| Bio10 | Mean temperature of warmest quarter | °C |
| Bio11 | Mean temperature of coldest quarter | °C |
| Bio12 | Mean annual precipitation | mm |
| Bio13 | Precipitation of wettest month | mm |
| Bio14 | Precipitation of the driest month | mm |
| Bio15 | Variation of precipitation seasonlity | - |
| Bio16 | Precipitation of wettest quarter | mm |
| Bio17 | Precipitation of driest quarter | mm |
| Bio18 | Precipitation of warmest quarter | mm |
| Bio19 | Precipitation of coldest quarter | mm |
| Model | TSS > 0.80 | AUC > 0.95 | Kappa Coefficient |
|---|---|---|---|
| Generalized Linear Models (GLMs) | 0.902 | 0.970 | 0.805 |
| Generalized Boosted Models (GBMs) | 0.906 | 0.979 | 0.808 |
| Generalized Additive Models (GAMs) | 0.775 | 0.890 | 0.487 |
| Classification Tree Analysis (CTA) | 0.842 | 0.899 | 0.723 |
| Artificial Neural Network (ANN) | 0.786 | 0.897 | 0.571 |
| one Rectilinear Envelope Similar to BIOCLIM (SRE) | 0.700 | 0.850 | 0.707 |
| Flexible Discriminant Analysis (FDA) | 0.894 | 0.967 | 0.820 |
| Multivariate Adaptive Regression Splines (MARSs) | 0.888 | 0.958 | 0.779 |
| Random Forest (RF) | 0.904 | 0.980 | 0.831 |
| Maximum Entropy Models (MaxEnt) | 0.724 | 0.862 | 0.731 |
| Optimaled Maximum Entropy Models (MaxEnt.2) | 0.903 | 0.977 | 0.788 |
| Full Ensemble Model (FEM) | 0.915 | 0.982 | 0.802 |
| Optimal Ensemble Model (OEM) | 0.919 | 0.982 | 0.822 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Guo, S.; Ma, C.; Yang, J.; Kang, X.; Li, R. Impact of Climate Change on the Potential Geographical Distribution Patterns of Luculia pinceana Hook. f. since the Last Glacial Maximum. Forests 2024, 15, 253. https://doi.org/10.3390/f15020253
Gao C, Guo S, Ma C, Yang J, Kang X, Li R. Impact of Climate Change on the Potential Geographical Distribution Patterns of Luculia pinceana Hook. f. since the Last Glacial Maximum. Forests. 2024; 15(2):253. https://doi.org/10.3390/f15020253
Chicago/Turabian StyleGao, Can, Shuailong Guo, Changle Ma, Jianxin Yang, Xinling Kang, and Rui Li. 2024. "Impact of Climate Change on the Potential Geographical Distribution Patterns of Luculia pinceana Hook. f. since the Last Glacial Maximum" Forests 15, no. 2: 253. https://doi.org/10.3390/f15020253





